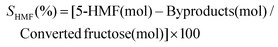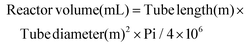Open Access Article
Open Access Article5-Hydroxymethylfurfural from fructose: an efficient continuous process in a water-dimethyl carbonate biphasic system with high yield product recovery†
Mahmoud
Sayed
 a,
Niklas
Warlin
a,
Niklas
Warlin
 b,
Christian
Hulteberg
b,
Christian
Hulteberg
 c,
Ian
Munslow
d,
Stefan
Lundmark
d,
Oleg
Pajalic
c,
Ian
Munslow
d,
Stefan
Lundmark
d,
Oleg
Pajalic
 d,
Per
Tunå
c,
Baozhong
Zhang
d,
Per
Tunå
c,
Baozhong
Zhang
 b,
Sang-Hyun
Pyo
b,
Sang-Hyun
Pyo
 a and
Rajni
Hatti-Kaul
a and
Rajni
Hatti-Kaul
 *a
*a
aDivision of Biotechnology, Department of Chemistry, Lund University, P.O. Box 124, SE-22100 Lund, Sweden. E-mail: rajni.hatti-kaul@biotek.lu.se
bCenter for Analysis and Synthesis, Department of Chemistry, Lund University, P.O. Box 124, SE-22100 Lund, Sweden
cDepartment of Chemical Engineering, Lund University, P.O. Box 124, SE-22100 Lund, Sweden
dPerstorp AB, Innovation, Perstorp Industrial Park, SE-284 80 Perstorp, Sweden
First published on 20th July 2020
Abstract
Bio-based 5-hydroxymethylfurfural (5-HMF) and its derivatives have attracted enormous attention due to their valuable market potential. Production of pure 5-HMF is challenging owing to the high reactivity of its functional groups and formation of by-products. In this study, an efficient continuous process for 5-HMF production in a biphasic system and its recovery at high yield and selectivity was developed. After an initial screening of different solvents, a water/dimethyl carbonate (DMC) system was selected for acid catalyzed fructose dehydration in a continuous mode using 0.23 M HCl as a catalyst. Effects of various reaction parameters on substrate conversion, product yield and selectivity, were determined. The process using 30% (w/v) fructose in water with three times the volume of DMC at 1 min residence time in a tube reactor at 200 °C provided 96.5% fructose conversion and 87.2% 5-HMF yield with a selectivity of 85.5% and 95.8% in aqueous and organic phases, respectively. Increasing the fructose concentration in the water phase to 52% gave 96.4% conversion and 74% 5-HMF yield. Using a fructose-glucose mixture as substrate had no effect on fructose conversion but affected slightly the selectivity of 5-HMF in the aqueous phase. Recovery of 5-HMF with ≥93% purity from DMC was achieved by solvent evaporation under vacuum, and improved by prior treatment with activated carbon, especially together with Na2CO3. Evaluation of the purified 5-HMF in a reaction with pentaerythritol showed comparable performance to the commercial 5-HMF in the production of a spirocyclic diol, a monomer for the production of polyesters and polyurethane.
Introduction
5-Hydroxymethylfurfural (5-HMF) has been included among the top 10 most valuable biobased products from biorefinery of carbohydrates by the US Department of Energy in 2010.1 It serves as a platform for the production of an array of fuel and chemical products such as dimethylfuran (DMF),2,3 2,5-furandicarboxylic acid (FDCA),4,5 levulinic acid, 2,5-diformylfuran (DFF),6,7 5-hydroxymethyl-5-furan carboxylic acid (HMFCA),8,9 dihydroxymethylfuran,10 5-formyl-2-furan carboxylic acid (FFCA),11 1,6-hexanediol, adipic acid, etc.12,13 Production of 5-HMF has thus attracted enormous attention, which is clearly evident by the large number of publications including several reviews appearing regularly since the early years of the 21st century although the compound has been known since the end of the 19th century.14–215-HMF is produced by acid-catalyzed elimination of three water molecules from hexoses. The reaction is accompanied by a number of other side reactions, resulting in the generation of soluble and insoluble humins by decomposition of the sugars or reactions between 5-HMF molecules and with sugar molecules, while organic acids including levulinic acid and formic acid are formed by rehydration of 5-HMF. Fructose is the most suitable substrate for the reaction in terms of reactivity and product selectivity which is attributed to the co-existence of the cyclic furanose tautomer, the preferred form for the production of 5-HMF, with pyranose form of the sugar in contrast to glucose that exists primarily as the pyranose form.22 As glucose is the preferred starting material due to its abundance in nature as a component of the polysaccharides, cellulose and starch, and also the disaccharide sucrose, dehydration to 5-HMF is often preceded or integrated with isomerization of glucose to fructose.14–16,23–26
Production of 5-HMF has been investigated in several solvent systems using a variety of homogeneous or heterogeneous acid catalysts.21,22,27 The solvent systems used may largely be divided into: (i) monophasic systems involving either water with a cosolvent or a polar aprotic solvent, and (ii) biphasic systems for in situ extraction of the 5-HMF from the reaction phase to the organic phase. Invariably, the focus has been on evaluating and optimizing the various systems and catalysts for achieving high 5-HMF yield, while reports covering separation and recovery of pure 5-HMF from the process are scant.
The highest yields of 5-HMF (60–91%) from fructose (10% w/v) in monophasic systems have been reported in dimethyl sulfoxide (DMSO), N-methyl-2-pyrrolidone (NMP), water-tetraethylammonium bromide (TEAB) at 95–110 °C.22,28 Good yields from higher fructose concentration have been reported by performing sugar dehydration in DMSO in a flow reactor.29,30 But solvents like DMSO or NMP have high boiling points, thus complicating the product recovery by distillation due to the reactive nature of 5-HMF. Moreover, sulphur and sulphur containing solvents have a toxic effect on most heterogeneous catalysts.15,26,30–32
Reactions in two-phase systems have been performed using many different solvents and combinations thereof as the extracting phase,15,21,22 which is invariably used in excess with respect to the reacting phase. Also, in many studies salts have been added to increase the partitioning of 5-HMF to the organic phase.21,33 Methyl isobutyl ketone (MIBK) has been the solvent of choice in several reports, the highest sugar conversion and 5-HMF yield being obtained with Ag3PW12O40 as the catalyst. The use of co-solvents either in the reacting phase (e.g. DMSO, NMP) or in the extracting phase containing MIBK (e.g. 2-butanol) has led to improved process performance.21,34–37 MIBK-water system has been used at kilogram scale production of 5-HMF from fructose, however with moderate conversion and yield.14,15 The use of THF as extracting phase has also been extensively reported and has yielded the best results so far but in systems with NaCl at high concentration.31,38,39 The drawback of using a mixture of solvents or salts presents a downstream problem for their separation if they are to be recycled in the process.19
Attempts have also been made with ionic liquids such as 1-butyl-3-methylimidazolium ([BMIM]+) and 1-ethyl-3-methylimidazolium ([BMIM]+) as reaction media, having the advantages of operation at low pressure and low temperature.15,40,41 But such systems require organic extracting phase in large excess as compared to the conventional biphasic systems. Deep eutectic solvents (DES) comprising a mixture of a hydrogen bond acceptor and a hydrogen bond donor such as choline chloride-citric acid are other systems when combined with a solvent (e.g. ethyl acetate) used in excess as the extracting phase, have provided high product yield.42,43 Both ionic liquids and biphasic systems have even been used for 5-HMF production directly from cellulose and several biomass materials, however with low 5-HMF yields.15,44 In a very recent review, careful consideration to the rational selection of the most suitable solvents for biphasic systems in terms of performance, environmental, health and safety (EHS) impacts, and subsequent downstream processing, has been highlighted.21 COSMO-RS (Conductor-like Screening Model for Real Solvents) was used as a tool for predicting the distribution coefficient of 5-HMF and furfural in solvents based on their structural information. Although the tool overestimated the presence of 5-HMF in the organic phase in general as compared to the experimental values, the study made a ranking of solvents based on their extraction ability for 5-HMF in a biphasic system. Combining this with scores from solvent selection guides based on EHS (environment-health-safety) considerations, ethyl acetate and methyl propionate were proposed as the most suitable solvents for in situ extraction of 5-HMF.21
