Open Access Article
Open Access ArticleMobility and adsorption of liquid organic hydrogen carriers (LOHCs) in soils – environmental hazard perspective†
Ya-Qi
Zhang
 ab,
Stefan
Stolte
ab,
Gizem
Alptekin
a,
Alica
Rother
a,
Michael
Diedenhofen
ab,
Stefan
Stolte
ab,
Gizem
Alptekin
a,
Alica
Rother
a,
Michael
Diedenhofen
 c,
Juliane
Filser
c,
Juliane
Filser
 d and
Marta
Markiewicz
d and
Marta
Markiewicz
 *ab
*ab
aUFT – Center for Environmental Research and Sustainable Technology, Department of Sustainable Chemistry, University of Bremen, Leobener Str. 6, D-28359 Bremen, Germany. E-mail: marta.markiewicz@tu-dresden.de
bInstitute of Water Chemistry, Technische Universität Dresden, Berg Str. 66, D-01069 Dresden, Germany
cBIOVIA, Dassault Systèmes Deutschland GmbH, D-51379 Leverkusen, Germany
dUFT – Center for Environmental Research and Sustainable Technology, Department of General and Theoretical Ecology, University of Bremen, Leobener Str. 6, D-28359 Bremen, Germany
First published on 17th September 2020
Abstract
Liquid organic hydrogen carriers (LOHCs) are an energy system that can be used to store and transport hydrogen under standard temperature and pressure chemically bound to a carrier. The LOHC systems show advantages over conventional energy systems (recyclability, higher sustainability and lower emissions) and other hydrogen-based systems (lower loses, ease of handling and higher safety), and are applied in stationary and mobile applications worldwide. The scale and type of use indicate that the release of LOHCs to the environment can be expected. Yet, their behaviour and fate have not been investigated especially with regard to assessment of exposure, mobility and possibility to reach surface water, groundwater or drinking water sources. To investigate that we studied the mobility of thirteen technologically promising LOHC candidates including indole, quinaldine, carbazole derivatives, benzyltoluene and dibenzyltoluene, and their (partially) saturated forms in soil, for the first time. The substances were classified into mobility classes based on their organic carbon–water partition coefficients (Koc) determined via in silico models and HPLC screening. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values increased in the order indoles < quinaldines < carbazole derivatives < benzyltoluenes < dibenzyltoluenes covering a full spectrum of mobility scale (from highly mobile to immobile). The behaviour of exemplary LOHC system – quinaldine including H2-unsaturated, partially and fully saturated forms – was further assessed by investigating the soil-water partition coefficients (Kd) via adsorption batch equilibrium and column leaching test. The study showed that some LOHCs can be expected to be very mobile in soils and have the potential to reach groundwater.
Koc values increased in the order indoles < quinaldines < carbazole derivatives < benzyltoluenes < dibenzyltoluenes covering a full spectrum of mobility scale (from highly mobile to immobile). The behaviour of exemplary LOHC system – quinaldine including H2-unsaturated, partially and fully saturated forms – was further assessed by investigating the soil-water partition coefficients (Kd) via adsorption batch equilibrium and column leaching test. The study showed that some LOHCs can be expected to be very mobile in soils and have the potential to reach groundwater.
Introduction
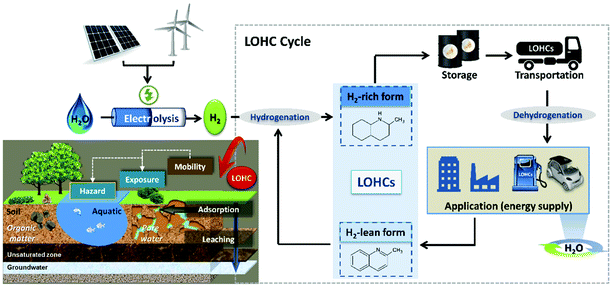
LOHC systems are promising alternatives developed in recent years to support and improve the current options for energy storage and transport.1–3 Unlike traditional energy systems in which fossil fuels are the main energy source, hydrogen that is covalently bound to LOHCs is used as the energy vector.1,2 Compared to fossil fuels, hydrogen shows higher gravimetric energy density (120 MJ kg−1)4–6 and inherently lower emissions (the only exhaust gas after combustion is water vapor, as opposed to SO2, NOx, CO, CO2 and volatile organic compounds produced from fossil fuels5,7). The hydrogen used in LOHC systems can be produced either from fossil fuels by steam reforming or by water electrolysis2,8 using renewable energy sources (Fig. 1), with the latter considered more attractive.9LOHC systems usually consist of a tandem of organic molecules, one of which is saturated (H2-rich, loaded), and the other is unsaturated (H2-lean, unloaded or spent). The compounds forming the LOHC tandem can be reversibly converted into each other by catalytic hydrogenation (e.g., catalysts Ru/Al2O3, 10–50 bar,8 150 °C (ref. 10)) of the unsaturated form and dehydrogenation (e.g., catalysts Pt/Al2O3, 1–5 bar,8 130–300 °C (ref. 8, 10 and 11)) of the saturated form (Fig. 1).9 The LOHC chemicals are not consumed in the process of energy generation and can be subject to multiple hydrogenation and dehydrogenation cycles, which is very different from fossil fuels.2 Due to the similarities in the physicochemical properties of LOHCs to those of fossil fuels,2,3 they can be implemented using the existing infrastructure, such as ships, ports,2 oil tanks, pipelines, and fuelling stations,1,12 that were developed for the transport, distribution and processing of fossil fuels. Use of existing infrastructure is economically attractive due to the lower investment costs3,4 and a smooth transition between conventional and LOHC energy systems.1,3
Indole,3,8 quinaldine,3,8 and carbazole derivatives,2,3,8,12 as well as isomeric mixtures of benzyltoluene or dibenzyltoluene13,14 with hydrogen storage capacities ranging from 5 to 7 wt%,3,4 have been recently proposed as the most promising potential candidates. The incorporation of nitrogen into the cyclic ring of LOHCs reduces the dehydrogenation enthalpy3 and improves the thermodynamics and kinetics of dehydrogenation.4,8 This renders nitrogen-substituted heterocycles particularly promising candidates.3 The technological advantages and disadvantages related to particular LOHC candidates, especially in automotive applications, have recently been reported in detail,3,12–16 showing that different LOHC systems can be chosen to fulfil requirements of different applications.
The considerable application potential of LOHC systems opens the global markets to this technology and relevant projects and product developments have been initiated in 2011, 2013, and 2014 by Chiyoda Corporation (Japan), Hydrogenious LOHC Technologies (Germany), and Hynertech (China), respectively.17 Accordingly, several large-scale pilot applications were launched around the world recently, for instance in Japan (global hydrogen supply chain),18 Germany (off-grid power supply8,19 and railway transport powered by LOHC20), and China (fuel cell bus fuelled by LOHC21 and mobility infrastructure22). In addition, the prospect of using LOHC technology in underground mining equipment and stationary power applications in South Africa has been recently announced.10 Moreover, some LOHC chemicals like benzyltoluene and dibenzyltoluene are already used, although on smaller scale, as heat transfer liquids23 while others are components of fossil fuels (e.g., quinaldine or unsubstituted carbazole).24
Usually a tank containing approximately 80 kg of LOHCs is required to achieve a driving range of approx. 700 km.8 The full-scale application of this technology and the complete replacement of fossil fuels would thus require approx. 2 × 1010–3 × 1010 tons of LOHCs to be handled, processed, stored and transported to satisfy the current energy demand worldwide.4,11 Due to such large volume in application and transport, the consequence of LOHC release (e.g., through leaks and accidental spills during production, transport and fuelling) into the environment should be considered. More specifically, the environmental hazard potential of these chemicals should ideally be known before the technology enters widespread commercial use to assure that the LOHC systems of both the best technological performance and least harm to the environment are chosen. However, experimental data regarding the environmental behaviour (in terms of mobility, adsorption and fate) of the LOHCs are still too scarce to make a reliable assessment.
