Inertial microfluidic cube for automatic and fast extraction of white blood cells from whole blood†
Abstract
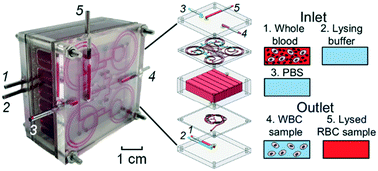
We report here a novel inertial microfluidic (IM) cube integrated with lysis, storage and extraction modules for extracting white blood cells (WBCs) from whole blood automatically, harmlessly and quickly. Lysis, storage, and extraction modules are designed to realize the purposes of complete mixing of whole blood and lysing buffer, thorough lysis of red blood cells (RBCs), and automatic extraction of WBCs from the lysed background RBCs, respectively. After demonstrating its conceptual design, we characterize the performances of the lysis and extraction modules. The results show that a high mixing efficiency of 94.2% can be achieved using our lysis modules for complete mixing of whole blood and lysing buffer. In the extraction module, an extraction efficiency of 88.1% can be achieved for the extraction of WBCs. Finally, we successfully apply our IM cube for the high throughput extraction of WBCs from human whole blood with an extraction efficiency of 83.9% and a cell viability of 96.6%, which are comparable to those using centrifugation and even better in some aspects. Our IM cube is based on passive secondary-flow mixing and inertial sorting, offers the advantages of small footprint, high stability and simple fabrication, and is a promising alternative method for extracting WBCs from human blood.


 Please wait while we load your content...
Please wait while we load your content...