Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Modeling iontophoretic drug delivery in a microfluidic device†
Maryam
Moarefian
a,
Rafael V.
Davalos
 b,
Danesh K.
Tafti
a,
Luke E.
Achenie
c and
Caroline N.
Jones‡
b,
Danesh K.
Tafti
a,
Luke E.
Achenie
c and
Caroline N.
Jones‡
 *de
*de
aDepartment of Mechanical Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA
bDepartment of Biomedical Engineering and Mechanics, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA
cDepartment of Chemical Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA
dDepartment of Biological Sciences, Virginia Polytechnic Institute and State University, Life Sciences 1 Bldg., Rm. 221, 970 Washington Street SW, Blacksburg, VA 24061, USA
eDepartment of Bioengineering, The University of Texas, Dallas, TX 75080, USA
First published on 30th July 2020
Abstract
Iontophoresis employs low-intensity electrical voltage and continuous constant current to direct a charged drug into a tissue. Iontophoretic drug delivery has recently been used as a novel method for cancer treatment in vivo. There is an urgent need to precisely model the low-intensity electric fields in cell culture systems to optimize iontophoretic drug delivery to tumors. Here, we present an iontophoresis-on-chip (IOC) platform to precisely quantify carboplatin drug delivery and its corresponding anti-cancer efficacy under various voltages and currents. In this study, we use an in vitro heparin-based hydrogel microfluidic device to model the movement of a charged drug across an extracellular matrix (ECM) and in MDA-MB-231 triple-negative breast cancer (TNBC) cells. Transport of the drug through the hydrogel was modeled based on diffusion and electrophoresis of charged drug molecules in the direction of an oppositely charged electrode. The drug concentration in the tumor extracellular matrix was computed using finite element modeling of transient drug transport in the heparin-based hydrogel. The model predictions were then validated using the IOC platform by comparing the predicted concentration of a fluorescent cationic dye (Alexa Fluor 594®) to the actual concentration in the microfluidic device. Alexa Fluor 594® was used because it has a molecular weight close to paclitaxel, the gold standard drug for treating TNBC, and carboplatin. Our results demonstrated that a 50 mV DC electric field and a 3 mA electrical current significantly increased drug delivery and tumor cell death by 48.12% ± 14.33 and 39.13% ± 12.86, respectively (n = 3, p-value <0.05). The IOC platform and mathematical drug delivery model of iontophoresis are promising tools for precise delivery of chemotherapeutic drugs into solid tumors. Further improvements to the IOC platform can be made by adding a layer of epidermal cells to model the skin.
1. Introduction
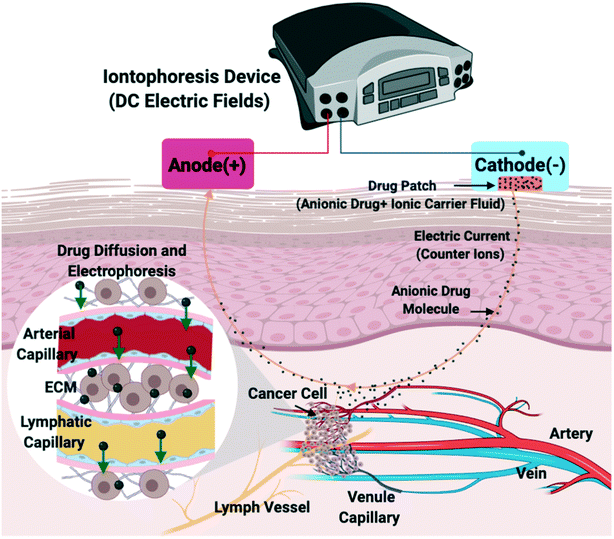
Intravenous chemotherapy is the traditional method for administering cytotoxic agents to cancer patients. Unfortunately, the full potential of anti-cancer drugs is limited because of systemic toxicity and poor tumor perfusion. In an attempt to improve the efficacy of anti-cancer drugs while mitigating their side effects, many groups have reported on the clinical use of electric fields to improve drug delivery. Most studies focus on the electropermeabilization of cells via high-intensity electric fields.1–7 More recently, however, the use of iontophoresis via low intensity electric fields8 was reported in animal models9–13 and in humans.14–17 Iontophoresis enhances drug delivery by electrophoresis, the movement of charged drug molecules in the tumor's extracellular matrix surrounding tumor capillaries. During cancer treatment, iontophoretic devices with external power and drug flow controls are implanted proximal to the tumor or onto the skin of the patient.17–19 However, iontophoretic delivery of chemotherapeutic drugs into the tumor microenvironment is still not well-defined.20,21 After passing the epidermis, iontophoresis enhances drug delivery via three mechanisms in the tumor vasculature (Fig. 1): 1) the electric field and current drives ions through the endothelial membrane of the blood vessels; 2) the ion-electric field interaction provides electrophoretic movement of ions, which increases ion delivery out of the blood vessel; and 3) electroosmotic flow produces bulk motion of the solvent itself, which carries ions or neutral species within the solvent ‘stream’.19,22 To date, most in vitro21,23–26 and in vivo27,28 studies focused on overcoming human epidermal membrane (HEM) drug resistance by using iontophoresis with a short delivery duration of the electric field. There is a need, however, to precisely control and predict the rate, direction, and distance of drug delivery in the tumor extracellular matrix (ECM) when using iontophoresis techniques. After passing through the HEM, iontophoretic drug delivery is still blocked by the ECM. Physiological and biological barriers within the ECM not only decrease the efficacy of chemical compounds, but also delay the compounds from reaching tumor cells in concentrations sufficient enough to exert a therapeutic effect.14,17 Barriers to iontophoretic drug delivery created by the ECM is a critical issue that must be addressed. Using a diffusion-based model, our groups has previously29 described that a 70% porosity, heparin-based hydrogel was a biomimetic scaffolding for modeling the chemoresistance of MDA-MB-231 triple-negative breast cancer (TNBC) ECM.29 Therefore, we chose to use a heparin-based hydrogel with 70% porosity to represent the ECM of an in vivo tumor.30,31Previous microfluidic in vitro studies have been conducted to mimic the three-dimensional microenvironments of tumors using on-chip technologies including the tumor vasculature network, which promotes drug resistance in the tumor microenvironment. Recent studies have utilized microfluidic platforms to obtain further insights into the mechanisms of electric field stimulation on cancer cells in 3D as well as the specific parameter values that affect tumor growth but do not harm surrounding, non-cancerous cells present in the tissue.32 The microfluidic approach facilitates numerous advantages including: (1) the precise control of the biomaterials' physical and chemical properties to resemble the physiological ECM; (2) nanoliter volumes reduce reagent usage and facilitates reproducibility;33 and (3) controlling the physics of drug transport phenomena in the presence of an applied electric field aids in the design and testing of new therapeutic approaches.34–36
In silico simulations are well-suited for testing combinations of multiple physical laws (e.g., diffusion and electrophoresis) and are used for estimating drug concentration profiles in the tumor.37–39 However, the fundamental mathematical model that incorporates the physics of electrophoresis transport is not well-defined. Based on the work of Pascal et al.,40 it was demonstrated in their electrokinetics drug delivery model41 that the application of an electric field enhances drug delivery at both micro- and macro-scales. However, a validated electrokinetics model42 that can be used for the prediction of the tumor response to chemotherapy in the presence of an applied low-intensity DC electric field has not been reported.