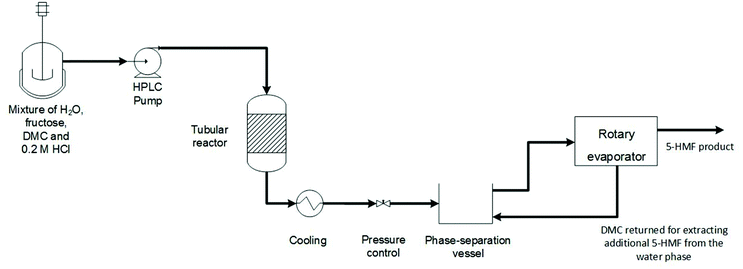
Based on the existing knowledge, the aim of the present study was to select a solvent that would enable a resource-efficient process for continuous sugar dehydration in a biphasic system providing high 5-HMF yield and selectivity, and allowing facile product purification, as well as complying with EHS requirements (Fig. 1). Effect of various reaction parameters on substrate conversion, product yield and selectivity was studied. Recovery of pure HMF from the process liquid was also investigated. The process performance was evaluated at a scale for processing about 1 kg fructose per day. The purified 5-HMF product was furthermore evaluated in a reaction with pentaerythritol to form a spirocyclic diol.45
Experimental
Materials
Fructose, 5-hydroxymethylfurfural (5-HMF), dimethyl carbonate (DMC, 99%), methyl isobutyl ketone (MIBK, 99%), 1-butanol (≥99.4%), 2-propanol (99%), tetrahydrofuran (THF, 99.9%), γ-valerolactone (GVL, ≥99%), HCl (37%), acetone-d6, chloroform-d6 (CDCl3), dimethyl sulfoxide-d6 and dimethyl-d6 carbonate were purchased from Sigma-Aldrich, while glucose, sodium carbonate, sodium hydroxide and active carbon were obtained from Merck. Pentaerythritol was a product of Perstorp AB, while fructose syrup was kindly provided by Nordic Sugar A/S.Screening of solvents in biphasic systems for batch fructose dehydration to 5-HMF
Fructose was dissolved in water at a concentration of 30% (w/v). Five hundred microliter of the fructose solution was taken in a 4 mL vial and supplemented with HCl to the final concentration of 0.23 M prior to adding 1.5 mL of a solvent, MIBK, DMC, 1-butanol, THF, 2-propanol or GVL. The vials were placed in a Thermomixer (HTMR 131, HLC BioTech, Germany) and heated at 95 °C and 500 rpm for 3 hours. Samples were collected every 20 min for analyses. This was done by letting the vials stand for 5 min at room temperature for phase separation to occur as well as to prevent the loss of organic solvent due to vaporization, and 20 μL samples each were collected from aqueous and organic phases, respectively, for determination of concentrations of fructose, 5-HMF and other by-products.Continuous dehydration of fructose into 5-HMF in a tube reactor
To a 30% (w/v) aqueous solution of fructose was added HCl (final concentration of 0.23 M) and three times the volume of DMC. The mixture was stirred on a magnetic stirrer and injected using a HPLC pump (Jasco PU-980, Jasco, Japan) at a flow rate of 2 mL min−1 and pressure of 20 bar into a 2.3 mL stainless steel tube reactor (4 mm diameter × 180 mm length) maintained at temperatures ranging from 150 to 200 °C using a heating jacket and a temperature control unit (Fig. 1). The process stream exiting the reactor was collected, allowed to phase separate, and 1 mL samples were withdrawn from the solvent and water phases, respectively, for analysis.The effect of the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC ratio of 1
DMC ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and of higher concentration of fructose (520 g L−1 water) at a water
5, and of higher concentration of fructose (520 g L−1 water) at a water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC ratio of 1
DMC ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 on 5-HMF production at 200 °C was also investigated. In other experiments, mixtures of fructose with glucose in weight ratios of 9
4 on 5-HMF production at 200 °C was also investigated. In other experiments, mixtures of fructose with glucose in weight ratios of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, at a total sugar concentration of 300 g L−1 in the water phase (in 1
1, respectively, at a total sugar concentration of 300 g L−1 in the water phase (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 water
3 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC system) were used for dehydration at 200 °C.
DMC system) were used for dehydration at 200 °C.
Subsequently, a tube reactor with about four times larger volume was set up, comprising a coiled tube (4 mm diameter × ∼800 mm length) inserted in a tube furnace to achieve the required process temperature, followed by another coil extension submerged in a chilled water bath for cooling the reaction mixture. A 30% (w/v) fructose solution in water, supplemented with 0.23 M HCl, was mixed with three times the volume of DMC in a total volume of 400 mL. The two phases were mixed by magnetic stirring and injected into the reactor maintained at 180 °C under pressure of 20–30 bars at flow rates of 7.5, 9, and 10 mL min−1, respectively, using a HPLC pump (515 HPLC pump, Waters, Massachusetts, USA) followed by a check valve (Swagelok, Ohio, USA) to keep the flow of the solution in one direction. Ten milliliter samples were collected from the reactor at the initial, middle and end points of the reaction for analysis of fructose, 5-HMF, and other by-products.
The biphasic mixture, having the same composition as above, was then pumped for a period of 7 hours at 10 mL min−1 (i.e. a total of 1080 g fructose per day) into the reactor in order to evaluate the process stability. A reaction was also done in the biphasic system using 30% w/v commercial fructose syrup (containing 90% fructose and 10% glucose) in water, which was pumped at a flow rate of 9 mL min−1 under the same conditions as above, and sample collection was performed as mentioned above. Moreover, in order to test reusability of the water phase after recovery of 5-HMF as described below, 100 g L−1 fructose and 0.12 M HCl were added and mixed with fresh DMC (3 times volume of the aqueous phase), and the mixture was subjected to dehydration in the tube reactor as described above. This procedure was repeated three times.
5-HMF recovery from the organic phase and aqueous phase
The recovery and purification of 5-HMF were tested from both organic and water phases after separation of the product mixture in a separation funnel. 5-HMF was recovered from the organic phase through evaporation of DMC at 40 °C under vacuum in a rotary evaporator. The effect of treatment of the organic phase with activated carbon on the purity of the recovered 5-HMF was tested. The activated carbon was mixed (2.5–10% w/v) in 50 mL organic phase for 5 min followed by centrifugation at 6000 rpm (Sorvall LYNX 4000, Thermo Scientific, Germany) for 10 min, and filtration of the supernatant through a 0.45 μm PTFE filter. The pretreated solution was further treated with 2% (w/v) NaOH pellets, and 2% (w/v) Na2CO3, respectively, to neutralize the residual HCl. One milliliter samples were collected for HPLC analysis.5-HMF from the aqueous phase was recovered into 3 times volume of DMC (the recycled organic phase after rotary evaporation above). The 5-HMF purity was compared with that of the commercial 5-HMF using HPLC, NMR and LC-MS. All the 5-HMF samples were stored overnight at −20 °C to obtain crystals.