There are several reasons that make the assessment of environmental impacts of the LOHC chemicals particularly interesting besides the large application scale. Firstly, many LOHC chemicals are organic bases with (predicted) pKb (base dissociation constant) values within environmentally relevant range, meaning they will be at least partially positively charged. Ionic and ionisable chemicals are outside the applicability domain of most models designed to predict affinity to soils.25,26 Consequently, these models deliver inadequate predictions, sometimes several orders of magnitude different from the measured value.27 Secondly, the lack of experimental data for Kow (n-octanol/water partition coefficient), Dow (pH-dependent distribution coefficient) or pKb that are needed for the models means that these values have to be estimated as well, which additionally increases the uncertainty of prediction. Lastly, our recent studies have indicated that some of the LOHC chemicals investigated here are poorly biodegradable (i.e. potentially persistent) or show considerable acute aquatic toxicity11 and chronic soil toxicity.28 Therefore, their continued release to the environment would lead to increasing concentrations which might adversely affect the biota.
Recently, in addition to Persistent, Bioaccumulative and Toxic (PBT) substances, a new class of substances of high concern was identified by regulatory agencies (German Environment Agency, UBA – Das Umweltbundesamt) namely Persistent, Mobile and Toxic (PMT) substances.29 These chemicals are polar and exhibit lower affinities to organic matter and solids in soils and sediments, as a result they might move through aquifers and various natural (e.g., riverbank and soil layers) or artificial (wastewater treatment plant29,30) barriers,29 implying increased probability to enter the water cycle and perhaps even to reach drinking water sources.29 Therefore, unlike PBT substances to which biota and humans are primarily exposed via diet, PMT chemicals are likely to exert negative effects via water sources.29 The UBA has recently proposed to qualify PMTs as Substances of Very High Concern (SVHC),29,31 which need to be treated with “equivalent level of concern” to those listed by REACH (e.g., PBTs) in terms of the direct impacts to human health or the environment29via e.g., drinking water supplies.
Due to the physicochemical properties of LOHCs as well as the types of use, all environmental compartments, i.e. soil, water and air, are likely to be affected. Among these compartments, soil is of particular importance since it usually acts as a barrier and sink for anthropogenically produced organic chemicals. Once in the soil, organic contaminants may undergo various processes, among which adsorption is particularly important in defining their mobility, i.e. how far they can spread from the point of release and what concentrations can be expected in adjacent ground and surface waters. The assessment of the chemicals’ affinity to soils is therefore crucial in determining their probability to reach the groundwater and drinking water sources. Adsorption also defines the extent of exposure of soil-dwelling organisms in which uptake is not based on the consumption of soil particles (i.e. porewater is the dominant route of exposure).
The present study therefore aimed to characterise the behaviour of several promising LOHC systems based on: quinaldine, indole, carbazole derivatives (ethylcarbazole, butylcarbazole, propylcarbazole), benzyltoluene and dibenzyltoluene, by investigating their adsorption to and mobility in standard soil. To this end, organic carbon–water partition coefficients (Koc) were measured using HPLC, and based on these values LOHC chemicals were assigned to mobility classes. Additionally, the soil-water partition coefficients (Kd) were measured using batch equilibrium and soil column leaching methods.
Materials and methods
A detailed description of the chemicals and the experimental procedures is given in the ESI.†Materials
The LOHC systems tested in the present study, including indoles, quinaldines (Quin), alkylcarbazole derivatives (Cars), benzyltoluenes (marketed as Marotherm LH® – MLH) and dibenzyltoluene (marketed as Marlotherm SH® – MSH) (Table 1), were provided by the research group of Prof. Dr Peter Wasserscheid, Institute of Chemical Reaction Engineering, Friedrich-Alexander University of Erlangen-Nürnberg, Germany. Two batches of standard soil RefeSol 01-A-004 (briefly named soil I and soil II), loamy sand (soil I: pH (CaCl2) = 5.4, Corg 1.21%, sand 76.7%, silt 17.2%, clay 6.1%; soil II: pH (CaCl2) = 5.3, Corg 0.80%, same texture as soil I) were ordered from Fraunhofer IME, Schmallenberg, Germany (Table S1†). The soils were dried for 24 h at room temperature and 2.0 mm-sieved before tests. Detailed information on all the materials is available in ESI-S1.†| LOHC name | Abbreviation | Formula | Chemical structure | MW [g mol−1] | Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sw Sw![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (25 °C) [mg L−1] a (25 °C) [mg L−1] |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow Kow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc Koc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
P.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c [%] c [%] |
|---|---|---|---|---|---|---|---|---|
“P.” indicates percentage of protonation; “n.a.” indicates values are out of the environmentally relevant range.a Sw: water solubility at 25 °C, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow: n-octanol/water partition coefficient, log Kow: n-octanol/water partition coefficient, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc: soil organic carbon–water partition coefficient. Values were predicted using in silico model COSMO-RS for the neutral forms of the compound (see ESI-S2†).b Mean values of cis and trans isomers.c Estimated at the pH 5.3 and pH 5.4 (the pH of test soil I and II) using MarvinSketch.35 Koc: soil organic carbon–water partition coefficient. Values were predicted using in silico model COSMO-RS for the neutral forms of the compound (see ESI-S2†).b Mean values of cis and trans isomers.c Estimated at the pH 5.3 and pH 5.4 (the pH of test soil I and II) using MarvinSketch.35 |
||||||||
| 2-Methyl-quinoline | Quin-2Me | C10H9N |
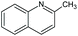
|
143.2 | 3.95 | 2.45 | 2.18 | 40 or 36 |
| Tetrahydro-2-methyl-quinoline | Quin-2Me-ph | C10H13N |
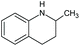
|
147.2 | 2.66 | 3.04 | 2.48 | 26 or 23 |
| Decahydro-2-methyl-quinolineb | Quin-2Me-H10 | C10H19N |
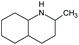
|
153.3 | 3.86 | 3.25 | 2.53 | 100 |
| Indole | Indole | C8H7N |
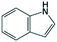
|
117.2 | 4.05 | 2.32 | 2.21 | n.a. |
| Indoline | Indoline | C8H9N |
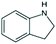
|
119.2 | 3.71 | 2.04 | 1.94 | 16 or 14 |
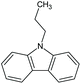
| 9-Ethyl-9H-carbazole | Car-2 | C14H13N |
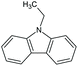
|
195.3 | 1.09 | 4.42 | 3.36 | n.a. |
| 9-Ethyl-hexahydro-carbazoleb | Car-2-ph | C14H19N |
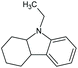
|
201.3 | 0.97 | 4.60 | 3.08 | 20 or 17 |
| 9-Ethyl-octahydro-carbazole | C14H21N |
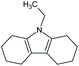
|
203.3 | 0.36 | 5.19 | 3.26 | n.a. | |
| 9-Ethyl-dodecahydro-carbazole | Car-2-H12 | C14H25N |
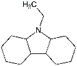
|
207.4 | 1.91 | n.a. | n.a. | 100 |
| 9-Propyl-9H-carbazole | Car-3 | C15H15N |
|
209.3 | 0.54 | 4.96 | 3.61 | n.a. |
| 9-Propyl-hexahydro-carbazoleb | Car-3-ph | C15H21N |
|
215.3 | 0.41 | 5.16 | 3.36 | 27 or 23 |
| 9-Propyl-octahydro-carbazole | C15H23N |
|
217.4 | −0.19 | 5.72 | 3.50 | n.a. | |
| 9-Propyl-dodecahydro-carbazole | Car-3-H12 | C15H27N |
|
221.4 | 1.40 | n.a. | n.a. | 100 |
| 9-Butyl-9H-carbazole | Car-4 | C16H17N |
|
223.3 | −0.001 | 5.50 | 3.89 | n.a. |
| 9-Butyl-hexahydro-carbazoleb | Car-4-ph | C16H23N |
|
229.4 | −0.04 | 5.58 | 3.62 | 27 or 24 |
| 9-Butyl-octahydro-carbazole | C16H25N |
|
231.4 | −0.77 | 6.27 | 3.79 | n.a. | |
| 9-Butyl-dodecahydro-carbazole | Car-4-H12 | C16H29N |
|
235.4 | 0.89 | n.a. | n.a. | 100 |
| Benzyltoluene | MLH | C14H14 |

|
182.3 | 0.69 | 4.78 | 3.34 | n.a. |
| Benzyltoluene (partially hydrogenated)b | MLH-ph | C14H20 |
|
188.3 | 0.02 | 5.46 | 3.65 | n.a. |
| Dibenzyltolueneb | MSH | C21H20 |

|
272.4 | −1.37 | 6.84 | 4.52 | n.a. |
Determination of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Koc
Koc
The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values of the LOHCs (Table 1) were first predicted by the Conductor-like Screening Model for Realistic Solvation (COSMO-RS, Leverkusen, Germany, see ESI-S2†) to estimate the retention times. Subsequently, the Koc values of the H2-lean and partially hydrogenated LOHC (-ph) chemicals were determined by high-performance liquid chromatography (HPLC) screening following OECD guideline 121.32 The H2-rich forms of the LOHC chemicals were not investigated due to their lack of UV activity. The test procedures in detail showing also the calibration and calculation of log
Koc values of the LOHCs (Table 1) were first predicted by the Conductor-like Screening Model for Realistic Solvation (COSMO-RS, Leverkusen, Germany, see ESI-S2†) to estimate the retention times. Subsequently, the Koc values of the H2-lean and partially hydrogenated LOHC (-ph) chemicals were determined by high-performance liquid chromatography (HPLC) screening following OECD guideline 121.32 The H2-rich forms of the LOHC chemicals were not investigated due to their lack of UV activity. The test procedures in detail showing also the calibration and calculation of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc are described in ESI-S3.† The LOHCs were then assigned to relative mobility classes based on the log
Koc are described in ESI-S3.† The LOHCs were then assigned to relative mobility classes based on the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc.