To address these issues, we developed both iontophoresis-on-chip (IOC) platform and mathematical model of iontophoretic drug delivery. Our iontophoresis-on-chip (IOC) platform allows for three unique applications: (1) acupuncture electrodes facilitate electrode insertion into the microfluidics and precise control over the location and direction of the applied electric field. (2) Incorporation of heparin-based hydrogels into fluidic channels to mimic and precisely control the physiology of the tumor ECM.43–51 (3) The open microfluidic platform facilitates exchange of the cell medium so as to maintain a constant pH and expose cancer cells to a drug of interest up to 48 h after 3D cell culture. Our mathematical model enabled us to predict optimal parameters (electric field intensity, direction), which were then validated in vitro. Our iontophoresis-on-chip (IOC) platform is the first microfluidic system in the literature to offer the opportunity to investigate the effects of electrophoretic carboplatin delivery into TNBC cells (MDA-MB-231) that are encapsulated in a heparin-based hydrogel.
2. Materials and methods
2.1. Design and fabrication of the microfluidic iontophoresis platform
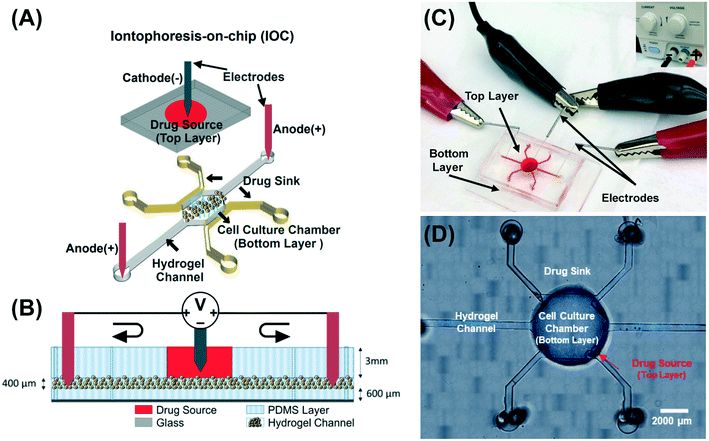
The iontophoresis-on-chip (IOC) platform was designed to mimic tumor vasculature by providing a top layer of arterial capillaries and two side channels of lymph capillaries in the bottom layer (Fig. 2A). The heparin-based hydrogel and cell culture medium (DMEM/F-12 + 10% FBS) is found between the top and the bottom layers and plays the role of a salt bridge. Sterile stainless-steel acupuncture needles with a diameter of 0.12 mm (Kingli, China) were used as electrodes to construct the DC electric field circuit (Fig. 2B and C). The negative electrode was placed in the top layer and the positive electrodes were placed in the inlet and outlet of the bottom layer's hydrogel channel (Fig. 2A and B). The master mold was patterned using two layers of photoresist: (i) the first layer (i.e., the top layer) consists of a central well (1.5 mm wide and 3 mm thick) and has the role of supplying media to cells that are encapsulated in hydrogel in the bottom layer; (ii) the second layer (i.e., the bottom layer) consists of a cell culture chamber that has the dimensions of 3 × 3 mm2, two sink channels with the dimensions of 0.5 × 3 mm2, 15 ports with the dimensions of 0.15 × 0.15 mm2 between the cell culture chamber and each sink channel, and two side hydrogel channels with the dimensions of 0.5 × 5 mm2 (Fig. 2A). The thickness of the bottom layer was optimized to 400 μm using the SU8-2100 photoresist and was fabricated according to the manufacturer's (MicroChem Corp.) instructions. Microfluidic devices were produced by replica molding using polydimethylsiloxane, (PDMS, Sylgard 184; Ellsworth Adhesives, Wilmington, MA, USA) on the master wafer, and fabricated using standard microfabrication techniques.52 We aligned and bonded the two PDMS layers of the microfluidic platform using a Nikon SMZ-1 stereo microscope and a Nordson MARCH (AP-300) oxygen plasma bonder, respectively. After bonding the two layers, the inlets and outlets were punched using a 0.75 mm biopsy puncher (Fig. 2D). Finally, a 6-well glass-bottom plate (MatTek, Ashland, MA, USA) was plasma-treated along with the PDMS IOC devices and the devices were bonded to the plate using a hot plate (85 °C for 10 min).2.2. Hydrogel preparation and dose response curve
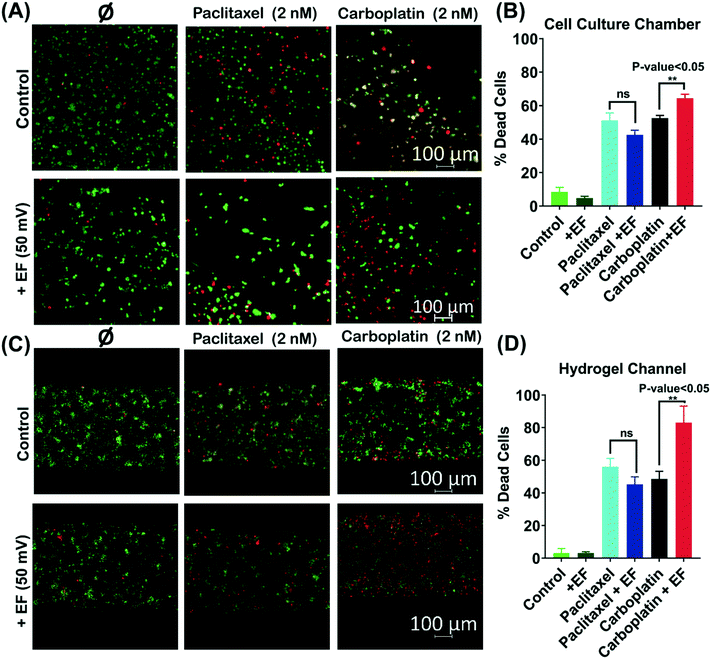
The HyStem-HP Hydrogel Kit w/ PEGSSDA (ESI BIO GS315P) was used to prepare the heparin hydrogel. The kit is composed of lyophilized solids of Heprasil® (thiol-modified sodium hyaluronate with thiol-modified heparin), Gelin-S® (thiol-modified gelatin), and PEGSSDA™ (disulfide containing polyethylene glycol di-acrylate), as well as degassed deionized water (DG Water). Gels were prepared as per manufacturer's directions. Cell seeding density and drug concentration optimization experiments were done in 384 well-plates with a 0.06 cm2 growth area, which is approximately the growth area of the fabricated microfluidic device (0.09 cm2). The drug was applied after 48 h of incubation. Cells were stained using a live and dead cell fluorescent assay (Abcam, Cambridge, MA) after 72 h of incubation. Dose–response curves of paclitaxel (Sigma-Aldrich, St. Louis, MO) and carboplatin (Sigma-Aldrich, St. Louis, MO) were obtained in single cells using a 10 K cell density in 30 μL of heparin-hydrogel. The paclitaxel dose response curve showed that 11 nM was the 90% effective dose (EC90) and the carboplatin dose response curve showed that 12 nM of carboplatin killed the maximum fraction of cells (85%) (Fig. S1A†). Because of these dose response curves, we chose to use 2 nM of the drugs to use doses less than the EC90 for both paclitaxel and carboplatin in the microfluidic in vitro environment. We tested the effect of electric field on the delivery of paclitaxel and carboplatin separately to examine this effect on both charged (carboplatin) and neutral (paclitaxel) drug delivery.2.3. Cell culture and iontophoresis-on-chip (IOC) assay
