Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A unique pathway to platform chemicals: aldaric acids as stable intermediates for the synthesis of furandicarboxylic acid esters†
Nicolaas
van Strien
 ,
Sari
Rautiainen
,
Sari
Rautiainen
 *,
Martta
Asikainen
,
David A.
Thomas
,
Juha
Linnekoski
,
Klaus
Niemelä
and
Ali
Harlin
*,
Martta
Asikainen
,
David A.
Thomas
,
Juha
Linnekoski
,
Klaus
Niemelä
and
Ali
Harlin
VTT Technical Research Centre of Finland Ltd, P.O. Box 1000, FI-02044 VTT, Finland. E-mail: sari.rautiainen@vtt.fi
First published on 24th August 2020
Abstract
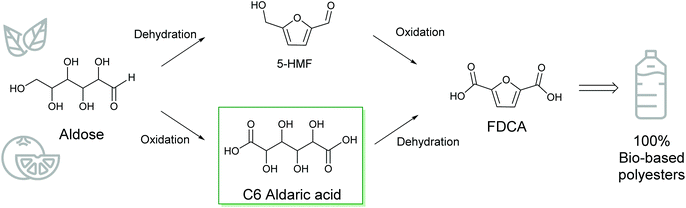
2,5-Furandicarboxylic acid (FDCA) has received attention as an emerging bio-based building block with many applications, especially in renewable polyesters. The common route to FDCA uses the unstable 5-hydroxymethylfurfural (HMF) as an intermediate. Here, we present an alternative route to FDCA and its esters using C6 aldaric acids as stable intermediates. Aldaric acids, or sugar diacids, can be obtained by the oxidation of C6 sugars or uronic acids from pectin. Subsequent dehydration of aldaric acids by solid acid catalysts in butanol produces furancarboxylates. Using silica-supported acid catalysts, over 90% yields of furancarboxylates were achieved with the selectivity to FDCA and its esters reaching 80%.
Introduction
The shift from fossil-based polymers to renewable plastics requires new efficient methods for the production of monomers from biomass. 2,5-Furandicarboxylic acid (FDCA) and its esters are promising bio-based substitutes for terephthalic acid in the production of polyesters.1,2 Compared to fossil-based polyethylene terephthalate (PET), polyethylene furanoate (PEF) produced from FDCA has about 50% lower carbon footprint.3 Furthermore, PEF polymers have superior gas barrier and mechanical properties compared to PET polymers.4 Of the PET components, mono-ethylene glycol (MEG) is currently available from renewable sources but no commercial production of bio-based terephthalic acid exists.5 Therefore, FDCA offers a compelling alternative for the production of 100% renewable polyesters. In addition, FDCA is rapidly gaining interest as a bio-based monomer for other applications such as polyurethanes6 and epoxy resins.7,8The current methods for producing FDCA use a two-step process with edible sugars like glucose or fructose as feedstock (Scheme 1).2,9 The sugar is first dehydrated using an acid catalyst into 5-hydroxymethylfurfural (HMF) which is further oxidized into FDCA. In addition to competing with the food chain, a serious disadvantage is that the intermediate HMF is unstable and readily reacts further under acidic conditions to produce levulinic acid and insoluble humins.10 HMF yields often remain low and furthermore, isolation and purification of HMF from the polar reaction media are challenging. Although extensive efforts have been made to suppress the side reactions and enable high yields of the isolated HMF, the inherent instability of HMF makes it a challenging molecule for biorefineries.11–14 One alternative route has been developed by Avantium; the dehydration is carried out in methanol, producing methoxymethylfurfural (MMF) as the intermediate.15,16 Good selectivity and yield are obtained; currently, this process is being run on a pilot scale with plans having been announced for a commercial-scale plant.4 The oxidation of the intermediate to FDCA can be performed with high yields using noble metal catalysts or the Amoco process employing the Mn–Co–Br catalyst.9 Promising results have also been achieved using biocatalytic oxidation of HMF or MMF to FDCA. High yields have been reported under mild conditions; however, further development is needed to increase feedstock concentrations and shorten reaction times.17
Sugars derived from lignocellulosic feedstock or agricultural residues would avoid competition with food production. High HMF yields (up to 66%) have been reported directly from cellulose using ionic liquids in combination with a Lewis acid catalyst.18 However, product separation and economical scale-up remain challenges. Recently, carboxylation of 2-furoic acid (FCA) into FDCA has also been reported.19 This presents an interesting alternative route for FDCA production, as furfural derived from lignocellulosic feedstock could be used as the intermediate for the previous steps in this process. Another recent example uses uronic acids derived from pectin for FDCA ester production via a three-step route including isomerisation, cyclodehydration and oxidation steps of the uronic acid.20 Although formation of HMF is avoided, the overall yield of FDCA remains at 45%.
