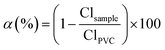Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dehydrochlorination of PVC in multi-layered blisterpacks using ionic liquids
Kamil
Oster
 *a,
Aleksander
Tedstone
*a,
Aleksander
Tedstone
 a,
Adam J.
Greer
a,
Adam J.
Greer
 a,
Nigel
Budgen
b,
Arthur
Garforth
*a and
Christopher
Hardacre
a,
Nigel
Budgen
b,
Arthur
Garforth
*a and
Christopher
Hardacre
 *a
*a
aDepartment of Chemical Engineering and Analytical Science, University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: kamil.oster@manchester.ac.uk; arthur.garforth@manchester.ac.uk; c.hardacre@manchester.ac.uk
bAstraZeneca, Global Environment, Alderley Park, Macclesfield, SK10 4TF, UK
First published on 13th July 2020
Abstract
PVC is often found in composite materials and dehydrochlorination has been shown to be a viable option to recover valuable carbon forms and HCl from PVC, however, in current processes, the high temperature of operation is still detrimental. Ionic liquids were shown to tackle this issue by reducing the temperature of dehydrochlorination and improving its yield. The aim of this work is to address the separation problems associated with mixed composite materials and provide insight into the use of dehydrochlorination as a potential feedstock recycling process, such as, cracking or hydrocracking. To this end, a multicomponent blisterpack containing PVC/aluminium/oPA was chosen to study the separation and dehydrochlorination of a mixed waste. Three ionic liquids with different anions were chosen for this purpose, i.e. trihexyl(tetradecyl)phosphonium chloride, bromide and hexanoate. The results showed that ionic liquids are capable of separating the layered components in the blisterpack and improving the dehydrochlorination degree significantly (up to 99%). The recyclability was shown to be reproducible with halide-based ionic liquids, whilst with the hexanoate anion, the recyclability was achieved by washing with sodium hydroxide, with simultaneous separation of the second polymer (namely oPA). Efficient separation of the layers in the blisterpack was observed using ionic liquids which also allows recycling of the PVC through dehydrochlorination.
1. Introduction
Polyvinyl chloride (PVC) is a commonly used plastic, accounting for around 10% of global plastic demand, and only surpassed by polyethylenes (29.7%) and polypropylene (19.3%), as reported by Plastics Europe.1 It is one of the plastics commonly released into the environment, with 40 Mt produced annually. The need for emerging recycling technologies is continuously increasing, as the current techniques (such as mechanical or feedstock recycling) are unable to provide sufficient recyclability of PVC, or deal with composite waste.1 The incineration of PVC generates hydrochloric acid (HCl), dioxins and dioxin-like compounds, which are incredibly environmentally persistent and toxic to humans/animals, showing high levels of bioaccumulation.2 PVC applications spread over building, construction and packaging, due to its superior properties, such as high durability, energy efficiency (caused by low thermal conductivity) and reusability.3 It is highly versatile, where changes in the formulation can improve the properties of the final product, conserving technical performance.3 PVC also has a lower carbon footprint than other thermoplastics; it is produced from rock salt (57%) and hydrocarbon from oil (43%) Nevertheless, the production of PVC from recycled feedstocks can result in vast CO2 waste savings; for example, replacing virgin PVC resin with PVC from post-consumer sources was estimated to save 2.056 tonnes of CO2 per 1 tonne of PVC.4Annually, only around 1.8% of PVC is recycled, 45% is landfilled and 15% is incinerated without energy recovery.1 There are two main issues with the practical recycling of PVC. Firstly, PVC usually contains additives based on heavy metals or phthalates (endocrine disruptors).5 Secondly, PVC contains high quantities of chlorine which imposes difficulties with end-of-life processing; pyrolysis produces HCl that must be captured, and if mixed with other polymer waste, can elevate the levels of char produced.6 PVC waste streams mostly contain other components such as glass, metals, and other plastics, which hinders the selective recycling of PVC.7,8 Commercially, VinyLoop offers recycling of PVC, however, the final product is of lower quality due to irreversible thermal degradation at PVCs melt temperature.9 Moreover, the process uses volatile solvents which do not circumvent the environmental difficulties with PVC.10 Landfill disposal and incineration are the least favoured options regarding PVC end-of-life treatment, due to production of corrosive HCl and problematic toxic chlorinated compounds emitted upon combustion.11,12
A further problem with plastics, in general, is that they are frequently found as composite materials, especially in packaging materials. In this case, much of the waste consists of flexible plastic films laminated with an aluminium foil, secured with layers of adhesive. Current recycling/separation techniques involve the use of harmful solvents, such as toluene or xylene, to dissolve the adhesive and separate the layered materials.13 A more sustainable solution has recently been investigated in the form of switchable solvents to improve the separation performance and recovery of the polymer, as well as increasing the overall recyclability of the process.14,15 However, these methods will not directly treat PVC.
One of the routes to treat PVC is dehydrochlorination, allowing the recovery of hydrogen deficient hydrocarbons and simultaneous recovery of HCl.16 The hydrogen deficient feedstock might then be co-mingled with other plastics, such as LDPE and PP, and hydrocracked to a naphtha-like product.17–19 Nevertheless, dehydrochlorination is an energy demanding reaction requiring a high processing temperature of >250 °C.20 This temperature can be lowered by using organic/inorganic bases or heavy metal catalysts, which do not circumvent the environmental issues of PVC end-of-life processing.21–23 Recently, Zhao et al. (2010) studied ionic liquid (IL) mediated dehydrochlorination.24 Therein, a significant decrease in the temperature of the reaction (<200 °C) and an improvement in the dehydrochlorination yield (ca. 90%) was observed.