The human TNBC cells used in this study were from an invasive ductal carcinoma cell line (MDA-MB-231, American Type Cell Culture HTB-26). Gibco™ Dulbecco's modified Eagle's medium F-12 (DMEM/F-12), containing high levels of glucose and GlutaMAX™ supplemented with 10% (v/v) fetal bovine serum (FBS, ATCC, Manassas, VA) and 1% (v/v) penicillin–streptomycin (100 units per mL penicillin and 0.1 mg per mL streptomycin), was used as a complete culture medium for the TNBC cells. The encapsulated breast cancer cells were studied using heparin hydrogel in complete media (DMEM + 10% FBS + 1% P/S). The cells were suspended in the Heprasil and Gelin-S mixture before adding the PEGSSDA crosslinker at a density of 10 K single cells in 30 μL of hydrogel. 2 nM concentrations of paclitaxel and carboplatin were used because they kill approximately 71% of cells without an applied electric field (Fig. S1A†). We used cells on top of the gel, and encapsulated cells inside the gel for 3D cell culture in the microfluidic device. The encapsulated cells in the hydrogel were introduced into the device through manual injection using a syringe and tubing until a uniform distribution of cells was attained in the cell culture chamber. In our study, we set the flow rate to zero (eliminating electroosmotic flow) and focused on electrophoresis transport only. To model the iontophoresis, we needed to define: 1) ECM porosity; 2) drug concentration; 3) cell density; and 4) drug charge. We set the drug concentration to be less than the effective dose of 90% (ED90), which we measured in both standard well plates and in the microfluidic device (Fig. S1†). Application of an external electric voltage resulted in faster delivery of anionic drugs compared to cationic drugs.35 We compared an anionic drug,53,54 carboplatin, to the gold standard non-ionized drug, paclitaxel. We also studied TNBC cells cultured in heparin-based hydrogel as a tumor ECM biomaterial.29,55 In our device, we validated the application of a 50 mV electric field to an ECM of 70% porosity to increase drug delivery to a tumor's single cells. We also varied the drug type (charge). In this study, we controlled low-intensity DC electric fields for electrophoretic drug delivery in the tumor's single cells. We quantified the effect of a 50 mV DC electric field and a 3 mA electric current on the percent of dead cells both mathematically and experimentally using a mass transfer model and a microfluidic platform, respectively. Our IOC mimics the tumor extracellular matrix through the use of MDA-MB-231 single cells in a heparin-based hydrogel, the lymph capillary (drug sink), and the blood capillary (drug source). We initially used a physics based mathematical model41 for sensitivity analysis and parameter optimization to reduce the number of in vitro experiments. To experimentally prove the chosen electric field intensity of 50 mV derived from the sensitivity analysis, we showed the effect of electrophoresis transport on increasing ionic macromolecule delivery in heparin-based hydrogel.The experiment was conducted for a total of 72 h where the cells were initially seeded and allowed to grow for 48 h and then treated with 2 nM of the drug and 50 mV of electric field for 5 h. The electric field was discharged after 5 h, however, the cells were left in the drug solution for an additional 19 h. Finally, the cells were stained with live and dead cell fluorescent assay medium (Abcam, Cambridge, MA) after 72 h (Fig. S3†).
2.4. Electrokinetics and mass transfer model
To reduce the number of in vitro experiments, we employed a physics-based model to predict the minimum electric field intensity needed to affect the maximum fraction of cells killed by simulating the effect of iontophoresis on carboplatin delivery. The model was then validated with a biomimetic three-dimensional microfluidic experiment by comparing the measured and predicted percentage of dead cells. See the ESI† for a more detailed description of the coupled electrokinetics and mass transfer model.2.5. Drug delivery model optimization and sensitivity analysis
Sensitivity analysis was used to eliminate a trial and error approach for determining the electric field intensity when conducting the IOC experiments. Sensitivity analysis of the electric field potential, between 0 and 70 mV, at the blood vessel wall showed 43.01 mV as the optimum electric field intensity to obtain a 79% cell death rate (Fig. 3B).Three non-dimensional numbers and the electric potential in the tumor region were optimized using GRG (generalized reduced gradient) nonlinear solver.56 The optimal value of φ2 (electric field intensity), 43.01 mV, was instrumental in eliminating multiple microfluidic in vitro experiments for electric field intensity optimization. Tables S1 and S2† show the dependent and independent variables, their sensitivity ranges, and optimum value to obtain the maximum fraction of cells killed.
