Open Access Article
Open Access ArticleDesign of a combined ionosolv-organosolv biomass fractionation process for biofuel production and high value-added lignin valorisation†
Meng
Chen
,
Francisco
Malaret
 ,
Anton E. J.
Firth
,
Pedro
Verdía
,
Aida R.
Abouelela
,
Yiyan
Chen
and
Jason P.
Hallett
,
Anton E. J.
Firth
,
Pedro
Verdía
,
Aida R.
Abouelela
,
Yiyan
Chen
and
Jason P.
Hallett
 *
*
Department of Chemical Engineering, Imperial College London, London, SW7 2AZ, UK. E-mail: j.hallett@imperial.ac.uk; Tel: +44 (0)2075945388
First published on 14th July 2020
Abstract
IonoSolv pretreatment using protic ionic liquids has shown impressive biomass fractionation performance and ionic liquid recyclability. Lignin condensation during ionoSolov pretreatment can lower its economic value and potentially limit the valorisation of lignin to produce high value materials. Organosolv pretreatment is known for generating a high-quality lignin fraction with a large potential for value-added applications. In this study, a hybrid fractionation process was designed based on ionoSolv and organosolv pretreatments, and was tested on two representative feedstocks, Miscanthus and pine. Compared to ionoSolv processing, the hybrid pretreatment displayed an improved fractionation performance by generating a cellulose-rich pulp which was more enzyme-accessible, and by removing a higher proportion of lignin. Saccharification yields reached 89% and 74% for Miscanthus and pine, respectively. The process was also able to maintain its high fractionation performance up to 50 wt% biomass loading. HSQC spectroscopy and GPC were used to characterise the isolated lignin. Alcohol induced α-alkoxylation took place during lignin fractionation and obstructed the lignin condensation, resulting in an improved quality lignin. A technoeconomic analysis was conducted for this new hybrid pretreatment, showing lower energy consumption for the IL regeneration step at a high organic solvent concentration, suggesting lower environmental impact and higher economic potential. This ionoSolv-organosolv pretreatment could be a milestone for the development of the current ionoSolv pretreatment at commercial scale.
Introduction
The development of biomass derived fuels and chemicals has been viewed as a key contributor to reducing society's reliance on petroleum.1,2 Environmental issues such as rapid growth of greenhouse gas (GHG) emissions have made this desire even more profound.3 1st generation biofuels, mainly bioethanol, have been able to achieve commercial-scale production in the US, Brazil, Canada and Europe;4–7 however, the production of these food crop-derived fuels is still regarded as uneconomical, environmentally harmful, and ethically problematic due to the intensive labour requirement, associated water pollution and soil erosion, and potential decrease in food security in certain regions.8–10 Thus, the vast majority of research in recent years has shifted its focus to inedible plant-derived fuels, e.g. cellulosic ethanol. Suitable cellulosic feedstocks for biorefineries include forest residues, agricultural wastes (e.g. rice straw, rice husk), municipal wastes and fast-growing energy crops, e.g. Miscanthus. These crops are particularly promising as most of them are waste products, having sufficient availability, and may be exploited sustainably.4,11–13 By replacing gasoline with cellulosic ethanol/butanol, up to 90% of GHG emissions can be eliminated, while only a 40% emission reduction can be achieved by corn-derived biofuels.14–16Lignocellulosic biomass is mainly made up by cellulose, hemicellulose and lignin, with its exact composition varying between species and growth stages.11 Compared to corn and sugarcane, the increased complexity of the feedstocks requires lignocellulosic materials to go through a pretreatment step prior to hydrolysis and microbial fermentation.3,17 Pretreatment is the costliest step in biorefinery, and hence becomes the economic bottleneck for building an industrially feasible biomass-to-biofuel/biomaterial conversion.17,18 The cost of the process includes direct energy inputs, and solvent and catalyst costs. These can be significantly reduced by using either cheap and environmental benign solvents, e.g. ethanol/butanol, or recyclable solvents, e.g. ammonia, ionic liquids. Technical lignins generated from current industrial biorefinery plants are estimated to have an annual production rate up to 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per plant (Kraft pulping plant) and only little is subjected to applications, with either low value or a limited market.19 However, several reviews have pointed out a wide range of potential value-added lignin utilisation options, including lignin-based carbon fibres, bio-based plasters, aromatic-based chemicals.20–22,23–27
000 tons per plant (Kraft pulping plant) and only little is subjected to applications, with either low value or a limited market.19 However, several reviews have pointed out a wide range of potential value-added lignin utilisation options, including lignin-based carbon fibres, bio-based plasters, aromatic-based chemicals.20–22,23–27
Both Miscanthus and pine are attractive feedstocks for advanced bioconversion processes. Miscanthus is a dedicated energy crop. Due its highly representative structure, many studies have been conducted using this feedstock.28–30 Pine is one of the dominant softwood resources with a significant biorefinery potential. Its high lignin content and high concentration of G subunits are problematic towards cost-effective biorefining.31 Both grassy biomass and softwoods have been intensively studied to develop suitable fractionation strategies.3,18,32 Physical processes like milling require high energy input.33 Dilute acid has a limited potential due to its corrosive reagent and inability to remove lignin.34 Ammonia fibre expansion (AFEX) process is ineffective for high lignin-content feedstocks such as softwoods.35 Using ionic liquids (IL) to pretreat grassy and woody feedstocks has a relatively short history but displays great potential. ILs, often referred as green solvents, are a group of low melting organic salts. They can fractionate biomass via two different mechanisms: dissolution and ionoSolv processing.11 Early studies have shown that 1-ethyl-3-methylimidazonium acetate, [EMIM][OAc], is particularly promising as a dissolution solvent due to its anion's high hydrogen-bond basicity, which enables cellulosic disruption.36,37 [EMIM][OAc] dissolves cellulose and the cellulose fibres are then regenerated in a less crystalline form. This yields a treated biomass possessing a better enzyme accessibility (i.e. a maximal cellulose digestibility >90%).38 However, [EMIM][OAc] has a low water tolerance (energy-intensive IL drying process), high prices (high operating cost) and low thermal stability (difficult to reuse), which has hindered its development in an industrially-viable biorefinery.39–41
Recently, a group of protic hydrogen sulfate ILs have been viewed as a suitable alternative to [EMIM][OAc].39 Instead of dissolving cellulose, these ILs dissolve lignin, remove hemicellulose, and leave cellulose largely intact. They are synthesised via a simple acid–base addition and their bulk production prices are estimated from $1.24 to $5.88 kg−1 (based on 2014 chemical commodity prices) compared to $20–$100 kg−1 for [EMIM][OAc].42 According to Baaqel et al.'s recent estimation, these protic IL prices are reduced to $0.78 kg−1 as the industrial production cost has lowered.43 They also exhibit reasonable thermal stabilities, with decomposition temperatures >277 °C (compared to 215 °C for [EMIM][OAc]), indicating IL cations that are comparatively harder to undergo side-chain dealkylation, which forms hazardous waste during pretreatment.39 One of the most promising ILs within this group is triethylammonium hydrogen sulfate, [TEA][HSO4]. [TEA][HSO4] containing 20 wt% water exhibits excellent fractionation ability towards Miscanthus, agriculture wastes, and hardwoods.44 Pretreatments using [TEA][HSO4] were able to obtain enzymatic saccharification yields ranging from 77% to 82%.29,44–47 Brandt-Talbot et al.'s recent work stated that [TEA][HSO4] can be recycled with no sign of degradation.48 They also demonstrated that this IL can be repeatedly used for four Miscanthus pretreatment cycles with a constant fractionation ability (i.e. zero decrease – even a slight increase – in sugar release yield). IL's ability of being reused without any compensations of the pretreatment outcome has a great significance for scaling up the process. However, [TEA][HSO4] is less effective than N,N-dimethyl-N-butylammonium hydrogen sulfate, [DMBA][HSO4] towards softwood biomass. Gschwend et al. reported that [DMBA][HSO4] was able to achieve an effective delignification and near quantitative (100%) glucose yield when pretreating a recalcitrant softwood feedstock, Pinus sylvestris.31 Lignin condensation was shown to take place during the process for both feedstocks, especially at severe pretreatment conditions required to achieve effective delignification. During IL delignification, β-O-4 ether linkages are most labile and potentially to break, then undergoing condensation.48 The severely condensed lignin structure is not ideal for lignin applications. Pretreatment technologies using organic solvents have been previously used in pulping industry, e.g. ALCELL™.49 Organosolv pretreatment is well known for its multi-product extraction capability: the process selectively dissolves lignin (which is later recovered as a solid), depolymerises hemicellulose into an aqueous fraction containing high-value products such as furfural, and leaves a high-purity cellulose pulp. Low boiling point solvents, including unbranched alcohols and ketones, are the few pretreatment agents reported to achieve effective fractionation performance independent of biomass type.3,50 Pan et al. used the Lignol process (40–60 wt% ethanol, catalysed by sulfuric acid, 185–198 °C, 30–60 min) to produce a series of low lignin-content softwood pulps.51 They also reported that these softwood pulps were highly accessible to enzymes and could be hydrolysed at a high rate, 98% cellulose conversion within 24 hours. A more recent work from Başakçılardan Kabakcı et al. compared the pretreatment effectiveness for ethanol, alkaline glycerol and formic acid organosolv processes in terms of recovered lignin.52 They suggested the ogranosolv lignins are all suitable for high value added applications such as active carbon production, but the lignin production rates varied between the organosolv processes. Meng et al. reported that ethanol organosolv process was able to significantly disrupt the crystallinity of the cellulose, achieving a highly accessible pulp.53 Acetone has been repeatedly suggested as a promising delignifying agent and hemicellulose solvent. Huijgen et al. reported a 79% delignification and 82% hemicellulose removal for wheat straw pretreated with 50 wt% aqueous acetone at 205 °C for 1 hour. Similar results for sweet sorghum bagasse were reported by Jafari et al.51,54,55 Comparatively, pretreatments in Sidiras et al.'s recent study were conducted at relatively milder conditions (50 wt% aqueous acetone/butanol/ethanol/methanol, catalysed by 0.045 N sulfuric acid, 160 °C for 20 min).56 Results showed butanol to be the best delignifying agent, with 63% lignin removal. Schmetz et al. investigated a butanol/sulphuric acid biphasic organosolv process for softwood, hardwood and grassy feedstocks.57 The hardwood and grassy pulps pretreated by the biphasic process at 180 °C were able to achieve glucose yields of 50% and 77%. The process was not effective towards softwood. Organic solvents may require expensive high-pressure equipment, but this may be considered a trade-off with the easy solvent recycling and high-quality by-products.