Furthermore, Glas et al. (2014) studied different types of cations and anions, and the effect of their structural properties (such as the β-parameter (hydrogen bonding accepting ability) and alkyl chain lengths of cations) on the dehydrochlorination yield.25 The authors showed that increasing the alkyl chain length of a cations resulted in a better degree of dehydrochlorination and a lower dehydrochlorination onset temperature. Further reductions in the dehydrochlorination onset temperature was achieved by introducing phosphonium-based ILs, such as [P4,4,4,4]+ and [P14,6,6,6]+. Nevertheless, the major influence on the dehydrochlorination onset temperature was observed from anion. The main feature which make ILs a more useful option for the recycling of PVC is their low vapour pressure, resulting in a low volatility and proven recyclability.26 However, many of the ILs studied, to date, are known to be difficult to prepare in a pure state and the process is not cost effective, particularly for imidazolium- and pyrrolidinium-based ILs,27 whilst phosphonium-based ILs are a cheaper and more readily available alternative.28
The trihexyl(tetradecyl)phosphonium cation, [P14,6,6,6]+, was chosen in this work due to the long alkyl chains in the cationic core which were shown to enhance the degree of dehydrochlorination.25 This cation was paired with hexanoate ([HexO]−), chloride, Cl−, and bromide, Br− anions. The overall aim of this work was to study the combined separation and dehydrochlorination of a PVC-containing blisterpack over an increasing temperature range (80–160) °C and duration (up to 24 hours). Currently, there are no known techniques in the literature that allow for simultaneous dehydrochlorination and component separation. The PVC composite material investigated was an unformed three-layer blisterpack containing orientated polyamide (oPA), aluminium and PVC. The study was also complemented by investigating the separation of PVC with ethyl acetate, a common alternative solvent, and performing the dehydrochlorination process on the separated PVC, to compare the use of ILs and common solvents. The effect of IL structure was discussed against dehydrochlorination degree (including reaction kinetics), IL recyclability/chemical stability, and the separation of blisterpack layers.
Glas et al. (2014) performed initial studies to examine the effect of cation/anion alkyl chain lengths, and the type of cation and anion.25 Phosphonium-based ILs showed a greater degree of dehydrochlorination, particularly those with long alkyl chain lengths, due to better permeation of the somewhat less polar IL in PVC. Hydrogen bond accepting properties (quantified in terms of the β-parameter) are dominant for the anion impact on the dehydrochlorination, i.e. higher β values reflect a higher basicity, thus, a higher dehydrochlorination efficiency, which is also in good correlation with the acid–base equilibrium pKa.31 Cl− and Br− paired with quaternary phosphonium cations were shown to reduce the temperature of dehydrochlorination down to 80 °C, and the acetate anion to 70 °C. Another issue highlighted in this study was the degradation of IL structure.
This paper reports ILs from an innovative perspective as universal solvent. Firstly, those can effectively separate different components in composites which then simplifies further processing in terms of recycling. Secondly, they induce dehydrochlorination of the PVC which enables its further recycling. Both factors determine ILs potential in recycling of composites, particularly those containing PVC. This is also supported based on currently available techniques, such as thermal dehydrochlorination,9 or use of harmful chemicals.10 Currently, there is no technique that allows simultaneous dehydrochlorination and components separation.
2. Experimental
Trihexyl(tetradecyl)phosphonium chloride, [P14,6,6,6]Cl, was purchased from Cytec Industries Inc. (Cyphos IL-101, purity ≥95%), and trihexyl(tetradecyl)phosphonium bromide, [P14,6,6,6]Br, was purchased from Merck (purity ≥95%). Trihexyl(tetradecyl)phosphonium hexanoate, [P14,6,6,6][HexO], was synthesised in accordance to a previously published procedure.29 Generally, the chloride-based precursor, [P14,6,6,6]Cl, was diluted with ethanol, subject to an anionic exchange to trihexyl(tetradecyl)phosphonium hydroxide, [P14,6,6,6][OH], by a strongly basic anion exchange resin. Subsequently, an acid–base reaction was carried out with hexanoic acid to obtain [P14,6,6,6][HexO]. All ILs were dried in vacuo (10−3) Pa at 65 °C for at least 72 h. The purity was checked with 1H, 13C and 31P NMR spectroscopy (B400 Bruker Avance III 400 MHz, solvent: CDCl3). The purity of [P14,6,6,6]Cl and [P14,6,6,6]Br was ≥0.95 mass fraction, and ≥0.98 for [P14,6,6,6][HexO]. The water content was measured by Karl Fisher titration (Metrohm 899 Coulometer with Hydranal Coulomat AG), and all ILs had a water content of ≤500 ppm.The unformed blisterpack material used in this work was supplied by AstraZeneca as a three-layer composite (oPA–Aluminum–PVC) secured with an adhesive (Degalan P24 as a model, methacrylate-based bead polymer supplied by Evonik Industries). The blisterpack was cut into 1 cm2 pieces and washed with ethanol in an ultrasonic cleaning bath for 5 minutes to remove contamination from the surface. Thereafter, the cleaned blisterpack was dried at 50 °C for at least 24 h.
The structure and elemental composition of the blisterpack was investigated using scanning electron microscopy using FEI Instruments Quanta 250 system equipped with an Oxford Instruments XMaxN detector coupled with energy-dispersive X-ray spectroscopy (SEM-EDXS). Blisterpack samples were mounted with the flat surface perpendicular to the SEM electron beam on 10 mm diameter aluminium stubs (Agar Scientific) with conductive carbon tabs (Agar Scientific) and sputter-coated with 5 nm carbon using a Quorum Q150 Rotary Pumped Coater. An accelerating voltage of 10 kV, working distance of 10.5 mm, and spot size 3.5 nm was used to excite C, N, O and Cl Kα transitions whilst minimising beam damage, and the resultant X-ray emission spectra were interpreted with the Oxford Instruments Aztec package. It can be seen in Fig. 1 that the studied blisterpack consists of 3 distinguishable layers, namely oriented polyamide (oPA), aluminium (Al) and polyvinyl chloride (PVC). The thicknesses of the layers were calculated based on the SEM image and were found to be (27, 53 and 59) μm for oPA, Al and PVC, respectively, constituting (19, 38 and 42)% of the blisterpacks three-layer structure. Thin layers of adhesive can be seen in the SEM image (between oPA–Al and Al–PVC), holding the layers together.
