Open Access Article
Open Access ArticleTemperature sensitive hydrogels cross-linked by magnetic LAPONITE® RD®: effects of particle magnetization†
Nikolai I.
Lebovka
 *,
Yurii M.
Samchenko
,
Liudmyla O.
Kernosenko
,
Tatiana P.
Poltoratska
,
Natalia O.
Pasmurtseva
,
Igor E.
Mamyshev
and
Vladimir A.
Gigiberiya
*,
Yurii M.
Samchenko
,
Liudmyla O.
Kernosenko
,
Tatiana P.
Poltoratska
,
Natalia O.
Pasmurtseva
,
Igor E.
Mamyshev
and
Vladimir A.
Gigiberiya
Institute of Biocolloidal Chemistry named after F. D. Ovcharenko, NAS of Ukraine, 42, blvr. Vernadskogo, Kyiv 03142, Ukraine. E-mail: lebovka@gmail.com
First published on 24th October 2020
Abstract
This work discusses the synthesis and the properties of magnetite modified LAPONITE® RD platelets (Lap). Magnetized Lap (mLap) nanoparticles were synthesized by a co-precipitation method with different weight ratios, X = Fe3O4/Lap (0–2). For characterization of the samples the particle size distributions and sedimentation behavior in an external magnetic field were studied. Temperature sensible hydrogels based on N-isopropylacrylamide cross-linked by mLap were synthesized. An increased aggregation of mLap particles in aqueous suspensions has been revealed, but all the systems demonstrated high sedimentation stability. Significant effects of the value of X on the rate of sedimentation mLap particles in magnetic fields and on the swelling ability of hydrogels have been revealed. For example at X = 2, an increase in swelling by ≈2.7 was observed as compared with the swelling of hydrogels based on pure Lap nanoparticles.
Introduction
Polymeric hydrogels are composed of hydrophilic polymer networks filled with large amounts of water (it can be more than 90%). Due to their ability to absorb significant amounts of water and biological fluids, softness, porosity and elasticity, hydrogels resemble the tissues of the human body more than any other synthetic biomaterial.1,2 In recent decades, due to their high biocompatibility, polymeric hydrogels have been successfully used in medicine and pharmaceutical industries.3–5 They are considered as intelligent materials6 for manufacturing of soft contact lenses,7 devices for targeted delivery and sustained release of drugs,8 biosensors,9 anti-burn and hemostatic dressings,10 materials for tissue engineering and plastic surgery.11Nowadays great attention has been paid to polymeric hydrogels with stimuli responsiveness (pH, ionic strength, temperature, light, electric, magnetic fields, etc.).12–14 For example, pH-sensitive hydrogels containing carboxylic or amine functional groups can be used for controlled release of selectively adsorbed drugs.15
The very popular are hydrogels based on acrylic monomers cross-linked using different chemical linkers.16,17 At room temperatures these temperature sensible hydrogels absorb a considerable amount of water and have an expanded hydrated conformation. However, under heating above ≈305 K they demonstrate transition into a compact dehydrated state.18,19 The transition temperature is close to the temperature of the human body and can be shifted to higher or lower temperatures by copolymerization with hydrophilic or hydrophobic monomers, respectively.19 This behaviour can be used to create a thermoregulatory drug delivery system, in particular photo-sensitizers.20 The main disadvantages of such hydrogels are related to their low optical transparency, mechanical strength, low response rate and swelling ability in water.
These disadvantages can be avoided using the additive of platelets of LAPONITE® (Lap).21 Hydrogels cross-linked by Lap nanoparticles have exhibited extraordinary mechanical toughness, tensile moduli and tensile strengths22 that were almost proportional to the platelet content. Such hydrogels have great potential for the fabrication of flexible pressure and strain sensors.23 The shape anisotropy of Lap nanoparticles and heterogeneity of surface charge distribution can lead to complex self-assembly and networking inside such systems.24 Lap-doped hydrogels based on acrylamide,25 acrylic acid,26N-isopropylacrylamide,27 and dimethyl-acrylamide28 have been already synthesized. As examples, Lap was used as an effective cross-linker for preparing the poly(N,N-dimethylaminoethyl methacrylate) based pH and temperature double-sensitive hydrogels with homogeneous distribution of the cross-link points along the gels.29 Lap has been used as a cross-linker for preparation of stimuli-responsive hydrogels and cryogels based on poly(N,N-dimethylaminoethyl methacrylate-co-2-acrylamido-2-methyl propanosulfonic acid) copolymeric networks.30 Lap based hydrogels demonstrated excellent mechanical strength that could be adjusted by controlling the content of Lap. Thermoresponsive hydrogel bioinks based on block copolymer poly(2-methyl-2-oxazoline)-b-poly(2-n-propyl-2-oxazine) and Lap were recently prepared.31 The materials demonstrated improved thermogelling and rheological properties beneficial for extrusion-based 3D printing.