The preparation of FDCA was reported in 1876 with galactaric acid (or mucic acid) as the substrate.21 In the original report, galactaric acid was heated in excess HBr and FDCA was obtained. Other authors have reported similar methods, where an excess of either H2SO4 or HBr was used to produce FDCA in around 50% yields.22,23 In more recent publications, an excess of benzene sulfonic acid or p-toluene sulfonic acid has been used for the dehydration of C6 aldaric acids to produce FDCA in around 50% yield.24–26 Zhao et al. reported a one-pot synthesis of diethyl furan-2,5-dicarboxylate using an excess of sulfonic acid followed by the addition of ethanol; 30% yield was obtained in 16 h after the two steps.26 With sub-stoichiometric amounts of the acid catalyst, up to 53% yields of FDCA were obtained.27,28 Taguchi et al. reported that heteropolyacids catalysed the dehydration at lower molar ratios compared to homogeneous acids; 44% FDCA ester was obtained with 10 mol% phosphotungstic acid in a 15 h reaction.28 These results show that aldaric acids are an interesting alternative for FDCA production, even though considerable improvements are needed for the methods to be industrially viable.
Aldaric acids have gained interest as value-added bio-based chemicals for the food, pharmaceutical and chemical industries; glucaric acid was listed by the DOE as one of the top value-added chemicals from biomass.29 Glucaric acid can be obtained by oxidation of glucose using a Pt catalyst30 or nitric acid31 in up to 60% or 45% yields, respectively. In a recent paper, Thaore et al. discussed the techno-economic and lifecycle assessments of the two methods.32 While both methods were shown to be economically viable, the heterogeneously catalysed route showed 22% lower environmental impact compared to the nitric acid-mediated route. An alternative route to aldaric acids is the oxidation of uronic acids obtained from pectin-containing waste streams. Au catalysts are highly selective in the oxidation of uronic acids to aldaric acids, giving up to quantitative yields under very mild conditions.33–36 Galactaric acid can also be produced directly from pectin without extensive purification using benign biotechnical means.37–40
The diacid functionality of aldaric acids is especially attractive for renewable polyesters and polyamides.2 Much of the research on aldaric acid valorisation has focused on producing adipic acid, one of the monomers of Nylon-66. Rennovia's two-step process includes the Pt-catalysed oxidation of glucose to glucaric acid followed by hydrogenation in the presence of a noble metal catalyst and HBr.30 Up to 60% and 89% yields were reported for the oxidation and hydrogenation steps, respectively. Adipic acid can also be produced from galactaric acid via muconic acid.41 Rhenium-catalysed deoxydehydration of galactaric acid can give up to quantitative yields of muconic acid in an alcohol solvent.42,43 Consecutive hydrogenation gives adipic acid in very high yields. In aqueous solutions, however, adipic acid yields remained low.44 Recycling of the precious Re catalyst was addressed by using an ionic liquid as a homogeneous support for the catalyst, giving an overall 91% yield of adipic acid.45
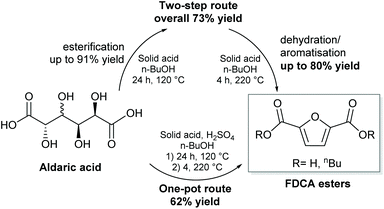
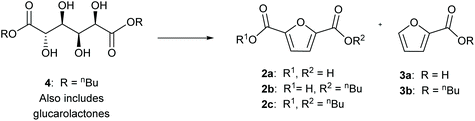
Here we show the efficient production of furancarboxylates from C6 aldaric acids (hexaric acids) using solid acid catalysts. In this recently patented process,46 over 95% total yields of furancarboxylates were obtained. The main products are the esters of FDCA and FCA, with the selectivity depending on the substrate, catalyst type and reaction conditions. Esterification prior to aromatisation increases the solubility and yield of the reaction. We show over 80% yield of FDCA esters, which to our knowledge is the highest reported yield of FDCA starting from aldaric acid esters (Scheme 2). The process uses an easily separable solid acid catalyst and n-butanol which is available from renewable sources. Furthermore, utilising pectin-derived galactaric acid expands the feedstock scope to many industry side streams previously unusable for FDCA production. Using sulfuric acid as the co-catalyst, FDCA esters can be obtained in a one-pot process starting from aldaric acid.