Koc.
Determination of Kd and adsorption isotherm
The quinaldine-based LOHC system was chosen as an example for cross-check. It was selected since its components are ionisable within the environmental pH range (Table 1), suggesting that their partitioning behaviour might not conform to the commonly used models. The adsorption isotherms in soil I were established for five concentrations (2.5, 5.0, 10, 25, and 50 mg L−1) following the batch equilibrium protocol given by OECD guideline 106.33 Two independent tests with triplicates of each concentration were conducted for every compound. Test details are described in ESI-S4.†The quinaldines were extracted using a liquid–liquid extraction and the amount was measured using gas chromatography mass spectrometry (GC/MS) analysis.
The amount of quinaldine adsorbed to the soil was calculated by subtracting the amount remaining in the aqueous phase at equilibration from the amount initially added. The concentrations of the test compounds in the soil phase (qe) were plotted against the concentrations in the liquid phase (Ce), and Kd (batch-Kd) was thus calculated as the slope of the linear portion of the adsorption curve. Freundlich model (eqn (1)) was used to fit the experimental data:
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = log qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf + 1/n Kf + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce | (1) |
Leaching in soil columns
The leaching of quinaldines in soil II was tested in soil columns according to OECD guideline 312.34 The column design and all the details on the soil column preparation and quinaldine spiking are in ESI-S5.† Additionally, atrazine was used as the reference compound (Table S2†) and two columns containing only atrazine were run for 648 h. The total concentrations of the LOHC and atrazine in the soil column were 100 and 20 mg kg−1 dw soil, respectively. Artificial rain (0.01 M CaCl2) was simulated by dropwise addition of water to the top of the column with the aid of a peristaltic pump (Spetec, Perimax 12, Erding, Germany) at a flow rate of 0.108 mL min−1 for 172 h (Quin-2Me-H10), 648 h (Quin-2Me) or 720 h (Quin-2Me-ph). The leachates were collected from the bottom of the column every 24 or 48 h and passed through filters made of glass wool packed in Pasteur pipettes. Two independent leaching tests were performed for each compound, with two replicates (columns) performed for each test. The compounds in the leachates were extracted by liquid–liquid extraction and analysed by GC/MS. The amount of the test compound in the leachate was calculated as percentage of the total mass (mass%) that was originally added and plotted against the multiples of the pore volume (PV). The breakthrough was determined as the PV point at which the substance first appeared at the outlet.Another set of Kd values (column-Kd) was thus determined from the column leaching experiments according to the method proposed by the Environmental Protection Agency (EPA).36 Here, the Kd values were directly calculated from the retardation factor (Rf) and factors related to the soil properties (n, total porosity; ρb, bulk density) using the equation (eqn (2)):
| Kd = [(Rf − 1) × n]/ρb | (2) |
The details on the determination of the parameters for the calculation of Kd are available in ESI-S5.†
Liquid–liquid extraction and GC/MS analysis
The concentrations of quinaldines were determined via liquid–liquid extraction (see ESI-S6†) followed by GC/MS analysis on a gas chromatograph (HP® GC system 6890N) equipped with a mass selective detector (HP® MS 5973, Agilent, Waldbronn, Germany). Further details of the setup and calibration parameters are given in ESI-S7.†Statistical analysis
Regression analysis was performed for the comparison between COSMO-RS and HPLC methods in terms of Koc values and for the evaluation of adsorption isotherms. Significance of difference between two data sets (i.e. the Kd values of H2-rich and H2-lean forms of the quinaldines that were obtained in adsorption batch test) were analysed by generalised linear models using R (i 3864.0.0).37P > 0.05 was considered not significant.Results
Mobility screening
As a first step we used in silico model COSMO-RS to predict log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow and log
Kow and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc of all tested compounds (Table 1). The log
Koc of all tested compounds (Table 1). The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values were then measured using cyanopropyl HPLC column.32 This HPLC column showed an excellent performance and comparability of log
Koc values were then measured using cyanopropyl HPLC column.32 This HPLC column showed an excellent performance and comparability of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values with those obtained using batch test for a wide variety of both polar and non-polar as well as ionisable and neutral compounds.39 The log
Koc values with those obtained using batch test for a wide variety of both polar and non-polar as well as ionisable and neutral compounds.39 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values determined for LOHC chemicals ranged from 1.17 to 5.41 (Table 2). The partially hydrogenated forms of the alkylcarbazoles, MLHs and MSH were found to exist as technical mixtures containing at least two components (Table S3†); therefore, the mean retention time of all the components was used to calculate Koc. The LOHCs were assigned to mobility classes according to their log
Koc values determined for LOHC chemicals ranged from 1.17 to 5.41 (Table 2). The partially hydrogenated forms of the alkylcarbazoles, MLHs and MSH were found to exist as technical mixtures containing at least two components (Table S3†); therefore, the mean retention time of all the components was used to calculate Koc. The LOHCs were assigned to mobility classes according to their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values and McCall's scale of mobility in soil.38 According to this scale, indoline, which had the lowest log
Koc values and McCall's scale of mobility in soil.38 According to this scale, indoline, which had the lowest log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, was classified as highly mobile in soils (class I: log
Koc, was classified as highly mobile in soils (class I: log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc 1.70–2.18). Quin-2Me, Quin-2Me-ph and indole fell into the “moderately mobile” category (class II: log
Koc 1.70–2.18). Quin-2Me, Quin-2Me-ph and indole fell into the “moderately mobile” category (class II: log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc 2.18–2.70). The remaining compounds were characterised by notably higher log
Koc 2.18–2.70). The remaining compounds were characterised by notably higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values, which increased in the following order: MLH < Car-2 < Car-2-ph < Car-3 < Car-3-ph < Car-4 ≈ Car-4-ph < MSH < MLH-ph. Thus, these compounds were assigned to the lowest mobility class (class V: log
Koc values, which increased in the following order: MLH < Car-2 < Car-2-ph < Car-3 < Car-3-ph < Car-4 ≈ Car-4-ph < MSH < MLH-ph. Thus, these compounds were assigned to the lowest mobility class (class V: log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc > 3.70) and labelled “immobile”. Moreover, the carbazoles containing extended alkyl chains tended to have higher log
Koc > 3.70) and labelled “immobile”. Moreover, the carbazoles containing extended alkyl chains tended to have higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values and lower mobility. The log
Koc values and lower mobility. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values determined using HPLC method correlated well with those predicted by COSMO-RS (R2 = 0.92, Fig. S1†).