Particularly, temperature sensitive hydrogels based on poly(N-isopropylacrylamide) (PNIPAAm) attract great attention as they exhibit a reversible volume phase transition at T ≈ 34 °C close to the physiological temperature range.32 The cross-linking of PNIPAAm by Lap allowed the preparation of hydrogels with high transparency, and excellent swelling and mechanical properties compared to chemically cross-linked hydrogels.33 The strong interaction between silicate platelets and PNIPAAm chains was explained by a combination of ionic interactions and hydrogen bonding.28 Recently, a technique for the preparation of anisotropic thermoresponsive hydrogels based on PNIPAAm cross-linked by Lap has been reported.34 The hydrogels demonstrated anisotropic mechanical performance and could be deformed anisotropically in response to a temperature change. The content of Lap incorporated in PNIPAAm can affect the storage modulus, phase transition temperature and swelling/deswelling rates.35
Application of magnetized Lap particles can improve the functionality of hydrogels. Different types of magnetite-covered clay nanoparticles e.g., Fe3O4-montmorillonite,36 Fe3O4-rectorite,37 and Fe3O4-Lap38 were synthesized. Magnetic interactions are very effective in controlling the spatial distribution of colloidal particles39 and, unlike electrostatic interactions, they can be carried out without direct contact between the medium and the field. The use of a magnetic field allows applying a number of strategies with various advantages to enhance the functional properties of hydrogels.40 Recently, temperature sensitive hydrogels based on PNIPAAm cross-linked by magnetically modified Lap (mLap) nanoparticles have been synthesized.41 The weight ratio of Fe3O4 and Lap was fixed at X = 1. The structure of mLap was characterized by scanning electron microscopy and infrared spectroscopy. However, detailed studies on the effects of magnetization Lap platelets (value of X) and properties of temperature sensitive hydrogels supported by magnetized Lap platelets have not yet been performed.
This work discusses the synthesis and properties of magnetic Lap platelets (mLap) with different feed weight ratios X = Fe3O4/Lap in the interval between 0 and 2. For characterization of the samples, particle size distribution and sedimentation of magnetic particles in an external magnetic field applied in a vertical direction were studied. The temperature-sensitive hydrogels based on poly(N-isopropylacrylamide) (PNIPAAm) physically cross-linked by mLap were synthesized. Their swelling was also discussed.
Materials and methods
Materials
LAPONITE® RD (Lap, Rockwood, Clay additives GmbH) is a synthetic clay of the general formula Si8−Mg5.45Li0.4H4O24Na0.7. Lap is composed of disk-shaped crystals with a thickness of h ≈ 1 nm (0.92 nm) and diameter of d ≈ 25 nm. It has density of ρ = 2.53 g cm−3 and its specific area can be theoretically estimated to be42S = 2/(ρh) ≈ 430 m2 g−1. In aqueous media, Lap has a tendency to degrade (dissolve), particularly under acidic conditions.43–46 Increase in pH, and concentration of Lap as well as that of salt resulted in a stabilizing effect against degradation.46 The faces are charged negatively and at pH 10, the edge surface is weakly positive.Iron(II) sulfate heptahydrate FeSO4·7H2O (Merck, 99%), iron(III) chloride FeCl3 (Merck, 98%), ammonium NH3·H2O (Merck, 25%), tetrasodium pyrophosphate Na4P2O7 (TSPP, Merck, 95%), ammonium persulfate (NH4)2S2O8, (APS, Sigma, 98%), N,N,N′,N′-tetramethylenediamine, (TEMED, Merck, 99%), and tetrasodium pyrophosphate (TSPP, Merck) were used as received without further purification. N-Isopropylacrylamide, NIPAAm, (Sigma-Aldrich, 97%) was recrystallized from hexane and dried under vacuum. More details on the chemicals used in this paper are presented in ESI,† Table S1.