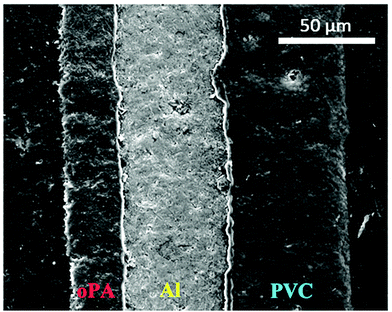 | ||
| Fig. 1 SEM image of blisterpack (oPA – oriented polyamide, Al – aluminium, PVC – polyvinyl chloride). | ||
To perform dehydrochlorination, 5% (w/w) of blisterpack squares were added to a 30 mL glass vial containing ca. 5 g of IL, and equilibrated at constant temperature (80, 100, 120, 140 and 160 °C). The open glass vial was kept at temperature for 15, 30, 60, 120, 240 and 1440 min whilst stirring at 300 rpm. After the reaction, the remaining solid was separated from the IL, and washed with ethanol 3 times in an ultrasonic cleaning bath for 5 min to minimise IL residue on the material. Thereafter, the samples were dried at 50 °C for at least 24 h. The ILs following reaction at 160 °C for 240 min were recycled, whereby the same IL was used in the same reaction another 4 times.
Ethyl acetate fits the green solvent criteria as it can be derived from biomass, and has better environmental,10 health and safety properties, relative to harsher organic solvents.26 It was assessed relative to the ILs as a solvent for swelling PVC and dissolving adhesive, thus, selectively separating PVC from the other blisterpack constituents.30 Afterwards, the PVC film was cast by evaporation of ethyl acetate at 50 °C. This form of PVC was also dehydrochlorinated by the above IL process, for comparison to the multilayer-form blisterpack. This was used as a standard process of PVC recycling which is proceeded by separation with organic solvents, and further dehydrochlorination. This process may be contrasted with the use of ILs for simultaneous separation–dehydrochlorination which may result in a reduction of the chemicals used.
The dehydrochlorination degree was measured by EDXS. The integrated peak area of Cl Kα (2.621 keV) in the EDS spectrum is equivalent to a relative concentration of the element in the sample. The dehydrochlorination degree, α, was determined by a ratio of the Cl Kα area in PVC before reaction (ClPVC) and the Cl Kα area in PVC after reaction (Clsample) which also limits the discussion to the degree of removing chlorine, instead of quantifying the accurate concentration of chlorine before/after reaction:
The spectrum was acquired in 5 different positions on each sample to examine the spatial distribution. The average (mean) peak area was taken for the above equation, whilst the average standard deviation was 3.24%.
3. Results and discussion
(a) De-HCl of PVC without ILs
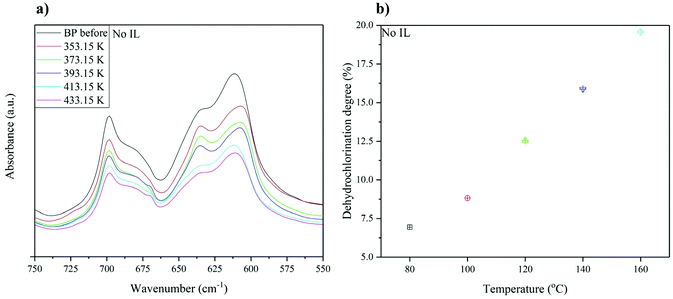
Conventionally, high yields of thermal dehydrochlorination require high temperatures (>250 °C).20 At lower temperatures, e.g. 180 °C, Zhao et al. (2010) reported a ca. 40% dehydrochlorination degree after 1 hour.24 To establish the baseline for the dehydrochlorination of the layered PVC material catalysed by ILs in this work, the blisterpack was first subjected to various temperatures for 4 h with no IL or solvent present. The results can be seen in Fig. 2. A clear decrease in C–Cl stretching in the 575–725 cm−1 infrared region can be observed as a result of dehydrochlorination occurring (Fig. 2a). Moreover, the dehydrochlorination degree quantified by EDXS can be seen in Fig. 2b. After 4 h at the lowest temperature of 80 °C, a conversion of 6.9% was achieved, whilst at the highest temperature of 160 °C, the conversion was 19.6%. The dehydrochlorination degree obtained in this work is lower than reported by Zhao et al. (2010),24 wherein, at 160 °C, ca. 30% dehydrochlorination (after 1 h) was found. This could be an effect of the fact that the PVC in this work is a component of a multi-layered material, which reduces the possibility of releasing HCl over the entire surface of PVC, as physical constraints are on one of the sides (i.e. adhesive/aluminium), hindering the dehydrochlorination yield. For this reason, it is advantageous to separate the layers in a concerted exfoliation–dehydrochlorination step which would result in increased surface area to initiate dehydrochlorination with enhanced reactivity. Nonetheless, no separation of the layers was observed during these experiments.(b) De-HCl of PVC in blisterpack with ILs