Koc values determined using HPLC method correlated well with those predicted by COSMO-RS (R2 = 0.92, Fig. S1†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc (n = 3, ±SD) determined by HPLC screening and the classification of mobility according to McCall's soil mobility scale38
Koc (n = 3, ±SD) determined by HPLC screening and the classification of mobility according to McCall's soil mobility scale38
| a Mean value ± SD of the components (include cis and trans isomers) in mixtures. |
|---|

|
K d and adsorption isotherms of the quinaldine LOHC system
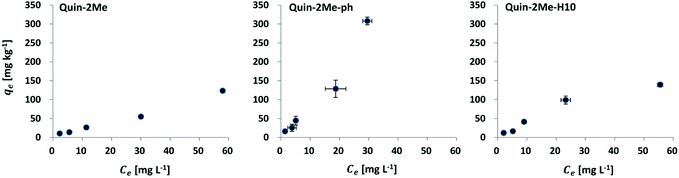
The batch-Kd of the quinaldine-based LOHC system followed the order: Quin-2Me-ph (6.57 mL g−1) > Quin-2Me-H10 (2.42 mL g−1) ≥ Quin-2Me (2.03 mL g−1) (Table 3), in which a difference of a factor of 2 to 3 existed between the partition coefficients of the first and the other two compounds, and the batch-Kd of the latter two compounds were very similar (no significant difference, with p = 0.77). The shapes of the adsorption isotherms (Fig. 2) provided some comparative insights into the interaction potentials of the three quinaldines in this test system. The isotherm of Quin-2Me and Quin-2Me-H10 had generally similar and lower slopes, whereas the isotherm of Quin-2Me-ph was characterised by a greater slope than the other two.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, log
Koc, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, and pKb values of the quinaldines
D, and pKb values of the quinaldines
| Quinaldine | Quin-2Me | Quin-2Me-ph | Quin-2Me-H10 | |
|---|---|---|---|---|
| Parameter | ||||
| a Determined in the adsorption batch equilibrium experiment in soil I (n = 6, ±SD). b Determined in the soil column leaching test in soil II (n = 4, ±SD). c Predicted using COSMO-RS. d Estimated using MarvinSketch.35 e Could not be calculated due to strong retention of the substance in soil column. f Not available in HPLC due to the lack of UV activity of the substance. | ||||
Batch-Kd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [mL g−1] a [mL g−1] |
Average | 2.03 ± 0.12 | 6.57 ± 0.39 | 2.42 ± 0.43 |
| R 2 | 0.99 | 0.99 | 0.91 | |
Column-Kd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b [mL g−1] b [mL g−1] |
1.00 ± 0.40 | n.a.e | 0.23 ± 0.03 | |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, HPLC Koc, HPLC |
2.19 | 2.36 | n.a.f | |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, batch Koc, batch |
2.23 | 2.73 | 2.30 | |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, column Koc, column |
2.10 | n.a.e | 1.46 | |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, COSMO-RS Koc, COSMO-RS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
2.18 | 2.48 | 2.53 | |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D (pH 5.4)d D (pH 5.4)d |
2.07 | 2.23 | -0.88 | |
| pKb (25 °C)d | 5.15 (5.7–5.8)40 | 4.88 | 10.75 | |
Furthermore, adsorption isotherms were fitted using Freundlich model (R2 = 0.95–0.97) (Table 4). The highest adsorption coefficient (Kf) estimated by the model was found for Quin-2Me-ph (9.20 mL g−1), which was followed by Quin-2Me-H10 (5.72 mL g−1) and Quin-2Me (4.19 mL g−1). Comparatively, low values of Freundlich parameter (1/n) were observed for Quin-2Me and Quin-2Me-H10 while higher 1/n approaching unity was found for Quin-2Me-ph (0.96, Table 4). This signifies that while the adsorption of the two former compounds is decreasing with concentration, the adsorption of Quin-2Me-ph is increasing almost linearly with concentration showing nearly no sign of approaching saturation. This is concurrent with the shape of the isotherms themselves (Fig. 2).
| Quinaldine | Model | K f [mL g−1] | 1/n | R 2 |
|---|---|---|---|---|
| Quin-2Me | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = 0.6218 + 0.7875 qe = 0.6218 + 0.7875![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce |
4.19 | 0.79 | 0.97 |
| Quin-2Me-ph | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = 0.9637 + 0.9629 qe = 0.9637 + 0.9629![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce |
9.20 | 0.96 | 0.96 |
| Quin-2Me-H10 | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = 0.7574 + 0.8296 qe = 0.7574 + 0.8296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce |
5.72 | 0.83 | 0.95 |
Since Koc is the soil-water partition coefficient normalised to the amount of soil organic matter according to the empirical equation Koc = Kd/foc, Koc can be easily calculated from the measured batch-Kd values in soil I, giving log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, batch values of 2.23, 2.73, and 2.30 for Quin-2Me, Quin-2Me-ph, and Quin-2Me-H10, respectively (Table 3).
Koc, batch values of 2.23, 2.73, and 2.30 for Quin-2Me, Quin-2Me-ph, and Quin-2Me-H10, respectively (Table 3).
Leaching of quinaldines in soil columns
Breakthrough was achieved the fastest (approx. at 2 PV) for Quin-2Me-H10 (Fig. 3C and S2C†), with approximately 65% of the total mass ultimately collected in the leachate. The breakthrough of Quin-2Me (Fig. 3A and S2A†) occurred later than that of Quin-2Me-H10 with a lag of approx. 7–9 PV, and approximately 45%–60% of this compound was ultimately collected in the effluent of the column. Quin-2Me-ph behaved very differently from the other quinaldines, with more than 99% of the total spiked mass retained on the column at the end of the test (Fig. 3B and S2B†). Atrazine, which was used as the reference compound and run either alone in a separate column (Fig. S3A and S4A†) or in the same column with the quinaldines (Fig. S3B–D and S4B–D†), showed a relatively constant leaching behaviour with breakthrough occurring at 4–6 PV (or approximately 48–72 h), and more than 90% was collected in the leachate at the end of the test.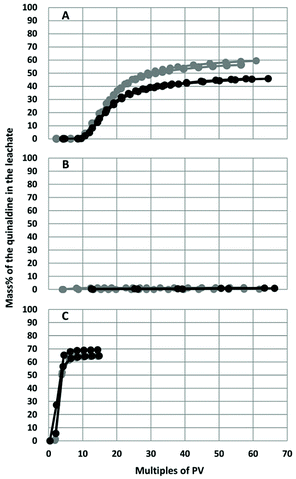 | ||
| Fig. 3 Breakthrough curves for Quin-2Me (A), Quin-2Me-ph (B) and Quin-2Me-H10 (C) in two independent experiments (grey and black), each with two columns packed with soil II. “PV”: pore volume. | ||
The column-Kd values calculated according to the EPA36 yielded values of 1.00 mL g−1 and 0.23 mL g−1 for Quin-2Me and Quin-2Me-H10, respectively (Table 3). The Kd obtained for Quin-2Me in the column experiment was within a factor of 2 compared to the value measured in the batch experiment. Quin-2Me-ph was strongly retained in the column, therefore no column-Kd value was calculated. This suggested however much higher affinity to soil than could have been expected based on batch-Kd and as compared to other two compounds. On the contrary, the column-Kd for Quin-2Me-H10 was an order of magnitude lower than that obtained in the batch experiment. Additionally, using the empirical equation for Koc, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, column values yielded 1.76–2.24 (avg. 2.01, Table S4†) were calculated for atrazine which were very close to literature values of 1.92. The log
Koc, column values yielded 1.76–2.24 (avg. 2.01, Table S4†) were calculated for atrazine which were very close to literature values of 1.92. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, column values for Quin-2Me and Quin-2Me-H10 was calculated to be 2.10 and 1.46, respectively (Table 3).