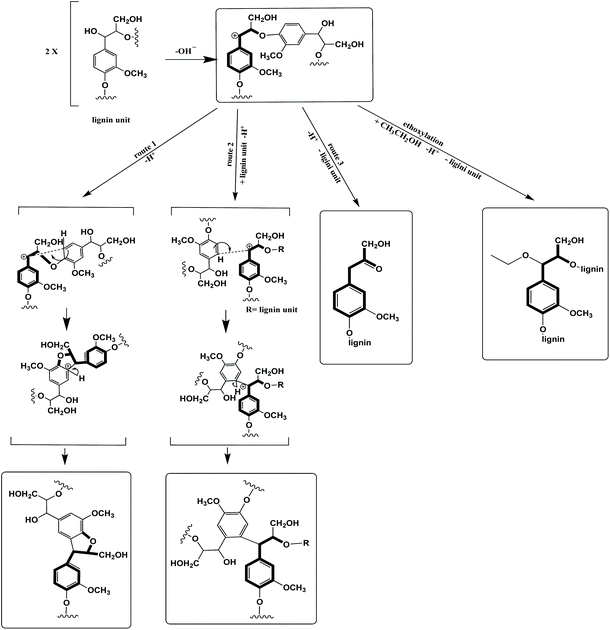
In the organosolv process, delignification takes place via breaking β-O-4 ether linkages and 4-O-methylglucuronic acid ester bonds between lignin and hemicellulose.58 The regenerated lignin has a beneficial chemical structure towards high value applications. It has high purity (low in carbohydrate, ash, and sulfur content) and low molecular weight with a narrow weight distribution.59 Lignin condensation is inevitable for organosolv pretreatments using harsh conditions, but Lancefield et al. and Bauer et al. suggested that by increasing the ethanol content in the pretreatment solvent, lignin degradation reactions can be largely suppressed and potentially eliminated. Four potential chemical modifications, including condensation, could take place at carbon α, presented in Scheme 1. During β-O-4 ether cleavage, monolignols are likely to transform into benzylic cations that can undergo intra- and intermolecular condensations and hydrolysis to give Hibbert ketone; ethanol can trap this cation via α-ethoxylation generating α-alkoxy ether units, preventing cations from being condensed to form new carbon single bonds; the α-alkoxy ether units also increase the lignin solubility, thus improving delignification.60,61
Combining two pretreatment strategies can improve results in biomass fractionation and is a promising alternative for the future of industrially viable biofuel/biomaterial conversion. Hybrid methods to date include several combined methods, e.g. alkaline and photocatalysis pretreatment, acid and microwave pretreatment.62,63 Limited work has been done to hybridise the organosolv process with other pretreatment strategies such as ionoSolv pretreatment, but this is worth investigating due to ionoSolv's excellent fractionation ability, recycling capability, and potential of delivering high value-added lignin products.64 To date, IL–organic mixtures, e.g. acetate based ILs with N-methyl-2 pyrrolidone and ethylene-glycol, used in pretreatments have focused on cellulose dissolving ILs. These solvent systems all face issues like high costs and low IL thermal stabilities when operating at high temperatures.65–68 Furthermore, an IL recycling step in an industrial ionoSolv process involves energy-intensive removal of water, which is added during pulp washing. Partially replacing water (and IL) in ionoSolv pretreatment solutions with low boiling point organic solvents can significantly reduce the energy input to recycle the IL, as it is easier to remove organic solvents from IL post-pretreatment mixtures than to remove water. The solvent cost can be further reduced by using ethanol or butanol produced within a biorefinery, subsequently reducing the capital cost of the biomass fractionation (through more intensified processing), making the ionoSolv-organosolv combined process more economically feasible.
In this work, we investigated the effectiveness of a newly developed pretreatment method combining key features of organosolv and ionoSolv processing on two feedstocks: Miscanthus and pine. [TEA][HSO4] and [DMBA][HSO4] ionic liquids were selected for Miscanthus and pine pretreatments, respectively. Ethanol, butanol and acetone were the organic solvent candidates for this hybrid method. The impacts of organic solvent concentration and biomass loading on Miscanthus fractionation were studied, while the effects of ethanol concentration and feedstock loading on the softwood deconstruction were investigated. Pretreatment effectiveness was evaluated by sugar yield after enzymatic hydrolysis of the treated biomass. Hemicellulose and lignin removals were determined by pulp composition analysis. Isolated lignins were analysed by HSQC NMR and GPC to gain insight about their chemical functionalities and structural changes, which are essential to evaluate their tunability and beneficial use as a starting material for high-value applications. We also conducted a high-level techno-economic evaluation to estimate the industrial feasibility of this new hybrid pretreatment technology.
Results and discussion
Different organic solvents were added to anhydrous [TEA][HSO4], in order to evaluate their effectiveness on the pretreatment of Miscanthus. The concentration of each organic solvent was varied from 0 wt% to 80 wt%. For the hybrid process using a mixture of organic solvent and IL, the pretreated biomass (pulp) was washed with the organic solvent in order to remove dissolved lignin and IL, and then allowed to air dry. The organic solvent was then removed from the pulp washings by rotary evaporation, leaving a solid mixture of lignin and IL. Water was used as an anti-solvent in order to separate the lignin from the IL, and then used to wash the lignin. The wet lignin was then freeze-dried in order to remove remaining water. IonoSolv pretreatments were performed for comparison, using aqueous [TEA][HSO4] (80 wt%), and using each organic solvent as a pulp washing solvent. The ionoSolv pretreatment using ethanol to wash the pretreated biomass is subsequently compared to the ethanol-[TEA][HSO4] fractionation process; the same comparison was made for butanol and acetone.Effect of organic solvents and their concentrations on biomass fractionation
The air-dried pulps were subjected to a saccharification assay and compositional analysis. Fig. 1 shows the composition of the pulps pretreated with 10 different ratios of ethanol and IL at 120 °C for 8 hours, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g g−1 biomass loading.
10 g g−1 biomass loading.
The glucan yields for this study's hybrid organosolv (ethanol)-ionoSolv processes flattened at 85% from 20 wt% to 60 wt% ethanol but were then halved at 80 wt% ethanol (to 45%), indicating a minimum amount of IL (at least 40 wt%) is required for effective pretreatment. Fig. 2a presents saccharification yields (detailed in the ESI Table S5†) for the pulps pretreated with different ethanol–[TEA][HSO4] mixtures. All glucose releasing yields are evaluated relative to the glucose content of untreated Miscanthus. The ionoSolv pretreatment (0 wt% ethanol, 20 wt% water) achieved a 75% glucose yield. However, by partially replacing the IL with ethanol, a maximum glucose yield of 85%, was observed (at 40% ethanol in the IL). This 10% increase is important for the potential commercialisation of IL-based pretreatments.2 Ethanol organosolv processing was able to achieve a similar glucan conversion, 78%, as the ionoSolv process, but at a higher temperature, 170 °C.69
An organosolv pretreatment with a competitive performance often requires high temperatures (>200 °C) or the use of acid catalysts, commonly sulfuric acid.54,55 In this hybrid pretreatment process, an acidic IL not only plays a role of dissolving hemicellulose and lignin, but also act as a catalyst, allowing fractionation of biomass at a low temperature. The combined pretreatment is more effective than using ethanol or IL alone; if the IL concentration is too low (≤20 wt%), there is insufficient catalytic activity to remove hemicellulose and lignin effectively. The study of Brandt-Talbot et al. has provided evidence for a positive correlation between lignin removal (delignification) and saccharification yield.48 Hence, the enzymatic hydrolysis of pulps pretreated with low IL-content organic mixtures will be hindered by the large amount of lignin preserved within the pulp.
Fig. 2b and c show the saccharification yields for the pulps pretreated with 5 different butanol–IL and acetone–IL mixtures (detailed in ESI Tables S6 and S7†). For butanol–IL mixtures, the glucose yield reached a maximum of 85%, and remained stable between 20 wt% and 40 wt% butanol. Peak yields were the same as those using ethanol–IL mixtures, suggesting that increasing the chain length of the organic solvent does not affect maximum sugar yield.
For acetone–IL mixtures, glucose yields peaked at 55%, significantly lower than butanol and ethanol. However, these results appear in line with those reported in literature. Acetone organosolv pretreatments carried out on sugarcane bagasse by Jafari et al. showed maximum glucose yields of 55% and 94% obtained at 150 °C and 180 °C, respectively.55 Another acetone organosolv pretreatment study for wheat straw recorded a glucose yield of 87% at 205 °C, but this study also showed the yields at 160 °C and 175 °C were less than 45%.54 The relatively low operating temperature (120 °C) could be responsible for the low sugar yield obtained by acetone–IL pretreatment in our study.
Three ionoSolv pretreatments (with 0 wt% organic solvent) were carried out, in which the only difference was the washing solvent. Glucose yields were highest for the pretreatment using ethanol as the washing solvent: 10% and 22% higher than those using butanol and acetone as washing solvents, respectively. It can be concluded that, compared to ethanol, using butanol and acetone as washing solvents negatively impacts delignification; therefore, non-ideal washing solvent usage could be another potential reason for the lower sugar yield of all acetone pretreatments.