2.6. Transport of macromolecules into heparin-based hydrogel
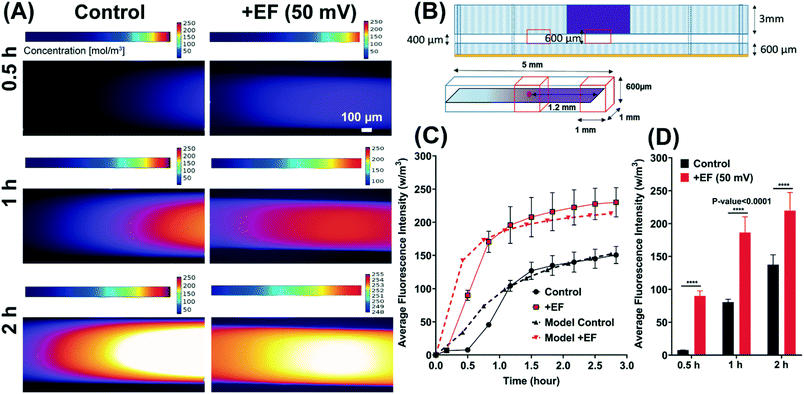
The first experimental setup was used to determine the ionized fluorescent dye concentration with and without applying a 50 mV of DC electric field. Sensitivity analysis and model optimization show that the electric potential of 43.01 mV resulted in the maximum fraction of tumor cells killed. Due to the restriction in the precision of our DC power supply (DC power supply must be >10 mV), we had to choose between 40 mV and 50 mV. We loaded a heparin-based hydrogel in the bottom layer, closed the outlets to eliminate gravitational flow, placed the device under a Nikon TiE time lapse microscope, and allowed the hydrogel to solidify (10–15 min). 30 μL of dye was added to the top layer and live imaging was started simultaneously. Live images were taken every 1 minute for 5 h in two 1 mm2x–y planes in the central chamber and the hydrogel channel over a 1.2 mm distance. The fluorescence intensity was measured over 10 different depths (40 μm) within the hydrogel (400 μm). The average intensity was determined over the 1 mm2x–y plane. The final average intensity is the average of x–y plane intensities at 10 different hydrogel depths. The calibration curve was used (Fig. S2†) to convert average dye intensity to dye concentration. We used Alexa Fluor 594® fluorescent dye with a molecular weight of 819.85 g mol−1, which is similar to paclitaxel's molecular weight (853.91 g mol−1) and close to carboplatin molecular weight (371.25 g mol−1). The experimental result of Alexa Fluor 594® concentration versus time validated the finite element model of dye concentration (diffusion and electrophoresis delivery with 50 mV electric potential gradient) in the porous hydrogel region.2.7. Confocal microscopy and image processing
Confocal microscopy was used to measure the fraction of cells killed with and without applying a 50 mV DC electric field. A confocal microscope (ZEISS LSM 880 with Airyscan) fitted with a ZEISS Axiocam 503 mono camera and a computerized stage was used to take fluorescent images. The microscope was controlled using ZEN software (Carl Zeiss Microscopy GmbH, Germany). Images were processed using ImageJ (NIH, Bethesda, MD). To calculate the fraction of cells killed (or the percentage of dead cells), the fluorescent light intensities from tetramethyl rhodamine iso-thiocyanate (TRITC, red: dead cells) were divided by the total fluorescent light intensities from TRITC (red: dead cells) and fluorescein isothiocyanate (FITC, green: live cells) in each 10 μm plane along the hydrogel's z-axis. Results from the confocal images were used for validation of the fraction of cells killed model41 (Fig. 6C).2.8. Microfluidics in vitro experiment statistical analysis
Statistical analyses were performed using Prism version 8.1.2 (332) software (GraphPad Software, La Jolla, CA) with a confidence level of α = 0.05. Two-way analysis of variance (ANOVA) was used to test for differences in the number of dead cells after electrophoresis treatment (n = 3) in our microfluidic device (n = 3). When results of ANOVA were significant, Tukey post hoc comparisons were used to examine differences among treatment groups. Data are presented as the arithmetic mean ± SD.3. Results and discussion
The combined mathematical and experimental approach in our study included four steps. First, we used a physics-based mathematical model,41 sensitivity analysis, and parameter optimization to eliminate trial and error in conducting in vitro experiments to determine the optimal electric voltage. Second, we showed the effect of electrophoresis transport on cationic macromolecule concentration (Alexa Fluor 594®) in the porous region (heparin-based hydrogel) using a transient electrokinetics drug release computational model, which was validated by a microfluidic in vitro experiment. Third, the IOC device was used to study carboplatin and paclitaxel delivery to TNBC cells in an in vitro experiment using 50 mV of electric field (EF) and 3 mA of electric current. Fourth, the in vitro experimental results were used to validate the fraction of cells killed in the mathematical model.3.1. Drug delivery mathematical model optimization and sensitivity analysis
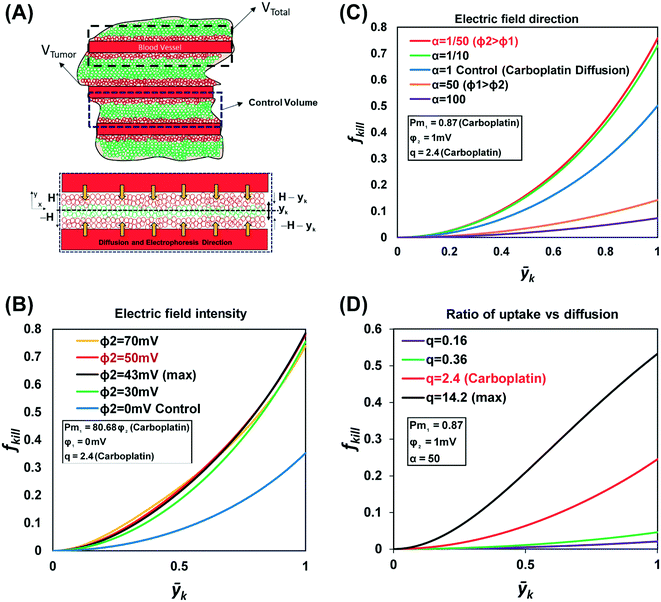
The physics-based model development is shown in the ESI.† In the final fraction of tumor killed function, eqn (24),† three non-dimensional numbers relate to the physics of uptake, diffusion, and electrophoresis drug transport and needed to be analyzed. Pm1 is the ratio between the electric potential and diffusivity, q is the ratio between the uptake rate of the carboplatin and diffusivity, and α is the ratio of electric potential in the drug source to the electric potential in the tumor. Three non-dimensional numbers and the electric potential in the tumor region were optimized using GRG (generalized reduced gradient) nonlinear solver.56The first non-dimensional number, 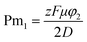 , is the ratio between the electric potential and the diffusion coefficient. We consider the problem of maximizing the fraction of cells killed subject to varying non-dimensional numbers. The Pm1 number is the function of the electric potential in the tumor region. Except for φ2, other terms in Pm1 are constant properties of carboplatin. We only look at the carboplatin property because it is a charged drug candidate for studying the effect of electric field on its delivery. At a large Pm1 (3.47), the electric potential φ2 has the highest value (43.01 mV); therefore, the fraction of cells killed is maximized (0.79) (Fig. 3B). Our results show the positive effect that electric potential has on the fraction of cells killed and that it can overcome carboplatin's low diffusion coefficient. As Pm1 decreases, the fraction of cells killed also decreases due to a decrease in φ2. When Pm1 is zero, the result indicates the fraction of cells killed (0.37) without applying an electric field.