The mechanism proposed by Zhao et al. (2010) is a one-step elimination reaction: the anion (such as Cl−) with hydrogen bond accepting properties weakens the C–H bond, whilst the cation complexes with the C–Cl bond, weakening the PVC structure (Fig. 4).24 Thus, dehydrochlorination is dependent on the cation–anion interaction, anion basicity, IL polarity, IL chemical stability over dehydrochlorination, and the activation energy barrier. Whilst the mechanism is plausible, sufficient experimental evidence was not provided to explain this phenomenon for ILs other than chloride-based ILs. Three ILs were tested for their impact on the dehydrochlorination of PVC in the blisterpack, as well as the separation, i.e. [P14,6,6,6]Cl, [P14,6,6,6]Br and [P14,6,6,6][HexO].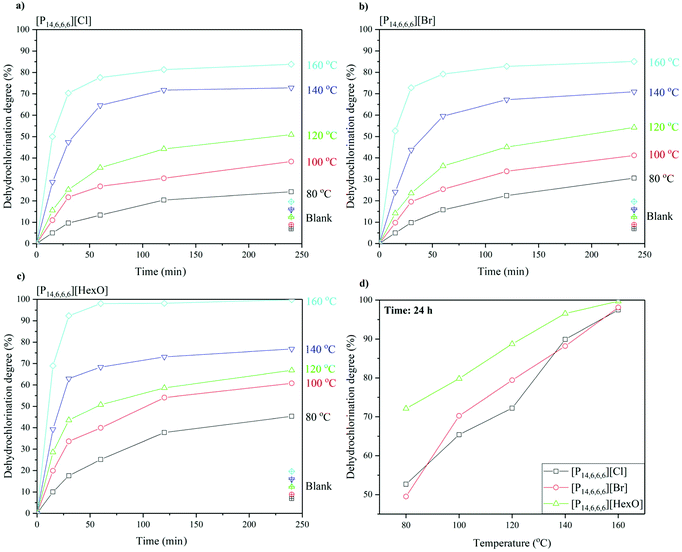 | ||
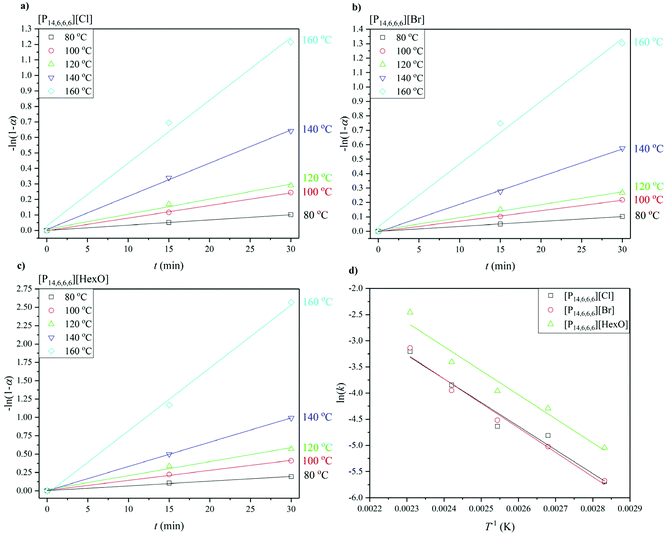
| Fig. 3 Dehydrochlorination degree as a function of time up to 4 hours for (a) [P14,6,6,6]Cl, (b) [P14,6,6,6]Br, (c) [P14,6,6,6][HexO], and for 24 hours as a function of temperature (d). | ||
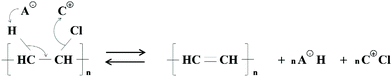 | ||
| Fig. 4 A mechanism of dehydrochlorination of PVC by ILs, proposed by Zhao et al. (2010).24 | ||
The results of the dehydrochlorination experiment in the chosen ILs over a 4 h period can be seen in Fig. 3a–c, and after 24 h reaction in Fig. 3d.
Dehydrochlorination is an elimination reaction and can be catalysed by Lewis bases. The differences in dehydrochlorination as a function of the anion can be explained based on acid–base equilibrium pKa. When HCl/HBr are created (in case of [P14,6,6,6]Cl and [P14,6,6,6]Br), the halide reacts with the unsaturated polymer formed to reform the halogenated polymer, e.g. PVC. Owing to the strength of the HCl/HBr, they can react with the unsaturated polymer, due to thermal effects. However, in the case of hexanoic acid, the anion (hexanoate) is much more basic (pKa 4.85 for hexanoic acid) and thus the proton is less available for the back reaction. This can be seen in Fig. 3a–c where the dehydrochlorination degree plateaus over a 4 h period, with the highest values achieved for [P14,6,6,6][HexO], i.e. at 160 °C after 4 hours (83.8, 85.1 and 99.8)% for [P14,6,6,6]Cl, [P14,6,6,6]Br and [P14,6,6,6][HexO], respectively. This can be also seen in the dehydrochlorination degree achieved after 24 h (Fig. 3d) for halide-based ILs and [P14,6,6,6][HexO] whereas [P14,6,6,6][HexO] produces systematically higher dehydrochlorination degree values than both [P14,6,6,6]Cl and [P14,6,6,6]Br, i.e. (52.6, 49.5 and 72.1)% at 80 °C for [P14,6,6,6]Cl, [P14,6,6,6]Br and [P14,6,6,6][HexO], respectively.
The dehydrochlorination degree depends on the reaction time and temperature. At the start of the process, there is a rapid increase in the dehydrochlorination degree, which also increases with temperature, i.e. at the highest temperature (160 °C), it reaches 50.1, 52.6 and 68.9% dehydrochlorination after 15 min using [P14,6,6,6]Cl, [P14,6,6,6]Br and [P14,6,6,6][HexO], respectively. In each case, the time-temperature profile is very similar, with the hexanoate anion showing a higher dehydrochlorination degree. After a rapid initial reaction, the dehydrochlorination degree levels off, which may be due to an equilibrium effect between the dehydrochlorinated PVC, formation of the free acid of the anion (HCl, HBr and hexanoic acid, in case of Cl−, Br− and hexanoate anion) and [P14,6,6,6]Cl (Fig. 4). Interestingly, considering the equilibrium model with the IL-catalysed reaction, no changes in the structure of [P14,6,6,6][HexO], e.g. replacement of hexanoate anion to chloride, were observed over the 4 h reaction.
Another factor to consider for the discussion on the mechanism and differences between the ILs impact on the dehydrochlorination, is the kinetics of the reaction. He et al. (2011) showed that the IL-induced dehydrochlorination (with 1-butyl-3-methylimidazolium hydroxide) is a first-order reaction:32
| ln(1 − α) = kt |
It can be seen in Fig. 6 that dehydrochlorination causes structural changes to the PVC. PVC after reaction exhibits rough, cracked surface. This structure can be caused by diffusion of HCl created during dehydrochlorination. Similar results were observed by Zhao et al. (2010).24 As the intention is to use this dehydrochlorinated material in a further feedstock recycling process, the perceived structural damage was not thought to be of concern.
