Degradable and recyclable bio-based thermoset epoxy resins†
Abstract
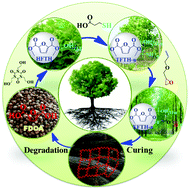
The design of a degradable high-performing thermoset without using organic solvents is critical for the understanding and sustainable development of homogeneous structures with simultaneous reinforcement and toughening functions. Here, we report a novel degradable and recyclable thermoset hyperbranched epoxy resin (EFTH-n) synthesized from bio-based 2,5-furandicarboxylic acid (FDCA). EFTH-n showed excellent performance on common diglycidyl ether of bisphenol-A (DGEBA) with simultaneous improvements in the impact strength, tensile strength, flexural strength, storage modulus and elongation by up to 181.84%, 60.22%, 24.08%, 32% and 58.0%, respectively. The homogeneous microstructure of EFTH-n/DGEBA composites was systematically analyzed using in situ Raman imaging, AFM, SEM, DMA, dynamic light scattering and the positron annihilation lifetime spectroscopy, which enabled us to attribute the improvements to the synergistic effect of the crosslinking density, free volume, intermolecular cavity, hyperbranched topological structure and good compatibility between the components, which was explained by an in situ reinforcing and toughening mechanism. We concluded that EFTH-n could significantly facilitate the degradation of the cured composites under mild conditions without using organic solvents together with a FDCA recycling yield of 56.8 wt%.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...