, is the ratio between the electric potential and the diffusion coefficient. We consider the problem of maximizing the fraction of cells killed subject to varying non-dimensional numbers. The Pm1 number is the function of the electric potential in the tumor region. Except for φ2, other terms in Pm1 are constant properties of carboplatin. We only look at the carboplatin property because it is a charged drug candidate for studying the effect of electric field on its delivery. At a large Pm1 (3.47), the electric potential φ2 has the highest value (43.01 mV); therefore, the fraction of cells killed is maximized (0.79) (Fig. 3B). Our results show the positive effect that electric potential has on the fraction of cells killed and that it can overcome carboplatin's low diffusion coefficient. As Pm1 decreases, the fraction of cells killed also decreases due to a decrease in φ2. When Pm1 is zero, the result indicates the fraction of cells killed (0.37) without applying an electric field.
The direction of electrophoresis can be opposite the direction of drug diffusion. The second non-dimensional number is α, which is the ratio of electric potential in the drug source to the electric potential in the tumor. The fraction of cells killed decreases when α is close to 1, i.e., when there is no electric potential gradient. Therefore, when α = (φ1 = φ2) the fraction of cells killed is minimized (0.44) (Fig. 3C). As α increases (φ1 > φ2), the electrical potential gradient is in the opposite direction of the concentration gradient, so the fraction of cells killed decreases. Note that α = 1/50 means φ2 is 50 times more than φ1, at which point the fraction of cells killed is maximized (0.79).
The non-dimensional number is analyzed to examine the fraction of cells killed based on drug uptake rate and diffusion. q is the ratio of the drug uptake rate to drug diffusion. Since q is always positive, the model always increases (monotonic) with different values of positive q. At small q values (0.16), carboplatin diffusion dominates over the uptake rate. High carboplatin diffusion can occur due to a decrease in the tumor drug uptake rate. Therefore, the number of cells killed at high carboplatin uptake (q = 14.2) leads to a maximized fraction of cells killed (0.55) and a decrease in tumor drug resistance (Fig. 3D).
When q is 2.4 for carboplatin, the uptake rate of carboplatin is smaller than its diffusion where the fraction of tumor cells killed is 0.25. The fraction of cells killed reaches an asymptote by increasing the value of q, which depends on the properties of the drug. Therefore, applying an electric field to enhance chemotherapy delivery of carboplatin is essential to kill the maximum fraction of cells (0.79). The graph shows that the primary sources of uncertainty in percent dead cells are the drug uptake rate and diffusivity, and this type of sensitivity analysis allows drug designers to assess the effects of the physical properties of the drugs in the interest of building robust drug delivery models. Our model investigated diffusion and electrophoresis drug transport into single cells. Increasing the electric field intensity increases the fraction of cells killed; however, the new physics of transport, such as joule heating and electroosmotic flow, should be added to the model for a more accurate prediction.
3.2. Delivery of cationic fluorescent dye into the hydrogel using a low intensity electric field
Sensitivity analysis and electric potential gradient optimization showed that an electric potential of 43.01 mV resulted in the maximum fraction of tumor cells being killed (0.7–0.8 or 70–80%). Based on mathematical model sensitivity analysis, we used a 50 mV electric field intensity for drug delivery experiments on single cells. Because of the restriction in the precision of our DC power supply (DC power supply must be >10 mV), we had to choose 50 mV instead of 43.01 mV. To test our hypothesis using a 50 mV electric field in transient condition, finite element modeling of macromolecule transport in hydrogel was computed. The model was validated with an electrophoresis microfluidic experiment using the IOC device by comparing the measured and predicted normalized fluorescence intensity of the dye. Alexa Fluor 594® cationic dye diffusion and electrophoretic delivery were investigated in a 3 h experiment and a transient continuum mass transfer model. The regions in the cell culture chamber and hydrogel channel of the device for the measurement of dye transient concentration (top: experimental regions, bottom: computational domain) are shown in Fig. 4B. The in silico simulation and in vitro experiment showed an increase (48.12%, n = 46, p-value <0.0001) in the average fluorescence intensity of the dye by applying 50 mV electric potential gradient in the hydrogel microchannel (Fig. 4A, C and D). The finite element model of the concentration profile in the microchannel was validated with an in vitro experiment of Alexa Fluor 594® cationic dye diffusion and electrophoretic delivery (Fig. 4C).3.3. A low-intensity electric field increases carboplatin delivery in breast cancer single cells
Based on model sensitivity analysis (section 3.2), we specified the low-intensity electric field to be 50 mV. Drug concentration optimization (Fig. S1A†) showed that 2 nM of paclitaxel and carboplatin have around a 59% and 71% tumor cell death rate, respectively. Therefore, we specified operating at 2 nM drug concentrations, which is less than the EC90 (effective concentration for 90% of the cells being dead). A significant difference was observed in the percent of dead cells by applying a 50 mV electric field and 3 mA electric current for 3 h (Fig. 5A and C). The percent of dead cells increased by 22% (n = 3, p-value <0.05) in the cell culture chamber (Fig. 5B) and 39% (n = 3, p-value <0.05) in the hydrogel channel (Fig. 5D).3.4. Fraction of cells killed model validation with in vitro experiment
The “fraction of cells killed” model was developed to facilitate the prediction of the iontophoresis outcome. The in vitro results of percent dead cells versus hydrogel depth were used for model validation (Fig. 6A). A summary of results of the percent of dead cells at different distances from the bottom of the chamber shows an increase in percent dead cells when delivering carboplatin using electrophoresis (Fig. 6B). The validated fraction of cells killed model shows an increase in the percent of dead cells when applying a 50 mV electric field and 3 mA electric current for 3 h experimentally and mathematically (Fig. 6C).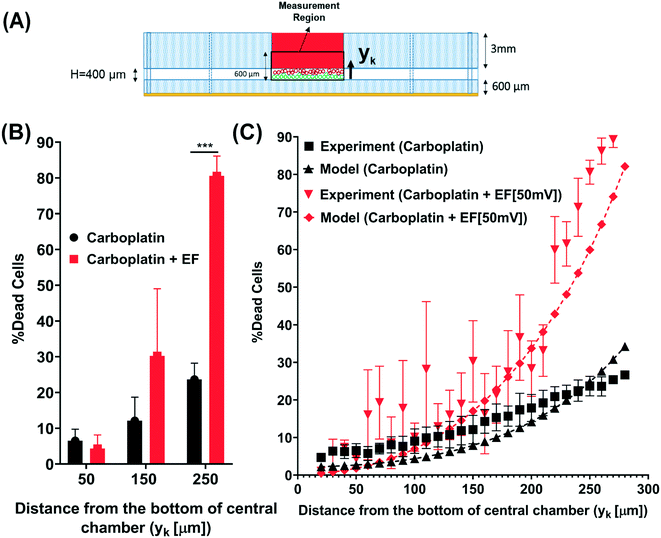 | ||
