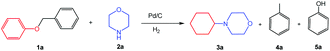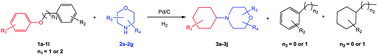Palladium-catalyzed synthesis of 4-cyclohexylmorpholines from reductive coupling of aryl ethers and lignin model compounds with morpholines†
Bingxiao
Zheng
a,
Jinliang
Song
 b,
Haihong
Wu
b,
Haihong
Wu
 *a,
Shitao
Han
a,
Jianxin
Zhai
a,
Kaili
Zhang
a,
Wei
Wu
a,
Caiyun
Xu
a,
Mingyuan
He
a and
Buxing
Han
*a,
Shitao
Han
a,
Jianxin
Zhai
a,
Kaili
Zhang
a,
Wei
Wu
a,
Caiyun
Xu
a,
Mingyuan
He
a and
Buxing
Han
 *ab
*ab
aShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, China. E-mail: hhwu@chem.ecnu.edu.cn; hanbx@iccas.ac.cn
bBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid and Interface and Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 17th November 2020
Abstract
This work describes the highly efficient Pd-catalyzed direct coupling of aryl ethers (including the typical lignin model compounds) and morpholines to produce 4-cyclohexylmorpholines, a useful class of fine chemicals. Without employing any acidic additives, various 4-cyclohexylmorpholines could be synthesized with good yields from a variety of aryl ethers using H2 as a hydrogen resource. A mechanism study revealed that the desired product was formed via the cleavage of the C(Ar)–O bonds to generate the corresponding cyclohexanones and subsequent reductive amination.
The transformation of renewable lignocellulose has been recognized as an alternative strategy to relieve the excessive reliance on depleting fossil resources (e.g., petroleum, coal and natural gas) for the production of chemicals and fuels.1–3 In this context, lignin, one of the three main components of lignocellulose, has gained significant research interest as a renewable aromatic carbon resource.4–9 Current research studies mainly focus on the synthesis of various oxygenates from lignin.10–17 In contrast, the strategies for the production of valuable nitrogen-containing compounds from lignin are still very limited.18–20 To convert lignin into small molecules, efficient cleavage of aromatic ether bonds (aryl C–O bonds) is the key. However, lignin has a very complex structure, resulting in difficulty in efficient and selective conversion of lignin. Alternatively, various aromatic ethers, including the motifs in lignin, were employed to study the cleavage of aromatic ether bonds, with the purpose of providing useful/potential methods for lignin conversion.
4-Cyclohexylmorpholines are an important class of N-containing compounds. As valuable fine chemicals, 4-cyclohexylmorpholines have been diversely employed as emulsifiers, corrosion inhibitors, and catalysts for polyurethane foam, etc. In general, 4-cyclohexylmorpholines can be synthesized through the reaction between diethylene glycol and cyclohexylamine or the reductive amination of cyclohexanone and morpholine, etc.21–26 Considering that the cyclohexanone could be potentially generated from the selective hydrogenolytic cleavage of the aryl ethers, some of which could be derived from lignin, it would be a sustainable strategy for the synthesis of 4-cyclohexylmorpholines from aryl ethers and morpholines by a successive process involving the cleavage of aryl ethers and reductive amination.15–17
It has been reported that various aryl amines or cyclohexylamines could be synthesized from the coupling of lignin-derived phenols with amines in the catalytic systems of Pd/C-sodium formate-trifluoroacetic acid27 or Pd/C-sodium formate.28,29 However, the use of sodium formate usually resulted in the generation of some salt waste. More importantly, there is a significant difference between the conversion of phenol and transformation of lignin into small molecules. Owing to the plenty of aryl C–O bonds in lignin, the synthesis of substituted amines by the reductive coupling of aryl ethers with amines would provide knowledge to study the catalytic systems for lignin conversion. In recent years, several catalytic systems have been developed for the synthesis of amines from aryl ethers (including the typical linkage in lignin).30,31 For example, various diaryl ethers, with 4-O-5 linkages in lignin, could be directly converted into amine derivatives (e.g., aryl amines and cyclohexylamines) over Pd(OH)2/C in the presence of NaBH4.30 Moreover, various cyclohexylamines could be prepared from aryl ethers in the catalytic system of Pd/C-Lewis acid using H2 as the hydrogen resource.31 In this