Cellulose digestibility, calculated based on glucan content of the raw biomass, was also evaluated for all conditions (detailed in ESI Fig. S1†). It is highly in line with the saccharification data, only differs numerically. The optimal ethanol/butanol–IL pulp digestibility (95% for 40 wt% ethanol, 84% for 40 wt% butanol) increased by 15% relative to ionoSolv pulps. Partially replacing IL with acetone in fractionation did not changed the pulp digestibility. An aqueous ethanol process achieved similar digestibilities as the ethanol–IL process, up to 98%, but only when it was performed as a second step of a 2-step pretreatment that involved a highly energy and time consuming pre-soaking step that employed diluted sulfuric acid for 17 hours under reflux.69
Delignification, also known as lignin removal, quantifies the degree of lignin dissolution into the pretreatment solvent, while lignin yield quantifies the amount of lignin precipitated from the pulp washing. Both are presented in Fig. 2. Delignification displayed a similar trend to saccharification yield, which is expected as lignin is the biggest cause of recalcitrance toward enzymatic hydrolysis for all biomass.48 Ethanol–IL pretreatments sustained high lignin removals (up to 89%) between 10 to 60 wt% ethanol, up to 10% higher than the ionoSolv pretreatment. A similar increase (11%) was observed for butanol–IL processing, with a maximum lignin removal of 82% observed at 20 wt% butanol. The use of acetone–IL mixtures did not lead to an obvious increase in delignification. IonoSolv pulps washed with acetone achieved a delignification of 55%. Delignification increased to 59% when 20 wt% acetone was incorporated with 80 wt% IL during pretreatment but dropped below 40% as the acetone content increased to 60 wt%. The reason for the relatively smaller increase of lignin removal (≤4%) between ionoSolv and acetone–IL processes could be due to the reduced solubility of lignin in acetone compared to ethanol and butanol and the higher operating temperatures required for effective pretreatment. Organosolv pretreatments using aqueous ethanol, butanol and acetone achieved delignifications of 42%, 63%, and 58% respectively.56 These pretreatments were conducted at a much higher temperature, 160 °C. The butanol/ethanol processes have lower delignifications than butanol/ethanol–IL ones, 26–47% lower in term of percentages. Organosolv process with acidic presoaking step achieved 70.3% delignification.69 It is worth mentioning that in the Organosolv study, butanol and acetone were the two organic solvents with the best performances. This is not the case for hybrid processes, where acetone–IL mixtures fractionated far less effectively than butanol/ethanol–IL mixtures.
It is interesting to note that for the three ionoSolv pretreatments with different pulp washing solvent choice (ethanol, butanol, acetone), the order of delignification was ethanol > butanol > acetone, with delignifications of 80%, 70% and 56%, respectively. This could be attributed to the solubilities of lignin in different washing solvents. It is believed that maximum lignin solubility occurs when the solubility parameters (δ) of the lignin and solvent are close to each other.70 Ni et al. estimated the δ value of lignin as 13.7 cal1/2 per cm3/2, and the δ values for ethanol (12.7 cal1/2 per cm3/2), butanol (11.4 cal1/2 per cm3/2) and acetone (9.9 cal1/2 per cm3/2) were reported by Yagi et al.49,71 Hence, the order of lignin solubility in these three washing solvents is expected to be ethanol ≥ butanol > acetone. During the pulp washing step, a lower lignin solubility in the washing solvent could lead to incomplete dissolution of the lignin dissolved in the IL during the pretreatment and leave a small fraction of lignin (possibly along with some residual IL) redeposited onto the cellulose surface. However, compositional analysis is unable to distinguish redeposited lignin from residual lignin. It is also worth noting that the current protocol for compositional analysis was developed for biomass rather than pretreated pulps, and it can give inaccurate acid-soluble lignin measurements when the samples have very low lignin content.
The lignin yield decreased as the ethanol content increased in the pretreatment solvent mixture. The lignin yields for pretreatments using 10 to 60 wt% ethanol were at least 30% lower than their corresponding delignification values, while lignin removal for the ionoSolv process was only lower than its delignification by 14%. Lower lignin yields could be attributed to increased lignin solubility in water, as the lignins extracted by organic solvents tend to be more water soluble than those extracted by ILs. This was further confirmed by experimental observation: pale yellow colloidal suspensions formed in the water washes in which the colloidal lignin could not be separated from water even after being centrifuged three times. A similar observation was recorded by Bauer et al.61
The highest lignin yield for butanol–IL pretreatments (20 wt% butanol) was 20% higher than that of the ionoSolv. This could be due to the increased carbohydrate impurities (arabinofuranose) of lignin, and increased lignin molecular weight (caused by lignin butoxylation), which was confirmed by the HSQC analysis (detailed in ‘Lignin characterisation’ section). During the butanol–IL pretreatments, lignin undergoes α-butoxylation as the α-hydroxyl group is replaced with a butoxy unit, hence the lignin's molecular weight is increased.60 Unlike the ethanol–IL pretreatment, lignin yields of most butanol–IL mixtures (20 wt%, 60 wt% and 80 wt%) exceeded their corresponding delignification. This could be explained by α-butoxy lignin being larger and less polar than α-ethoxy lignin, therefore less water-soluble and not forming colloidal suspensions during the washing step. The dissolved and recovered lignin analysis (detailed in ESI Fig. S4†) shows that the sum of acid-soluble, acid-insoluble and recovered lignin contents for treated biomass exceeded the raw biomass lignin content. Furthermore, the lignin HSQC analysis suggested the butanol–IL lignin were not highly condensed and shows the incorporation of butoxylated ether and hemicellulose units (Fig. 7). Little or no mass increase due to lignin degradation was observed on the HSQC.
For acetone–IL pretreatments in Fig. 2c, the lignin yields for 20 wt%, 40 wt% and 60 wt% acetone were 63%, 62% and 54%, respectively, which were all above the yield of the corresponding ionoSolv process at 50%. Lignin yields for all acetone concentrations exceeded their delignification. The reason for this is not fully understood yet but the formation of condensed lignin oligomers and pseudo-lignin is suspected to play a role. Condensed lignin oligomers are large and water insoluble, formed by aggregation of short water-soluble lignin oligomers. Carbohydrate degradation products, such as 5-HMF and furfural, can react with lignin oligomers, forming lignin-like polymers. These lignin-like polymers can be detected together with other acid-insoluble lignin during the pulp compositional assay. Acetone can interact with hemicellulose via forming acetonide, in which the acetone modified sugar units could potentially further degraded, forming lignin-like motif, playing a part in this pseudo-lignin formation.61 Brandt et al. reported the presence of pseudo-lignin units and lignin condensation for ionoSolv lignin when treated at high severity conditions (long pretreatment time and high temperature), though those conditions were much harsher than those employed here.48
Fig. 2 also shows glucan recovery and hemicellulose removal for all pretreatment solvent mixtures, expressed in percentages of the theoretical maximum. Glucan recovery remained around 90% for all pretreatment mixtures, indicating that only slight glucan degradation occurred for all organic–IL mixtures. This is in agreement with the ionoSolv and organosolv processes reported in the literature, indicating the new hybrid pretreatment performs similarly, dissolving hemicellulose and lignin and leaving a cellulose-rich pulp.48,56 A clear decreasing trend of hemicellulose removal with increasing organic solvent content was observed. The highest removal achieved by the hybrid pretreatments (20 wt% ethanol, butanol and acetone) were 85%, 80%, and 82%, respectively, which are 12%, 11%, and 11% lower than the corresponding ionoSolv pretreatments. Organosolv pretreatments could remove up to 88%, 93%, and 94% of hemicellulose using ethanol, butanol, and acetone respectively. However, these pretreatments were conducted at a higher temperature, in the presence of sulfuric acid.56 In summary, the optimal pretreatment solvent composition for Miscanthus was 40 wt% ethanol with 60 wt% [TEA][HSO4] as it achieved the highest lignin removal and subsequently the highest saccharification yield.
Effect of biomass loading on biomass fractionation
Current pretreatments used in lignocellulosic ethanol plants often require high capital costs, usually accounting for 20% of the overall plant capital cost.72 Increasing the biomass loading could lead to a reduced reactor size and a reduced solvent cost, potentially decreasing the capital expenditure (CAPEX) up to 40%.31 In order to be industrially viable, it is important for the pretreatment process developed here to operate at a high biomass loading. Therefore, ionoSolv-organosolv pretreatments with 5 different biomass loadings, ranging from 10 to 50 wt% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 1
10 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g g−1) were conducted to evaluate the performance of this newly developed process at high loadings. Fig. 3 presents the key indicators for the performance of these ionoSolv-organosolv processes. All pretreatments were carried out for Miscanthus at 120 °C for 8 h with an IL
2 g g−1) were conducted to evaluate the performance of this newly developed process at high loadings. Fig. 3 presents the key indicators for the performance of these ionoSolv-organosolv processes. All pretreatments were carried out for Miscanthus at 120 °C for 8 h with an IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol mass ratio of 60
ethanol mass ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, which are the optimal conditions identified earlier.