Results and discussion
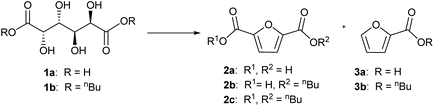
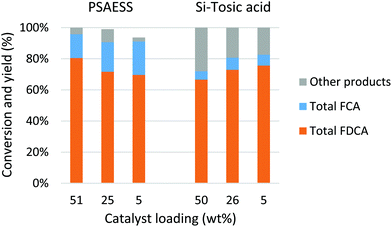
The dehydration of aldaric acid and consecutive formation of the furan ring take place under acidic conditions. Several families of solid acid catalysts were selected for the synthesis of furancarboxylates; acids supported on polymers, silica, alumina and zirconia were tested as well as acidic zeolites and clays. Many of the tested catalysts were obtained from commercial sources and some were prepared using methods previously reported in the literature (see the ESI† for more details). Previously, we have shown that also some transition metals, such as rhenium, can form furancarboxylates from aldaric acids but these catalysts favour deoxydehydration to linear muconic acid.46,47Both glucaric acid and galactaric acid are stable crystalline compounds and poorly soluble in most solvents. The initial screening was performed with galactaric acid (1a) in n-butanol to improve the solubility by in situ ester formation. At 210 °C, low conversions were achieved with only up to 7% furancarboxylates (Fig. S3†). Increasing the temperature to 230 °C increased the conversions; catalysts showing the desired activity towards aromatisation are shown in Fig. S4.† With sulfated zirconia, the furancarboxylate yields were very low. Sulfated alumina as well as Nafion NR50 clearly favoured the formation of furanmonocarboxylates, as was the case with silicotungstic acid. The most promising results were obtained with acids on silica carriers; phenyl sulfonic acid ethyl sulfide silica (PSAESS) gave furancarboxylates with 41% selectivity at 82% conversion (Table 1, entry 1). GC analyses of the silylated reaction mixtures showed that the main furan products were 2,5-furandicarboxylic acid 2a and its esters (2b, 2c) and 2-furanmonocarboxylates (3a, 3b). While both acid and ester forms of the furans were obtained, the ester forms were prevalent. It should be noted that all of the furandicarboxylates 2 can be used for polymerisation into PEF. On the other hand, the monocarboxylates 3 act as chain-terminating agents in polymerisation lowering the molecular weight of the polymer. For clarity, we have listed the selectivity to the separate products as well as the combined selectivity to furandicarboxylates 2 and furanmonocarboxylates 3 in Table 1. Other products include isomers of the furandicarboxylates (Fig. S6†), probably 2,3-furandicarboxylic acid and its esters.28
| Entry | Catalyst | Cat. amount (wt%) | Substrate conc. (M) | Conv. (mol%) | Selectivity (mol%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 3a | 3b | 2 | 3 | |||||
| Reaction conditions: substrate 1b, 10 ml n-butanol, 220 °C, 4 h, 5 bar N2.a 1a as the substrate, 20 ml n-butanol, 230 °C, 2 h.b 20 ml n-butanol.c One-pot reaction: 9.6 mmol 1a, 12 ml n-butanol, 2.0 mmol H2SO4, 24 h at 120 °C followed by 4 h at 220 °C. | |||||||||||
| 1a | PSAESS | 50 | 0.48 | 82 | 2 | 10 | 16 | 5 | 8 | 28 | 13 |
| 2b | PSAESS | 50 | 0.16 | 85 | 0 | 13 | 53 | 2 | 17 | 66 | 19 |
| 3 | PSAESS | 50 | 0.31 | 95 | 2 | 18 | 54 | 2 | 15 | 74 | 17 |
| 4 | Si-Propylsulfonic acid | 50 | 0.31 | 100 | 14 | 39 | 21 | 0 | 0 | 74 | 0 |
| 5 | Si-Tosic acid | 50 | 0.31 | 100 | 1 | 10 | 46 | 2 | 11 | 57 | 13 |
| 6 | PSAESS | 50 | 0.62 | 100 | 1 | 13 | 66 | 3 | 13 | 80 | 16 |
| 7 | Si-Propylsulfonic acid | 50 | 0.62 | 100 | 51 | 0 | 0 | 0 | 0 | 51 | 0 |
| 8 | Si-Tosic acid | 50 | 0.62 | 100 | 6 | 19 | 42 | 1 | 4 | 67 | 5 |
| 9 | PSAESS | 5 | 0.62 | 94 | 2 | 19 | 54 | 3 | 20 | 75 | 23 |