Reaction of the purified 5-HMF with pentaerythritol
The purified 5-HMF was tested in a reaction with pentaerythritol to form the spirocyclic diol as described by Warlin et al.45 The reaction was compared with HMF (98%) obtained from a commercial source (Nanjing Confidence Chemical Co., China).Analytical procedures
The concentrations of fructose, 5-HMF and by-products were determined using HPLC (JASCO, Tokyo, Japan) equipped with a RI detector (ERC, Kawaguchi, Japan), a JASCO UV detector operating at 215 nm and a JASCO intelligent autosampler. Separation of the compounds was carried out on a fast acid analysis chromatographic column connected to a guard column (Biorad, Richmond, CA, USA). The column temperature was maintained at 65 °C using a chromatographic oven (Shimadzu, Tokyo, Japan). Samples were diluted with Milli-Q quality water and mixed with 10% v/v sulfuric acid (25 μL mL−1 sample) and then filtered through 0.45 μm filters. A 40 μL aliquot was injected in 10 mM H2SO4 mobile phase flowing at a rate of 0.6 mL min−1. The peaks for the different compounds were confirmed and quantified using external standards.The volumetric productivity (Qp), yield (Yp/s) and total selectivity (ST), selectivity (S) of 5-HMF production, residence time, and reactor volume were calculated for all experiments as follows:
| Conversion (%) = (Xfinal/Xinitial) × 100 |
| Qp (g L−1 h−1) = (Pfinal − Pinitial)/dt |
| Yp/s (%) = [(Pfinal − Pinitial)/(Xinitial − Xfinal)] × 100 |
| ST HMF (%) = [Yield (%)/Conversion (%)] × 100 |
| Residence time (s) = Reactor volume/Flow (mL min−1) × 60 |
where P is the product, X is the substrate and t is the reaction time.
The purity of 5-HMF and the diol monomer produced from HMF was further confirmed using UHPLC-MS with UV–VIS detection on a Waters Acquity UHPLC + Waters XEVO-G2 QTOF mass spectrometer using a Waters Acquity CSH C18, 1.7 μm, 2.1 × 100 mm column. Samples were run using a gradient with water (0.1% formic acid) and acetonitrile at a flow rate of 0.50 mL min−1 and a column temperature of 60 °C. Gradient parameters were 0–1 min: 3% acetonitrile, 1–9 min: 3–50% acetonitrile, 9–9.5 min 50–95% acetonitrile, 9.5–11.0 min 95% acetonitrile, 11.0–11.1 min: 95–3% acetonitrile, and 11.1–13 min: 3% acetonitrile. The sample injection volume was 2 μL, and detection wavelength used was 190–300 nm. MS parameters: cap voltage 3.0 kV, cone voltage 40 kV, ext 4, source temperature 120 °C, desolvation temperature 500 °C, cone gas 50, desolvation gas 800, centroid resolution mode, m/z interval 100–1200, using Leucine Enkephalin for lock mass correction. 1H and 13C NMR measurements were performed on a Bruker DR X400 spectrometer at 400.13 MHz and 100.61 MHz, respectively.
5-HMF stability in DMC, DMSO, 1-butanol, GVL, MIBK and water was investigated by determining the red and blue-shifts of 5-HMF C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and H–O, using Fourier-transform infrared spectroscopy (FT-IR).47 The spectra of samples were obtained in a region of 500–4000 cm−1 using Nicolet-iS5 (Thermo Scientific, USA). An air background spectrum was collected before the analysis of the sample, and subtracted from each sample spectrum.
O and H–O, using Fourier-transform infrared spectroscopy (FT-IR).47 The spectra of samples were obtained in a region of 500–4000 cm−1 using Nicolet-iS5 (Thermo Scientific, USA). An air background spectrum was collected before the analysis of the sample, and subtracted from each sample spectrum.
Results and discussion
Small-scale experiments on fructose dehydration to 5-HMF in biphasic systems
Inspite of the large number of studies reported on 5-HMF production in several solvent systems, we initiated our studies with screening of a limited number of solvents for 5-HMF partitioning and the efficiency of acid-catalyzed fructose dehydration. The solvents chosen were GVL, THF, MIBK, DMC, 1-butanol and 2-propanol; the main considerations in the choice were low boiling point (except for GVL) for enabling facile 5-HMF separation in terms of low energy expense and recycling to the process, reasonable distribution coefficient for 5-HMF, low toxicity, and potentially renewable origin. Although ethyl acetate has been suggested as a suitable solvent for a biphasic system for 5-HMF production,21 our preliminary studies showed poor fructose conversion as well as 5-HMF yield, hence the solvent was not included in the study.Table 1A lists some properties of the solvents including boiling point, miscibility with water, and partition coefficients of 5-HMF in the biphasic systems. While GVL, 2-propanol and THF are miscible with water, DMC, 1-butanol and MIBK form two phases but have varying miscibilities with water at 22 °C. DMC has the highest miscibility (139 g L−1) and MIBK the lowest (19.1 g L−1). According to Dibenedetto et al.,48 although the solubility of DMC increases further with the increase in temperature (14.3% at 80 °C), water/DMC system existed as a two-phase system even at 150 °C. On the other hand, the partition coefficient (ratio of concentration in organic phase to the concentration in water phase) of 5-HMF, determined by partitioning 5-HMF in the biphasic systems made with equal volumes of solvent and water phases at 22 °C, was the highest in water/1-butanol (1.5) followed by water/DMC (1.2) and water/MIBK (0.6) systems. This seems to be in agreement with the order of ranking of the solvents according to their extraction ability proposed by Esteban et al.,21 wherein 1-butanol was ranked number 8, DMC 15 and MIBK 28 in contrast to ethyl acetate that was ranked number 1.21 The low partitioning of 5-HMF in MIBK containing system is attributed to the high polarity of 5-HMF and low solubility of the solvent in water, and that could also explain the formation of humins observed during 5-HMF production in this system.
| A | |||
|---|---|---|---|
| Solvent | Boiling point (°C) | Miscibility with H2O (g L−1) | Partition coefficient |
| GVL | 207 | Miscible | — |
| 2-Propanol | 82.6 | Miscible | — |
| THF | 66 | Miscible | — |
| DMC | 90 | 139 | 1.2 |
| 1-Butanol | 117 | 73 | 1.5 |
| MIBK | 116 | 19.1 | 0.6 |
| B | |||
|---|---|---|---|
| System | Fructose conversion (%) | 5-HMF yield (%) | Productivity (g L−1 h−1) |
| a 5-HMF yield and productivity were not determined due to overlapping of the chromatographic peaks with GVL. | |||
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GVL GVL |
92 ± 10.1 | ||
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol 2-propanol |
48.8 ± 2.4 | 67.1 ± 0.6 | 22.9 ± 0.6 |
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF THF |
65.5 ± 13.7 | 51.5 ± 4.4 | 23.6 ± 0.4 |
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC |
50.4 ± 9.3 | 87.6 ± 6.2 | 30.9 ± 0.3 |
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-butanol 1-butanol |
36.4 ± 9.8 | 73.5 ± 23.3 | 16.0 ± 3.4 |
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MIBK MIBK |
37.4 ± 3.5 | 85.9 ± 12.6 | 22.5 ± 2.6 |
Considering the scores for waste, environmental impact, health and safety in the GlaxoSmithKline (GSK) solvent sustainability guide, 1-butanol, DMC and MIBK are ranked among the highest based on the overall characterization of the holistic sustainability.49 The major environmental concerns were air impact for 1-butanol, recycling for DMC (which would be related to its high water solubility), and air impact and life cycle analysis (LCA) for MIBK. 2-Propanol was slightly lower in the ranking and the major concerns were recycling and biotreatment, while THF had major issues concerning aquatic impact, biotreatment and VOC emissions. GVL was among the newly assessed solvents with the main concern being its high boiling point.49
Yet another consideration made in this study was not to use a heterogeneous catalyst to avoid the risk of blocking the reactor with humins during continuous operation; instead a low concentration (0.23 M) of HCl was used as the catalyst. The dehydration reactions in the above systems were performed in vials at 95 °C for 3 hours. Fructose was used at a concentration of 300 g L−1 of the water phase, and aqueous fructose solution was used as a control reaction system. As shown in Table1B, the level of fructose conversion followed the order: GVL > THF > DMC > 2-propanol > MIBK > 1-butanol.