40, which are the optimal conditions identified earlier.
 | ||
| Fig. 3 Effect of biomass loading on fractionation effectiveness for Miscanthus ethanol–[TEA][HSO4] pretreatment with 60 wt% IL and 40 wt% ethanol at 120 °C. | ||
Fractionation effectiveness of the ethanol–IL pretreatment was less affected than for the ionoSolv when increasing biomass loading. Saccharification yield was 87% at 10 wt% loading and 71% at 50 wt% loading, only a 16% drop in glucose yield as the loading increased 5-fold. This result is very promising as it has been reported that the ability of both organic and ionic solvents to delignify biomass drops significantly at higher biomass loading.73 A drop in saccharification yield of 52% was reported for the ionoSolv process, 78% for 10 wt% loading, but only 26% for 50 wt% loading.73 The reason behind this drop is usually related to mass transfer limitations, where at high biomass loading there is not enough solvent in contact with the biomass and the solvents only wet the surface, resulting in poor delignification. Here, exceptionally high delignification was achieved at a solids loading of 50 wt% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g g−1), indicating that mass transfer does not pose a serious challenge for this hybrid organic-ionic pretreatment medium.74 This could be a combined effect of several factors: (1) compared to ionoSolov pretreatments, the hybrid pretreatment uses a medium of lower viscosity due to the incorporation of 40 wt% ethanol as a co-solvent instead of 20 wt% water; and (2) compared to organosolv pretreatments, the use of 60 wt% ionic liquid helps maintain effective fractionation, as the [HSO4]− based ionic liquid has shown effective delignification performance at high solids loading with challenging feedstocks such as pine wood (and lignin solubility is higher than in organic solvents).32
2 g g−1), indicating that mass transfer does not pose a serious challenge for this hybrid organic-ionic pretreatment medium.74 This could be a combined effect of several factors: (1) compared to ionoSolov pretreatments, the hybrid pretreatment uses a medium of lower viscosity due to the incorporation of 40 wt% ethanol as a co-solvent instead of 20 wt% water; and (2) compared to organosolv pretreatments, the use of 60 wt% ionic liquid helps maintain effective fractionation, as the [HSO4]− based ionic liquid has shown effective delignification performance at high solids loading with challenging feedstocks such as pine wood (and lignin solubility is higher than in organic solvents).32
Glucan recovery remained constant for all biomass loadings. Hemicellulose removal dropped from 62% to 37% when the solid loading increased from 10 wt% to 50 wt%; this is likely due to the decreased acidity of the partially organic medium. Higher hemicellulose content does not appear to negatively affect the saccharification yield in this study. As previously mentioned, lignin hinders the enzymatic saccharification of the pulp more than other biomass components. Lignin removal was observed to track saccharification yield, decreasing by only 16% at 50 wt% solids loading. By comparison, the delignifications recorded for ionoSolv processing were 80% for 10 wt% loading and 47% for 50 wt% loading.73
Lignin recovery dropped more than delignification, 60% and 12%, for 10 wt% and 50 wt% solids loading, respectively. Two reasons may explain this substantial drop. Firstly, increased amounts of the water-soluble lignin fraction formed colloidal suspensions instead of being precipitated. Second, the amount of lignin dissolved in ethanol–IL mixtures during the pretreatment substantially increased when biomass loading was increased by 5 times. Extracted lignin could not be fully dissolved by ethanol in the pulp washing step due to the limited lignin solubility of ethanol at room temperature, thus a small fraction of dissolved lignin was trapped with pulp and compositional analysis could not distinguish this with the residual lignin fraction of the pulp.
Fractionation of softwood using the hybrid process
It is necessary for an ideal pretreatment to be feedstock-independent, as a broad range of biomass feedstocks have been identified as having biorefinery potential. Pinus sylvestris (pine) softwood was subjected to this ionoSolv-organosolv pretreatment, to examine its performance for more recalcitrant feedstocks. [DMBA][HSO4] has been reported to be one of the best performing protic ILs for pine, and so was used with ethanol to prepare four different organic–IL mixtures (0 wt%, 20 wt%, 40 wt%, 80 wt% ethanol).34 All pretreatments were conducted in triplicate at 170 °C for 80 minutes in hydrothermal autoclave reactors instead of pressure tubes, due to the higher operational pressures at the temperatures required to pretreat pine. A comparison of the saccharification yields using pressure tubes and reactors was first performed, with results showing that pretreatments using reactors for 80 minutes have the same fractionation performance as using pressure tubes for 30 minutes, due to significant differences in heating rates (detailed in ESI Table S11†).Compared to the ionoSolv process (0 wt% ethanol, 20% wt% water), the ethanol–IL process displayed improved fractionation when 20 to 40 wt% IL was replaced by ethanol (Fig. 4a, detailed in ESI Table S9†). Saccharification yield reached a maximum of 74% at 20 wt% ethanol, 12% higher than the value for the ionoSolv process. The glucose yield was reduced to only 5% when the ethanol content increased to 80 wt%. Cellulose digestibility also suggested pretreating pine with ethanol–IL mixture could potentially lead to a maximum 7% increase (detailed in ESI Fig. S2†). For organosolv pretreatments (using a water–ethanol mixture 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 w/w, 1% sulfuric acid), a maximum glucose yield, 75%, was reported for pitch pine.75 However, this comparable glucose yield was reached at much higher temperature, 210 °C. Agnihotri et al. reported an aqueous ethanol softwood pretreatment for two longer operational durations, 90 min.76 They reported that at 170 °C, the glucose yield was merely 10%, which is up to 64% lower than our hybrid processes. All of this suggests that hybrid processes can achieve reasonable glucose yields for recalcitrant feedstocks at milder pretreatment conditions than those needed for Organosolv processes.
50 w/w, 1% sulfuric acid), a maximum glucose yield, 75%, was reported for pitch pine.75 However, this comparable glucose yield was reached at much higher temperature, 210 °C. Agnihotri et al. reported an aqueous ethanol softwood pretreatment for two longer operational durations, 90 min.76 They reported that at 170 °C, the glucose yield was merely 10%, which is up to 64% lower than our hybrid processes. All of this suggests that hybrid processes can achieve reasonable glucose yields for recalcitrant feedstocks at milder pretreatment conditions than those needed for Organosolv processes.
80% glucan recovery was observed for the pine pulp treated with aqueous IL, i.e. 20% glucose was degraded during the ionoSolv pretreatment. This is much more severe than for Miscanthus (96% glucan recovery, shown in Fig. 3). For pine, the degree of glucose degradation was inversely correlated with the ethanol content of the pretreatment. Quantitative glucan recovery was reported for the process using 80 wt% ethanol. Both Pan et al. and Del Rio et al. aqueous ethanol processes produced a highly digestible pulp, but both suffered from significant glucose degradation.77,78 Delignification displayed the same trend as the saccharification yield. It peaked at 20 wt% ethanol, with a lignin removal of 80%, whereas the ionoSolv process only achieved 70% of lignin removal. With the facilitation of the mineral acid, the organosolv delignification of Loblolly pine was able to achieve 61%, with a glucose recovery of 79%.79 The degree of glucose degradation in aqueous ethanol pretreatment is similar to the hybrid process, but the lignin removal was less effective, i.e. Agnihotri et al. reported a lignin removal below 10% at 170 °C.76 Complete hemicellulose removal was achieved by ionoSolv pretreatment, while a maximum removal of 85% was achieved by the ethanol–IL process with 20 wt% ethanol. The oragnosolv processes reported a hemicellulose removal of 89%.77,78
For the ionoSolv process, the lignin yield exceeded delignification, indicating the presence of the undesired pseudo-lignin and a severe lignin degradation. It was not the case for ethanol–IL pretreatment, the lignin yields were lower than their delignification, suggesting the degree of lignin condensation could be potentially less severe than for the ionoSolv process. The value of the lignin yield peaked at 74% at 20 wt% ethanol and kept decreasing as the ethanol concentration increased.
As we have seen, an improved pretreatment effectiveness for ethanol–IL mixtures relative to the aqueous IL was found. A further evaluation of the ethanol–IL pretreatment was carried out to investigate the pretreatment performance relative to different biomass loadings. Three biomass-to-liquid loadings (10 wt%, 30 wt%, 50 wt%) were investigated (Fig. 4b). Pretreatments were conducted using an ethanol-[DMBA][HSO4] mixture with 40 wt% ethanol.
Unlike with Miscanthus (Fig. 3), the fractionation strength of the ethanol–IL mixture was significantly weakened for pine at high biomass loadings. This was evidenced by the large drop in the saccharification yield: which was 70% at 10% biomass loading, but it was halved when the loading increased by 3 times and dropped to 17% at 50 wt% solid loading. This massive drop in the glucose yield could be attributed to the decreasing delignification. The lignin removal was 70% at 10 wt% biomass loading but declined to 43% as the loading increased 5-fold. Comparatively, the ionoSolv pretreatment was able to achieve a much better fractionation at high loadings but a worse performance at low loadings in terms of enzymatic saccharification. Glucose releasing yields of 40% and 55% were recorded for ionoSolv process using 50 wt% and 10 wt% solids loading.31
In summary, using ethanol–IL mixtures instead of an aqueous IL to pretreat pine with a low biomass loading could effectively reduce glucose degradation and enhance lignin extraction, consequently improving the overall pretreament effectiveness. However, due to the recalcitrant nature of the feedstock, the ethanol–IL process is less effective at high loadings, and more severe pretreatment conditions may be required.