| 10 | Si-Tosic acid | 5 | 0.62 | 100 | 0 | 15 | 61 | 0 | 7 | 76 | 7 |
| 11c | Si-Tosic acid | 5 | 0.81 | 100 | 0 | 22 | 40 | 0 | 11 | 62 | 11 |
The yields of 2 starting from 1a were quite modest, and the reason for this might be the low solubility of galactaric acid in the solvent n-butanol. Therefore, we decided to improve the solubility of the starting material by esterification with n-BuOH prior to aromatisation. After refluxing in n-BuOH with an acid catalyst, the mixture was hot-filtered and evaporated to give 91% isolated yield of galactaric di-butyl ester 1b with 91% purity, with 5% monobutyl ester as the minor product (see the ESI†). The resulting mixture was soluble in n-butanol at 70 °C, whereas the free acid 1a did not dissolve even at 150 °C. We used this mixture in aromatisation without further purification.
The aromatisation reactions were carried out in stainless steel pressure reactors charged with 5 bar nitrogen and stirred with magnetic stirring at 300 rpm. During the reaction, the pressure increased to a maximum of 30 bar at 220 °C. When using 1b as the substrate, the selectivity to furandicarboxylates 2 doubled; PSAESS gave 66% selectivity at 85% conversion in 4 h (Table 1, entry 2). FDCA dibutyl ester 2c was the major product with 53% selectivity and the monoester 2b was formed with 13% selectivity. In addition, furanmonocarboxylates 3 were formed with 19% selectivity.
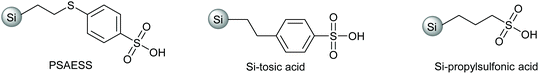
The higher solubility of 1b enabled increasing the substrate concentration, which is beneficial from both environmental and economic viewpoints. When the substrate concentration was doubled, 74% selectivity to 2 was achieved at 95% conversion (Table 1, entry 3). In addition to PSAESS, two other silica-supported sulfonic acid catalysts, Si-propylsulfonic acid and Si-tosic acid, were studied under similar conditions (Scheme 3). These catalysts gave full conversion and furandicarboxylates 2 were obtained with 74% and 57% selectivity, respectively (Table 1, entries 4 and 5). Interestingly, no decarboxylation products 3 were detected when using Si-propylsulfonic acid. Further doubling the substrate concentration gave full conversion with all three catalysts. In the case of PSAESS, 80% of furandicarboxylates 2 were obtained (entry 6), which is to our knowledge the highest FDCA yield reported starting from aldaric acid esters. Taking into account the esterification, the overall yield from galactaric acid over the two steps is 73%. Si-Propylsulfonic acid gave only 51% selectivity to 2 (entry 7), much lower than in the more dilute solution. With Si-tosic acid, the selectivity to 2 increased to 67% (entry 8).
Differences between the catalysts are mainly related to the acid strength and hydrophobicity; the density of acid sites of the three catalysts is in a similar range, 0.6–0.9 mmol g−1 (Scheme 3). Si-Propylsulfonic acid is slightly less acidic and more hydrophobic compared to PSAESS and Si-tosic acid,48 which could reduce interactions with the polar reactants, e.g. reducing the amount of decarboxylation. However, with higher substrate concentration, this also leads to lower selectivity. The difference between PSAESS and Si-tosic acid is a thioether bridge, which has been shown to have a promoting effect in fructose dehydration.49 In this work, PSAESS gives the highest selectivity to 2 but also more of unfavourable decarboxylation. In a blank experiment without an acid catalyst, 7% yield of 2 was obtained, confirming the crucial role of the catalyst (Table S1†).