| Fig. 6 Percent dead tumor cells model validation shows that low-intensity electric field promotes iontophoretic delivery of carboplatin into breast cancer cells. (A) Representative of the experimental measurement region in the cell culture chamber of the device, for calculating the percent dead tumor cells when treated with 2 nM of carboplatin with and without 50 mV of electric field. (B) Graph shows increase in percent dead tumor cells that are seeded at 50 μm,150 μm, and 250 μm distance from the bottom of the cell culture chamber and treated with 2 nM of carboplatin with and without 50 mV of electric field; the difference in the percent dead cells when treated with 2 nM of carboplatin with electric field is significantly higher at 250 μm (closest to drug source) compared to other distances. (C) Percent dead tumor cells model validation with the in vitro experiment shows an accurate correlation between model and experiment (Table S3†). | ||
Our results demonstrate that 50 mV of DC electric field and 3 mA of electric current increases drug delivery by 48.12% and increases cell death by 39.13%. Our obtained experimental results validated our recent drug transport model.41
The correlation between the fraction of cells killed model and the in vitro experiment was measured using two different methods (Table S3†):57 1. RNMSE (root normalized mean square error) and 2. FB (fraction of bias). The correlation between cationic dye concentration in hydrogel experimental and theoretical values was calculated using the same methodologies. We found that the FB (fractional bias) value was equal to 0.02, where a positive FB indicated that the model is under prediction, and an RNMSE (root normalized mean square error)54 equal to 0.3 indicated low scattering from the mean. Overall, the statistical analysis indicated a strong correlation between the model and the microfluidic experiment.
4. Conclusion
This paper demonstrates, for the first time, an iontophoresis-on-chip platform that mimics the outcome of iontophoretic drug delivery for the treatment of breast cancer. The mathematical model for the carboplatin concentration profile between two blood vessels in a confined tumor volume was able to accurately predict our in vitro microfluidic results. In this study, we varied the drug type (charge) and cell density as the two main parameters for the on-chip experiments. As expected, iontophoresis was only effective in increasing the charged drug (carboplatin) delivery to the individual tumor cells. Our device could potentially be used for high-throughput screening of charged drug candidates for iontophoretic treatment for breast cancer. Our modeling and experimental results show that an applied electric field intensity of 50–70 mV of DC electric fields and 3 mA of electric current led to the maximum percentage of dead cells (70–90%). This low intensity electric field has been reported to have minimal side effects on healthy tissues and is significantly lower than that following electroporation. The on-chip platform allows us to precisely control the physics of transport phenomena by adjusting the device geometries, boundary conditions, and initial values of drug dose and electric field intensity to match the mathematical model. The predictive models that we developed in this manuscript define the influence of DC electrical fields and electric current on iontophoretic drug delivery to tumors and may possibly assist physicians in designing an effective treatment regimen for breast cancer patients.58 We proved the synergy between our mathematical models and our in vitro experiments, which led to a reduction in the number of electric field intensity trials using the mathematical model sensitivity analysis. We then proved the accuracy of the chosen electric field intensity values from the sensitivity analysis by macromolecule electrophoretic delivery into the heparin-based hydrogel microfluidics experiment. The results of the macromolecule electrophoresis delivery into the porous hydrogel was used to validate a transient electrokinetics mass transfer model.In the future, our device also has the capability to vary flow, ECM porosity, electric field intensity, tumor size, and tumor type. This model is the first step towards generating a predictive model for in vivo applications. Further improvements to the model for transdermal iontophoretic drug delivery can be made and validated by adding additional components in the microfluidic device, mimicking the human epidermis. Currently, iontophoresis is only used for transdermal drug delivery to accessible tumors. In the future, endoscopic surgeries may enable the implantation of 3D printed hydrogels and electrodes59–62 for electrically-controlled drug delivery into inaccessible solid breast tumors.
5. Author contributions
MM, and CNJ designed the experiments and device, conceived the experiment(s), edited the manuscript, and the work was done under CNJ's supervision. MM designed iontophoresis-on-chip, conducted the experiments, developed the electrokinetics models, and analyzed the data. DKT, RVD, and LEA contributed to the microfluidic device design and computational model. All authors have read and approved the article.6. Funding
Research materials and equipment for this study were funded by CNJ startup funds from the Department of Biological Sciences at Virginia Tech and The National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM133610. MM was supported by the VT-Initiative for Maximizing Student Development (IMSD) (NIGMS 2R25GM072767-05A1) and the Department of Mechanical Engineering at Virginia Tech.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Dr. Jiangtao Cheng for kindly providing us with the electrical instruments required for the experimental work. We also acknowledge support from Fralin Life Sciences Institute of Virginia Tech for facilitating confocal imaging and the Micro & NanoFabrication Laboratory at Virginia Tech's Bradley Department of Electrical and Computer Engineering for master mold fabrication. We would also like to thank Dr. Janet Webster for critically reading and editing the manuscript. We would like to also thank Udaya Sree Datla and Nidhi Menon for their input and help with the hydrogel experimental design.References
- E. Neumann, et al., Gene transfer into mouse lyoma cells by electroporation in high electric fields, EMBO J., 1982, 1(7), 841 CrossRef PubMed.