Lignin characterisation
For both ionoSolv and ionoSolv-organosolv pretreatments, lignin removal is a key indicator of pretreatment effectiveness. Typically, lignin is chemically modified while being extracted from biomass. The degree of this modification and the nature of the pretreatment medium have a critical influence on the lignin's potential use for high value-added applications such as lignin-based carbon fibers and aromatic platform chemicals. A detailed characterisation of the extracted lignin is required to understand how the lignin was chemically modified by different pretreating solvent mixtures. 1H–13C heteronuclear quantum coherence (HSQC) NMR spectroscopy was applied to discover changes in the lignin's key functionalities or major subunits (listed in Fig. 5); Gel Permeation Chromatography (GPC) was also conducted to investigate lignin's molecular weight changes with different pretreatment conditions.Grassy biomass
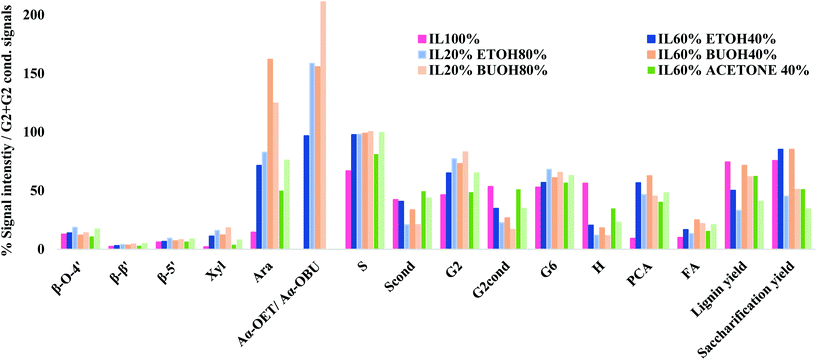
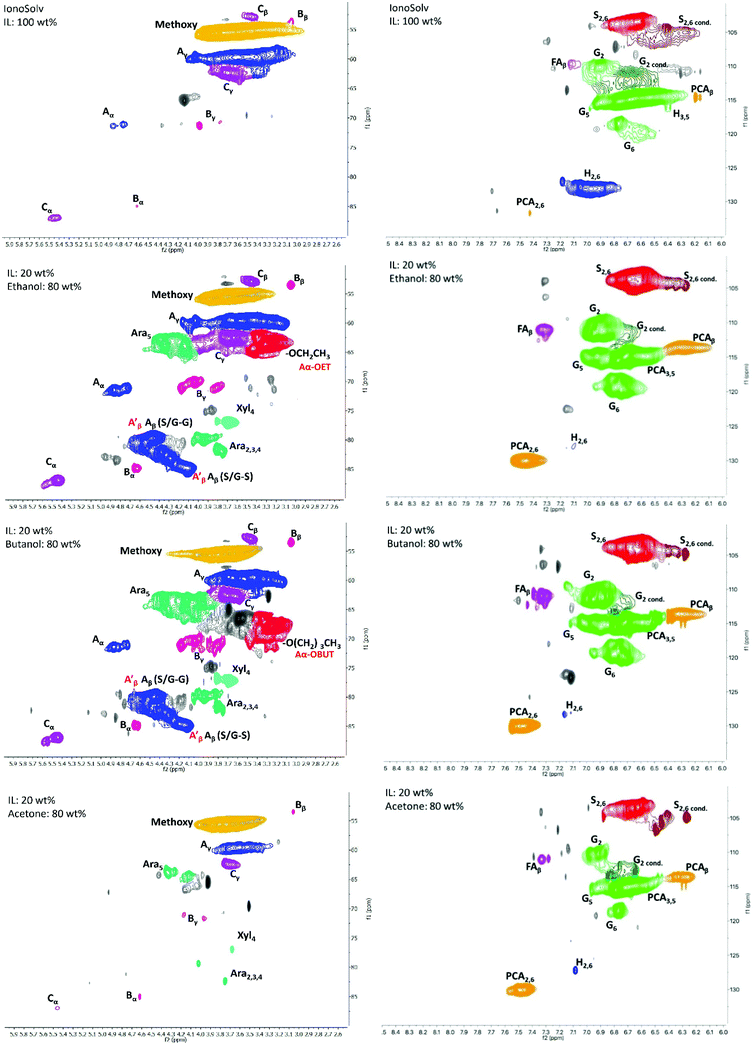
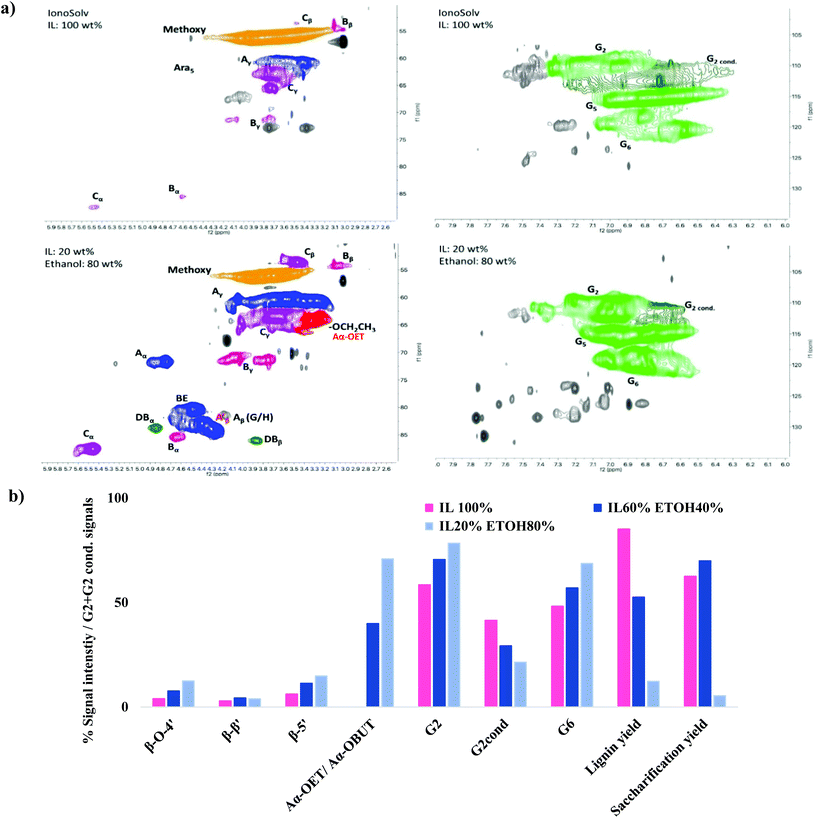
HSQC NMR analysis characterised the ionoSolv lignin and 6 organic–IL lignins (ethanol 40 wt% and 60 wt%, butanol 40 wt% and 60 wt%, acetone 40 wt% and 60 wt%). According to Fig. 6, the most common subunits appearing in the side chain region are β-O-4 ether (A), β–β resinol (B), β-5 phenylcoumaran (C), and α-alkoxy ether (A′). Lignin-carbohydrate linkages are also detected, with arabinose (Ara) and xylose (Xyl) in evidence. The major subunits recorded in the aromatic region are uncondensed and condensed guaiacyl (G2, G5, G6, G2cond.), uncondensed and condensed syringyl (S2,6, S2,6 cond.), p-coumaric acid (PCA) and p-hydroxyphenyl (H). Each of these subunits for both regions are highlighted in Fig. 7. A semi-quantitative analysis was conducted to quantify the abundance of these major subunits via peak volume integration. All peak signals intensities were presented in Fig. 6 as percentages to the sum of G2 and G2cond integrals, which were believed to remain constant for most of the lignin recovered from pretreatments regardless of the conditions.48 The degree of condensation could be quantified by the signal intensity of the integrals of G2cond relative to G2 and G2cond.46For ionoSolv lignin and organic–IL lignins, the most important findings in the side chain region are: (1) the rise of the α-butoxylated/ethoxylated β-O-4 ether signals; and (2) the increased signal intensities for carbohydrates which originate from the hemicellulose fraction. Lancefield et al. have demonstrated that butanol and ethanol are involved in lignin chemical modification during organosolv via α-butoxylation/ethoxylation.60 In Dong et al.'s work about diol oragnosolv process, they suggested that various diols e.g. 1,4 butanediol, could graft lignin α-hydroxyl group in similar fashion as aliphatic primary alcohols, which subsequently changed the lignin solubilty.80 The presence of α-alkoxylation is believed to largely inhibit the lignin condensation at the α-carbon position and to improve the lignin solubility during pretreatment, leading to better delignification. The appearance of α-butoxylated/ethoxylated β-O-4 ether linkages confirms that the butanol/ethanol functions in the same fashion in the organic–IL process as in the organosolv process. The intensities of these α-alkoxylated ether linkages increased proportionally to the organic content of the pretreatment solvent mixtures. As acetone does not directly interact with any lignin subunits but does form acetonide with selective cis-vicinal hydroxyl group in the carbohydrate subunits, α-alkoxylated ether linkages were not detected for all lignin recovered from the acetone–IL process.61 Unlike ionoSolv lignin, carbohydrate subunits like arabinose and xylose were detected for lignins recovered from organic–IL pretreatments. This is particularly relevant for the arabinose signal, as its intensity increased dramatically with increasing organic content in the pretreatment mixture: up to 162% relative to G2 and G2cond integrals for organic–IL lignins compared to 15% for ionoSolv lignin. As the nature of lignin-carbohydrate linkage in Miscanthus is the bridge between ferulic acid/p-coumarate acid and arabinosyl unit on xylan backbone, more intense sugar peaks (relative to ionoSolv lignin) suggest the lignin-carbohydrate linkages were only partially cleaved and lignin was extracted with a trace of hemicellulose during pretreatment. Highly similar lignin-carbohydrate linkages were observed in an aqueous ethanol process, in which the major carbohydrate units, arabinofuranose, was connected to the p-coumarate units of lignin.61 The signals for β-O-4 ethers were under 20% for both ionoSolv and organic–IL lignins, compared with 40% β-O-4 ether abundance for native lignin, indicating β-O-4 linkages were the most readily cleaved during the lignin fractionation regardless of the pretreatment conditions.29 Increasing the organic content in the pretreatment solvent resulted in a slight increase in the signal intensities of β–β and β-5 linkages. This means that fewer resinol and phenylcoumaran units were chemically modified by the organic–IL mixture comparing to the case of ionoSolv pretreatment, as these units are fairly chemical stable and unlikely to be cleaved during this mild pretreatment.29
The major differences in the aromatic region between ionoSolv lignin and organic–IL lignins are (1) the degree of lignin condensation and (2) the degree of PCA conversion to H units. Less intense signals for the G2cond integral were observed in Fig. 7 for organic–IL lignins, indicating that organic–IL lignins are less condensed. This is further81 confirmed by the degree of condensation of lignin (detailed in the ESI†). For ionoSolv lignin, 53% of the signal intensity of the G2 and G2cond integrals belonged to G2cond, signifying that half of the G2 units were involved in the condensation. On the other hand, this signal intensity was reduced to 22% and even 16%, when lignins were extracted by an 80 wt% ethanol or butanol–IL mixture. The S/G ratio of the lignins was not directly correlated with the degree of condensation of lignin (detailed in ESI†). The ionoSolv lignin had an S/G ratio of 0.55, the values for the ionoSolv-organosolv lignins ranged from 0.65 to 0.72, while those for organosolv lignins extracted by aqueous ethanol or acetone ranged from 0.54 to 0.61.61 Several ionoSolv process studies have demonstrated that the PCA units in the lignin tend to convert into hydroxyphenyl groups in the presence of aqueous IL.29,46,48 This is reflected by a large signal intensity decrease of the PCA units and the increase of the signal for H units.
During the organic–IL pretreatments, instead of polymerizing into H units (like in ionoSolv pretreatments), the PCA units that link the hemicellulose residue (arabinose) to the lignin polymers remained significantly unchanged during the fractionation. Therefore, the PCA integrals were found to be more intense for organic–IL lignins, relative to ionoSolv lignin.