Fructose conversion was as high as 92% in water/GVL system but the amount of 5-HMF formed could not be estimated by the analytical conditions used due to overlapping of the chromatographic peaks of 5-HMF and GVL. Nearly complete fructose (10 wt%) conversion with 84% yield of 5-HMF has in fact been reported earlier in a GVL-water system saturated with potassium bromide.50 Nevertheless, the main drawback with GVL is the similarity in structure with 5-HMF and its high boiling point, which would make the separation and recovery of 5-HMF very difficult. While THF and 2-propanol have low boiling points (66 and 82.6 °C, respectively), the yield of 5-HMF from fructose in the corresponding systems was low. Earlier reports have reported high product selectivity in THF based biphasic systems containing high NaCl concentrations (up to 35 wt%) and with hafnyl phosphate as catalyst.38,39,51 In contrast, the selectivity and productivity of HMF formation were significantly higher in water/DMC system (DMC boiling point = 90 °C) although at moderate fructose conversion (Table 1B). On the other hand, the water/MIBK system showed selectivity close to that in the DMC-containing system, but the fructose conversion and productivity were much lower, and humin formation was clearly visible at the end of the reaction. Moreover, the low LCA score of MIBK does not favor its choice as the solvent.49 The results obtained with the water/1-butanol seem to be in agreement with the earlier report, wherein the addition of over 13 wt% NaCl significantly improved the fructose conversion and HMF yield.51,52
Water/DMC system was thus selected for further studies based on the above results. In a recent report on fructose dehydration in biphasic systems made with organic carbonates and using cerium phosphate as the catalyst, highest 5-HMF yield of over 67% with selectivity of 93.2% was achieved in DMC, and a highly pure solid 5-HMF was obtained by evaporating the solvent under vacuum.48 Increase in the alkyl chain length from methyl to ethyl and allyl led to a decrease in 5-HMF yield. Besides the low boiling point of DMC, a further advantage of this system for 5-HMF production is the insolubility of fructose in the solvent allowing clean and efficient separation of the untransformed substrate from the product as well as that of the product from the solvent and efficient solvent recycling. Moreover, DMC has low toxicity and environmental impact and has very good LCA credentials.49,53,54 According to the CHEM21 selection guide for solvents, DMC is grouped among the less classical-solvents and is regarded as the greenest carbonate and a potential replacement for methyl ethyl ketone (MEK), ethyl acetate, MIBK, butyl acetate and most other ketones and glycol ethers.54 DMC is already used as a solvent in different processes, as an electrolyte in ion batteries and as a reagent for the transformation of functional groups.55,56
Continuous dehydration of fructose into 5-HMF in water/DMC system in a tube reactor: evaluation of reaction parameters
Production of 5-HMF from fructose in the water/DMC system was then investigated in a tube reactor with a volume of 2.3 mL, using a flow rate of 2 mL min−1. As suggested earlier,16 temperature and water content are the critical factors for enhancing fructose dehydration and 5-HMF selectivity. Table 2A shows that increasing the reaction temperature from 150 to 200 °C improved the conversion of fructose from 62.2 to 96.5%, and the yield of 5-HMF from 34.8 to 87.2%. The effect of temperature is most likely related to increased solubility of DMC in water, making the system more like a single liquid phase system with improved mass transfer properties. On the other hand, high temperature promotes humin formation and rehydration reactions in a batch process containing water,15,16,57 but these undesired reactions were reduced by performing the reaction in a continuous mode with DMC as the extracting phase. A short residence time of about 1 min (based on the flow rate) in the tube reactor minimizes the risk of unspecific reactions and hence yields higher product selectivity (Fig. 1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose ratio
glucose ratio
| Fructose load (g l−1 water phase) | Temperature [°C] | Aqueous![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) organic phase ratio organic phase ratio |
Fructose conversion [%] | Total 5-HMF yield [%] | 5-HMF in aqueous phase [% of total] | Selectivity of 5-HMF formation [%] | 5-HMF selectivity in aqueous phase [%] | 5-HMF selectivity in organic phase [%] | 5-HMF [g h−1] |
|---|---|---|---|---|---|---|---|---|---|
| a Low selectivity due to the accumulation of difructose anhydrides (DFAs), the cyclic intermediate that is converted to 5-HMF with prolonged incubation. b The yield calculation is based on fructose concentration only. | |||||||||
| (A) Temperature | |||||||||
| 300 | 200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
96.5 | 87.2 | 18.9 | 90.4 | 85.5 | 95.8 | 21.2 |
| 300 | 180 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
89.5 | 85.7 | 18.1 | 95.8 | 80.0 | 94.3 | 19.3 |
| 300 | 170 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
85.6 | 71.7 | 17.4 | 83.8 | 72.4 | 95.1 | 15.5 |
| 300 | 150 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
62.2 | 34.8 | 18.2 | 56.0 | 19.4 | 92.3 | 5.5 |
| (B) Aqueous–organic phase ratio | |||||||||
| 300 | 200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
96.0 | 69.0 | 36.8 | 71.9 | 68.3a | 96.8 | 16.75 |
| 300 | 200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
96.5 | 87.2 | 18.9 | 90.4 | 85.5 | 95.8 | 21.2 |
| 300 | 200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
96.6 | 87.2 | 16.1 | 90.3 | 88.9 | 94.8 | 21.5 |
| 300 | 200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
98.3 | 89.1 | 11.1 | 90.6 | 93.2 | 94.8 | 22.4 |
| (C) Fructose concentration | |||||||||
| 520 | 200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
96.4 | 74.0 | 12.0 | 76.8 | 74.0 | 89.4 | 31.1 |
(D) Fructose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) glucose (g glucose (g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) g) g)
|
|||||||||
270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
90.2 | 90.9 | 20.2 | ND | 84.6 | 95.0 | 16.8 |
150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
96.5 | 96.7b | 20.5 | ND | 65 | 92.7 | 10.13 |
Around 18% of the total 5-HMF formed was found in the aqueous phase, which was attributed to the solubility of both 5-HMF and DMC in water. This implies that the aqueous phase would need to be processed in order to recover all the 5-HMF formed. While the selectivity of 5-HMF formation was generally high in the organic phase over the entire temperature range (over 90%), there was a dramatic improvement in selectivity in the water phase from 19.4% at 150 °C to 85.5% at 200 °C. This is ascribed to the accumulation of difructose anhydrides (DFAs), the cyclic intermediate that is converted to 5-HMF, at a higher temperature.