- M. Okino and H. Mohri, Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors, Jpn. J. Cancer Res., 1987, 78(12), 1319–1321 Search PubMed.
- S. Orlowski, et al., Transient electropermeabilization of cells in culture: increase of the cytotoxicity of anticancer drugs, Biochem. Pharmacol., 1988, 37(24), 4727–4733 CrossRef PubMed.
- G. Sersa, et al., Electrochemotherapy in treatment of tumours, Eur. J. Surg. Oncol., 2008, 34(2), 232–240 CrossRef PubMed.
- M. Wichtowski, et al., Electrochemotherapy in Breast Cancer - Discussion of the Method and Literature Review, Breast Care, 2017, 12(6), 409–414 CrossRef PubMed.
- M. G. Bourke, et al., Effective treatment of intractable cutaneous metastases of breast cancer with electrochemotherapy: Ten-year audit of single centre experience, Breast Cancer Res. Treat., 2017, 161(2), 289–297 CrossRef CAS PubMed.
- M. Wichtowski, et al., Electrochemotherapy in the Treatment of Breast Cancer Metastasis to the Skin and Subcutaneous Tissue - Multicenter Experience, Oncol. Res. Treat., 2019, 42(1–2), 47–51 CrossRef CAS PubMed.
- A. K. Banga, S. Bose and T. K. Ghosh, Iontophoresis and electroporation: comparisons and contrasts, Int. J. Pharm., 1999, 179(1), 1–19 CrossRef CAS PubMed.
- A. Banerjee, et al., Intestinal iontophoresis from mucoadhesive patches: a strategy for oral delivery, J. Controlled Release, 2019, 297, 71–78 CrossRef CAS PubMed.
- L. F. Dalmolin and R. F. V. Lopez, Nanoemulsion as a Platform for Iontophoretic Delivery of Lipophilic Drugs in Skin Tumors, Pharmaceutics, 2018, 10(4), 214 CrossRef CAS PubMed.
- H. Liang, et al., Effects of methycobal iontophoresis combined with balance acupuncture on peripheral facial paralysis, Zhongguo Zhenjiu, 2018, 38(9), 955–960 Search PubMed.
- M. J. Pikal, The role of electroosmotic flow in transdermal iontophoresis, Adv. Drug Delivery Rev., 2001, 46(1), 281–305 CrossRef CAS PubMed.
- S. R. Patel, et al., In vitro and in vivo evaluation of the transdermal iontophoretic delivery of sumatriptan succinate, Eur. J. Pharm. Biopharm., 2007, 66(2), 296–301 CrossRef CAS PubMed.
- J. D. Byrne, J. J. Yeh and J. M. DeSimone, Use of iontophoresis for the treatment of cancer, J. Controlled Release, 2018, 284, 144–151 CrossRef CAS PubMed.
- J. B. Sloan and K. Soltani, Iontophoresis in dermatology: a review, J. Am. Acad. Dermatol., 1986, 15(4), 671–684 CrossRef CAS PubMed.
- J. D. Byrne, et al., Impact of formulation on the iontophoretic delivery of the FOLFIRINOX regimen for the treatment of pancreatic cancer, Cancer Chemother. Pharmacol., 2018, 81(6), 991–998 CrossRef CAS PubMed.
- J. D. Byrne, et al., Local iontophoretic administration of cytotoxic therapies to solid tumors, Sci. Transl. Med., 2015, 7(273), 273ra14 CrossRef PubMed.
- J. B. Wolinsky, Y. L. Colson and M. W. Grinstaff, Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers, J. Controlled Release, 2012, 159(1), 14–26 CrossRef CAS.
- T. Karpiński, Selected Medicines Used in Iontophoresis, Pharmaceutics, 2018, 10(4), 204 CrossRef PubMed.
- E. Eljarrat-Binstock and A. J. Domb, Iontophoresis: A non-invasive ocular drug delivery, J. Controlled Release, 2006, 110(3), 479–489 CrossRef CAS PubMed.
- E. Eljarrat-Binstock, et al., In Vitro and In Vivo Evaluation of Carboplatin Delivery to the Eye Using Hydrogel-Iontophoresis, Curr. Eye Res., 2008, 33(3), 269–275 CrossRef CAS PubMed.
- B. J. Kirby, Micro-and nanoscale fluid mechanics: transport in microfluidic devices, Cambridge university press, 2010 Search PubMed.
- S. K. Li, et al., In vitro and in vivo comparisons of constant resistance AC iontophoresis and DC iontophoresis, J. Controlled Release, 2003, 91(3), 327–343 CrossRef CAS.
- J.-P. Sylvestre, R. H. Guy and M. B. Delgado-Charro, In Vitro Optimization of Dexamethasone Phosphate Delivery by Iontophoresis, Physical Therapy, 2008, 88(10), 1177–1185 CrossRef PubMed.
- E. Eljarrat-Binstock, et al., Charged nanoparticles delivery to the eye using hydrogel iontophoresis, J. Controlled Release, 2008, 126(2), 156–161 CrossRef CAS PubMed.
- E. Eljarrat-Binstock, et al., Methylprednisolone delivery to the back of the eye using hydrogel iontophoresis, J. Ocul. Pharmacol. Ther., 2008, 24(3), 344–350 CrossRef CAS PubMed.
- H.-Z. Jia and X.-J. Peng, Efficacy of iontophoresis-assisted epithelium-on corneal cross-linking for keratoconus, International Journal of Ophthalmology, 2018, 11(4), 687–694 Search PubMed.
- J. H. Jung, et al., Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle, J. Controlled Release, 2018, 277, 14–22 CrossRef CAS.
- N. Menon, H. X. Dang, U. S. Datla, M. Moarefian, C. B. Lawrence, C. A. Maher and C. N. Jones, Heparin-based hydrogel scaffolding alters the transcriptomic profile and increases the chemoresistance of MDA-MB-231 triple-negative breast cancer cells, Biomater. Sci., 2020, 8(10), 2786–2796 RSC.