The average molecular weight of all Miscanthus lignins and their molecular weight distributions were measured by GPC and are presented in the ESI Section 3.4.† For lignin recovered from the ethanol–IL and butanol–IL mixtures, the number average molar mass (Mn) remained constant regardless of the organic content, while the weight average molar mass (Mw) increased steadily from 0 wt% to 60 wt% ethanol/butanol, 4047 Da to 4525 Da for ethanol, 3114 Da to 3527 Da for butanol, and then increased more dramatically, reaching 7691 Da and 6971 Da at 80 wt% for ethanol and butanol, respectively. Mw for ionoSolv lignins was below 4000 Da. The large rise in the molecular weight after 60 wt% of organics could be attributed to a small growing fraction of α-alkoxylated lignin oligomers, which have higher molecular weight than the native lignin units. Assuming molecular weights of ∼200 Da for each lignin unit, α-ethoxylation/butoxylation could lead to a 14% and 28% increase in the molecular weight of the lignin units. When the organic content is fairly high during pretreatment (>60 wt%), more α-ethoxylation/butoxylation is taking place, generating a significant amount of α-alkoxylated lignin oligomers which are then precipitated. No clear correlation was observed between IL acidities and lignin molecular weight. Increasing the biomass loading did not affect the recovered lignin's molecular weight or its weight distribution.
Softwood
Lignin recovered from three pine pretreatments using different ethanol–[DMBA][HSO4] mixtures (ethanol 0 wt%, 40 wt%, 80 wt%) were subjected to HSQC NMR and GPC analysis (presented in ESI Section 3.3†) to get a better understanding of the chemical modification that happened to the lignin during the ionoSolv-organosolv fractionation process. All the major lignin subunits including dibenzodioxocin (DB), presented in Fig. 5, are highlighted in the spectra shown in Fig. 8a and a semi-quantitative analysis is presented in Fig. 8b, providing more insights for the changes in the abundance of these major linkages in pine lignin.According to the semi-quantitative integral analysis for pine lignin, more β-O-4 ether linkages were removed during the ionoSolv process, compared to the ionoSolv-organosolv process. The degree of chemical modification to resinol (β–β) units were the same for both processes, while the degree of lignin modification taking place at phenylcoumaran (β-5) units decreased when the ethanol content of the ionoSolv-organosolv pretreatment increased. As in the case of Miscanthus lignin, α-ethoxylation was the major chemical reaction happening during the lignin fractionation when ethanol was used as a co-solvent in ionoSolv pretreatment for pine. The signal intensity for the α-ethoxylated β-O-4 linkages rose proportionally with increasing ethanol content in the pretreating solvent. The enhanced α-ethoxylation hindered the condensation to certain extent. The degree of condensation was reduced from 41% (ionoSolv) to 21% (ethanol 80 wt%). In softwood, it is reported that mannose units in hemicellulose bond to lignin side chains via α-ether linkages.81 Here, no carbohydrate peaks were found, suggesting the lignin–hemicellulose bonding was broken during fractionation and recovered lignin is sugar-free.
High-level technoeconomic analysis of the ionoSolv-organosolv process
Brandt et al. have highlighted that the IL regeneration step can potentially be the most energy intensive step in the ionoSolov process.11,82 The energy requirements for drying the IL have been modelled as a flash distillation in HYSYS V8.8 with the following assumptions: (1) the diluted solution contains 3 equivalents of water per equivalent of IL on a mass basis as per the laboratory scale protocol and (2) the solution is dried to 20 wt% water for the ionoSolv case (0 wt% of organic solvent) and to 2 wt% for the other cases. Further details are provided in the ESI.† The energy requirements to regenerate the IL (water removal only), normalized to the energy content (HHV basis) in the ethanol produced with a theoretical yield of 100% glucose conversion to ethanol, are shown in Fig. 9. It can be seen that the energy requirements using organic solvent contents below 30–40 wt% are higher than the IonoSolv process due to the extreme drying required. However, beyond this point, the addition of organic solvents shows a clear reduction of the energy required for the IL thermal regeneration. It is important to note that detailed equilibrium data for the IL–water-organics systems are not available, and that the process have not been optimized in terms of water needed to precipitate the lignin, the regeneration technology nor heat integration. Therefore, the calculated values need to be considered only as a trend. Ethanol shows the highest energy consumption out of the three organic solvents, at high organic–solvent content. However, the savings in energy from the use of butanol or acetone will likely be offset by an increase in the operating cost (OPEX) due to the logistics of importing these substances into the biorefinery, given that the facility is already producing ethanol. Furthermore, if there is excess heat available, there will not be any benefits of using other solvents unless they are already being produced or used in the industrial facility, as there would not be a need to reduce the energy consumption.44,83The energy that could be obtained from lignin if used as a fuel was calculated assuming 100% efficiency in the boiler (24.6 ± 0.9 MJ kg−1, average HHV value for ionoSolv Miscanthus lignins).29 This was normalized to the energy required to regenerate the IL under the simulation assumptions and shown as triangles in Fig. 9. As expected, as the addition of organic solvents reduces the overall IL-regeneration energy, the process becomes more energy efficient at high organic content. However, the simulated process scheme is not fully energetically autonomous at any organic solvent content, which shows the importance of process optimization when scaling-up lab protocols. Once again, these values should be seen as a trend. Interestingly, the two alcohols show similar trends between 20–60 wt% content, and in all cases more energy could be recovered from the lignin obtained with these solvents than acetone. At 80 wt% organic content, the ethanol shows a clear advantage over the other solvents.
The impact on CAPEX of the different cases cannot be determined without a detailed techno-economic analysis. The reactor and solid handling facilities are expected to remain unchanged as the solid loading, temperatures and pulp yields are very similar. If flash distillation is used to dry the IL, it is expected that the heat exchanger cost will follow the trend of the energy consumption. Therefore, this part of the process should be less expensive at high organic content. However, the organic solvent–water needs to be separated, probably though azeotropic distillation, to recycle the organic solvent. This will add complexity and will increase both the CAPEX and the OPEX. However, if the plant is already producing ethanol, the ethanol recovery unit could be used to separate this mixture. Another point to be considered for OPEX calculation when comparing the ionoSolv process with the ionoSolv-organosolv process is organic solvent losses due to their relative high volatilities. If the aim of the biorefinary is to produce ethanol, a detailed techno-economic analysis taking into account all of the above is required to determine the minimum ethanol selling price (MESP).
It is important to emphasize that the above analysis is based on a flash distillation system for the removal of water from the IL. The boiling point temperature and the energy of vaporization of the different organic solvents used in this work are given in the ESI.† The differences in these properties may lead to different conclusions if other process schemes, heat integration or order drying technologies are used to regenerate the IL. For this reason, a detail techno-economic analysis will be performed and presented in a future dedicated paper. The water tolerance. i.e. the amount of water that can be left in the IL–organic solvent recycle for the ionoSolv-organosolv process also needs to be investigated.
Experimental
Materials and methods
Miscanthus × giganteus was harvested from Silwood Park campus Imperial College London, UK. Softwood chips, Pinus sylvestris, originated from Bedfordshire, UK, supplied by Bark UK Online. Both Feedstocks were air-dried, ground and sieved to 180–850 μm (20 + 80 US mesh scale) before used. All chemicals were purchased from Sigma Aldrich or VWR international and used as received, unless mentioned otherwise. In this work, the Karl-Fisher titrator and the analytical balance used were a V20 volumetric Titrator (Mettler-Toledo) and a Sartorius CPA 1003 S balance (±0.001 g). For ionic liquid synthesis, 1H NMR was recorded on a Bruker 400 MHz spectrometer. Chemical shifts are reported in ppm, with the solvent (DMSO) signal at 2.500 (1H spectrum). Electrospray mass spectrometry experiments were conducted by Dr Lisa Haigh (Imperial College London, Chemistry department) on a Micromass Premier spectrometer. Pretreatments were conducted in Ace Pressure Tubes (Miscanthus) and Hydrothermal Autoclave Reactors with Teflon Chamber (pine).Ionic liquid synthesis
1H NMR, 400 MHz, DMSO-d6, δH 8.98 (1 H, s, N–H+), 7.03 (1 H, br s, HSO4−), 3.09 (6 H, qd, J = 7.3, 4.9 Hz, N–CH2), 1.18 (9 H, t, J = 7.3 Hz, N–CH2CH3). MS (Magnet FB+) m/z: 102.13 ([TEA]+, 100%), (Magnet FB−) m/z: 96.96 ([HSO4]−, 100%).
1H NMR, 400 MHz, DMSO-d6, δH 9.44 (2 H, br, s, N–H+, HSO4−), 3.04–2.99 (2 H, m, N–CH2), 2.76 (6 H, s, N–(CH3)2), 1.64–1.50 (2 H, m, N–CH2CH2), 1.30 (2 H, h, J = 7.4 Hz, N–CH2CH2CH2), 0.90 (3 H, t, J = 7.4 Hz, N–CH2CH2CH2CH3). MS (Magnet FB+) m/z: 102.13 ([DMBA]+, 100%), (Magnet FB−) m/z: 96.96 ([HSO4]−, 100%).