Koc, column values for Quin-2Me and Quin-2Me-H10 was calculated to be 2.10 and 1.46, respectively (Table 3).
Discussion
Interaction of LOHCs with soil
In this study, we characterised the adsorption and mobility of LOHCs taking into account different levels of hydrogen saturation.It is often assumed that the hydrophobic interactions with soil organic matter are the main forces driving adsorption of organic compounds to soils. For that reason, if experimentally determined Koc values are not available, Kow is often used to predict parameters defining affinity to soils e.g., Koc. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values obtained using HPLC showed a good correlation with the COSMO-RS-predicted log
Koc values obtained using HPLC showed a good correlation with the COSMO-RS-predicted log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values (R2 = 0.94, Fig. S5†). Yet the Kow seems to underestimate the Koc value obtained by HPLC, especially for more hydrophobic compounds with a log
Kow values (R2 = 0.94, Fig. S5†). Yet the Kow seems to underestimate the Koc value obtained by HPLC, especially for more hydrophobic compounds with a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow > 3.0 (Fig. S5†).
Kow > 3.0 (Fig. S5†).
The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values for the LOHC candidates and some of their partially hydrogenated forms measured using HPLC spanned five orders of magnitude (1.71–5.41) (Table 2). The potential mobility of these compounds in soil, ranged from highly mobile to immobile, proving that they have vastly different potentials to contaminate drinking water sources. Partitioning into soil organic matter plays an important role in the retention of many chemicals in soil41–43 and the mobility decreases with increasing Koc.44,45 The compounds with the highest Koc values are more likely to be retained and accumulated at the soil surface where they were released. Therefore, it seems that indoline, Quin-2Me, Quin-2Me-ph and indole would likely be transported further and deeper crossing natural barriers such as soil layers and aquifers than the carbazole derivatives, MLHs and MSH.
Koc values for the LOHC candidates and some of their partially hydrogenated forms measured using HPLC spanned five orders of magnitude (1.71–5.41) (Table 2). The potential mobility of these compounds in soil, ranged from highly mobile to immobile, proving that they have vastly different potentials to contaminate drinking water sources. Partitioning into soil organic matter plays an important role in the retention of many chemicals in soil41–43 and the mobility decreases with increasing Koc.44,45 The compounds with the highest Koc values are more likely to be retained and accumulated at the soil surface where they were released. Therefore, it seems that indoline, Quin-2Me, Quin-2Me-ph and indole would likely be transported further and deeper crossing natural barriers such as soil layers and aquifers than the carbazole derivatives, MLHs and MSH.
In spite of the role of organic matter in controlling chemicals’ adsorption in soils, among the test set are compounds that can be charged to a different extent depending on the pH of the solution (see Table 1 for potential protonation and Table 3 for pKb values). Hydrophobic interactions with soil organic carbon dominating the sorption is often true for neutral organic compounds but might not be the case for ionisable compounds like quinaldine derivatives.46 Ionisation influences not only the compounds’ hydrophobicity but also the ability to interact with soil via electrostatic interactions. This type of interaction is not present in a cyanopropyl HPLC column that is recommended for estimation of Koc according to OECD guideline.32 It was also reported that for permanently charged or ionisable compounds their affinity to soils may be largely underestimated by Kow.25 Additionally, the degree of saturation of cyclic rings also influences the ability to interact through pi–pi interactions47,48 between aromatic rings present in the solute and those present in soil organic matter. We therefore chose a quinaldine-based system to investigate in more detail on how these structural differences will influence the affinity to model soils. To that end the obtained Kd values in the batch equilibrium and column leaching tests were recalculated into Koc to provide basis for comparisons based on the amount of organic matter present in model soils (Table 3). As we progressed from the least (in silico model) to the more complex and realistic scenario (soil columns) for assessing soil affinity a markable difference in the behaviour of the three members of quinaldine-based LOHC systems became apparent.
For Quin-2Me, the Koc obtained using modelling (COSMO-RS) and different experimental methods (HPLC, batch and column) were similar (Table 3), with the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc values ranging from 2.10 (log
Koc values ranging from 2.10 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, column) to 2.23 (log
Koc, column) to 2.23 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, batch) thus Quin-2Me can be classified as “highly mobile” (column leaching) to “moderately mobile” (batch test).38 For the saturated form, Quin-2Me-H10, more significant difference in Koc values (of nearly an order of magnitude) was observed with the log
Koc, batch) thus Quin-2Me can be classified as “highly mobile” (column leaching) to “moderately mobile” (batch test).38 For the saturated form, Quin-2Me-H10, more significant difference in Koc values (of nearly an order of magnitude) was observed with the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, column apparently lower than the log
Koc, column apparently lower than the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, batch (i.e. 1.46 vs. 2.30) implying that the compound's mobility ranges from “very high” (log
Koc, batch (i.e. 1.46 vs. 2.30) implying that the compound's mobility ranges from “very high” (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc < 1.7) to “moderate”.38 In other words, the fully saturated quinaldine (Quin-2Me-H10) might be more mobile than could have been expected based on Koc values predicted by COSMO-RS or obtained experimentally in the batch test system. Quin-2Me-ph was retained strongly in the soil column with less than 1% of the spiked mass being leached out. Quin-2Me-ph thus seems to be rather “immobile” in soil which was quite unexpected taking into account its rather low predicted and measured Koc values obtained in HPLC and batch systems.
Koc < 1.7) to “moderate”.38 In other words, the fully saturated quinaldine (Quin-2Me-H10) might be more mobile than could have been expected based on Koc values predicted by COSMO-RS or obtained experimentally in the batch test system. Quin-2Me-ph was retained strongly in the soil column with less than 1% of the spiked mass being leached out. Quin-2Me-ph thus seems to be rather “immobile” in soil which was quite unexpected taking into account its rather low predicted and measured Koc values obtained in HPLC and batch systems.
It is noteworthy that soil in the environment is highly heterogeneous. Large variation of soil composition, from texture to minerals to varied quantity and quality of organic carbon, has to be taken into consideration. In other words, adsorption and mobility of the LOHCs and their accessibility to organisms can vary according to local soil49,50 and climatic conditions.51
Mechanistic insight into the interaction of quinaldines with soil
All three quinaldines are organic bases and can be present either in their neutral or protonated forms45,52,53 depending on their pKb values (Table 3) and the pH of the surroundings. The ionised fractions of the quinaldines, at the pH values of the tests (pH 5.3 and 5.4), are in the order following Quin-2Me-ph (23%–26%) < Quin-2Me (36%–40% or 66%–75%) ≪ Quin-2Me-H10 (100%) (Table 1). Models available so far perform poorly in describing adsorption of organic ions. This is mostly due to the fact that they usually neglect the interaction potentials of the ionised fraction by assuming that only the neutral portion of the compound is interacting with soil through hydrophobic interactions. Nevertheless, especially organic cations are able to interact strongly with soils. The exact molecular mechanisms of their sorption is still elusive but ion-exchange was recognised as one most important interaction.26 Droge et al. have recently proposed a model describing adsorption of organic bases (carrying positive charge at test pH) to soil taking into account both hydrophobic interactions with soil organic matter (SOM) as well as ion exchange interactions with both clay and SOM fractions.26 Applying this modelling frame to our test system shows that despite extensive protonation of the test compounds we should be expecting sorption dominated by hydrophobic interactions. This is due to the fact that both model soils have low CEC (cation-exchange capacity) values (Table S1†), therefore, the contribution of ion-exchange mechanism could be low. This shows that the soil affinity of these ionic compounds cannot be predicted easily and there is a need for better predictive models with applicability domain covering ionic and ionisable compounds.Although the column leaching experiment was not performed for the other potentially ionisable LOHC compounds in this study, i.e. indoline and alkylcarbazoles, it is probable that their mobility in soils would also deviate from the Koc obtained using HPLC. Further confirmation of the affinities of these compounds by batch equilibrium and leaching experiments is recommended if these compounds were to be implemented as LOHCs on larger scale.