Hence, the selectivity of 5-HMF formation can be enhanced by increasing the reaction time or temperature.16
As the presence of DMC was shown to limit the formation of side products, the amount of DMC used was varied to give aqueous/organic phase ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for dehydration of 30% (w/v) fructose at 200 °C (Table 2B). The low 5-HMF yield and selectivity, 69% and 68.3%, respectively, at a ratio of 1
5 for dehydration of 30% (w/v) fructose at 200 °C (Table 2B). The low 5-HMF yield and selectivity, 69% and 68.3%, respectively, at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 suggested lower partitioning of 5-HMF into DMC, and consequently further reactions of 5-HMF in the water phase into humins and other by-products. The increase in phase ratio to 1
2 suggested lower partitioning of 5-HMF into DMC, and consequently further reactions of 5-HMF in the water phase into humins and other by-products. The increase in phase ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 improved 5-HMF yield to 87.2, 87.2, and 89.1%, respectively. Even 5-HMF selectivity in the water phase was increased to over 93% at water/DMC phase ratio of 1
5 improved 5-HMF yield to 87.2, 87.2, and 89.1%, respectively. Even 5-HMF selectivity in the water phase was increased to over 93% at water/DMC phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (Table 2B). These results are a significant improvement over the 5-HMF production using either homogeneous or heterogeneous catalysts in different biphasic systems reported to date (Table S1†).15 For example, dehydration of fructose (100 g L−1) catalyzed by 0.25 M HCl in water/MIBK system at a phase ratio of 1
5 (Table 2B). These results are a significant improvement over the 5-HMF production using either homogeneous or heterogeneous catalysts in different biphasic systems reported to date (Table S1†).15 For example, dehydration of fructose (100 g L−1) catalyzed by 0.25 M HCl in water/MIBK system at a phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in a continuous biphasic flow reactor gave 5-HMF yield of 74%.15,58
3 in a continuous biphasic flow reactor gave 5-HMF yield of 74%.15,58
The possibility to use high substrate concentrations without leading to the formation of by-products is crucial for scaling up the process of 5-HMF production.15 The fructose concentration of 30% w/v (in water phase) used in the present study is already higher than the majority of the studies reported in the literature so far. Increasing the concentration further to 520 g L−1 water in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 water/DMC system at 200 °C and 2 mL min−1 flow rate led to 96.4% conversion of fructose and 5-HMF yield of 75%, and selectivity of 89.4 and 74% in DMC and water phase, respectively, but with productivity increasing to 31 g h−1 (Table 2C). The decrease in 5-HMF yield compared to that with 30% w/v fructose is due to the insufficient amount of DMC used to recover all the produced 5-HMF, and also the limited residence time to allow complete conversion of the DFA intermediate to 5-HMF in a continuous mode. These limitations can be easily overcome by further optimization of the operational conditions.
4 water/DMC system at 200 °C and 2 mL min−1 flow rate led to 96.4% conversion of fructose and 5-HMF yield of 75%, and selectivity of 89.4 and 74% in DMC and water phase, respectively, but with productivity increasing to 31 g h−1 (Table 2C). The decrease in 5-HMF yield compared to that with 30% w/v fructose is due to the insufficient amount of DMC used to recover all the produced 5-HMF, and also the limited residence time to allow complete conversion of the DFA intermediate to 5-HMF in a continuous mode. These limitations can be easily overcome by further optimization of the operational conditions.
Since biomass streams are more abundant in glucose, which is even co-existing with fructose in sugar, molasses, and high fructose syrup, all potential raw materials for 5-HMF production,25 experiments were performed with fructose/glucose mixtures of varying weight ratios of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 50
10 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, at an initial sugar concentration of 30% (w/v in water), in the 1
50, at an initial sugar concentration of 30% (w/v in water), in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 water/DMC at 200 °C in the tube reactor. Fructose conversion of 90.9 and 96.7%, and glucose conversion of only 17 and 36.6% was observed in the 90
3 water/DMC at 200 °C in the tube reactor. Fructose conversion of 90.9 and 96.7%, and glucose conversion of only 17 and 36.6% was observed in the 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 50
10 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 fructose/glucose mixtures, respectively (Table 2D). Total 5-HMF yield of 81 and 83%, respectively, was achieved in the two cases.
50 fructose/glucose mixtures, respectively (Table 2D). Total 5-HMF yield of 81 and 83%, respectively, was achieved in the two cases.
While the 5-HMF selectivity was high in the organic phase there was a significant decrease from 84.6% in the system with the higher fraction of fructose to 65% in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture. These observations are in agreement with the hypothesis of the furanose tautomer yielding higher 5-HMF selectivity especially at a higher temperature and in polar solvents.16 Addition of CaCl2 as a phase modifier to the water/MIBK system has earlier been shown to dramatically enhance 5-HMF yield from glucose in comparison to the system with NaCl or without salt.59
50 mixture. These observations are in agreement with the hypothesis of the furanose tautomer yielding higher 5-HMF selectivity especially at a higher temperature and in polar solvents.16 Addition of CaCl2 as a phase modifier to the water/MIBK system has earlier been shown to dramatically enhance 5-HMF yield from glucose in comparison to the system with NaCl or without salt.59
Keeping in view the subsequent step of 5-HMF separation and purification, it would appear that the maximum resource efficiency and thus economic performance would be achieved by using primarily fructose as the substrate for the dehydration reaction. Hence, it may be more economical to separate glucose and fructose, e.g. by simulated moving bed chromatography of the mixture obtained as a result of glucose isomerization or sucrose inversion,60,61 and utilize the separated glucose for another application or further isomerization.
5-HMF stability in DMC
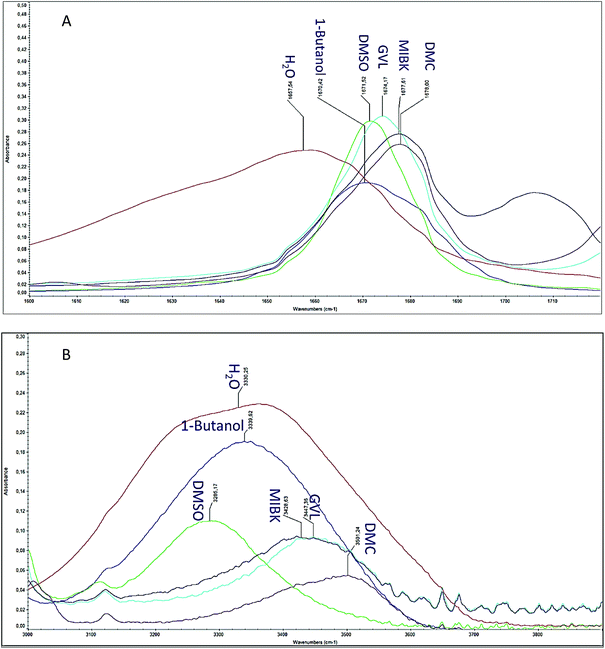
From the results above, it is clear that DMC has a stabilizing effect on reducing the non-specific rehydration and aldol condensation reactions of 5-HMF. In order to understand the underlying reason, FTIR measurements were done to determine the strength of the interaction between 5-HMF and different solvents by the red- and the blue shifts of 5-HMF carbonyl- and hydroxyl group absorption bands in the FTIR spectra as reported earlier for 5-HMF in DMSO.47 The peak shift of 5-HMF carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and hydroxyl (O–H) groups in the DMSO was suggested to be an evidence of hydrogen bond formation between the furan functional groups and DMSO. As depicted in Fig. 2A and Table S2,† a blue shift for 5-HMF carbonyl (C
O) and hydroxyl (O–H) groups in the DMSO was suggested to be an evidence of hydrogen bond formation between the furan functional groups and DMSO. As depicted in Fig. 2A and Table S2,† a blue shift for 5-HMF carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) was observed in all the organic solvents including DMC, DMSO, 1-butanol, GVL and MIBK compared to that in the aqueous solution of 5-HMF (1657 cm−1); MIBK and DMC revealing the highest shift. The absorption band of 5-HMF hydroxyl (O–H) groups (at 3300 cm−1 in water) exhibited the highest blue shift in DMC compared to all other tested solvents, while DMSO led to a red shift in accordance with the earlier report47 (Fig. 2B and Table S2†). It is not possible to differentiate the hydroxyl groups of 5-HMF from that of the hydroxyl groups in water and 1-butanol. These observations may suggest higher stability of 5-HMF in DMC due to strength of hydrogen bonding between DMC and both 5-HMF functionalities and thereby reducing the side reactions of furan molecules with each other and with sugar.