Lignocellulosic biomass fractionation
Pretreatments, determination of ionic liquid water contents, and biomass oven dried weight (ODW) were carried out by following our laboratory standard operating procedure,84 in triplicate. All Miscanthus pretreatments were performed at 120 °C in Ace Pressure Tubes, as the operational pressure was within the working range of the tubes. For pine pretreatments the operational temperature was 170 °C and Hydrothermal Autoclave Reactors were used, as the internal pressure generated during pretreatment was above the rating of the Ace Pressure Tubes. For control (ionoSolv) pretreatments, a biomass to stock solution A/B of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g g−1 was applied on ODW basis. For Miscanthus organic solvent concentration experiments, the biomass to solvent ratio was 1
10 g g−1 was applied on ODW basis. For Miscanthus organic solvent concentration experiments, the biomass to solvent ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g g−1 and organic solvent to [TEA][HSO4] ratios were 1
10 g g−1 and organic solvent to [TEA][HSO4] ratios were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 8
9 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g g−1 for ethanol, and 2
2 g g−1 for ethanol, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to 8
8 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g g−1 for butanol and acetone. A detailed description of the solvent compositions can be found in the ESI.† For pine organic solvent concentration experiments, ethanol to [DMBA][HSO4] ratios were 2
2 g g−1 for butanol and acetone. A detailed description of the solvent compositions can be found in the ESI.† For pine organic solvent concentration experiments, ethanol to [DMBA][HSO4] ratios were 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 4
8, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 8
6 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g g−1. For biomass loading experiments, 5 biomass to solvent ratios, ranging from 1
2 g g−1. For biomass loading experiments, 5 biomass to solvent ratios, ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 5
10 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g g−1 were used for Miscanthus; and 3 biomass to solvent ratios, 1
10 g g−1 were used for Miscanthus; and 3 biomass to solvent ratios, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 3
10, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 5
10, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g g−1 were tested on pine. The ethanol to IL ratio was 4
10 g g−1 were tested on pine. The ethanol to IL ratio was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 g g−1 for all feedstocks.
6 g g−1 for all feedstocks.
For pretreatments without air-drying of the treated biomass (wet pulp), the procedure was kept unchanged before the Soxhlet extraction. After the extraction step, the pulps were transferred from the Soxhlet to 50 mL centrifuge tubes. Each sample was washed with 50 mL DI water and left for one hour. The water-pulp suspensions were centrifuged for 30 min at 2000 rpm and the supernatant decanted. The washing step was repeated once. All washed pulps were store at 4 °C. Their moisture content measurements and saccharification analysis were conducted within the next 3 days.
Pulp characterisation
 | (1) |
 | (2) |
Isolated lignin characterisation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were used for instrument calibration. The column set was eluted with a mixture of GPC grade DMSO and LiBr (1 g L−1) with a flow rate of 0.4 mL min−1 at 60 °C. Each Sample (20 mg) was dissolved in 1 mL eluent mixture overnight and filtered.
000) were used for instrument calibration. The column set was eluted with a mixture of GPC grade DMSO and LiBr (1 g L−1) with a flow rate of 0.4 mL min−1 at 60 °C. Each Sample (20 mg) was dissolved in 1 mL eluent mixture overnight and filtered.
Conclusions
In this study, a new hybrid pretreatment process was developed by incorporating an organic co-solvent into the ionoSolv process in place of water. The newly developed process using a mixture of organic solvent (ethanol, butanol or acetone) and [TEA][HSO4] was tested on Miscanthus and its fractionation effectiveness and the composition of the pulps generated were compared with the ionoSolv process. Pretreatments using 40 wt% ethanol or butanol with 60 wt% IL had a glucose yields of 85%, 10% higher than that of ionoSolv process. This could be explained by the improved delignification of the biomass during fractionation, confirmed by compositional analysis of the pulps. Incorporating acetone with IL to pretreat biomass did not change the overall process effectiveness, as the glucose releasing yield and delignification remained at the same level as the ionoSolv process.This pretreatment also demonstrated that it could keep a decent fractionation effectiveness even up to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g g−1 biomass loading. The glucose yield for 50 wt% biomass loading was 71%, only 10% lower than at a 10 wt% biomass loading.
10 g g−1 biomass loading. The glucose yield for 50 wt% biomass loading was 71%, only 10% lower than at a 10 wt% biomass loading.
An ionoSolv-organosolv pretreatment using an ethanol–[DMBA][HSO4] mixture was conducted for pine, and a 12% increase in glucose releasing yield and a 10% increase in lignin removal was observed, indicating that this hybrid process also has a successful performance towards more recalcitrant feedstocks compared to ionoSolv pretreatment.
Recovered lignin from the organic–IL fractionation for both Miscanthus and pine were subjected to the HSQC NMR and GPC analysis for more detailed characterisation. According to HSQC NMR analysis, organic alcohols, like ethanol, butanol, induced α-alkoxylation during lignin fractionation and turned β-O-4 ether units into α-alkoxylated ether units. This modification not only tunes lignin solubility for a more efficient delignification, but also hinders lignin condensation, which can potentially improve the economic value of the lignin fraction as a side-product of the pretreatment.
This ionoSolv-organosolv pretreatment demonstrates that a biomass fractionation process can simultaneously generate a highly enzyme accessible cellulose fraction and high-quality lignin with excellent potential for high value-added uses.
Preliminary technoeconomic analysis of the process showed that the energy consumption of the IL regeneration step can be reduced, minimizing the operating costs and the environmental footprint of the hybrid process when operated at a high organic solvent content. Additionally, it is believed that after optimization of the process scheme, the economic profitability can be maximized when compared to other pretreatment options. This could be a breakthrough for applying current level of the ionoSolv pretreatment technology in a commercial scale biorefinery.
Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
The authors acknowledge funding from the Engineering and Physical Sciences Research Council (EP/S000771/1) and the Department of Chemical Engineering at Imperial College London.Notes and references
- L. Santarelli, M. Saxe, C. Gross, A. Surget, S. Dulawa, N. Weisstaub, J. Lee, R. Duman, O. Arancio, F. Battaglia, C. Beizung and R. Hen, Science, 2014, 301, 805–809 CrossRef PubMed.
- J. P. H. Van Wyk, Trends Biotechnol., 2001, 19, 172–177 CrossRef CAS PubMed.
- V. B. Agbor, N. Cicek, R. Sparling, A. Berlin and D. B. Levin, Biotechnol. Adv., 2011, 29, 675–685 CrossRef CAS PubMed.
- B. D. Solomon, Ann. N. Y. Acad. Sci., 2010, 1185, 119–134 CrossRef PubMed.
- J. Goldemberg, Science, 2007, 315, 808–810 CrossRef CAS PubMed.
- J. Fargione, J. Hill, D. Tilman, S. Polasky and P. Hawthorne, Science, 2008, 319, 1–3 CrossRef PubMed.
- C. Vezzoli, F. Ceschin, L. Osanjo, M. K. M'Rithaa, R. Moalosi, V. Nakazibwe and J. C. Diehl, Green Energy Technol, 2018, pp. 3–22 Search PubMed.
- S. Ulgiati, M. Giampietro and D. Pimentel, Bio. Sci., 1997, 47, 587–600 CrossRef.
- M. Giampietro and S. Ulgiati, CRC Crit. Rev. Plant Sci., 2005, 24, 365–384 CrossRef CAS.
- M. Giampietro, K. Mayumi and J. Ramos-Martin, Int. J. Environ. Res., 2006, 1, 51–87 Search PubMed.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- L. R. Lynd, P. J. Weimer, W. H. Van Zyl and I. S. Pretorius, Bioresour. Technol., 2002, 66, 506–577 CAS.
- J. L. Espinoza-Acosta, P. I. Torres-Chavez, E. Carvajal-Millan, B. Ramirez-Wong, L. A. Bello-Perez and B. Montano-Leyva, BioResources, 2014, 9, 3660–3687 CrossRef.
- I. D. C. Macedo, M. R. L. V. Leal and J. E. A. R. Da Silva, Assessment of greenhouse gas emissions in the production and use of fuel ethanol in Brazil, Secretariat of the Environment of the State of São Paulo, Gov. of State São Paulo, Brazil, 2004 Search PubMed.
- J. Hill, E. Nelson, D. Tilman, S. Polasky and D. Tiffany, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11206–11210 CrossRef CAS PubMed.
- A. E. Farrell, R. J. Plevin, B. T. Turner, A. D. Jones, M. O'hare and D. M. Kammen, Science, 2006, 311(5760), 506–509 CrossRef CAS PubMed.
- N. Mosier, C. Wyman, B. Dale, R. Elander, Y. Y. Lee, M. Holtzapple and M. Ladisch, Bioresour. Technol., 2005, 96, 673–686 CrossRef CAS PubMed.
- P. Binod, R. Sindhu, R. R. Singhania, S. Vikram, L. Devi, S. Nagalakshmi, N. Kurien, R. K. Sukumaran and A. Pandey, Bioresour. Technol., 2010, 101, 4767–4774 CrossRef CAS PubMed.
- C. E. Wyman, Biotechnol. Prog., 2003, 19(2), 254–262 CrossRef CAS.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- M. Norgren and H. Edlund, Curr. Opin. Colloid Interface Sci., 2014, 19, 409–416 CrossRef CAS.
- I. Norberg, Y. Nordström, R. Drougge, G. Gellerstedt and E. Sjöholm, J. Appl. Polym. Sci., 2013, 128, 3824–3830 CrossRef CAS.
- J. F. Kadla, S. Kubo, R. A. Venditti, R. D. Gilbert, A. L. Compere and W. Griffith, Carbon, 2002, 40, 2913–2920 CrossRef CAS.
- J. H. Lora, in Monomers, Polymers and Composites from Renewable Resources, Elsevier, Oxford, UK, 2008 Search PubMed.
- H. Chung and N. R. Washburn, Green Mater., 2013, 1, 137–160 CrossRef CAS.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843 CrossRef PubMed.
- J. D. P. Araújo, C. A. Grande and A. E. Rodrigues, Chem. Eng. Res. Des., 2010, 88, 1024–1032 CrossRef.
- R. El Hage, N. Brosse, L. Chrusciel, C. Sanchez, P. Sannigrahi and A. Ragauskas, Polym. Degrad. Stab., 2009, 94, 1632–1638 CrossRef CAS.
- F. J. V. Gschwend, F. Malaret, S. Shinde, A. Brandt-Talbot and J. P. Hallett, Green Chem., 2018, 20, 3486–3498 RSC.
- C. Vanderghem, Y. Brostaux, N. Jacquet, C. Blecker and M. Paquot, Ind. Crops Prod., 2012, 35, 280–286 CrossRef CAS.
- F. J. V. Gschwend, C. L. Chambon, M. Biedka, A. Brandt-Talbot, P. S. Fennell and J. P. Hallett, Green Chem., 2019, 21, 692–703 RSC.
- X. Zhang, M. Tu and M. G. Paice, Bioenergy Res., 2011, 4, 246–257 CrossRef.