Synthesis procedures
Initially, Lap was dispersed in distilled water degassed by N2 at 20 °C. The concentration of Lap was 1.5 wt% (hereinafter %). The suspension was carefully mixed for 10 min using an ultrasound clean bath operating at 25 kHz with a power of 140 W (Nitecore NFF01, China). A careful stirring procedure was applied for good exfoliation of Lap tacktoids in water.24,48 Then, the temperature was increased up to 70 °C, the solutions containing certain amounts of FeSO4·7H2O and FeCl3 of distilled water were added to the suspension of Lap, and the mixtures were stirred at 250 rpm by using a magnetic stirrer (Heidolp MR 3004 S) in an N2 atmosphere for 10 min. Ammonia solution (NH3H2O) was added drop-wise to prepare iron oxides. The pH of the final mixture was about 10. The obtained mixtures were aged at 70 °C for 2 hours. Finally, the mLap particles were separated from the aqueous suspension using a magnet. After magnetic separation the suspensions of mLap particles (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were washed several times with distilled water. Commonly after the first three washings the suspension pH attained the neutral value pH 7. Then the samples were dried at room temperature until the final moisture content of ≤1% and the obtained mLap particles were used for the preparation of thermosensitive NIPAA-based hydrogels.
1) were washed several times with distilled water. Commonly after the first three washings the suspension pH attained the neutral value pH 7. Then the samples were dried at room temperature until the final moisture content of ≤1% and the obtained mLap particles were used for the preparation of thermosensitive NIPAA-based hydrogels.
The mLap synthesis procedure is schematically presented in Fig. 1a. It can be assumed that because of the high ion exchange capacity of Lap, the iron ions are adsorbed on Lap surfaces. It can result in the formation of nanomagnetite spots on the surface of Lap (Fig. 1a). However, formation of some quantity of Fe3O4 nanoparticles in partially exfoliated galleries of Lap and individual Fe3O4 nanoparticles cannot be excluded.
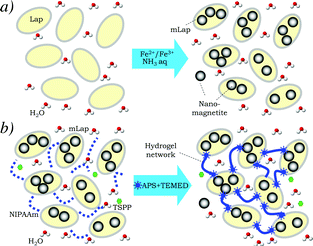 | ||
| Fig. 1 Preparation schemes of magnetic Lap (mLap) nanoparticles (a) and temperature-sensitive hydrogels based on poly(N-isopropylacrylamide) (PNIPAAm) physically cross-linked by mLap (b). Structural formulae of NIIPAAm, TSPP, APS and TEMED are presented in ESI,† Table S1. | ||
The interactions between Fe3O4 and Lap species in magnetized mLap samples were supported by a noticeable shift of the Fe–O vibration band of magnetite in FTIR spectra and changes in TGA traces (Fig. S1 and S2 in ESI†).
The previous SEM studies of Fe3O4 + Lap composites in powder state revealed the presence of spherical particles of 5–10 nm in size attributed to the formed structure of magnetite in magnetic composites.41
The sedimentation stability of mLap aqueous suspensions in the absence and presence of external magnetic fields was evaluated by the spectrophotometric method using a photoelectrocolorimeter KFK-2MP (Zagorsky Optical-Mechanical Plant, Russia) at a wavelength of 540 nm. The magnetic field was induced by a AlNiCo500 magnet (50 × 25 × 8 mm, residual flux density 1210 mT, Poland) placed at the bottom of the cuvette. The time dependence of optical transmission, Tr(t), was measured.
The swelling ability S of hydrogels, defined as S = ms/md − 1 (here, ms, and md are the masses of the swollen (after 24 hours of swelling) and dry hydrogels, respectively) was studied in distilled water by the gravimetric method. The samples were weighed with an accuracy up to 10−4 g using an analytical balance “Axis” (Poland).
All measurements were conducted at approximately the same value, pH ≈ 6.