Estimating and measuring soil affinity – method comparison
Three typical methods for determining the adsorption of organic contaminants in soils were applied in this study. The HPLC screening method is the least complex, but the assumption that it adequately represents the potential for interaction with the soil was not supported.Both column leaching and batch equilibrium tests are commonly conducted to evaluate sorption. The duration of the batch equilibrium test is much shorter than that of the column test, especially when investigating strongly adsorbing substrates (such as Quin-2Me-ph in this study).54 Moreover, due to efficient mixing, the adsorption equilibrium is generally achieved faster in the batch equilibrium test because macroscopic mass transfer is not hindered.54 Therefore, the batch equilibrium test is often treated as the “worst case scenario”.54 However, the mobility (leachability) in soils can be underestimated by the batch equilibrium test,55 as what has been shown here particularly for Quin-2Me-H10. Column leaching represents a dynamic system that is supposed to simulate the downward movement of chemicals through soil.54 Although the column experiment is more time-consuming, it provides better simulation of the water flow through the porous soil profile due to a more realistic solid-to-liquid ratio.54 Processes that occur in nature, such as particle-associated transport,44 are also accounted for in column leaching tests.54 In such a test, the adsorption equilibrium is not necessarily achieved in the dynamic process, even at the point of breakthrough,54 due to insufficient mixing and the adsorption/desorption processes of various, often competing, ions.54,56 In addition, column leaching test could provide more information for the assessment of the potential for groundwater contamination, which can eventually be correlated to the risk of human exposure via drinking water or contaminated crops.44
The concentration used to spike the soil columns in the study was 100 mg kg−1 being the worst-case scenario, which might occur in the environment only through heavy contamination resulting from accidental spills. However, the localised leakage or spillage of these compounds is possible since they may eventually be produced and transported on a large scale and handled by citizens in the same way fossil fuels currently are.
Assessment of persistence, mobility and toxicity
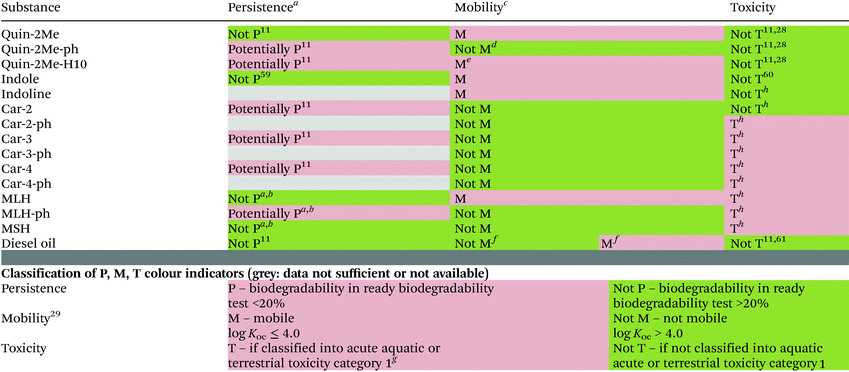
Recently compounds that are mobile in soils and therefore are able to reach surface water, groundwater or even drinking water are causing increasing concern and may be a subject of regulatory actions in the future. Since there is a concern that some LOHCs possess traits of PMT substances we have performed a PMT-screening using recently proposed cut-off values.29,57,58 We have adapted these values to the data that are available for LOHCs, which means in most cases we have used screening-level data. This resulted in following threshold values: (a) log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc < 4.0 for classification as mobile (M), (b) classification into aquatic toxicity acute category 1 or terrestrial toxicity category 1 for fulfilling the “toxic” criterion (T), and (c) less than 20% biodegradation in ready biodegradability test for classification as persistent (P). Based on results gathered in this study two components of the quinaldine-based LOHC system (i.e. Quin-2Me and Quin-2Me-H10), indole-based LOHC system (indoline and indole) and MLH fulfil the ‘M’ criterion (Table 5). The rather low affinity of these compounds to soils indicates a risk of groundwater contamination, especially for Quin-2Me-H10, which is considered “very mobile”. All the other compounds are expected to be retained in soil to higher extent and therefore present a lower risk towards groundwater contaminations.
Koc < 4.0 for classification as mobile (M), (b) classification into aquatic toxicity acute category 1 or terrestrial toxicity category 1 for fulfilling the “toxic” criterion (T), and (c) less than 20% biodegradation in ready biodegradability test for classification as persistent (P). Based on results gathered in this study two components of the quinaldine-based LOHC system (i.e. Quin-2Me and Quin-2Me-H10), indole-based LOHC system (indoline and indole) and MLH fulfil the ‘M’ criterion (Table 5). The rather low affinity of these compounds to soils indicates a risk of groundwater contamination, especially for Quin-2Me-H10, which is considered “very mobile”. All the other compounds are expected to be retained in soil to higher extent and therefore present a lower risk towards groundwater contaminations.
a Determined using ready biodegradability test according to OECD 301F.11
b Unpublished results.
c Mobility classified based on log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc determined by HPLC in this study unless indicated otherwise.
d Re-classified as not mobile based on no leaching in soil column experiment.
e Classified based on batch and leaching test.
f Mobility determined based on log Koc determined by HPLC in this study unless indicated otherwise.
d Re-classified as not mobile based on no leaching in soil column experiment.
e Classified based on batch and leaching test.
f Mobility determined based on log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc of different components of diesel oil.62
g Category 1: for aquatic toxicity EC50 < 1.0 mg L−1, for terrestrial toxicity EC50 ≤ 10 mg per kg dry weight.
h Based on predicted acute aquatic toxicity (see Table S5† for details). Koc of different components of diesel oil.62
g Category 1: for aquatic toxicity EC50 < 1.0 mg L−1, for terrestrial toxicity EC50 ≤ 10 mg per kg dry weight.
h Based on predicted acute aquatic toxicity (see Table S5† for details).
|
|---|
|
In our previous studies we have shown that Quin-2Me-H10 and Quin-2Me-ph are resistant to biodegradation in ready biodegradability test whereas Quin-2Me is biodegradable to high extent (i.e. not persistent)11 (Table 5). This warrants classification of the two former substances as ‘P’ and the latter as ‘not P’. Additionally, all H2-lean and H2-rich carbazole derivatives showed no degradation in ready biodegradability test (partially hydrogenated forms were not tested) and were therefore classified as ‘P’ (Table 5).11 On the contrary, H2-lean forms of MLH and MSH were significantly degraded in that test system and classified as ‘not P’ (unpublished results). Indole was shown to be susceptible to biodegradation59 and was assessed as readily biodegradable.