- A. T. W. M. Hendriks and G. Zeeman, Bioresour. Technol., 2009, 100, 10–18 CrossRef CAS PubMed.
- J. Sumphanwanich, N. Leepipatpiboon, T. Srinorakutara and A. Akaracharanya, Ann. Microbiol., 2008, 58, 219–225 CrossRef CAS.
- J. D. McMillan, Enzym. Convers. Biomass Fuels Prod, 1994, vol. 566, pp. 292–324 Search PubMed.
- N. Sathitsuksanoh, K. M. Holtman, D. J. Yelle, T. Morgan, V. Stavila, J. Pelton, H. Blanch, B. A. Simmons and A. George, Green Chem., 2014, 16, 1236–1247 RSC.
- A. Brandt, J. P. Hallett, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2010, 12, 672–679 RSC.
- S. H. Lee, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2009, 102, 1368–1376 CrossRef CAS PubMed.
- A. George, A. Brandt, K. Tran, S. M. S. N. S. Zahari, D. Klein-Marcuschamer, N. Sun, N. Sathitsuksanoh, J. Shi, V. Stavila, R. Parthasarathi, S. Singh, B. M. Holmes, T. Welton, B. A. Simmons and J. P. Hallett, Green Chem., 2015, 17, 1728–1734 RSC.
- D. Klein-Marcuschamer, B. A. Simmons and H. W. Blanch, Biofuels, Bioprod. Biorefin., 2011, 5, 562–569 CrossRef CAS.
- M. T. Clough, K. Geyer, P. A. Hunt, J. Mertes and T. Welton, Phys. Chem. Chem. Phys., 2013, 15, 20480–20495 RSC.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Green Chem., 2014, 16, 3098–3106 RSC.
- H. Baaqel, I. Díaz, V. Tulus, B. Chachuat, G. Guillén-gosálbez and J. P. Hallett, Green Chem., 2020, 22, 3132–3140 RSC.
- F. Malaret, F. J. V. Gschwend, J. M. Lopes, W.-C. Tu and J. P. Hallett, RSC Adv., 2020, 10, 16050–16060 RSC.
- C. L. Chambon, T. Y. Mkhize, P. Reddy, A. Brandt-Talbot, N. Deenadayalu, P. S. Fennell and J. P. Hallett, Biotechnol. Biofuels, 2018, 11, 247 CrossRef CAS PubMed.
- C. L. Chambon, M. Chen, P. S. Fennell and J. P. Hallett, Front. Chem., 2019, 7, 246 CrossRef CAS PubMed.
- L. Weigand, S. Mostame, A. Brandt-Talbot, T. Welton and J. P. Hallett, Faraday Discuss., 2017, 202, 331–349 RSC.
- A. Brandt-Talbot, F. J. V. Gschwend, P. S. Fennell, T. M. Lammens, B. Tan, J. Weale and J. P. Hallett, Green Chem., 2017, 19, 3078–3102 RSC.
- Y. Ni and Q. Hu, J. Appl. Polym. Sci., 1995, 57, 1441–1446 CrossRef CAS.
- X. Zhao, K. Cheng and D. Liu, Appl. Microbiol. Biotechnol., 2009, 82, 815–827 CrossRef CAS PubMed.
- X. Pan, C. Arato, N. Gilkes, D. Gregg, W. Mabee, K. Pye, Z. Xiao, X. Zhang and J. Saddler, Biotechnol. Bioeng., 2005, 90, 473–481 CrossRef CAS PubMed.
- S. Başakçılardan Kabakcı and M. H. Tanış, Biomass Convers. Biorefin. DOI:10.1007/s13399-020-00677-2.
- X. Meng, S. Bhagia, Y. Wang, Y. Zhou, Y. Pu, J. R. Dunlap, L. Shuai, A. J. Ragauskas and C. G. Yoo, Ind. Crops Prod., 2020, 146, 112144 CrossRef CAS.
- W. J. J. Huijgen, J. H. Reith and H. Den Uil, Ind. Eng. Chem. Res., 2010, 10132–10140 CrossRef CAS.
- Y. Jafari, H. Amiri and K. Karimi, Appl. Energy, 2016, 168, 216–225 CrossRef CAS.
- D. K. Sidiras and I. S. Salapa, Thünen Inst. Wood Res. ECI Symp. Ser, october, 2015 Search PubMed.
- Q. Schmetz, H. Teramura, K. Morita, T. Oshima, A. Richel, C. Ogino and A. Kondo, ACS Sustainable Chem. Eng., 2019, 7, 11069–11079 CrossRef CAS.
- Z. Zhang, M. D. Harrison, D. W. Rackemann, W. O. S. Doherty and I. M. O'Hara, Green Chem., 2016, 18, 360–381 RSC.
- J. H. Lora, C. F. Wu, E. K. Pye and J. J. Balatinecz, Lignin Properties and Materials, 2009, pp. 312–323 Search PubMed.
- C. S. Lancefield, I. Panovic, P. J. Deuss, K. Barta and N. J. Westwood, Green Chem., 2017, 19, 202–214 RSC.
- S. Bauer, H. Sorek, V. D. Mitchell, A. B. Ibáñez and D. E. Wemmer, J. Agric. Food Chem., 2012, 60, 8203–8212 CrossRef CAS PubMed.
- K. Niu, P. Chen, X. Zhang and W. S. Tan, J. Chem. Technol. Biotechnol., 2009, 84, 1240–1245 CrossRef CAS.
- Z. Shengdong, W. Yuanxin, Z. Yufeng, T. Shaoyong, X. Yongping, Y. Ziniu and Z. Xuan, Chem. Eng. Commun., 2006, 193, 639–648 CrossRef.
- J. Rughani and G. D. McGinnis, Biotechnol. Bioeng., 1989, 33, 681–686 CrossRef CAS.
- L. Wu, S. H. Lee and T. Endo, Bioresour. Technol., 2013, 140, 90–96 CrossRef CAS PubMed.
- S. Wang, W. Zhao, T. S. Lee, S. W. Singer, B. A. Simmons, S. Singh, Q. Yuan and G. Cheng, ACS Sustainable Chem. Eng., 2018, 6, 4354–4361 CrossRef CAS.
- N. L. Mai, S. H. Ha and Y. M. Koo, Process Biochem., 2014, 49, 1144–1151 CrossRef CAS.
- A. Asakawa, T. Oka, C. Sasaki, C. Asada and Y. Nakamura, Ind. Crops Prod., 2016, 86, 113–119 CrossRef CAS.
- N. Brosse, P. Sannigrahi and A. Ragauskas, Ind. Eng. Chem. Res., 2009, 48, 8328–8334 CrossRef CAS.
- J. Sameni, S. Krigstin and M. Sain, BioResources, 2017, 12, 1548–1565 CrossRef CAS.
- Y. Yagi, H. Inomata and S. Saito, Macromolecules, 1992, 25, 2997–2998 CrossRef CAS.
- B. Yang and C. E. Wyman, Biofuels, Bioprod. Biorefin., 2008, 2, 26–40 CrossRef CAS.
- C. L. Chambon, PhD thesis, Imperial College London, 2017.
- A. G. Cruz, C. Scullin, C. Mu, G. Cheng, V. Stavila, P. Varanasi, D. Xu, J. Mentel, Y. De Chuang, B. A. Simmons and S. Singh, Biotechnol. Biofuels, 2013, 6, 52 CrossRef CAS PubMed.
- N. Park, H. Y. Kim, B. W. Koo, H. Yeo and I. G. Choi, Bioresour. Technol., 2010, 101, 7046–7053 CrossRef CAS PubMed.
- S. Agnihotri, I. A. Johnsen, M. S. Bøe, K. Øyaas and S. Moe, Wood Sci. Technol., 2015, 49, 881–896 CrossRef CAS.
- L. F. Del Rio, R. P. Chandra and J. N. Saddler, Appl. Biochem. Biotechnol., 2010, 161, 1–21 CrossRef CAS.
- X. Pan, D. Xie, R. W. Yu, D. Lam and J. N. Saddler, Ind. Eng. Chem. Res., 2007, 46, 2609–2617 CrossRef CAS.
- M. N. Borand and F. Karaosmanoğlu, J. Renewable Sustainable Energy, 2018, 10, 033104 CrossRef.
- C. Dong, X. Meng, C. S. Yeung, H. Y. Tse, A. J. Ragauskas and S. Y. Leu, Green Chem., 2019, 21, 2788–2800 RSC.
- H. Nishimura, A. Kamiya, T. Nagata, M. Katahira and T. Watanabe, Sci. Rep., 2018, 8, 1–11 CrossRef CAS PubMed.
- A. R. Abouelela, F. V. Gschwend, F. Malaret and J. P. Hallett, Commercial Aspects of Biomass Deconstruction with Ionic Liquids, Chapter 5 in Commercial Applications of Ionic Liquids, ed. M. B. Shiflett, Springer, 2020 Search PubMed.
- A. Firth, B. Zhang and A. Yang, Appl. Energy, 2019, 235, 1314–1334 CrossRef.
- F. J. V. Gschwend, A. Brandt, C. L. Chambon, W. C. Tu, L. Weigand and J. P. Hallett, JoVE, 2016, e54246 Search PubMed.
- A. Sluiter, B. Hames, D. Hyman, C. Payne, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and J. Wolfe, Determination of total solids in biomass and total dissolved solids in liquid process samples, Laboratory Analytical Procedure (LAP), 2008 Search PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Biomass Anal. Technol. Team Lab. Anal. Proced, 2004, vol. 2011, pp. 1–14 Search PubMed.
- M. Selig, N. Weiss and Y. Ji, Enzymatic Saccharification of Lignocellulosic Biomass, Laboratory Analytical Procedure (LAP), 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Pretreatment solvent compositions, detailed protocol of compositional analysis and enzymatic hydrolysis, proton and mass spectra of ionic liquids, HSQC NMR spectra of recovered lignin and details on the energy consumption for the technoeconomic analysis. See DOI: 10.1039/d0gc01143f |
| This journal is © The Royal Society of Chemistry 2020 |