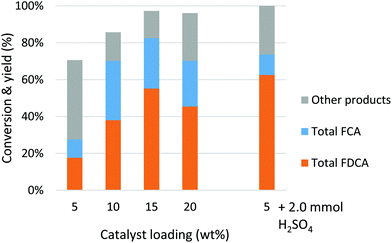
As quite high catalyst amounts were used in the previous experiments, we wanted to optimise the process by reducing the catalyst amounts. Using PSAESS and Si-tosic acid, we decreased the catalyst loading from 50 wt% to 5 wt% (Fig. 1). With PSAESS, the conversion and selectivity to 2 dropped slightly and 75% yield of 2 was obtained with 5 wt% (corresponding to ca. 1 mol%) catalyst (Table 1, entry 9). In addition, decarboxylation increased giving up to 23% of 3 as the side product. Remarkably, Si-tosic acid behaved the opposite; the selectivity to furandicarboxylates increased with decreased catalyst amount and 76% yield of 2 was achieved with 5 wt% (1 mol%) catalyst (entry 10). Furthermore, decarboxylation into 3 occurred to a lesser extent with Si-tosic acid compared to PSAESS.
 | ||
| Fig. 1 Optimising the catalyst amount in 1b aromatisation using PSAESS and Si-tosic acid. Reaction conditions: 6.2 mmol 1b, 10 ml n-butanol, 220 °C, 4 h. | ||
To reduce the number of steps needed, we wanted to combine the esterification and aromatisation into a one-pot, two-step process. Based on the previous results, we chose to concentrate on Si-tosic acid as the preferred catalyst. Knowing that Si-tosic acid can also catalyse the esterification of aldaric acids, we used the conditions for esterification (24 h at 120 °C) in the first stage, followed by the aromatisation stage at higher temperature (4 h, 220 °C). The substrate 1a, catalyst and solvent were all loaded into the autoclave at the start of the reaction. In our first attempt, both the conversion and selectivity were considerably lower than when starting from 1b (Fig. 2); less than 20% yield of 2 was obtained using 5 wt% Si-tosic acid. Increasing the catalyst amount to 15 wt% gave 55% yield of 2 at 97% conversion. However, decarboxylation was more pronounced and up to 30% of 3 was formed. The blank experiment with no catalyst gave only 7% diester 1b under similar conditions (Table S1†). To aid the reaction as well as to increase the solubility of 1a,42 we used sulfuric acid as the co-catalyst in the reaction. Indeed, 62% of 2 was formed at full conversion with 21 mol% sulfuric acid and 5 wt% Si-tosic acid (Table 1, entry 11). Furthermore, the selectivity to monocarboxylates 3 decreased to 11%. We are currently improving the one-pot reaction conditions e.g. by further optimising the esterification step. However, this is to our knowledge the highest amount of furandicarboxylates obtained from aldaric acid in a one-pot catalytic process.
Next, we wanted to expand the substrate scope to glucaric acid, which can be produced by oxidation of glucose. Attempts to use the commercially available glucaric acid potassium salt directly in the aromatisation step failed. Glucaric acid readily forms lactones under acidic conditions, which could prevent further reaction.50 Esterification of glucaric acid potassium salt with n-BuOH using sulfuric acid gave a mixture containing 39% dibutyl glucarate and 51% monobutyl glucarolactones (see the ESI†). Aromatisation of this mixture 4 gave 2 with ca. 50% selectivity at full conversion with both PSAESS and Si-tosic acid as catalysts (Table 2, entries 1 and 2). Reducing the catalyst amount to 5 wt% Si-tosic acid gave furanmonocarboxylates 3 as the main product with 37% selectivity and 2 with only 27% selectivity (entry 3). The glucarolactones in 4 probably cause lower reactivity compared to 1b. In addition, decarboxylation is clearly more pronounced starting from 4 than in the case of 1b. Gratifyingly, the addition of a catalytic amount of sulfuric acid increased the selectivity and decreased decarboxylation considerably; up to 70% of furandicarboxylates were obtained using Si-tosic acid and 27 mol% H2SO4 (entries 4 and 5). An experiment with only sulfuric acid as the catalyst gave 50% of 2 (entry 6). Similar yields were reported by Taguchi et al. with 2 equivalents of H2SO4 in an 8 h reaction.28 However, we also detected the formation of 24 wt% of insoluble char which had not been detected in the previous experiments. Evidently, the use of a solid acid together with sulfuric acid is beneficial for the reaction.