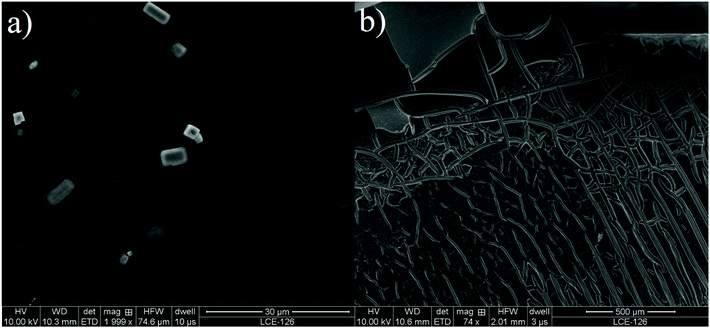 | ||
| Fig. 6 SEM image of (a) PVC before dehydrochlorination, (b) PVC after dehydrochlorination with [P14,6,6,6][Cl] at 140 °C and 240 minutes. | ||
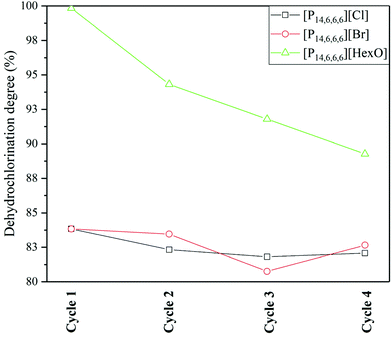
(c) Recyclability and chemical stability of ILs
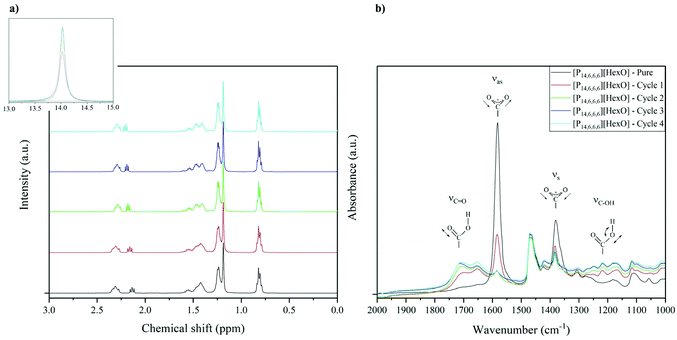
Chloride based ILs were previously shown to exhibit chemical stability on being reused for the dehydrochlorination process.24 The chemical stability is due to the mechanism of dehydrochlorination, involving the release of HCl from the PVC structure, and regeneration of the IL anion/catalyst. Therefore, as expected, no observable changes were found in the dehydrochlorination degree for [P14,6,6,6]Cl over 4 cycles (Fig. 7). In addition, [P14,6,6,6]Br and [P14,6,6,6]Cl showed similar dehydrochlorination degree profiles over a wide range of temperatures and reaction times, indicating similar molecular effects regarding the dehydrochlorination.Carboxylate-based ILs (i.e. with acetate anion) in the literature were shown to improve the dehydrochlorination with a higher initial conversion than [P14,6,6,6]Cl and [P14,6,6,6]Br; however, their chemical stability was a limiting factor, i.e. long reaction times causing conversion to the chloride-based IL and/or degradation, due to the creation of carboxylic acid (i.e. acetic acid) with a low boiling point, thus, escaping the system.25 [P14,6,6,6][HexO] was used in this work as hexanoic acid has a boiling point of 205 °C. However, it can be seen in Fig. 7 that the dehydrochlorination degree with this IL decreased after each cycle.
The NMR spectra of [P14,6,6,6]Cl and [P14,6,6,6]Br did not show any changes before and after reaction. 1H NMR spectra for [P14,6,6,6][HexO] before and after reaction (in 4 cycles) can be found in Fig. 8a. The appearance of a singlet peak at ca. 14 ppm (–COO![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) can be seen after the first cycle, confirming the presence of carboxylic acid in the system. Moreover, this hexanoic acid peak is increasing in intensity which indicates an increasing amount of carboxylic acid in the system relative to the carboxylate anion. As can be seen in Fig. 8a, a downfield shift of the peak at ca. 2.2 ppm (H3C–(CH2)2–CH2–C
) can be seen after the first cycle, confirming the presence of carboxylic acid in the system. Moreover, this hexanoic acid peak is increasing in intensity which indicates an increasing amount of carboxylic acid in the system relative to the carboxylate anion. As can be seen in Fig. 8a, a downfield shift of the peak at ca. 2.2 ppm (H3C–(CH2)2–CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–COO−) indicates a deshielding effect from protonating the carboxylate group, i.e. a result of carboxylate–carboxylic acid transition.
2–COO−) indicates a deshielding effect from protonating the carboxylate group, i.e. a result of carboxylate–carboxylic acid transition.
Normalisation of the spectrum to the 15 1H multiplet at ca. 0.82 ppm (as a sum of terminal –CH3 groups protons in the cation and anion, excluding subsequent overlap of hexanoic acid), the ratio of hexanoic acid/hexanoate is 0.34, 0.71, 0.82, and 0.83 for cycles 1–4, respectively. This correlates with the results of decreasing dehydrochlorination degree after each recycling phase (Fig. 7), and approaching equilibrium of carboxylate–carboxylic acid.
The FTIR spectra of [P14,6,6,6]Cl and [P14,6,6,6]Br also did not show any changes regarding the chemical stability (before and after reaction). The FTIR spectrum for [P14,6,6,6][HexO] before and after reaction can be seen in Fig. 8b. The presence of carboxylic acid created during dehydrochlorination can be differentiated from the carboxylate group, through an asymmetric stretch of the –COOH group at 1738 cm−1 and symmetric stretch at 1216 cm−1. Moreover, a decrease in absorbance of the peaks at 1577 cm−1 (asymmetric CO stretching) and 1375 cm−1 (symmetric CO stretching) reflects a loss of carboxylate groups in the IL.
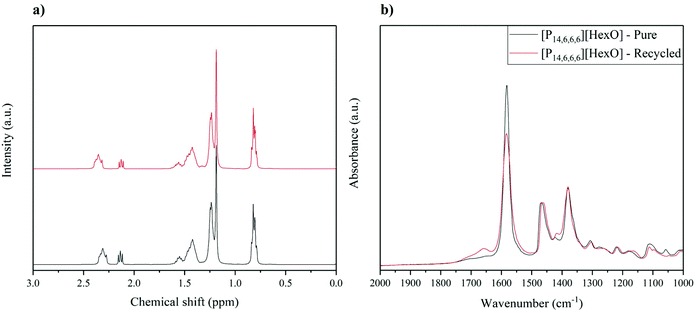
As shown above, the dehydrochlorination degree decreases after each cycle, which is associated with conversion of the hexanoate anion to hexanoic acid and replacing it with a chloride anion. The [P14,6,6,6][HexO] after the 4th cycle was separated and washed with a 2 M aqueous solution of NaOH for 16 h, forming NaCl and converting hexanoic acid back to the hexanoate anion. Afterwards, [P14,6,6,6][HexO] was washed with water until no positive result in AgNO3 test (for Cl− detection), to remove residuals of Cl− (from NaCl) in the IL. The IL was then dried in in vacuo (10−3) Pa at 65 °C for 24 h. The structure of recycled [P14,6,6,6][HexO] was confirmed and compared to pure [P14,6,6,6][HexO] before any dehydrochlorination (Fig. 9). As expected, there was no singlet at ca. 14 ppm observed indicating conversion of hexanoic acid to the hexanoate anion (Fig. 9a), with the number of protons in the cation and anion equal to 78 (as in pure IL), reflecting in ≥0.98 weight purity. Moreover, the FTIR spectrum indicates recovery of the carboxylate anion was 97.5%, based on stretching band at 1577 cm−1.