catalytic system, the role of acidic additives was to activate the C–O bond, which was helpful for its cleavage. These results imply that the syntheses of 4-cyclohexylmorpholines from the direct reaction of morpholines and aryl ethers are feasible. However, these developed catalytic systems were hindered by the use of salt-type reductants (e.g., NaBH4) accompanied by the generation of salt waste30 or homogeneous acidic additives that are difficult to be separated or recycled.31 Therefore, it is highly desirable to develop simple and robust catalytic systems for the syntheses of 4-cyclohexylmorpholines by direct conversion of aryl ethers, including the typical lignin model compounds.
Inspired by the achievements in the synthesis of amines from lignin derivatives, herein, we report for the first time that 4-cyclohexylmorpholines could be efficiently synthesized via the direct reaction of morpholines with various aryl ethers, including several lignin model compounds, e.g., benzyl phenyl ether, phenethyl phenyl ether, and 2-phenoxyacetophenone, over Pd/C using molecular H2 at moderate pressure (1 MPa) in the absence of any acidic additives (Scheme 1). The fact that using H2 at the feasible pressure (e.g., 1 MPa) to generate more active H could promote the hydrogenolysis of the C–O bond, which could replace the role of homogeneous acidic additives, thereby having some obvious advantages from the green chemistry points of view. The new class of useful N-containing compounds (4-cyclohexylmorpholines) and more applicability of lignin model compounds further confirm the great potential of this catalytic system.
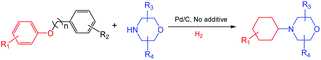 | ||
| Scheme 1 Synthesis of 4-cyclohexylmorpholines from the direct coupling of lignin model compounds and morpholines. | ||
Initially, the conversion of benzyl phenyl ether (the typical α-O-4 linkage in lignin) with morpholine to synthesize 4-cyclohexylmorpholine was selected as a model reaction to evaluate the catalytic activity of various catalysts (Table 1). No reaction occurred in the absence of a catalyst (Table 1, entry 1). Pt/C was inactive for the reaction (Table 1, entry 2) because it generally showed lower activity in the hydrogenolytic cleavage of C–O ether bonds.16 Moreover, the yield of 4-cyclohexylmorpholine was very low over Ru/C (Table 1, entry 3) because of the low activity of Ru/C for both the cleavage of the ether bonds in benzyl phenyl ether and the hydrogenation of phenol under the employed reaction conditions, which could be verified by several control experiments (Scheme S1†). To our delight, the yield of 4-cyclohexylmorpholine could reach 99% with a complete conversion of benzyl phenyl ether over Pd/C (Table 1, entry 4). Additionally, although a small amount of benzyl phenyl ether was converted into toluene and phenol over homogeneous Pd(acac)2, no 4-cyclohexylmorpholine was generated (Table 1, entry 5) because the generated phenol could not be transformed into cyclohexanone (the intermediate for the desired product) in this catalytic system. From the above discussion, we could find that the intrinsic nature of the catalyst affected the reactivity of the reaction, and Pd/C was the best choice among the studied catalysts.
| Entry | Catalyst | Conversionb (%) | Yieldb (%) | ||
|---|---|---|---|---|---|
| 3a | 4a | 5a | |||
| a Reaction conditions: 1a, 1.0 mmol; 2a, 3.5 mmol; catalyst, 6 mol% metal based on 1a; m-xylene, 3.5 mL; reaction temperature, 90 °C; H2 pressure, 3 MPa; and reaction time, 8 h. b The conversion and yield were determined by GC using dodecane as a standard. | |||||
| 1 | — | — | — | — | — |
| 2 | Pt/C | — | — | — | — |
| 3 | Ru/C | 18.4 | 9.8 | 18 | 8.5 |
| 4 | Pd/C | 100 | >99 | >99 | — |
| 5 | Pd(acc)2 | 10.9 | — | 9.6 | 9.8 |
Various reaction parameters were subsequently optimized with Pd/C as the catalyst. First, it was found that the molar ratio of morpholine and benzyl phenyl ether could affect the product distribution (Fig. 1A). With a molar ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or more, the products were 4-cyclohexylmorpholine and toluene. In contrast, phenol or methylcyclohexane would be detected when the molar ratio is decreased to less than 1.5
1 or more, the products were 4-cyclohexylmorpholine and toluene. In contrast, phenol or methylcyclohexane would be detected when the molar ratio is decreased to less than 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (e.g., 1