| Entry | Catalyst | Cat. amount (wt%) | Conv. (mol%) | Selectivity (mol%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 3a | 3b | 2 | 3 | ||||
| Reaction conditions: 7.4 mmol mixture 4 (calculated based on average Mw, see the ESI†), 10 ml n-butanol, 220 °C, 4 h.a 2.0 mmol H2SO4 added.b 2.0 mmol H2SO4 added, 24 wt% insoluble char produced. | ||||||||||
| 1 | PSAESS | 50 | 100 | 1 | 12 | 35 | 3 | 15 | 48 | 18 |
| 2 | Si-Tosic acid | 50 | 100 | 0 | 14 | 38 | 0 | 8 | 52 | 8 |
| 3 | Si-Tosic acid | 5 | 91 | 0 | 0 | 27 | 0 | 37 | 27 | 37 |
| 4a | Si-Tosic acid | 5 | 100 | 0 | 14 | 50 | 0 | 11 | 64 | 11 |
| 5a | Si-Tosic acid | 10 | 100 | 0 | 12 | 59 | 0 | 11 | 71 | 11 |
| 6b | — | 100 | 0 | 9 | 41 | 0 | 10 | 50 | 10 | |
Conclusions
We have shown here that aldaric acids are an attractive starting material for producing FDCA as a renewable building block. Aldaric acids can be obtained from currently underutilised pectin-containing side-streams or from glucose by oxidation. Subsequent dehydration of the aldaric acids using solid acid catalysts produces furancarboxylates in high yields. Importantly, this route avoids the use of unstable HMF as the intermediate in FDCA production. We carried out the dehydration of galactaric and glucaric acid with silica-supported solid acid catalysts in n-butanol at temperatures above 200 °C. Esterification of aldaric acids with an alcohol solvent prior to aromatisation increases the solubility and facilitates the reaction. The best results were obtained with phenyl sulfonic acid ethyl sulfide silica (PSAESS) and Si-tosic acid; over 80% yields of FDCA and its esters were achieved from galactaric acid ester. Considering the esterification of galactaric acid, an overall yield of 73% FDCA esters was achieved. Esterification of glucaric acid gave a mixture of dibutyl glucarate and glucarolactones, which reduced the selectivity to FDCA esters and increased decarboxylation. However, addition of a catalytic amount of sulfuric acid as the co-catalyst gave FDCA in up to 70% yield. To our knowledge, these are the highest reported yields of FDCA starting from aldaric acids. Finally, we also showed that esterification and aromatisation can be combined in a one-pot, two-step reaction.Experimental
Materials
Galactaric acid (97% purity), Nafion NR50 (≥90% assay, beads) and phenyl sulfonic acid ethyl sulfide silica (PSAESS, 95% assay, ≥45 μm particle size, 0.6–0.9 mmol g−1 loading) were obtained from Sigma Aldrich. Glucaric acid potassium salt (≥98% purity) was obtained from Santa Cruz. Silicotungstic acid (83.4% WO3 assay) was obtained from Alfa Aesar. Si-Tosic acid (0.62 meq g−1, 40–63 μm particle size) and Si-propylsulfonic acid (0.88 meq g−1, 40–63 μm particle size) were obtained from SiliCycle.General method for the synthesis of aldaric acid esters
Esterification of galactaric acid was carried out using Si-tosic acid and esterification of glucaric acid was performed using sulfuric acid. In a typical procedure, aldaric acid and n-butanol were placed in a three-neck flask and stirred with a magnetic stirrer. To this was added the acid catalyst, and the reaction mixture was heated to reflux for 24 h. Once complete, the reaction mixture was hot-filtered (80 °C) over a porosity 3 sinter. Evaporation of the solvent (45 °C, less than 20 mbar) afforded the esterified aldaric acid. See the ESI† for detailed procedures and analyses.General method for producing furancarboxylates
