Towards practical sample preparation in point-of-care testing: user-friendly microfluidic devices
Abstract
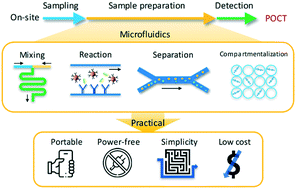
Microfluidic technologies offer a number of advantages for sample preparation in point-of-care testing (POCT), but the requirement for complicated external pumping systems limits their wide use. To facilitate sample preparation in POCT, various methods have been developed to operate microfluidic devices without complicated external pumping systems. In this review, we introduce an overview of user-friendly microfluidic devices for practical sample preparation in POCT, including self- and hand-operated microfluidic devices. Self-operated microfluidic devices exploit capillary force, vacuum-driven pressure, or gas-generating chemical reactions to apply pressure into microchannels, and hand-operated microfluidic devices utilize human power sources using simple equipment, including a syringe, pipette, or simply by using finger actuation. Furthermore, this review provides future perspectives to realize user-friendly integrated microfluidic circuits for wider applications with the integration of simple microfluidic valves.


 Please wait while we load your content...
Please wait while we load your content...