1 (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5
1, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the amount of phenol or methylcyclohexane increased with the decrease of the molar ratio. These results indicated that 1.5
1), and the amount of phenol or methylcyclohexane increased with the decrease of the molar ratio. These results indicated that 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was the optimal molar ratio of morpholine and benzyl phenyl ether in our reaction system. Second, the influence of Pd usage was investigated. There was no obvious difference in the yield when the amount of Pd decreased from 6 to 3 mol% (Fig. 1B). However, the yield of the product decreased on reducing the amount of the catalyst when the Pd amount was less than 3 mol%, suggesting that the suitable Pd usage was 3 mol%. Third, too low H2 pressure was not favorable to the reaction (Fig. 1C). For example, the yield of 4-cyclohexylmorpholine was only 58.6% when the reaction was conducted at 0.5 MPa. When the reaction was carried out at 1 MPa, the benzyl phenyl ether would be completely converted with a high yield of 4-cyclohexylmorpholine (>99%). These results were probably caused by the amount of active H formed on the Pd surface under different H2 pressure conditions. Generally, higher H2 pressure was beneficial for the formation of active H, which could improve the reactivity of the hydrogenolysis of the C–O bond to form phenol and toluene. Thus, 1 MPa was selected as the optimal pressure for subsequent studies. Through a moderate H2 pressure (1 MPa), the use of homogeneous acidic additives was avoided. Fourth, kinetic experiments indicated that the reaction could be completed in 6 h (Fig. 1D). In the reaction process, the yields of 4-cyclohexylmorpholine and toluene increased with the prolonging reaction time, while the yield of phenol sharply increased first and then gradually decreased. Finally, it was observed that the reaction temperature had a significant impact on the reactivity (Fig. 1E). When the reaction temperature increased from 60 to 90 °C, both the conversion and yield increased, while the yield of the desired 4-cyclohexylmorpholine was constant when the reaction temperature was higher than 90 °C. Therefore, 90 °C was the optimal reaction temperature for our reaction system. Additionally, the recyclability of the catalyst was studied under both 99% yield and lower yield (about 40%) of the product. The results indicated that Pd/C could be reused for at least three catalytic cycles with no obvious change in both substrate conversion and product (i.e., 4-cyclohexylmorpholine and toluene) yields with a reaction time of 6 h (Fig. 1F) or 1.6 h (Fig. 1G), indicating that Pd/C was stable under the reaction conditions. As characterized by TEM (Fig. S1†), the recovered Pd/C had the same morphology as the original Pd/C.
1 was the optimal molar ratio of morpholine and benzyl phenyl ether in our reaction system. Second, the influence of Pd usage was investigated. There was no obvious difference in the yield when the amount of Pd decreased from 6 to 3 mol% (Fig. 1B). However, the yield of the product decreased on reducing the amount of the catalyst when the Pd amount was less than 3 mol%, suggesting that the suitable Pd usage was 3 mol%. Third, too low H2 pressure was not favorable to the reaction (Fig. 1C). For example, the yield of 4-cyclohexylmorpholine was only 58.6% when the reaction was conducted at 0.5 MPa. When the reaction was carried out at 1 MPa, the benzyl phenyl ether would be completely converted with a high yield of 4-cyclohexylmorpholine (>99%). These results were probably caused by the amount of active H formed on the Pd surface under different H2 pressure conditions. Generally, higher H2 pressure was beneficial for the formation of active H, which could improve the reactivity of the hydrogenolysis of the C–O bond to form phenol and toluene. Thus, 1 MPa was selected as the optimal pressure for subsequent studies. Through a moderate H2 pressure (1 MPa), the use of homogeneous acidic additives was avoided. Fourth, kinetic experiments indicated that the reaction could be completed in 6 h (Fig. 1D). In the reaction process, the yields of 4-cyclohexylmorpholine and toluene increased with the prolonging reaction time, while the yield of phenol sharply increased first and then gradually