Results and discussion
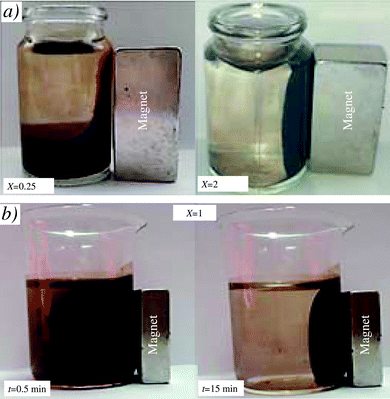
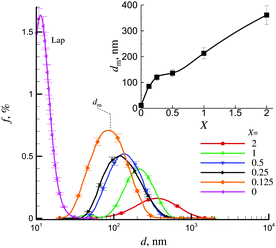
Fig. 2 shows different examples of the separation of mLap aqueous suspensions by interaction with a magnet. The preliminary studies have shown that there exists a certain critical threshold of X (≈0.05) above which the effects of magnet on the separation of the suspension become observable. However, for X ≥ 0.125 after relatively long time (t ≈ 15 min), practically all mLap particles can be separated from the aqueous suspension using a magnet.Fig. 3 shows the examples of particle size distribution functions f(d) for samples with different contents of magnetite X (Fe3O4/Lap). For the highest content of magnetite (X = 2), the largest aggregates of mLap nanoparticles were formed with the peak of f(d) function located at dm ≈ 361 nm. A decrease in X resulted in a decrease in dm and finally, for the smallest content of magnetite (X = 0.125) the peak of f(d) function was located at dm ≈ 85 nm. It corresponded to the formation of aggregates of several mLap nanoparticles. Inset in Fig. 3 shows the dm(X) dependence.
Therefore, the coverage of Lap particles by nanomagnetite can cause formation of relatively large aggregates of magnetic particles. Moreover, the heterocoagulation between individual Lap platelets and magnetite can also result in the formation of large aggregates. This heterocoagulation may be supported by differences in surfaces charges of Lap49 and nanomagnetite50 particles. Such heterocoagulation was observed in the sedimentation behavior in magnetic fields for mechanical mixtures of nanometric magnetite and micron-sized sodium montmorillonite particles.36
Nevertheless all these aggregates in mLap suspensions were relatively small and overall, the suspensions preserved their sedimentation stability owing to the thermal energy.
The magnetic properties of the magnetized mLap nanoparticles were investigated indirectly using sedimentation analysis. The effects of a magnetic field on sedimentation stability can be illustrated by changes in the optical transmission of the suspension. Similar experiments were performed for mixtures of nanomagnetite and sodium montmorillonite.36
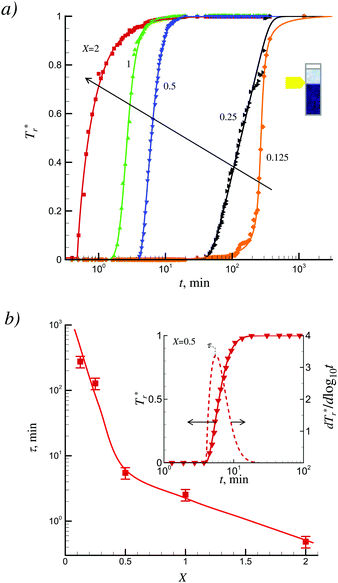
Examples of time dependences of the normalized optical transmission Tr*(t) = Tr(t)/Tmr (here Tmr is a maximum (saturation) transmission at long time of sedimentation) for different X are presented in Fig. 4a. The presented dependencies evidenced the existence of a long delay time before starting sedimentation. The derivative dTr*(t)/d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t can be used for the calculation of the characteristic sedimentation time τ (Fig. 4b).
t can be used for the calculation of the characteristic sedimentation time τ (Fig. 4b).
Note that in the absence of a magnetic field, the suspensions of mLap were rather stable and very slow sedimentation for several days was observed. In the presence of a magnetic field the sedimentation was more accelerated at high values of X. For example, the characteristic sedimentation time was ≈4.6 hours at X = 0.125 and decreased up to ≈30 s at X = 2.
Such a significant effect of the magnetic field on sedimentation can reflect a direct effect of magnetic field on the magnetic particles and formation of big flocculi in the suspension due to the dipole–dipole magnetic attractions between magnetite-covered clay platelets.36 The noticeable retardation in sedimentation for X below 0.5 (Fig. 4b) can reflect the high stability of suspensions for small-sized aggregates (<100 nm, Fig. 3) and weakening of the magnetic field effects on the deposition processes.
