When robotics met fluidics
Abstract
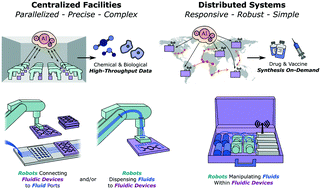
High-throughput fluidic technologies have increased the speed and accuracy of fluid processing to the extent that unlocking further gains will require replacing the human operator with a robotic counterpart. Recent advances in chemistry and biology, such as gene editing, have further exacerbated the need for smart, high-throughput experimentation. A growing number of innovations at the intersection of robotics and fluidics illustrate the tremendous opportunity in achieving fully self-driving fluid systems. We envision that the fields of synthetic chemistry and synthetic biology will be the first beneficiaries of AI-directed robotic and fluidic systems, and largely fall within two modalities: complex integrated centralized facilities that produce data, and distributed systems that synthesize products and conduct disease surveillance.


 Please wait while we load your content...
Please wait while we load your content...