Furthermore, low to moderate toxicity of the three quinaldines as well as H2-rich forms of alkylcarbazoles was observed, showing the effects categorised as “acute 2” or “acute 3” in aquatic11 and “harmful” in terrestrial28 organisms – none of which fulfils the ‘T’ criterion in current ‘PMT’ assessment approach (Table 5).29,57,58 Based on this screening-level data Quin-2Me-H10 would be considered potentially “PM” (persistent and mobile) substance.29 Quin-2Me does not raise concerns at the moment due to its biodegradability, while the risk of Quin-2Me-ph to contaminate drinking water would be low because of the immobility. No experimental data are currently available for H2-lean or intermediate forms of alkylcarbazoles, MLH- or MSH-based LOHC as well as indoline that could be used for preliminary hazard classification. In order to fill that gap we used a mathematical model (ECOSAR part of US EPA EPI Suite package63) to predict acute toxicity towards algae, invertebrates and fish. The exact values are presented in Table S5.† Based on these predicted values all alkylcarbazole- as well as MLH- and MSH-derivatives except ethylcarbazole (Car-2) would be classified as aquatic acute toxicity category 1 and therefore would fulfil the ‘T’ criterion (Table 5). Based on model values Car-2 belongs to acute category 2 and indoline to acute category 3, meaning none of them fulfils the ‘T’ criterion. So far, the accuracy of that predictions cannot be verified. Nevertheless, based on results obtained in vitro tests (in mammalian cell line) partially hydrogenated alkylcarbazoles seem to be approximately two orders of magnitude more toxic than H2-rich/lean quinaldine suggesting generally high toxicity.11
The components of each LOHC system tested present different characteristics in terms of persistence, mobility and toxicity (Table 5). More data are needed for a full-scale evaluation of the environmental and human risk, and for an overall hazard assessment at least two compound forms (H2-rich and H2-lean) of each LOHC system should be taken into account, since they can differ considerably as was shown here for members of quinaldine-based LOHC system. So far based on available data the LOHC systems do not seem to be clearly less hazardous than fossil fuels such as diesels regarding the “P, M, T” criterion (Table 5). However, the fact that LOHCs contribute to safer, high-capacity and long-term storage and transport of renewable energy, make them superior to fossil fuels. The less complex composition than that of fossil fuels11 would be another benefit for the hazard monitoring and management of these compounds in the environment.
Conclusions
Novel energy systems based on liquid organic hydrogen carrier (LOHC) technology have been developed recently for storage and transport of conventional or renewable energy. In many geographical locations, applications of LOHCs in stationary and mobile (vehicular) energy supply as well as in long-distance energy transport have been initiated. Given large scale of application and considerable release potential the environmental impact assessment of this technology is necessary. This study evaluated the adsorption and mobility in soil of a series of LOHC chemicals (quinaldines, indoles, alkylcarbazoles, benzyltoluenes and dibenzyltoluene in different H2-loading forms) which are considered most promising candidates. In silico model COSMO-RS is a useful tool for the prediction and screening of compounds’ adsorption. Further investigations using HPLC-screening suggested the mobility based on the Koc ranging from highly to not mobile with particular LOHC systems in following order: indoles < quinaldines < carbazole derivatives < benzyltoluenes < dibenzyltoluene. Additionally, soil affinity of quinaldine-based LOHC system was investigated in more detail using adsorption batch equilibrium and column leaching test (28 days). In combination with the findings in our previous studies on persistence and toxicity (aquatic and terrestrial), the fully saturated quinaldine – Quin-2Me-H10 – was classified as “PM” (persistent and mobile) chemical, which has potential of contaminating water sources due to lack of degradability and low retention in soils. Many other LOHC chemicals tested in this study, i.e. Quin-2Me-ph, carbazole derivatives, benzyltoluene (the partially saturated form) and dibenzyltoluene, appeared to be rather immobile. For these compounds an accumulation close to the point of release may be expected if no significant degradation occurs and no imminent danger of contaminating water resources exist. Considering that within the LOHC system H2-rich and -lean forms are paired in reaction cycles and accompanied with intermediates (e.g., -ph), LOHCs released into the environment are very likely mixtures of the different forms. Therefore, the environmental hazard assessment of LOHC system needs to be based on the most hazardous compound (form) that might be used/produced within the cycle. In addition, the environmental hazard profile should be weighed against the socio-economic and technological improvements they offer in terms of reduced emissions, possibility of flexible long-term (renewable) energy storage and lower cost (economic/environmental) than many other options.Conflicts of interest
The authors declare the following competing interest: Michael Diedenhofen is a scientist at BIOVIA, Dassault Systèmes Deutschland GmbH, who is the developer of the COSMOtherm and distributor of the TURBOMOLE software packages.Acknowledgements
This work was financially supported by the University of Bremen and the European Union FP7 COFUND within Marie Curie Actions BremenTrac Program (grant agreement No. 600411) and M8 Postoc-Initiatve PLUS (funded by the German Excellence Initiative) as well as the German Federal Foundation for the Environment (Deutsche Bundesstiftung Umwelt (DBU), Osnabrück/Germany). We would like to thank the entire UFT Team for the interdisciplinary cooperation. We acknowledge the working group of Prof. Dr Peter Wasserscheid for providing the LOHC chemicals. The authors would like to thank Dr Jan Köser for the support on MATLAB.References
- D. Teichmann, W. Arlt, P. Wasserscheid and R. Freymann, Energy Environ. Sci., 2011, 4, 2767–2773 RSC.
- D. Teichmann, W. Arlt and P. Wasserscheid, Int. J. Hydrogen Energy, 2012, 37, 18118–18132 CrossRef CAS.
- Q. L. Zhu and Q. Xu, Energy Environ. Sci., 2015, 8, 478–512 RSC.
- M. Markiewicz, Y.-Q. Zhang, A. Bösmann, N. Brückner, J. Thöming, P. Wasserscheid and S. Stolte, Energy Environ. Sci., 2015, 8, 1035–1045 RSC.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS.
- I. Dincer, Renewable Sustainable Energy Rev., 2000, 4, 157–175 CrossRef.
- P. Preuster, C. Papp and P. Wasserscheid, Acc. Chem. Res., 2017, 50, 74–85 CrossRef CAS.
- D. Geburtig, P. Preuster, A. Bösmann, K. Müller and P. Wasserscheid, Int. J. Hydrogen Energy, 2016, 41, 1010–1017 CrossRef CAS.
- P. M. Modisha, C. N. M. Ouma, R. Garidzirai, P. Wasserscheid and D. Bessarabov, Energy Fuels, 2019, 33, 2778–2796 CrossRef CAS.
- M. Markiewicz, Y.-Q. Zhang, M. T. Empl, M. Lykaki, J. Thöming, P. Steinberg and S. Stolte, Energy Environ. Sci., 2019, 12, 366–383 RSC.
- K. M. Eblagon, D. Rentsch, O. Friedrichs, A. Remhof, A. Zuettel, A. J. Ramirez-Cuesta and S. C. Tsang, Int. J. Hydrogen Energy, 2010, 35, 11609–11621 CrossRef CAS.
- A. Fikrt, R. Brehmer, V.-O. Milella, K. Müller, A. Bösmann, P. Preuster, N. Alt, E. Schlücker, P. Wasserscheid and W. Arlt, Appl. Energy, 2017, 194, 1–8 CrossRef CAS.
- K. Müller, K. Stark, V. N. Emel'yanenko, M. A. Varfolomeev, D. H. Zaitsau, E. Shoifet, C. Schick, S. P. Verevkin and W. Arlt, Ind. Eng. Chem. Res., 2015, 54, 7967–7976 CrossRef.
- M. Niermann, A. Beckendorff, M. Kaltschmitt and K. Bonhoff, Int. J. Hydrogen Energy, 2019, 44, 6631–6654 CrossRef CAS.
- P. Hu, E. Fogler, Y. Diskin-Posner, M. A. Iron and D. Milstein, Nat. Commun., 2015, 6, 1–7 Search PubMed.
- B. Verheul, Overview of hydrogen and fuel cell developments in China, Shanghai, 2019 Search PubMed.
- Japan launches first global hydrogen supply chain demo project; liquid organic hydrogen carrier (LOHC) technology, https://www.greencarcongress.com/2017/07/20170728-ahead.html, (accessed 27 August 2020).
- G. Ozin, Hydrogen Storage in Liquid Organic Hydrogen Carriers, https://www.advancedsciencenews.com/hydrogen-storage-liquid-organic-hydrogen-carriers/ Search PubMed, (accessed 27 August 2020).