decreased. Finally, it was observed that the reaction temperature had a significant impact on the reactivity (Fig. 1E). When the reaction temperature increased from 60 to 90 °C, both the conversion and yield increased, while the yield of the desired 4-cyclohexylmorpholine was constant when the reaction temperature was higher than 90 °C. Therefore, 90 °C was the optimal reaction temperature for our reaction system. Additionally, the recyclability of the catalyst was studied under both 99% yield and lower yield (about 40%) of the product. The results indicated that Pd/C could be reused for at least three catalytic cycles with no obvious change in both substrate conversion and product (i.e., 4-cyclohexylmorpholine and toluene) yields with a reaction time of 6 h (Fig. 1F) or 1.6 h (Fig. 1G), indicating that Pd/C was stable under the reaction conditions. As characterized by TEM (Fig. S1†), the recovered Pd/C had the same morphology as the original Pd/C.
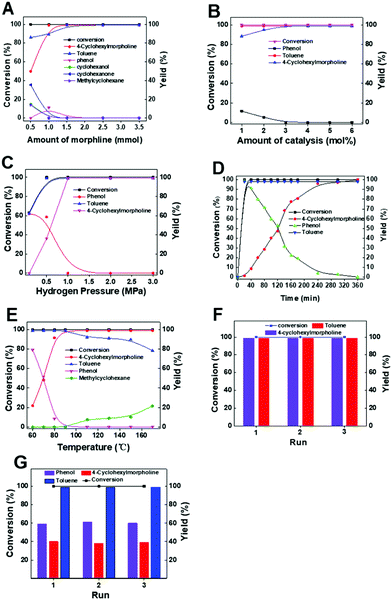 | ||
| Fig. 1 Effect of various reaction parameters. (A) Morpholine amount, (B) catalyst amount, (C) H2 pressure, (D) reaction time, (E) effect of reaction temperature, (F) reusability of Pd/C (6 h) and (G) reusability of Pd/C (1.6 h). Other conditions were the same as in entry 4 of Table 1. | ||
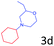
Delighted by the excellent result of the reaction of benzyl phenyl ether and morpholine, the scope of the substrates (both morpholines and ethers) was explored to show the applicability of this strategy to synthesize 4-cyclohexylmorpholines (Table 2). First, it was found that various morpholines with different substituents could efficiently react with benzyl phenyl ether (1a) to generate the corresponding 4-cyclohexylmorpholines (Table 2, entries 1–7), and the position of the substituent would affect the reactivity of the morpholines. The yields of the desired products would be quantitative when the substituent is at the meta-position of the amine group (Table 2, entries 2–5). In comparison, when the substituent was at the ortho-position of the amine group, the reactivity of the morpholines decreased, and the reaction temperature was increased to 120 °C to achieve a good yield (about 86%) of the desired products (Table 2, entries 6 and 7). Second, the reactivities of various ethers with morpholine (2a) were investigated (Table 2, entries 8–19). When the substituents were on the benzyl group (Table 2, entries 9–14), there was no significant difference in the yield of 4-cyclohexylmorpholine. High yields (>93%) were obtained for all the substrates with substituents on the benzyl group when the reactions were conducted at 90 °C with a reaction time of 6 h. In contrast, when the substituents were on the phenyl part (Table 2, entries 15–18), 4-cyclohexylmorpholines were not generated at 90 °C because the cleavage of the C–O bond to form the corresponding phenols and subsequent hydrogenation of these substituted phenols were both difficult at a low temperature like 90 °C. To obtain satisfactory yields (>85%) of the corresponding 4-cyclohexylmorpholine, high reaction temperature (150 °C) should be employed for the substrates with substituents on the phenyl part (Table 2, entries 15–18). Herein, it should be pointed out that the products 3f and 3g–3j were a mixture of cis/trans isomers. Furthermore, phenethyl phenyl ether and 2-phenoxyacetophenone (Table 2, entries 19 and 20), the most abundant C–O linkage (β-O-4) in lignin, could react with morpholine to generate 4-cyclohexylmorpholine with yields of 39% and 35%. These results above indicated that the developed catalytic system was highly applicable in the synthesis of 4-cyclohexylmorpholine through the direct cleavage of the ether bonds.
| Entry | Substrates | Products | Yieldsb (%) |
|---|---|---|---|
| a Reaction conditions: 1, 1.0 mmol; 2, 1.5 mmol; catalyst, 3 mol% metal based on 1; m-xylene, 3.5 mL; reaction temperature, 90 °C; H2 pressure, 1 MPa; and reaction time, 6 h. b The yield was determined by GC using dodecane as a standard. c Reaction temperature, 120 °C; reaction time, 12 h. d Catalyst, 6 mol% metal based on 1, reaction temperature, 150 °C; H2 pressure, 3 MPa; and reaction time 12 h. e Catalyst, 9 mol% metal based on 1, reaction temperature, 170 °C; H2 pressure, 3 MPa; and reaction time, 24 h. | |||
| 1 |
|
|
>99 |
| 2 |
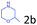
|