- P. Karuppuswamy, et al., Functionalized hybrid nanofibers to mimic native ECM for tissue engineering applications, Appl. Surf. Sci., 2014, 322, 162–168 CrossRef CAS.
- Q. L. Loh and C. Choong, Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size, Tissue Eng., Part B, 2013, 19(6), 485–502 CrossRef CAS PubMed.
- A. Pavesi, et al., Engineering a 3D microfluidic culture platform for tumor-treating field application, Sci. Rep., 2016, 6, 26584 CrossRef CAS PubMed.
- I. C. Lee, Cancer-on-a-chip for Drug Screening, Curr. Pharm. Des., 2018, 24(45), 5407–5418 CrossRef CAS.
- S. Murdan, Electro-responsive drug delivery from hydrogels, J. Controlled Release, 2003, 92(1), 1–17 CrossRef CAS.
- M. Curcio, et al., On demand delivery of ionic drugs from electro-responsive CNT hybrid films, RSC Adv., 2015, 5(56), 44902–44911 RSC.
- S. M. Rahman, J. M. Campbell, R. N. Coates, K. M. Render, C. E. Byrne, E. C. Martin and A. T. Melvin, Evaluation of intercellular communication between breast cancer cells and adipose-derived stem cells via passive diffusion in a two-layer microfluidic device, Lab Chip, 2020, 20(11), 2009–2019 RSC.
- J. Aroesty, et al., Tumor growth and chemotherapy: Mathematical methods, computer simulations, and experimental foundations, Math. Biosci., 1973, 17(3–4), 243–300 CrossRef CAS.
- J. Siepmann and F. Siepmann, Modeling of diffusion controlled drug delivery, J. Controlled Release, 2012, 161(2), 351–362 CrossRef CAS PubMed.
- C. Mircioiu, et al., Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems, Pharmaceutics, 2019, 11(3), 140 CrossRef CAS PubMed.
- J. Pascal, et al., Mechanistic patient-specific predictive correlation of tumor drug response with microenvironment and perfusion measurements, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(35), 14266–14271 CrossRef CAS PubMed.
- M. Moarefian and J. A. Pascal, Fundamental mathematical model shows that applied electrical field enhances chemotherapy delivery to tumors, Math. Biosci., 2016, 272, 1–5 CrossRef PubMed.
- J. H. Sung, C. Kam and M. L. Shuler, A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip, Lab Chip, 2010, 10(4), 446–455 RSC.
- M. F. McGuire, et al., Formalizing an Integrative, Multidisciplinary Cancer Therapy Discovery Workflow, Cancer Res., 2013, 73(20), 6111 CrossRef PubMed.
- H. Enderling and K. A. Rejniak, Simulating Cancer: Computational Models in Oncology, Front. Oncol., 2013, 3, 233 Search PubMed.
- S. C. Choe, et al., Model for in vivo progression of tumors based on co-evolving cell population and vasculature, Sci. Rep., 2011, 1, 31 CrossRef PubMed.
- H. B. Frieboes, et al., Physical Oncology: A Bench-to-Bedside Quantitative and Predictive Approach, Cancer Res., 2011, 71(2), 298 CrossRef CAS.
- C. Liu, et al., Use of mathematical models to understand anticancer drug delivery and its effect on solid tumors, Pharmacogenomics, 2011, 12(9), 1337–1348 CrossRef CAS.
- F. Michor, et al., What does physics have to do with cancer?, Nat. Rev. Cancer, 2011, 11, 657 CrossRef CAS PubMed.
- H. M. Byrne, Dissecting cancer through mathematics: from the cell to the animal model, Nat. Rev. Cancer, 2010, 10, 221 CrossRef CAS PubMed.
- A. Swierniak, M. Kimmel and J. Smieja, Mathematical modeling as a tool for planning anticancer therapy, Eur. J. Pharmacol., 2009, 625(1), 108–121 CrossRef CAS PubMed.
- P. Dogra, et al., Mathematical modeling in cancer nanomedicine: a review, Biomed. Microdevices, 2019, 21(2), 40 CrossRef.
- S. Brittain, et al., Soft lithography and microfabrication, Phys. World, 1998, 11(5), 31 CrossRef CAS.
- A. J. Di Pasqua, et al., Activation of Carboplatin by Carbonate, Chem. Res. Toxicol., 2006, 19(1), 139–149 Search PubMed.
- A. Ciancetta, C. Coletti, A. Marrone and N. Re, Activation of carboplatin by carbonate: A theoretical investigation, Dalton Trans., 2012, 41(41), 12960–12969 RSC.
- M. Kim, et al., Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes, Biomaterials, 2010, 31(13), 3596–3603 CrossRef CAS.
- L. S. Lasdon, R. L. Fox and M. W. Ratner, Nonlinear optimization using the generalized reduced gradient method. Revue française d'automatique, informatique, recherche opérationnelle, Oper. Res., 1974, 8(V3), 73–103 Search PubMed.
- M. Soni, et al., A performance evaluation of WRF model using different physical parameterization scheme during winter season over a semi-arid region, India, International Journal of Earth and Atmospheric Science, 2014, 1(3), 104–114 Search PubMed.
- A. Calvert, et al., Carboplatin dosage: prospective evaluation of a simple formula based on renal function, J. Clin. Oncol., 1989, 7(11), 1748–1756 CrossRef CAS PubMed.
- A. Simeunovic and D. J. Hoelzle. Coupled Dynamics of Material Delivery and Robotic Manipulator Axes in Endoscopic Additive Manufacturing, in 2019 American Control Conference (ACC), 2019 Search PubMed.
- A. A. Adib and D. Hoelzle. Hybrid System Model of Microextrusion-Based Direct-Write Additive Manufacturing, in 2019 American Control Conference (ACC), 2019 Search PubMed.
- C. J. Bloomquist, et al., Controlling release from 3D printed medical devices using CLIP and drug-loaded liquid resins, J. Controlled Release, 2018, 278, 9–23 CrossRef CAS PubMed.
- H. J. Oh, et al., 3D Printed Absorber for Capturing Chemotherapy Drugs before They Spread through the Body, ACS Cent. Sci., 2019, 5(3), 419–427 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0lc00602e |
| ‡ Current address: The University of Texas at Dallas Bioengineering and Sciences Building, 12.806, 800 N Loop Rd, Richardson, TX 75080 E-mail: Caroline.Jones@UTDallas.edu |
| This journal is © The Royal Society of Chemistry 2020 |