A structure-free digital microfluidic platform for detection of influenza a virus by using magnetic beads and electromagnetic forces†‡
Abstract
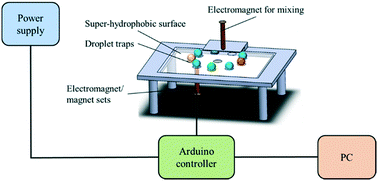
H1N1, a subtype of influenza A virus, has emerged as a global threat in the past decades. Due to its highly infectious nature, an accurate and rapid detection assay is urgently required. Therefore, this study presents a new type of digital microfluidic platform for H1N1 virus detection by utilizing a one-aptamer/two-antibodies assay on magnetic beads. The droplets containing magnetic beads were driven by electromagnetic forces on a structure-free, super-hydrophobic surface to automate the entire assay within 40 min. With different levels of hydrophobic modification, the droplets could be easily controlled and positioned without any assisted microstructure. The tunable electromagnetic forces could be adjusted for three kinds of operating modes for the manipulations of beads and droplets, including movement of droplets containing magnetic beads, mixing of two droplets and beads extraction out of droplets. When compared with previous studies, the manipulations of droplets and magnetic particles in this study are more flexible as they can be easily adjusted by fine-tuning the magnetic flux density. Furthermore, the magnetic beads also served as three-dimensional substrates for the new enzyme-linked immunosorbent assay (ELISA)-like assay. The magnetic beads were conjugated with aptamers, which have high specificity towards H1N1 viruses such that they could be specifically captured and detected. The horseradish peroxidase-conjugated secondary antibody was then used to activate tyramide-tetramethylrhodamine (TTMR) such that fluorescent signals could be amplified. With this approach, the limit of detection was experimentally found to be 0.032 hemagglutination units/reaction, which is sensitive enough for clinical diagnostics. This kind of digital microfluidic platform with the ELISA-like assay could effectively reduce the consumption of samples and reagents such that the volume of all droplets including the H1N1 sample, antibodies, TTMR and wash buffers was only 20 μL. This is the first time that a digital microfluidic platform was demonstrated such that the entire diagnostic process for influenza A H1N1 viruses could be performed by using electromagnetic forces, which could be promising for rapid and accurate diagnosis of influenza.
- This article is part of the themed collection: Coronavirus articles - free to access collection


 Please wait while we load your content...
Please wait while we load your content...