|
>99 |
| 3 |
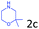
|
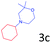
|
>99 |
| 4 |
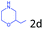
|
|
>99 |
| 5 |
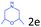
|

|
>99 |
| 6c |
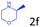
|
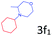
|
86.1 |
| 7c |
|
|
85.9 |
| 8 |

|
|
>99 |
| 9 |

|
|
97.0 |
| 10 |

|

|
97.4 |
| 11 |
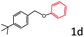
|

|
98.1 |
| 12 |

|

|
93.3 |
| 13 |

|

|
98.7 |
| 14 |

|

|
>99 |
| 15d |

|
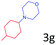
|
>99 |
| 16d |

|

|
>99 |
| 17d |

|

|
84.7 |
| 18d |
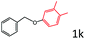
|
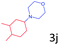
|
96.5 |
| 19e |
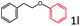
|

|
39.4 |
| 20e |
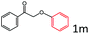
|

|
35.0 |
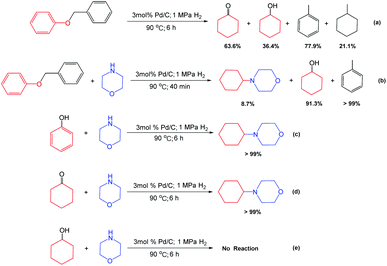
To reveal the reaction mechanism, several control experiments were conducted. First, benzyl phenyl ether could be transformed into cyclohexanone, cyclohexanol, toluene, and methylcyclohexane over Pd/C (3 mol% Pd) under the above optimal reaction conditions (90 °C, 6 h, and 1 MPa H2) in the absence of morpholine (Scheme 2a), indicating that the C–O in the benzyl phenyl ether could be cleaved under the used conditions. Second, in the presence of morpholine, the hydrogenation of toluene could not occur (Scheme 2b) due to the negative effect of nitrogen-containing compounds. This result also provided the reason why there was no hydrogenation of toluene observed in the process to produce 4-cyclohexylmorpholine. Third, phenol and cyclohexanone could be quantitatively converted to generate (Scheme 3) 4-cyclohexylmorpholine (Scheme 2c and d), while no product was detected when using cyclohexanol (Scheme 2e). These results indicated that cyclohexanone from the hydrogenation of phenol could be rapidly consumed, and thus, no cyclohexanol was formed in the process with morpholine.
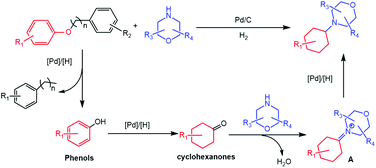
Based on the above experimental results and some reported information, a possible reaction mechanism was proposed for the formation of 4-cyclohexylmorpholines from the reaction of aryl ethers and morpholines (Scheme 3). Initially, H2 was activated to form active H on the surface of Pd particles. Then, hydrogenolytic transformation of the aryl ethers to form phenols and substituted benzene occurred because of the higher bonding energy of the aromatic C(sp2)–O bond than that of the alkyl C(sp3)–O bond.32 The generated phenols would be hydrogenated to generate the corresponding cyclohexanones, which could react with morpholines to form the intermediate A. Finally, the desired 4-cyclohexylmorpholines were formed by the hydrogenation of the intermediate A.