The recycled and dried [P14,6,6,6][HexO] was reused for the dehydrochlorination of PVC (160 °C, 4 h). The dehydrochlorination degree achieved with recycled [P14,6,6,6][HexO] was 96.5%, as compared to 99.8% and 94.3% after the 1st (with pure [P14,6,6,6][HexO]) and 2nd cycle, respectively. The above results show that despite the limiting decrease of dehydrochlorination degree with cycles, in the case of [P14,6,6,6][HexO], it is possible to recycle the IL to restore activity for dehydrochlorination.
(d) Separation of the layered material
A significant constraint in the feedstock or mechanical recycling of PVC is separation of the components from mixed waste streams. In the case of the blisterpack, oPA, aluminium, and adhesive residue needs to be separated from PVC, so the PVC can then be taken for recycling.35 ILs can provide a solution in this case, where the presence of aluminium and oPA do not impose difficulties for the dehydrochlorination reaction as a feedstock recycling process, due to the peeling off effect observed during the reaction. It was noticed that both the temperature and time of the reaction influences the delamination, or more specifically de-gluing PVC and oPA from Al. The effect of these two factors can be seen in Table 1. The results for all three ILs were the same, suggesting that it is a time and temperature effect, and the presence of IL, rather than related to the IL anion type. At the lowest temperature, 80 °C, delamination was achieved after 2 h, whilst at the highest temperature, 160 °C, it was observed after 15 min.| 80 °C | 100 °C | 120 °C | 140 °C | 160 °C | |
|---|---|---|---|---|---|
| 15 min | − | − | − | − | + |
| 30 min | − | − | − | + | + |
| 60 min | − | − | + | + | + |
| 120 min | + | + | + | + | + |
| 240 min | + | + | + | + | + |
| 1440 min | + | + | + | + | + |
Each batch of blisterpack contains 19% of oPA (5 g IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
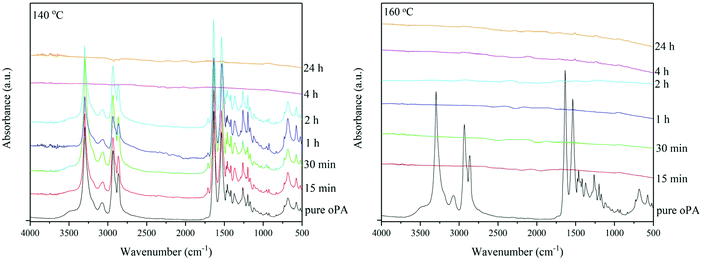
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0475 g oPA, resulting in 1% of oPA in IL). Melting–dissolution of oPA in the IL, during or after delamination, is expected based on the results observed by Zheng et al. (2018) where the melting–solubility of nylon 6,6 occurred below 220 °C in low IL concentrations.36 The FTIR spectrum of the blisterpack (oPA-side) is shown in Fig. 10. It can be seen that at 140 °C, a flat line is observed after 4 hours, indicating no oPA is present on the blisterpack, whilst at 160 °C this happens after 15 min. At temperatures below 140 °C, no effect was observed. This is also summarised in Table 2 (the results for all ILs are the same).
0.0475 g oPA, resulting in 1% of oPA in IL). Melting–dissolution of oPA in the IL, during or after delamination, is expected based on the results observed by Zheng et al. (2018) where the melting–solubility of nylon 6,6 occurred below 220 °C in low IL concentrations.36 The FTIR spectrum of the blisterpack (oPA-side) is shown in Fig. 10. It can be seen that at 140 °C, a flat line is observed after 4 hours, indicating no oPA is present on the blisterpack, whilst at 160 °C this happens after 15 min. At temperatures below 140 °C, no effect was observed. This is also summarised in Table 2 (the results for all ILs are the same).
| 80 °C | 100 °C | 120 °C | 140 °C | 160 °C | |
|---|---|---|---|---|---|
| 15 min | − | − | − | − | + |
| 30 min | − | − | − | − | + |
| 60 min | − | − | − | − | + |
| 120 min | − | − | − | − | + |
| 240 min | − | − | − | + | + |
| 1440 min | − | − | − | + | + |
To confirm the solubility of oPA in the ILs, the FTIR spectrum of [P14,6,6,6]Cl and [P14,6,6,6]Br is shown in Fig. 11. [P14,6,6,6][HexO] has not been presented as the vibration bands for adhesive and oPA overlapped with the carboxylic acid/[HexO]−, thus masking the effect of solubility. The appearance of two bands at 1730 cm−1 and 1220 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) indicate the presence of adhesive (Degalan bead polymer), and the two bands at 1650 cm−1 (C
O stretching) indicate the presence of adhesive (Degalan bead polymer), and the two bands at 1650 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and 1550 cm−1 (
O stretching) and 1550 cm−1 (![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –C
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), indicate the presence of oPA (nylon).
O), indicate the presence of oPA (nylon).
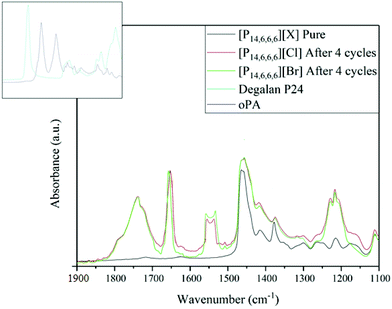 | ||
| Fig. 11 FTIR spectrum for [P14,6,6,6]Cl and [P14,6,6,6]Br before reaction and after the 4th cycle (160 °C, 4 h). | ||
It can be seen in Fig. 12 that the oPA, initially present in [P14,6,6,6][HexO], can be transferred to the solution of NaOH/H2O during the washing process. This highlights the possibility to fully recover all components of the blisterpack with use of only ILs: (1) PVC delaminated and dehydrochlorinated efficiently; (2) oPA delaminated and dissolved in IL, with further separation with NaOH/H2O; (3) Al can be easily separated with commonly used techniques, for example by using magnets.