O) was observed in all the organic solvents including DMC, DMSO, 1-butanol, GVL and MIBK compared to that in the aqueous solution of 5-HMF (1657 cm−1); MIBK and DMC revealing the highest shift. The absorption band of 5-HMF hydroxyl (O–H) groups (at 3300 cm−1 in water) exhibited the highest blue shift in DMC compared to all other tested solvents, while DMSO led to a red shift in accordance with the earlier report47 (Fig. 2B and Table S2†). It is not possible to differentiate the hydroxyl groups of 5-HMF from that of the hydroxyl groups in water and 1-butanol. These observations may suggest higher stability of 5-HMF in DMC due to strength of hydrogen bonding between DMC and both 5-HMF functionalities and thereby reducing the side reactions of furan molecules with each other and with sugar.
In the same context, the effective solvation of 5-HMF in DMC due to hydrogen bonding was confirmed by 1H NMR spectroscopy investigations with varied concentrations. As reported in the literature, hydrogen bonding between an alcohol and a solvent can lead to better solvation so that the O–H chemical shift is less concentration dependent (i.e. solute molecules are less aggregated at relatively higher concentration).62 As shown in Table S3 and Fig. S1–S4,† the chemical shift of the O–H proton of 5-HMF showed insignificant concentration dependence in DMSO-d6, acetone-d6, and DMC-d6 in the range of 8.5–68 mg mL−1,† indicating the existence of strong hydrogen bonding between these solvents and 5-HMF. On the contrary, there was a clear concentration dependence of the O–H chemical shifts in CDCl3 (Table S3 and Fig. S5†), a non-hydrogen bonding solvent. However, we believe that there might be other factors affecting the stability of 5-HMF in H2O/DMC system, which need further investigations.
Validating the 5-HMF production process in a larger reactor
The 5-HMF production in kilogram scale reported in literature is characterized by low fructose conversion, 5-HMF yield and selectivity.15,63 In this work, we attempted to determine the scalability of the 5-HMF production process using a tube reactor with over 4 times larger volume (9.4 mL) (Table 3A). Three different flow rates, 7.5, 9 and 10 mL min−1 were tested for pumping in the biphasic system with 30% w/v fructose (in water phase) into the flow reactor at 180 °C (higher temperature could not be used due to technical problems with the equipment). The fructose conversion was >98% at all flow rates, but 5-HMF yield (83.6 and 84.8%) and selectivity (84.5 and 85.9%) were higher at higher flow rates, i.e. 9 and 10 mL min−1, respectively. Furthermore, the biphasic system was processed continuously for 7 hours (4000 mL volume of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of water/DMC, respectively, with 320 g fructose in the aqueous phase) in the flow reactor at a flow rate of 10 mL min−1, which resulted in an overall fructose conversion of 95.1% and 5-HMF yield and selectivity of 74.4% and 78.2%, respectively (Table 3B). The HPLC chromatograms showed the presence of difructose anhydrides (DFAs), indicating that 5-HMF yield and selectivity can be further improved through increasing the reaction temperature and residence time.16,26 Subsequently, dehydration of fructose syrup (90% purity) was studied at a flow rate of 9 mL min−1 under otherwise similar reaction conditions. The results showed 98.8% conversion of fructose, and 5-HMF yield and selectivity of 76.8% and 77.7%, respectively (Table 3C).
3 ratio of water/DMC, respectively, with 320 g fructose in the aqueous phase) in the flow reactor at a flow rate of 10 mL min−1, which resulted in an overall fructose conversion of 95.1% and 5-HMF yield and selectivity of 74.4% and 78.2%, respectively (Table 3B). The HPLC chromatograms showed the presence of difructose anhydrides (DFAs), indicating that 5-HMF yield and selectivity can be further improved through increasing the reaction temperature and residence time.16,26 Subsequently, dehydration of fructose syrup (90% purity) was studied at a flow rate of 9 mL min−1 under otherwise similar reaction conditions. The results showed 98.8% conversion of fructose, and 5-HMF yield and selectivity of 76.8% and 77.7%, respectively (Table 3C).
| Fructose load (g L−1 water phase) | Flow rate (ml min−1) | Aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) org phase ratio org phase ratio |
Fructose conversion [%] | Total HMF yield (%) | HMF selectivity (%) |
|---|---|---|---|---|---|
| (A) Flow rate | |||||
| 300 | 7.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
98.8 | 80.7 | 81.7 |
| 300 | 9.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
98.8 | 83.6 | 84.6 |
| 300 | 10.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
98.7 | 84.8 | 85.9 |
| (B) Process operation for 7 hours | |||||
| 300 | 9.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
95.1 | 74.4 | 78.2 |
| (C) Fructose syrup | |||||
| 226 | 9.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
98.8 | 76.8 | 77.7 |
The 5-HMF yield and fructose conversion were compared to the length of the reactor for the comparable residence time of about 67 seconds. In both cases (180 mm and 800 mm reactor) there is no plug-flow in the tubular reactor and tube velocities were about the same. One can clearly see from the two experiments that the longer reactor gives higher fructose conversion than the shorter one (Fig. S6A†). Comparing the data from both reactors for the same conditions: water/DMC ratio, temperature and sugar concentration, one can conclude that maximum conversion of the fructose is reached before 60 s residence time and 5-HMF yield decreases with time (i.e. longer residence time gives lower 5-HMF yield). For these conditions one can argue that an optimum would be a reactor shorter than 800 mm and residence time about 60 s (Fig. S6B†).
Recovery of 5-HMF and recycling of DMC and water phases
The low boiling point of DMC (90 °C) facilitates its removal from the product under relatively mild conditions. The DMC phase containing HMF (32.9 g L−1 DMC) was separated from the aqueous phase in a separation funnel. Removal of the solvent by rotary evaporation resulted in a dark solution with >98% 5-HMF yield and 93% purity. However the product purity was reduced to about 91% and 82% on letting the solution stand for 24 h and 72 h, respectively, at room temperature (Table 4B), which was assumed to be due to the residual acid from the reaction in the concentrated solution.15,57 Effect of treating the organic phase with activated carbon (AC) and an inorganic base either individually or in combination prior to the evaporation on 5-HMF purity and stability was tested. Treatment with AC only resulted in a clear yellow-brown colored solution, which turned brighter with larger amount (7.5 and 10 wt%) used (Fig. S7†), while the 5-HMF yield decreased from 97.9% to 83.7% with increasing the AC amount from 2.5 to 10 wt% (Table 4A). The 5-HMF purity remained unaffected (94–97%) even after leaving the samples at room temperature for 72 h (Table 4B).| (A) | ||
|---|---|---|
| Sample treatment | 5-HMF conc. (g L−1) | 5-HMF recovery (%) |
| 1. Untreated DMC phase of reaction product | 32.9 ± 0.0 | 100 |
| 2. Treatment with 2.5 wt% AC | 32.2 ± 0.9 | 97.9 |
| 3. 5 wt% AC | 30.0 ± 1.8 | 91.0 |
| 4. 7.5 wt% AC | 30.1 ± 0.2 | 91.4 |
| 5. 10 wt% AC | 27.6 ± 1.0 | 83.7 |
| 6. 2.5 wt% AC + 2 wt% NaOH | 25.9 ± 0.0 | 78.6 |
| 7. 5 wt%AC + 2 wt% NaOH | 28.5 ± 0.0 | 86.7 |
| 8. 7.5 wt% AC + 2 wt%NaOH | 27.6 ± 0.0 | 83.9 |
| 9. 10 wt% AC + 2% Na2CO3 | 27.2 ± 0.0 | 82.7 |
| (B) | |||
|---|---|---|---|
| Sample | 5-HMF purity % during storage at room temperature | ||
| Directly after purification | After 24 h | After 72 h | |
| Direct recovery | 93 | 91 | 82 |
| 5 wt% AC | 94 | 94 | 97 |
| 5 wt% AC + 2 wt% Na2CO3 | 96 | 90 | 78 |
| (C) | |||
|---|---|---|---|
| 5-HMF recovery from aqueous phase | 5-HMF conc (g L−1) | Recovery (%) | |
| H2O | DMC | ||
| Initial HMF | 38.1 | 0 | 100 |
| 1st extraction | 9.1 | 29.1 | 76.4 |
| 2nd extraction | 1.9 | 5.7 | 15 |
| Total recovery % | 34.8 | 91.34 | |
Addition of NaOH pellets (2 wt%) after treatment with AC led to slight darkening of the 5-HMF containing solvent as well as significant reduction of the 5-HMF yield (Table 4A and Fig. S2†). On the other hand, with Na2CO3, a weak base, 5-HMF of higher purity (96%) with a yield of 82.7% and only a slight color change was obtained (Table 4A and Fig. S7†), but the product purity was decreased with time to 78% after 72 h (Table 4B). These observations suggested that even the alkali triggers conversion of 5-HMF to other by-products.46,64
Analysis of the product purity using 1H NMR spectroscopy and comparing the spectra with that of the commercial HMF sample confirmed the high purity of 5-HMF obtained in the samples. In the 5-HMF recovered directly by evaporation from DMC phase signals for the hydroxyl proton (a) and water were significantly broadened (Fig. S8†). This was most likely caused by proton exchange between HMF and water. In all samples, a signal at 3.72 ppm was observed, which was assigned to the residual dimethyl carbonate. By comparing the integral of the signal corresponding to the DMC protons (DMC) to the aromatic 5-HMF proton (c), the DMC-content was roughly estimated to 8 mol% compared to 5-HMF. This seems to be in line with the purity estimated by HR-LCMS (Fig. S9–S12†). In addition, minor signals were observed at 4.75 ppm, 6.8 ppm and 8.0 ppm (marked by 1, 2 and 3 in Fig. S8†). Since these impurities were present in trace amounts they proved difficult to assign with absolute certainty.