In a typical procedure, the weighed solvent, substrate and solid catalyst were added in a Hastelloy C-276 pressure reactor (75 ml) equipped with a magnetic stirring bar. The reactor was sealed and flushed with nitrogen before pressurising to approximately 5 bar with nitrogen. The reactor was then heated to the required reaction temperature (measured internally) for the indicated time. Magnetic stirring was used for mixing at 300 rpm. The reaction mixture was cooled to room temperature and filtered. Solvents were evaporated from the liquid phase (fraction 1) using a rotary evaporator and the residue was weighed. The solids from the first filtration were washed with 20 ml of hot n-butanol. The solution from the second filtration (fraction 2) was evaporated using a rotary evaporator and then dried in a vacuum oven and weighed. Both isolated product fractions were analysed quantitatively with GC-FID using a Shimadzu GC-1020 Plus Gas Chromatograph equipped with a ZB-5HT Inferno column. GC-MS was used for product identification. Further purification of the products for NMR identification was carried out using Kugelrohr distillation.51Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
Funding from Business Finland is gratefully acknowledged.References
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- B. M. Stadler, C. Wulf, T. Werner, S. Tin and J. G. de Vries, ACS Catal., 2019, 9, 8012–8067 CrossRef CAS.
- A. J. J. E. Eerhart, A. P. C. Faaij and M. K. Patel, Energy Environ. Sci., 2012, 5, 6407–6422 RSC.
- Avantium YXY Technology, https://www.avantium.com/technologies/yxy/, (accessed June 2020).
- J. K. Ogunjobi, T. J. Farmer, C. R. McElroy, S. W. Breeden, D. J. MacQuarrie, D. Thornthwaite and J. H. Clark, ACS Sustainable Chem. Eng., 2019, 7, 8183–8194 CrossRef CAS.
- L. Zhang, X. Luo, Y. Qin and Y. Li, RSC Adv., 2017, 7, 37–46 RSC.
- J. Deng, X. Liu, C. Li, Y. Jiang and J. Zhu, RSC Adv., 2015, 5, 15930–15939 RSC.
- A. Marotta, V. Ambrogi, P. Cerruti and A. Mija, RSC Adv., 2018, 8, 16330–16335 RSC.
- M. Sajid, X. Zhao and D. Liu, Green Chem., 2018, 20, 5427–5453 RSC.
- R. J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS.
- A. Mukherjee, M. J. Dumont and V. Raghavan, Biomass Bioenergy, 2015, 72, 143–183 CrossRef CAS.
- I. K. M. Yu and D. C. W. Tsang, Bioresour. Technol., 2017, 238, 716–732 CrossRef CAS.
- L. T. Mika, E. Cséfalvay and Á. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef CAS.
- K. I. Galkin and V. P. Ananikov, ChemSusChem, 2019, 12, 2976–2982 CrossRef CAS.
- A. S. V. De Sousa Dias, G. J. M. Gruter and R. J. van Putten, US9090581B2, 2015.
- C. Thoma, J. Konnerth, W. Sailer-Kronlachner, P. Solt, T. Rosenau and H. W. G. van Herwijnen, ChemSusChem, 2020, 13, 3544–3564 CrossRef CAS.
- H. Yuan, H. Liu, J. Du, K. Liu, T. Wang and L. Liu, Appl. Microbiol. Biotechnol., 2020, 104, 527–543 CrossRef CAS.
- Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS.
- G. R. Dick, A. D. Frankhouser, A. Banerjee and M. W. Kanan, Green Chem., 2017, 19, 2966–2972 RSC.
- F. van der Klis, J. van Haveren, D. S. van Es and J. H. Bitter, ChemSusChem, 2017, 10, 1460–1468 CrossRef CAS.
- R. Fittig, Chem. Ber., 1876, 9, 1189–1199 CrossRef.
- R. Kuhn and K. Dury, Justus Liebigs Ann. Chem., 1951, 571, 44–68 CrossRef CAS.