In addition, a control experiment indicated that substituted benzenes could not be hydrogenated under the standard conditions (90 °C with morpholine, Scheme S2†). Thus, the yields of the substituted benzenes were quantitative in the reactions conducted under standard conditions. However, a very small part of the substituted benzenes would be hydrogenated when more extreme conditions (e.g., 120, 150, and 170 °C) were involved (Table 2, entries 5, 6 and 13–20). Thus, their yields were no longer quantitative (Table S1†).
Conclusions
In conclusion, we have developed a direct route to synthesize 4-cyclohexylmorpholines from morpholines and aryl ethers over Pd/C with H2 as the hydrogen resource. Various aryl ethers, including several lignin model compounds, e.g., benzyl phenyl ether (α-O-4 linkage in lignin), phenethyl phenyl ether and 2-phenoxyacetophenone (β-O-4 linkage in lignin), could be efficiently and directly transformed into the corresponding 4-cyclohexylmorpholines without using any acidic additives. More importantly, it was found that ethers with substituents on the benzyl group showed higher reactivity than those with substituents on the phenyl part. We believe that this methodology has great potential in the cleavage of C–O bonds in aryl ethers, and also shows a promising application in upgrading renewable lignin derivatives into highly valuable nitrogen-containing chemicals. However, it should be pointed out that there is significant difference between the used aryl ethers and raw lignin, although they both contain aryl C–O bonds. This strategy shows very low reactivity when using raw lignin as the reactant, which is probably caused by the complex structure of raw lignin. Direct and efficient conversion of raw lignin into N-containing compounds is a great challenge, and more efforts still need to be paid to developing robust catalytic methods for efficient lignin transformation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Key Research and Development Program of China (2017YFA0403103), Beijing Municipal Science & Technology Commission (Z191100007219009), the Chinese Academy of Sciences (QYZDY-SSW-SLH013), and Youth Innovation Promotion Association of CAS (2017043).Notes and references
- C. L. Chen, J. Y. Xin, L. L. Ni, H. X. Dong, D. X. Yan, X. M. Lu and S. J. Zhang, Green Chem., 2016, 18, 2341–2352 RSC.
- Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS.
- K. Sanderson, Nature, 2011, 474, S12–S14 CrossRef CAS.
- (a) A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843 CrossRef; (b) E. Furimsky, Appl. Catal., A, 2000, 199, 147–190 CrossRef CAS.
- Y. Yang, H. Fan, J. Song, Q. Meng, H. Zhou, L. Wu, G. Yang and B. Han, Chem. Commun., 2015, 51, 4028–4031 RSC.
- C. F. Zhang, J. M. Lu, X. C. Zhang, K. MacArthur, M. Heggen, H. J. Li and F. Wang, Green Chem., 2016, 18, 6545–6555 RSC.
- M. Wang, L. H. Li, J. M. Lu, H. J. Li, X. C. Zhang, H. F. Liu, N. C. Luo and F. Wang, Green Chem., 2017, 19, 702–706 RSC.
- L. Dong, L. L. Yin, Q. N. Xia, X. H. Liu, X. Q. Gong and Y. Q. Wang, Catal. Sci. Technol., 2018, 8, 735–745 RSC.
- F. Mauriello, E. Paone, R. Pietropaolo, A. M. Balu and R. Luque, ACS Sustainable Chem. Eng., 2018, 6, 9269–9276 CrossRef CAS.