Moreover, the DMC recovered above after separation of 5-HMF by rotary evaporation was examined using NMR. Comparison of the NMR spectrum with that of commercial DMC showed high similarity indicating that DMC was stable during process conditions (Fig. S13 and S14†). The recovered DMC was recycled for the recovery of 5-HMF from the aqueous phase at the same volume ratio used in production, giving a total recovery yield of 91.3% in two extraction cycles (Table 4C). The recovered DMC can be recycled further in subsequent production cycles, as demonstrated earlier by Dibenedetto and coworkers.48 It must be stressed that product extraction and recovery is more efficiently carried out at large scale using continuous extractors, and our results confirm the 5-HMF recovery by extraction into DMC to be a simple and cost-effective operation that should be easy to scale up.
Even recycling of the residual water phase, after extraction of 5-HMF, was tested by supplementing with fructose and HCl (at half the concentration used in the previous reaction cycle) and performing dehydration in the tube reactor. The procedure was repeated 3 times; nearly complete fructose conversion was observed each time with similar efficiency and no by products were observed.
Evaluating the purified 5-HMF for the production of a polymer building block
The purified 5-HMF was tested in an acid-catalyzed acetalation reaction with bio-based pentaerythritol to form a spirocyclic diol product that has recently been used for the synthesis of polyesters and poly(urethane-urea)s.45 Preliminary LCA of the diol showed considerably lower greenhouse gas emissions as compared to 1,3-propanediol. 5-HMF conversion of 40%, 63%, and 66% (Table 5) to the spirocyclic diol product was achieved from the samples (from Table 4B) recovered from the process in the water/DMC system by solvent removal directly after the dehydration reaction, after treatment with activated carbon, and after treatment with activated carbon and sodium carbonate, respectively. The identity of the spirocyclic diol product formed was confirmed by 1H NMR (Fig. S15†). In comparison, the commercial 5-HMF (98% purity, Nanjing Confidence Chemical Co.) underwent 69% conversion under identical conditions.45| Type of 5-HMF | Commercial 5-HMF | 5-HMF recovered after different treatments | ||
|---|---|---|---|---|
| Direct concentration | AC | AC + Na2CO3 | ||
| 5-HMF conversion (%) | 69 | 42 | 63 | 66 |
Conclusions
This study presents a promising method for rapid and facile production of 5-HMF from pure fructose and commercial fructose syrup at high selectivity and yield using a continuous process in a biphasic system followed by simple downstream processing. A process with a residence time of only about 1 min is far superior than most of the systems described in the literature so far. We believe that the outstanding performance of the system is related to the features of DMC – that of relative high solubility in water especially at high temperature, its effect on enhancing the stability of 5-HMF, and its low boiling point. The high solubility, on the other hand, would impact the recyclability of the solvent. DMC is assessed to be a green, sustainable solvent used in many industrial processes,49,65 and there are ongoing efforts for developing alternative routes for the production of DMC from CO2 so as to move away from fossil-based production.66 It is of course attractive to be able to produce 5-HMF directly from cellulose or glucose but the relatively low product purity and the extensive downstream processing to obtain the pure product makes upscaling of the process challenging and costly. The system presented here shows potential for scaling up into a cost-effective and resource-efficient process with minimal waste in contrast to the several systems reported so far (Table S1†). The efficiency of solvent recycling will most likely be the main cost determinant for the process. Besides process scale up, further studies are also needed to determine the most optimal conditions for a long shelf life of 5-HMF.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support of the Swedish Research Agency FORMAS (grant no. 942-2016-33 for Farm2Furan project) and the Swedish Foundation for Strategic Environmental Research (MISTRA, grant no. 2016/1489 for the research programme STEPS). We thank John P. Jensen at Nordic Sugar A/S for providing us with pure fructose and fructose syrup, and for fruitful discussions.Notes and references
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- F. Yang, J. Mao, S. Li, J. Yin, J. Zhou and W. Liu, Catal. Sci. Technol., 2019, 9, 1329–1333 RSC.
- Y. Yang, H. Liu, S. Li, C. Chen, T. Wu, Q. Mei, Y. Wang, B. Chen, H. Liu and B. Han, ACS Sustainable Chem. Eng., 2019, 7, 5711–5716 CrossRef CAS.
- H. Zhou, H. Xu and Y. Liu, Appl. Catal., B, 2019, 244, 965–973 CrossRef CAS.
- H. Xia, J. An, M. Hong, S. Xu, L. Zhang and S. Zuo, Catal. Today, 2019, 319, 113–120 CrossRef CAS.
- H. Liu, X. Cao, J. Wei, W. Jia, M. Li, X. Tang, X. Zeng, Y. Sun, T. Lei and S. Liu, ACS Sustainable Chem. Eng., 2019, 7, 7812–7822 CrossRef CAS.
- H. Zhang, Z. Feng, Y. Zhu, Y. Wu and T. Wu, J. Photochem. Photobiol., A, 2019, 371, 1–9 CrossRef CAS.
- X.-Y. Zhang, M.-H. Zong and N. Li, Green Chem., 2017, 19, 4544–4551 RSC.
- M. Sayed, S.-H. Pyo, N. Rehnberg and R. Hatti-Kaul, ACS Sustainable Chem. Eng., 2019, 7, 4406–4413 CrossRef CAS.
- N. Li, Y. Li and Z. Minhua, US Pat, 20190040430A1, 2019 Search PubMed.
- C. Zhang, X. Chang, L. Zhu, Q. Xing, S. You, W. Qi, R. Su and Z. He, Int. J. Biol. Macromol., 2019, 128, 132–139 CrossRef CAS PubMed.
- B. Xiao, M. Zheng, X. Li, J. Pang, R. Sun, H. Wang, X. Pang, A. Wang, X. Wang and T. Zhang, Green Chem., 2016, 18, 2175–2184 RSC.