- From the Lab to the Rails, https://www.fz-juelich.de/SharedDocs/Pressemitteilungen/UK/EN/2018/2018–04-19-lohc-zug.html;jsessionid=44F4EA2C24CF9E831CC0FDA63FB4E06D, (accessed 27 August 2020).
- V. Leung, LOHC technology implemented in China, https://www.gasworld.com/lohc-technology-implemented-in-china/2014010.article Search PubMed, (accessed 27 August 2020).
- J. Sampson, Hydrogen mobility infrastructure for China, https://www.gasworld.com/hydrogen-mobility-infrastructure-for-china/2014052.article Search PubMed, (accessed 27 August 2020).
- N. Brückner, K. Obesser, A. Bösmann, D. Teichmann, W. Arlt, J. Dungs and P. Wasserscheid, ChemSusChem, 2014, 7, 229–235 CrossRef.
- A.-K. Reineke, A. Preiss, M. Elend and J. Hollender, Chemosphere, 2008, 70, 2118–2126 CrossRef CAS.
- S. T. J. Droge and K.-U. Goss, Environ. Sci. Technol., 2013, 47, 798–806 CrossRef CAS.
- S. T. J. Droge and K.-U. Goss, Environ. Sci. Technol., 2013, 47, 14233–14241 CrossRef CAS.
- M. Hörsing, A. Ledin, R. Grabic, J. Fick, M. Tysklind, J. L. C. Jansen and H. R. Andersen, Water Res., 2011, 45, 4470–4482 CrossRef.
- Y.-Q. Zhang, M. Markiewicz, J. Filser and S. Stolte, Environ. Sci. Technol., 2018, 52, 258–265 CrossRef CAS.
- M. Neumann and I. Schliebner, Protecting the sources of our drinking water-a revised proposal for implementing criteria and an assessment procedure to identify persistent, mobile and toxic (PMT) and very persistent, very mobile (vPvM) substances registered under REACH, German Environment Agency, Dessau-Rosslau, 2017 Search PubMed.
- H. P. H. Arp, T. N. Brown, U. Berger and S. E. Hale, Environ. Sci.: Processes Impacts, 2017, 19, 939–955 RSC.
- H. P. H. Arp, Preliminary assessment of substances registered under REACH that could fulfil the proposed PMT/vPvM criteria, Norwegian Geotechnical Institute, Oslo, 2018 Search PubMed.
- OECD, Test No. 121: Estimation of the Adsorption Coefficient (Koc) on Soil and on Sewage Sludge using High Performance Liquid Chromatography (HPLC), OECD Guidelines for the Testing of Chemicals, Section 1, OECD Publishing, Paris, 2001, DOI:10.1787/9789264069909-en.
- OECD, Test No. 106: Adsorption – Desorption Using a Batch Equilibrium Method, OECD Guidelines for the Testing of Chemicals, Section 1, OECD Publishing, Paris, 2000, DOI:10.1787/9789264069602-en.
- OECD, Test No. 312: Leaching in Soil Columns, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris, 2004, DOI:10.1787/9789264070561-en.
- ChemAxon, MarvinSketch v 14.10.6.0, 2014 Search PubMed.
- Environmental Protection Agency, Understanding variation in partition coefficient, Kd, values - Volume I: The Kd Model, Methods of Measurement, and Application of Chemical Reaction Codes, 1999, vol. I Search PubMed.
- The R Foundation, The R Project for Statistical Computing R-4.0.0 for Microsoft® Windows, 2020 Search PubMed.
- P. J. McCall, R. L. Swann, D. A. Laskowski, S. M. Unger, S. A. Vrona and H. J. Dishburger, Bull. Environ. Contam. Toxicol., 1980, 24, 190–195 CrossRef CAS.
- W. Kördel, G. Kotthoff and J. Müller, Chemosphere, 1995, 30, 1373–1384 CrossRef.
- G. Jones, The Chemistry of Heterocyclic Compounds, Quinolines, Wiley-Interscience, 1991, vol. 32 Search PubMed.
- J. Tolls, Environ. Sci. Technol., 2001, 35, 3397–3406 CrossRef CAS.
- M. Kuśmierz, P. Oleszczuk, P. Kraska, E. Pałys and S. Andruszczak, Chemosphere, 2016, 146, 272–279 CrossRef.
- W. D. Weissenfels, H.-J. Klewer and J. Langhoff, Appl. Microbiol. Biotechnol., 1992, 36, 689–696 CrossRef CAS.
- D. M. Revitt, T. Balogh and H. Jones, Environ. Sci. Pollut. Res., 2014, 21, 4209–4219 CrossRef CAS.
- K. Kobetičová, J. Bezchlebová, J. Lána, I. Sochová and J. Hofman, Ecotoxicol. Environ. Saf., 2008, 71, 650–660 CrossRef.
- European Centre for Ecotoxicology and Toxicology of Chemicals, Environmental Exposure Assessment of Ionisable Organic Compounds - Technical Report No. 123, Brussels, 2013 Search PubMed.
- T. A. T. Aboul-Kassim and B. R. T. Simoneit, in Pollutant-Solid Phase Interactions Mechanisms, Chemistry and Modeling, Springer Berlin Heidelberg, 2001, pp. 107–167 Search PubMed.
- D. Liu, J. Gui and Z. Sun, J. Mol. Catal. A: Chem., 2008, 291, 17–21 CrossRef CAS.
- M. Matzke, K. Thiele, A. Müller and J. Filser, Chemosphere, 2009, 74, 568–574 CrossRef CAS.
- B. Styrishave, T. Hartnik, P. Christensen, O. Andersen and J. Jensen, Environ. Toxicol. Chem., 2010, 29, 1084–1090 CAS.
- K. Vancampenhout, K. Wouters, B. De Vos, P. Buurman, R. Swennen and J. Deckers, Soil Biol. Biochem., 2009, 41, 568–579 CrossRef CAS.
- I. N. Anyanwu and K. T. Semple, Environ. Technol. Innov., 2015, 3, 108–120 CrossRef.
- A. B. Thomsen, K. Henriksen, C. Grøn and P. Møldrup, Environ. Sci. Technol., 1999, 33, 2891–2898 CrossRef CAS.
- M. Markiewicz, C. Jungnickel, C.-W. Cho and S. Stolte, Environ. Sci.: Processes Impacts, 2015, 17, 1462–1469 RSC.
- P. Grathwohl and B. Susset, Waste Manage., 2009, 29, 2681–2688 CrossRef CAS.
- W. Kördel, C. Bernhardt, K. Derz, K. Hund-Rinke, J. Harmsen, W. Peijnenburg, R. Comans and K. Terytze, J. Hazard. Mater., 2013, 261, 854–862 CrossRef.
- H. Rüdel, W. Körner, T. Letzel, M. Neumann, K. Nödler and T. Reemtsma, Environ. Sci. Eur., 2020, 32, 1–11 CrossRef.
- U. Berger, N. Ost, D. Sätteler, I. Schliebner, R. Kühne, G. Schüürmann, M. Neumann and T. Reemtsma, Umweltbundesamt, Texte, 2018, 9, 1–61 Search PubMed.
- S. Fetzner, Appl. Microbiol. Biotechnol., 1998, 49, 237–250 CrossRef CAS.
- J. Kim, H. Hong, A. Heo and W. Park, FEMS Microbiol. Lett., 2013, 343, 89–99 CrossRef CAS.
- A. J. Reinecke, M. Van Wyk and S. A. Reinecke, Bull. Environ. Contam. Toxicol., 2016, 96, 804–809 CrossRef CAS.
- European Chemicals Agency (ECHA), Fuels, diesel – Adsorption/desorption, https://echa.europa.eu/registration-dossier/-/registered-dossier/7243/5/5/2, (accessed 27 August 2020).
- US EPA Estimation Programs Interface SuiteTM for Microsoft® Windows EPI Suite™ v 4.1, 2014.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc02603d |
| This journal is © The Royal Society of Chemistry 2020 |