- W. N. Haworth, W. G. M. Jones and L. F. Wiggins, J. Chem. Soc., 1945, 1, 1–4 RSC.
- G. Brǎtulescu, Rev. Roum. Chim., 2000, 45, 883 Search PubMed.
- J. Lewkowski, Pol. J. Chem., 2001, 75, 1943 CAS.
- D. Zhao, F. Delbecq and C. Len, Molecules, 2019, 24, 8–10 Search PubMed.
- G. Bratulescu, J. Soc. Alger. Chim., 2000, 10, 135–137 CAS.
- Y. Taguchi, A. Oishi and H. Iida, Chem. Lett., 2008, 37, 50–51 CrossRef CAS.
- T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass: Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas, United States, 2004 Search PubMed.
- R. Archer, E. L. Dias, V. J. Murphy, T. R. Boussie, Z. M. Fresco, J. Shoemaker and H. Jiang, WO2010144862A2, 2010.
- T. N. Smith, K. Hash, C.-L. Davey, H. Mills, H. Williams and D. E. Kiely, Carbohydr. Res., 2012, 350, 6–13 CrossRef CAS.
- V. B. Thaore, R. D. Armstrong, G. J. Hutchings, D. W. Knight, D. Chadwick and N. Shah, Chem. Eng. Res. Des., 2020, 153, 337–349 CrossRef CAS.
- S. Rautiainen, P. Lehtinen, J. Chen, M. Vehkamäki, K. Niemelä, M. Leskelä and T. Repo, RSC Adv., 2015, 5, 19502–19507 RSC.
- F. van der Klis, A. E. Frissen, J. van Haveren and D. S. van Es, ChemSusChem, 2013, 6, 1640–1645 CrossRef CAS.
- D. S. J. van Esvan Haveren, H. W. C. Raaijmakers, F. van der Klis, G. P. F. M. van Engelen and A. E. Frissen, US9079844B2, 2015.
- F. van der Klis, L. Gootjes, J. van Haveren, D. S. van Es and J. H. Bitter, React. Chem. Eng., 2018, 3, 540–549 RSC.
- D. Mojzita, M. Wiebe, S. Hilditch, H. Boer, M. Penttilä and P. Richard, Appl. Environ. Microbiol., 2010, 76, 169–175 CrossRef CAS.
- H. Boer, S. Hildich, M. Penttilä and P. Richard, US8895273B2, 2015.
- S. Kambourakis, B. M. Griffin and K. V. Martin, US9528133B2, 2017.
- T. Paasikallio, A. Huuskonen and M. G. Wiebe, Microb. Cell Fact., 2017, 16, 119 CrossRef.
- I. Khalil, G. Quintens, T. Junkers and M. Dusselier, Green Chem., 2020, 22, 1517–1541 RSC.
- M. Shiramizu and F. D. Toste, Angew. Chem., Int. Ed., 2013, 52, 12905–12909 CrossRef CAS.
- X. Li, D. Wu, T. Lu, G. Yi, H. Su and Y. Zhang, Angew. Chem., Int. Ed., 2014, 53, 4200–4204 CrossRef CAS.
- B. Hočevar, M. Grilc and B. Likozar, Catalysts, 2019, 9, 286 CrossRef.
- N. Shin, S. Kwon, S. Moon, C. H. Hong and Y. G. Kim, Tetraherdon, 2017, 73, 4758–4765 CrossRef CAS.
- D. Thomas, A. Harlin and M. Asikainen, US10301276B2, 2019.
- D. Thomas, A. Harlin and M. Asikainen, US9969669B2, 2018.
- Shagufta, I. Ahmad and R. Dhar, Catal. Surv. Asia, 2017, 21, 53–69 CrossRef CAS.
- A. J. Crisci, M. H. Tucker, M. Lee, S. G. Jang, J. A. Dumesic and S. L. Scott, ACS Catal., 2011, 1, 719–728 CrossRef CAS.
- J. M. Brown, M. Manley–Harris, R. J. Field and D. E. Kiely, J. Carbohydr. Chem., 2007, 26, 455–467 CrossRef CAS.
- D. Thomas and J. Linnekoski, WO2019155127A1, 2019.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc02293d |
| This journal is © The Royal Society of Chemistry 2020 |