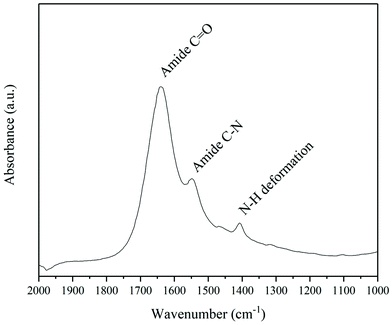 | ||
| Fig. 12 FTIR spectrum of NaOH/H2O solution used for washing [P14,6,6,6][HexO] after 4th cycle, indicating presence of oPA in NaOH/H2O. | ||
(e) De-HCl of PVC separated by ETAC
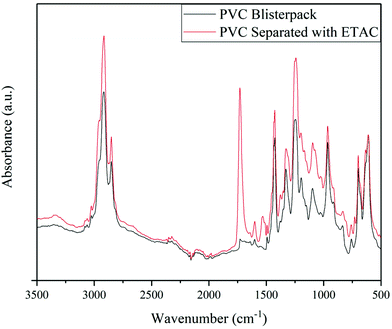
Feedstock recycling relies on the separation of plastic components, to avoid the generation of corrosive products in a pyrolysis reactor, such as HCl.37 Ethyl acetate (ETAC) was used as a comparison, to selectively separate PVC from the rest of the blisterpack. It is classified as a green solvent, and moreover, it has the tendency to swell PVC which would enable separation through delamination.30,38 The procedure for the separation of PVC from the rest of the blisterpack was described in the Experimental section. To assess the chemical quality of the separated PVC after the solvent was removed, the FTIR spectra was compared to the PVC in the blisterpack before processing (Fig. 13). The appearance of the band at 1730 cm−1 indicates the presence of adhesive in the reformed PVC during the separation at 50 °C. The same dehydrochlorination reaction as for the blisterpack was performed on the separated PVC (using the three ILs at 160 °C and 4 h). Both dehydrochlorination degree values for the PVC in blisterpack and separated are similar (Table 3), showing that the separation process prior to the dehydrochlorination reaction does not produce higher dehydrochlorination of PVC, allowing for a concerted separation/dehydrochlorination recycling process in one step by using ILs. Moreover, it indicates that the separation process is not the rate determining step. The rate of the dehydrochlorination is slower than the rate of separation of the blisterpack. Using ILs simplifies the recycling process where separation and/or purification would otherwise be an essential pre-processing step.| Ionic liquid | Dehydrochlorination degree (%) | |
|---|---|---|
| Blisterpack | Separated PVC | |
| [P14,6,6,6]Cl | 83.8 | 82.9 |
| [P14,6,6,6]Br | 85.1 | 86.7 |
| [P14,6,6,6][HexO] | 99.8 | 98.4 |
It is important to consider the scale up of such a technology, and this can limit the complexity of the chosen system for this application. For example, the synthesis of ILs with complex ions can increase the number of reaction steps required to reach the final product, therefore, focusing on simpler materials, such as [P6,6,6,14]Cl (synthesised from the reaction of trihexylphosphine with 1-chlorotetradecane) that is commercially available in bulk, can reduce associated costs considerably.39 However, while cost is an important factor, safety and environmental implications must also be considered in this process, when compared with thermal dehydrochlorination. Furthermore, hazards can be reduced considerably by using non-flammable and non-volatile materials. The use of ILs in commercial processes by BASF (and many other companies), shows that ILs can be successfully scaled, handled, and effectively recycled for many processes.40 It should be noted that there is currently no known industry process for chemically recycling PVC in multi-layered packaging e.g. blisterpacks, and waste is simply sent to landfill or incinerated. The separation of the individual layers is a complex challenge, and in this case, further work is required to conclusively determine the fate of other blisterpack components, such as the oPA layer and the adhesive, as well as the HCl produced, however early results are promising, indicating that the IL can be recycled up to five times with no loss in activity.
4. Conclusions
Phosphonium based IL-assisted exfoliation/dehydrochlorination was performed on a PVC-containing laminate composite, to study the effect of changing the IL anion, and to explore temperature and time as parameters. [P14,6,6,6]Cl and [P14,6,6,6]Br demonstrated retention of dehydrochlorination efficacy (83–85% dehydrochlorination degree) over a 4-cycle solvent recyclability test (4 h, 160 °C), and although [P14,6,6,6][HexO] had a higher initial efficacy (>99% dehydrochlorination degree) at these conditions, its ability to facilitate the dehydrochlorination reaction decreased with repeated use. This loss is thought to be due to protonation of the hexanoate anion, and its subsequent neutralisation to hexanoic acid, with [P14,6,6,6]Cl generated from the chloride anions released from PVC. The [P14,6,6,6][HexO] was thus recycled with a NaOH solution (ca. 97.5%), recovering dehydrochlorination affinity (degree of 96.5%, as compared to after the 1st cycle 99.8%). Washing with a NaOH solution was also shown to separate oPA from ILs which additionally indicates the possibility of circularity of the dehydrochlorination with ILs.The varying type and density of the components of a laminated material can only be exploited for recycling (e.g. by flotation, magnetic separation of metals) once delamination has occurred, and all the ILs tested demonstrated this capability, with reaction temperature a factor in the rate of delamination. By combining this with dehydrochlorination, a material is produced that is suitable for feedstock recycling processes that PVC would otherwise be incompatible with, and advantageous over a standard solvent treatment that would only separate the layers of the composite.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The results in this manuscript are part of the RE3 – Rethinking Resources and Recycling project, funded by the Engineering and Physical Sciences Research Council (EPSRC) (EP/S025200/1). The project was also supported by the UK Catalysis Hub (EP/R026939/1). The authors would like to thank the Resource Efficiency segment at Evonik Industries AG for supplying adhesive samples, as well as AstraZeneca for their ongoing support.References
-
Plastics Europe, Plastics – the Facts 2019, An analysis of European plastics production, demand and waste data, 2019 Search PubMed
.