Stimuli-sensitive hydrogels (e.g., chemical, pH and temperature sensitive) have attracted considerable attention in the pharmaceutical field due to the possibility of their applications as a biosensor and in the drug release process.3,51 Physical cross-linking of hydrogels by magnetically modified Lap platelets can open new perspectives for supplementary physical stimuli in these promising applications.
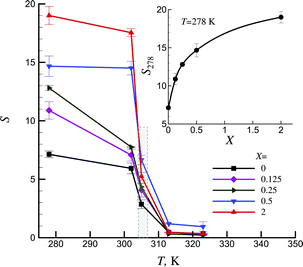
The studied physically cross-linked by Lap platelet hydrogel is negative temperature-sensitive;52 it has a lower critical solution temperature and contracts upon heating in the range of 304–308 K (dashed area in Fig. 5). It reflects the ability of the system to exhibit hydrophilic nature at low temperatures and hydrophobic nature above some critical temperatures. Such hydrogels can be used for temperature-modulated drug release by bulk squeezing and surface regulation.53 Magnetic modification of Lap significantly affected the hydrophilic properties of NIPAA-based temperature sensitive hydrogels. It should be noted that magnetic modification practically has no effect on the transition temperature.
Inset in Fig. 5 shows that magnetization of Lap led to an increase in the swelling ability in the low temperature range. For example, at T = 278 K and X = 2 an increase by ≈2.7 was observed as compared with the swelling ability of hydrogels based on pure Lap nanoparticles. Therefore, the degree of magnetization of Lap particles can significantly affect the hydrophilic properties of the hydrogel.
The swelling behavior of magnetic hydrogels can reflect the mechanism of hydrogel formation and nature of magnetic nanoparticles. For example, in magnetic alginate hydrogels (consisting of magnetic iron particles embedded within the alginate network), prepared by a special two-step protocol, the swelling capacity was not affected by the presence or content of the magnetic particles.54 This was explained by the insignificant effects of magnetic particles on the porosity of the hydrogels. Similar insignificant effects of magnetic particles on the swelling ability of poly(2-oxazoline)-based magnetic hydrogels were also observed.55 However, the mechanism of Lap magnetization on the swelling of magnetic hydrogels is not completely clear. It can be speculated that the observed effects can reflect the significant effects of Lap magnetisation on the structure and porosity of hydrogels.
Note that the physical cross-linking supported by silicate platelets enhance the mechanical properties of the hydrogels.21 Covering Lap by nanomagnetite and formation of aggregates of mLap can affect the rigidity of the polymeric chains without breakdown of the total networks. Therefore, more thorough studies are desirable in future to elucidate the effects of mLap on mechanical and rheological properties of magnetic hydrogels.
Conclusions
Hybrid magnetic particles based on the platelets of Lap (mLap particles) covered by magnetite with different weight ratios X = Fe3O4/Lap (X = 0–2) have been prepared and used for the synthesis of the temperature-sensitive hydrogels (PNIPAAm) physically cross-linked by mLap. The analysis of the particle size distributions revealed an increased aggregation of mLap particles in aqueous suspensions, but all the systems demonstrated high sedimentation stability. Sedimentation behavior in magnetic fields of magnetite-covered Lap particles evidenced the presence of a significant effect of the value of X on the rate of sedimentation. Moreover, temperature sensitive hydrogels cross-linked by mLap particles demonstrated the presence of a significant increase in the swelling ability. For example at X = 2 an increase by ≈2.7 was observed as compared with the swelling ability of hydrogels based on pure Lap nanoparticles.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding from the National Academy of Sciences of Ukraine, Projects 7/9/3-f-4-1230-2020 #0120U100226, # 0120U102372/20-N, and #0120U102343/20-N.Notes and references
- Q. Chai, Y. Jiao and X. Yu, Gels, 2017, 3, 6 CrossRef.
- J. Fu and M. in het Panhuis, J. Mater. Chem. B, 2019, 7, 1523–1525 RSC.
- N. A. Peppas, Hydrogels in medicine and pharmacy: fundamentals, CRC press, 2019, vol. 1 Search PubMed.
- S. Rafieian, H. Mirzadeh, H. Mahdavi and M. E. Masoumi, Sci. Eng. Compos. Mater., 2019, 26, 154–174 CAS.