- S. Song, J. Zhang, G. Gözaydın and N. Yan, Angew. Chem., Int. Ed., 2019, 58, 4934–4937 CrossRef CAS.
- L. Jiang, H. W. Guo, C. Z. Li, P. Zhou and Z. H. Zhang, Chem. Sci., 2019, 10, 4458–4468 RSC.
- M. Hua, J. L. Song, C. Xie, H. R. Wu, Y. Hu, X. Huang and B. Han, Green Chem., 2019, 21, 5073–5079 RSC.
- N. Yan, Y. Yuan, R. Dykeman, Y. Kou and P. J. Dyson, Angew. Chem., Int. Ed., 2010, 49, 5549–5553 CrossRef CAS.
- K. L. Luska, P. Migowski, S. E. Sayed and W. Leitner, Angew. Chem., Int. Ed., 2015, 54, 15750–15755 CrossRef CAS.
- L. Dong, L. Lin, X. Han, X. Si, X. Liu, Y. Guo, F. Lu, S. Rudć, S. F. Parker, S. Yang and Y. Wang, Chem, 2019, 5, 1521–1536 CAS.
- Q. Meng, M. Hou, H. Liu, J. Song and B. Han, Nat. Commun., 2017, 8, 14190–14197 CrossRef CAS.
- Z. Zhang, M. Liu, J. Song, H. Liu, Z. Xie, S. Liu, Q. Meng, P. Zhang and B. Han, Green Chem., 2018, 20, 4865–4869 RSC.
- J. Julis and W. Leitner, Angew. Chem., Int. Ed., 2012, 51, 8615–8619 CrossRef CAS.
- K. L. Luska, J. Julis, E. Stavitski, D. N. Zakharov, A. Adams and W. Leitner, Chem. Sci., 2014, 5, 4895–4905 RSC.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51, 2564–2601 CrossRef CAS.
- T. E. Muller and M. Beller, Chem. Rev., 1998, 98, 675–703 CrossRef.
- D. Crozet, M. Urrutigoity and P. Kalck, ChemCatChem, 2011, 3, 1102–1118 CrossRef CAS.
- (a) M. Stein and B. Breit, Angew. Chem., Int. Ed., 2013, 52, 2231–2234 CrossRef CAS; (b) T. J. Barker and E. R. Jarvo, Angew. Chem., Int. Ed., 2011, 50, 8325–8328 CrossRef CAS; (c) T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef.
- M. H. S. A. Hamid, C. L. Allen, G. W. Lamb, A. C. Maxwell, H. C. Maytum, A. J. A. Watson and J. M. J. Williams, J. Am. Chem. Soc., 2009, 131, 1766–1774 CrossRef CAS.
- H. Alinezhad, M. Tajbakhsh, F. Salehian and K. Fazli, Tetrahedron Lett., 2009, 50, 659–661 CrossRef CAS.
- N. M. Patil and B. M. Bhanage, Catal. Today, 2015, 247, 182–189 CrossRef CAS.
- Z. W. Chen, H. Y. Zeng, S. A. Girard, F. Wang, N. Chen and C. J. Li, Angew. Chem., Int. Ed., 2015, 54, 14487–14491 CrossRef CAS.
- Z. W. Chen, H. Y. Zeng, H. Gong, H. N. Wang and C. J. Li, Chem. Sci., 2015, 6, 4174–4178 RSC.
- V. R. Jumde, E. Petricci, C. Petrucci, N. Santillo, M. Taddei and L. Vaccaro, Org. Lett., 2019, 21, 7033–7037 CrossRef.
- H. Y. Zeng, D. W. Cao, Z. H. Qiu and C. J. Li, Angew. Chem., Int. Ed., 2018, 130, 3814–3819 CrossRef.
- X. J. Cui, K. Junge and M. Beller, ACS Catal., 2016, 6, 7834–7838 CrossRef CAS.
- (a) C.-C. Chiu, A. Genes, A. Borgna and N. Rösch, ACS Catal., 2014, 4, 4178–4188 CrossRef CAS; (b) H. Wu, J. Song, C. Xie, C. Wu, C. Chen and B. Han, ACS Sustainable Chem. Eng., 2018, 6, 2872–2877 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed Experimental section with materials, general procedures for the coupling of morpholines with ethers, NMR data and HR-MS data. See DOI: 10.1039/d0gc03188g |
| This journal is © The Royal Society of Chemistry 2021 |