- A. Buekens and K. Cen, J. Mater. Cycles Waste Manage., 2011, 13, 190–197 CrossRef CAS
.
-
C. E. Wilkes, J. W. Summers, C. A. Daniels and M. T. Berard, PVC handbook, Hanser Munich, 2005, vol. 184 Search PubMed
.
- H. Stichnothe and A. Azapagic, Resour., Conserv. Recycl., 2013, 71, 40–47 CrossRef
.
- A. Buekens and A. Sevenster, J. Mater. Cycles Waste Manage., 2010, 12, 184–192 CrossRef CAS
.
- P. T. Williams and E. A. Williams, Energy Fuels, 1999, 13, 188–196 CrossRef CAS
.
- K. Ragaert, S. Huysveld, G. Vyncke, S. Hubo, L. Veelaert, J. Dewulf and E. Du Bois, Resour., Conserv. Recycl., 2020, 155, 104646 CrossRef
.
- N. Singh, D. Hui, R. Singh, I. P. S. Ahuja, L. Feo and F. Fraternali, Composites, Part B, 2017, 115, 409–422 CrossRef CAS
.
- M. A. Keane, J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol., 2007, 82, 787–795 CAS
.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC
.
- I. Mersiowsky, Prog. Polym. Sci., 2002, 27, 2227–2277 CrossRef CAS
.
- R. Miranda, H. Pakdel, C. Roy, H. Darmstadt and C. Vasile, Polym. Degrad. Stab., 1999, 66, 107–125 CrossRef CAS
.
- A. Cervantes-Reyes, A. Núñez-Pineda, C. Barrera-Díaz, V. Varela-Guerrero, G. Martínez-Barrera and E. Cuevas-Yañez, Waste Manag., 2015, 38, 61–64 CrossRef CAS PubMed
.
- T. Mumladze, S. Yousef, M. Tatariants, R. Kriūkienė, V. Makarevicius, S.-I. Lukošiūtė, R. Bendikiene and G. Denafas, Green Chem., 2018, 20, 3604–3618 RSC
.
- S. Yousef, T. Mumladze, M. Tatariants, R. Kriūkienė, V. Makarevicius, R. Bendikiene and G. Denafas, J. Cleaner Prod., 2018, 197, 379–392 CrossRef CAS
.
-
G. Wypych, PVC degradation and stabilization, Elsevier, 2015 Search PubMed
.
- A. Akaha, J. Hernandez-Martinezb, C. Rallanb and A. A. Garforth, Chem. Eng., 2015, 43, 2395–2400 Search PubMed
.
- A. Bin Jumah, V. Anbumuthu, A. A. Tedstone and A. A. Garforth, Ind. Eng. Chem. Res., 2019, 58, 20601–20609 CrossRef CAS
.
- A. A. Garforth, Y.-H. Lin, P. N. Sharratt and J. Dwyer, Appl. Catal., A, 1998, 169, 331–342 CrossRef CAS
.
- J. Yu, L. Sun, C. Ma, Y. Qiao and H. Yao, Waste Manag., 2016, 48, 300–314 CrossRef CAS PubMed
.
- Y. Uemichi, K. Takuma, M. Sugioka and T. Kanazuka, Bull. Chem. Soc. Jpn., 1991, 64, 735–737 CrossRef CAS
.
- L. Guo, G. Shi and Y. Liang, Polymer, 2001, 42, 5581–5587 CrossRef CAS
.
- Q. Zhou, C. Tang, Y.-Z. Wang and L. Zheng, Fuel, 2004, 83, 1727–1732 CrossRef CAS
.
- T. Zhao, Q. Zhou, X.-L. He, S.-D. Wei, L. Wang, J. M. N. van Kasteren and Y.-Z. Wang, Green Chem., 2010, 12, 1062–1065 RSC
.
- D. Glas, J. Hulsbosch, P. Dubois, K. Binnemans and D. E. De Vos, ChemSusChem, 2014, 7, 610–617 CrossRef CAS PubMed
.
- T. Welton, Proc. R. Soc. A, 2015, 471(2183), 20150502 Search PubMed
.
- L. Andreani and J. D. Rocha, Braz. J. Chem. Eng., 2012, 29, 1–13 CrossRef CAS
.
- K. J. Fraser and D. R. MacFarlane, Aust. J. Chem., 2009, 62, 309–321 CrossRef CAS
.
- K. Oster, P. Goodrich, J. Jacquemin, C. Hardacre, A. P. C. Ribeiro and A. Elsinawi, J. Chem. Thermodyn., 2018, 121, 97–111 CrossRef CAS
.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2015, 18, 288–296 RSC
.
- R. Lungwitz and S. Spange, New J. Chem., 2008, 32, 392–394 RSC
.
- X.-L. He, Q. Zhou, X.-Y. Li, P. Yang, J. M. N. van Kasteren and Y.-Z. Wang, Polym. Degrad. Stab., 2012, 97, 145–148 CrossRef CAS
.
- R. Miranda, J. Yang, C. Roy and C. Vasile, Polym. Degrad. Stab., 1999, 64, 127–144 CrossRef CAS
.
- T. Kameda, K. Imai, G. Grause, T. Mizoguchi and T. Yoshioka, Polym. Degrad. Stab., 2009, 94, 1595–1597 CrossRef CAS
.
-
VinylPlus, PVC Recycl. Technol. Rep Search PubMed
.
- X. Zheng, Q. Lin, P. Jiang, Y. Li and J. Li, Polymer, 2018, 10, 562 Search PubMed
.
- F. Perugini, M. L. Mastellone and U. Arena, Environ. Prog., 2005, 24, 137–154 CrossRef CAS
.
- V. J. Ducruet, A. Rasse and A. E. Feigenbaum, J. Appl. Polym. Sci., 1996, 62, 1745–1752 CrossRef CAS
.
- R. M. Cuéllar-Franca, P. García-Gutiérrez, S. F. R. Taylor, C. Hardacre and A. Azapagic, Faraday Discuss., 2016, 192, 283–301 RSC
.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC
.
| This journal is © The Royal Society of Chemistry 2020 |