- H. Chang, C. Li, R. Huang, R. Su, W. Qi and Z. He, J. Mater. Chem. B, 2019, 7, 2899–2910 RSC.
- M. Mahinroosta, Z. J. Farsangi, A. Allahverdi and Z. Shakoori, Mater. today Chem., 2018, 8, 42–55 CrossRef CAS.
- O. Wichterle and D. Lim, Nature, 1960, 185, 117–118 CrossRef.
- R. Narayanaswamy and V. P. Torchilin, Molecules, 2019, 24, 603 CrossRef.
- X. Yu, Y. Jiao and Q. Chai, Nano LIFE, 2016, 6, 1642001 CrossRef CAS.
- R. Z. Murray, Z. E. West, A. J. Cowin and B. L. Farrugia, Burn. trauma, 2019, 7, 2 Search PubMed.
- J. M. Shapiro and M. L. Oyen, JOM, 2013, 65, 505–516 CrossRef CAS.
- S. Gao, G. Tang, D. Hua, R. Xiong, J. Han, S. Jiang, Q. Zhang and C. Huang, J. Mater. Chem. B, 2019, 7, 709–729 RSC.
- B. S. Gomes, P. M. Mendes and Q. Li, in Functional Organic and Hybrid Nanostructured Materials, ed. Q. Li, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2018, pp. 203–243 Search PubMed.
- J. Hoque, N. Sangaj and S. Varghese, Macromol. Biosci., 2019, 19, 1800259 CrossRef.
- R. Kouser, A. Vashist, M. Zafaryab, M. A. Rizvi and S. Ahmad, ACS Appl. Bio Mater., 2018, 1, 1810–1822 CrossRef CAS.
- N. Shah and K. Patel, J. Pharm. Sci. Biosci. Res., 2014, 4, 114–120 Search PubMed.
- D. Qureshi, S. K. Nayak, S. Maji, A. Anis, D. Kim and K. Pal, Eur. Polym. J., 2019, 120, 109220 CrossRef CAS.
- N. A. Platé, T. L. Lebedeva and L. I. Valuev, Polym. J., 1999, 31, 21 CrossRef.
- U. G. Spizzirri, I. Altimari, F. Puoci, O. I. Parisi, F. Iemma and N. Picci, Carbohydr. Polym., 2011, 84, 517–523 CrossRef CAS.
- Y. Samchenko, G. Dolinsky, N. Pasmurtseva, T. Poltoratskaya, Z. Ulberg and M. Gamalia, Nauk. Sci. Notes. - Chem. Sci. Technol., 2015, 170, 34–39 Search PubMed.
- K. Haraguchi, T. Takehisa and S. Fan, Macromolecules, 2002, 35, 10162–10171 CrossRef CAS.
- J. Yang, S. Liu, Y. Xiao, G. Gao, Y. Sun, Q. Guo, J. Wu and J. Fu, J. Mater. Chem. B, 2016, 4, 1733–1739 RSC.
- Z. Wang, Y. Cong and J. Fu, J. Mater. Chem. B, 2020, 8, 3437–3459 RSC.
- N. Lebovka, L. Lisetski and L. A. Bulavin, in Modern Problems of the Physics of Liquid Systems, ed. L. Bulavin and L. Xu, Springer Nature, Cham, Switzerland AG, 2019, pp. 137–164 Search PubMed.
- O. Okay and W. Oppermann, Macromolecules, 2007, 40, 3378–3387 CrossRef CAS.
- H. Li, R. Gu, S. Xu, A. Abudurman and J. Wang, Appl. Clay Sci., 2014, 101, 335–338 CrossRef CAS.
- M. Shibayama, J. Suda, T. Karino, S. Okabe, T. Takehisa and K. Haraguchi, Macromolecules, 2004, 37, 9606–9612 CrossRef CAS.
- K. Haraguchi, R. Farnworth, A. Ohbayashi and T. Takehisa, Macromolecules, 2003, 36, 5732–5741 CrossRef CAS.
- Y. Chen, W. Xu, Y. Xiong, C. Peng, W. Liu, G. Zeng and Y. Peng, J. Mater. Res., 2013, 28, 1394–1404 CrossRef CAS.
- T. Boyaci and N. Orakdogen, Appl. Clay Sci., 2016, 121, 162–173 CrossRef.