- S.-H. Pyo, J. H. Park, V. Srebny and R. Hatti-Kaul, Green Chem., 2020, 22, 4450–4455 RSC.
- R.-J. Van Putten, J. C. Van Der Waal, E. De Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- A. Mukherjee, M.-J. Dumont and V. Raghavan, Biomass Bioenergy, 2015, 72, 143–183 CrossRef CAS.
- G. S. Svenningsen, R. Kumar, C. E. Wyman and P. Christopher, ACS Catal., 2018, 8, 5591–5600 CrossRef CAS.
- W. Fan, C. Verrier, Y. Queneau and F. Popowycz, Curr. Org. Synth., 2019, 16, 583–614 CrossRef CAS PubMed.
- B. Agarwal, K. Kailasam, R. S. Sangwan and S. Elumalai, Renewable Sustainable Energy Rev., 2018, 82, 2408–2425 CrossRef CAS.
- Y. Dou, S. Zhou, C. Oldani, W. Fang and Q. Cao, Fuel, 2018, 214, 45–54 CrossRef CAS.
- T. Tongtummachat, N. Akkarawatkhoosith, A. Kaewchada and A. Jaree, Front. Chem., 2020, 7, 951 CrossRef.
- J. Esteban, A. J. Vorholt and W. Leitner, Green Chem., 2020, 22, 2097–2128 RSC.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- R. Huang, W. Qi, R. Su and Z. He, Chem. Commun., 2010, 46, 1115–1117 RSC.
- H. Huang, C. A. Denard, R. Alamillo, A. J. Crisci, Y. Miao, J. A. Dumesic, S. L. Scott and H. Zhao, ACS Catal., 2014, 4, 2165–2168 CrossRef CAS.
- S. P. Teong, G. Yi and Y. Zhang, Green Chem., 2014, 16, 2015–2026 RSC.
- G. Svenningsen, UC Riverside, PhD thesis, Univ. California Riverside, 2018 Search PubMed.
- A. A. Rosatella, S. P. Simeonov, R. F. Frade and C. A. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- J. Wang, W. Xu, J. Ren, X. Liu, G. Lu and Y. Wang, Green Chem., 2011, 13, 2678–2681 RSC.
- C. Aellig and I. Hermans, ChemSusChem, 2012, 5, 1737–1742 CrossRef CAS PubMed.
- S.-H. Pyo, M. Sayed and R. Hatti-Kaul, Org. Process Res. Dev., 2019, 23, 952–960 CrossRef CAS.
- Z. Cao, M. Li, Y. Chen, T. Shen, C. Tang, C. Zhu and H. Ying, Appl. Catal., A, 2019, 569, 93–100 CrossRef CAS.
- A. J. McCue and J. A. Anderson, Catal. Sci. Technol., 2014, 4, 272–294 RSC.
- Y. Román-Leshkov and J. A. Dumesic, Top. Catal., 2009, 52, 297–303 CrossRef.
- J. N. Chheda, Y. Román-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342–350 RSC.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef PubMed.
- J. N. Chheda and J. A. Dumesic, Catal. Today, 2007, 123, 59–70 CrossRef CAS.
- P. Yan, M. Xia, S. Chen, W. Han, H. Wang and W. Zhu, Green Chem., 2020 10.1039/d0gc01446j.
- S. Xu, D. Pan, F. Hu, Y. Wu, H. Wang, Y. Chen, H. Yuan, L. Gao and G. Xiao, Fuel Process. Technol., 2019, 190, 38–46 CrossRef CAS.
- Z. Cao, Z. Fan, Y. Chen, M. Li, T. Shen, C. Zhu and H. Ying, Appl. Catal., B, 2019, 244, 170–177 CrossRef CAS.
- S. Hu, Z. Zhang, Y. Zhou, B. Han, H. Fan, W. Li, J. Song and Y. Xie, Green Chem., 2008, 10, 1280–1283 RSC.
- J. Y. G. Chan and Y. Zhang, ChemSusChem, 2009, 2, 731–734 CrossRef CAS PubMed.
- M. Zuo, K. Le, Z. Li, Y. Jiang, X. Zeng, X. Tang, Y. Sun and L. Lin, Ind. Crops Prod., 2017, 99, 1–6 CrossRef CAS.
- T. Kobayashi, M. Yoshino, Y. Miyagawa and S. Adachi, Sep. Purif. Technol., 2015, 155, 26–31 CrossRef CAS.
- B. Saha and M. M. Abu-Omar, Green Chem., 2014, 16, 24–38 RSC.
- N. Warlin, M. N. G. Gonzalez, S. Mankar, N. Valsange, M. Sayed, S.-H. Pyo, N. Rehnberg, S. Lundmark, R. Hatti-Kaul and P. Jannasch, Green Chem., 2019, 21, 6667–6684 RSC.
- M. Mednick, J. Org. Chem., 1962, 27, 398–403 CrossRef CAS.
- G. Tsilomelekis, T. R. Josephson, V. Nikolakis and S. Caratzoulas, ChemSusChem, 2014, 7, 117–126 CrossRef CAS PubMed.
- A. Dibenedetto, M. Aresta, L. di Bitonto and C. Pastore, ChemSusChem, 2016, 9, 118–125 CrossRef CAS PubMed.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC.
- P. Wrigstedt, J. Keskiväli and T. Repo, RSC Adv., 2016, 6, 18973–18979 RSC.
- C. V. McNeff, D. T. Nowlan, L. C. McNeff, B. Yan and R. L. Fedie, Appl. Catal., A, 2010, 384, 65–69 CrossRef CAS.
- N. Jiang, W. Qi, R. Huang, M. Wang, R. Su and Z. He, J. Chem. Technol. Biotechnol., 2014, 89, 56–64 CrossRef CAS.
- G. Fiorani, A. Perosa and M. Selva, Green Chem., 2018, 20, 288–322 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2015, 18, 288–296 RSC.
- S.-H. Pyo, J. H. Park, T.-S. Chang and R. Hatti-Kaul, Curr. Opin. Green Sustain. Chem., 2017, 5, 61–66 CrossRef.
- M. Selva, A. Perosa, D. Rodríguez-Padrón and R. Luque, ACS Sustainable Chem. Eng., 2019, 7, 6471–6479 CrossRef CAS.
- Y. Muranaka, H. Nakagawa, R. Masaki, T. Maki and K. Mae, Ind. Eng. Chem. Res., 2017, 56, 10998–11005 CrossRef CAS.
- M. Brasholz, K. Von Kaenel, C. H. Hornung, S. Saubern and J. Tsanaktsidis, Green Chem., 2011, 13, 1114–1117 RSC.
- C. García-Sancho, I. Fúnez-Núñez, R. Moreno-Tost, J. Santamaría-González, E. Pérez-Inestrosa, J. Fierro and P. Maireles-Torres, Appl. Catal., B, 2017, 206, 617–625 CrossRef.
- G. Dünnebier, J. Fricke and K.-U. Klatt, Ind. Eng. Chem. Res., 2000, 39, 2290–2304 CrossRef.
- L. Hobbs, in Starch, Elsevier, 2009, pp. 797–832 Search PubMed.
- M. A. Wendt, J. Meiler, F. Weinhold and T. C. Farrar, Mol. Phys., 1998, 93, 145–152 CrossRef CAS.
- R. J. van Putten, A. S. Dias and E. de Jong, Wiley Online Library, 2013, pp. 81–117 Search PubMed.
- A. Sınaǧ, A. Kruse and V. Schwarzkopf, Ind. Eng. Chem. Res., 2003, 42, 3516–3521 CrossRef.
- F. Aricò and P. Tundo, Russ. Chem. Rev., 2010, 79, 479 CrossRef.
- T. Kläusli, Green Process. Synth., 2014, 3, 235–236 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc01422b |
| This journal is © The Royal Society of Chemistry 2020 |