A high-throughput cell culture system based on capillary and centrifugal actions for rapid antimicrobial susceptibility testing†
Abstract
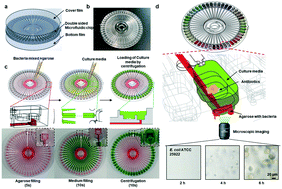
Antibiotic resistance is a global threat to modern society. Rapid determination of suitable antibiotics that inhibit bacterial growth can effectively reduce antibiotic resistance and improve clinical treatment. The conventional methods of antimicrobial susceptibility testing (AST) depend on optical density measurements, which require long-time incubation. Various kinds of rapid AST systems which utilize various technologies from the field of lab on a chip have promised a great reduction in measurement time, but cannot achieve high-throughput, user-friendly testing due to the complexity of the testing system. Here, we introduce a capillary and centrifuge-based rapid AST system that reduces the time of loading the sample and culture media while achieving a high-throughput testing capacity. The capability of the proposed system is validated in a systematic analysis that includes sample loading characteristics and AST trials with standard strains. The proposed system provides a useful tool for drug testing in cell-culture systems with user-friendly and high-throughput analysis.
- This article is part of the themed collection: Lab on a Chip Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...