- C. Hu, L. Hahn, M. Yang, A. Altmann, P. Stahlhut, J. Groll and R. Luxenhofer, J. Mater. Sci., 2020, 1–15 Search PubMed.
- K. László, K. Kosik and E. Geissler, Macromolecules, 2004, 37, 10067–10072 CrossRef.
- S. S. Das, K. Hussain, S. Singh, A. Hussain, A. Faruk and M. Tebyetekerwa, et al. , Curr. Pharm. Des., 2019, 25, 424–443 CrossRef CAS.
- L. Chen, Q. Wu, J. Zhang, T. Zhao, X. Jin and M. Liu, Polymer, 2020, 192, 122309 CrossRef.
- I. Mirković, M. S. Nikolić, S. Ostojić, J. Maletaškić, Z. Petrović and J. Djonlagić, J. Serb. Chem. Soc., 2020, 85(9), 1197–1221 CrossRef.
- C. Galindo-Gonzalez, J. De Vicente, M. M. Ramos-Tejada, M. T. Lopez-Lopez, F. Gonzalez-Caballero and J. D. G. Duran, Langmuir, 2005, 21, 4410–4419 CrossRef CAS.
- D. Wu, P. Zheng, P. R. Chang and X. Ma, Chem. Eng. J., 2011, 174, 489–494 CrossRef CAS.
- G. R. Mahdavinia, Z. Rahmani, A. Mosallanezhad, S. Karami and M. Shahriari, Desalin. Water Treat., 2016, 57, 20582–20596 CrossRef CAS.
- K. Komaee, B. Yellen, G. Friedman and N. Dan, J. Colloid Interface Sci., 2006, 297, 407–411 CrossRef CAS.
- W. Shi, J. Huang, R. Fang and M. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 5177–5194 CrossRef CAS.
- O. Goncharuk, Y. Samchenko, D. Sternik, L. Kernosenko, T. Poltorats’ka, N. Pasmurtseva, M. Abramov, E. Pakhlov and A. Derylo-Marczewska, Appl. Nanosci., 2020 DOI:10.1007/s13204-020-01388-w.
- E. S. H. Leach, A. Hopkinson, K. Franklin and J. S. van Duijneveldt, Langmuir, 2005, 21, 3821–3830 CrossRef CAS.
- A. Mourchid and P. Levitz, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1998, 57, R4887 CrossRef CAS.
- S. Jatav and Y. M. Joshi, Appl. Clay Sci., 2014, 97, 72–77 CrossRef.
- D. W. Thompson and J. T. Butterworth, J. Colloid Interface Sci., 1992, 151, 236–243 CrossRef CAS.
- R. P. Mohanty and Y. M. Joshi, Appl. Clay Sci., 2016, 119, 243–248 CrossRef CAS.
- G. R. Mahdavinia, M. Soleymani, M. Sabzi, H. Azimi and Z. Atlasi, J. Environ. Chem. Eng., 2017, 5, 2617–2630 CrossRef CAS.
- S. Ali and R. Bandyopadhyay, Langmuir, 2013, 29, 12663–12669 CrossRef CAS.
- M. Manilo, N. Lebovka and S. Barany, Colloids Surf., A, 2014, 462, 211–216 CrossRef CAS.
- M. Manilo, S. Netreba, V. Prokopenko, N. Lebovka and S. Barany, Colloids Surf., A, 2016, 506, 291–297 CrossRef CAS.
- Z. Qiao, M. Cao, K. Michels, L. Hoffman and H.-F. Ji, Polym. Rev., 2019, 60(3), 1–22 Search PubMed.
- N. A. Peppas, P. Bures, W. S. Leobandung and H. Ichikawa, Eur. J. Pharm. Biopharm., 2000, 50, 27–46 CrossRef CAS.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2012, 64, 18–23 CrossRef.
- C. Gila-Vilchez, A. B. Bonhome-Espinosa, P. Kuzhir, A. Zubarev, J. D. G. Duran and M. T. Lopez-Lopez, J. Rheol., 2018, 62, 1083–1096 CrossRef CAS.
- M. Cvek, A. Zahoranova, M. Mrlik, P. Sramkova, A. Minarik and M. Sedlacik, Colloids Surf., B, 2020, 110912 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: 10.1039/d0ma00687d |
| This journal is © The Royal Society of Chemistry 2020 |