Cleaving protected peptides from 2-chlorotrityl chloride resin. Moving away from dichloromethane†
Abstract
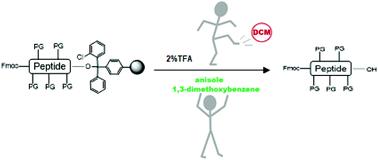
In recent years, the work of various research groups has allowed the substitution of the hazardous solvents most widely used in solid-phase peptide synthesis, namely DMF, NMP, DCM, DEE, among others, by several much less hazardous solvents. Indeed, greener alternatives have been found for almost all steps of the process, with the exception of the cleavage of protected peptides from 2-chlorotrityl chloride resin. Here, after careful screening of several of the so-called green solvents, we propose 2% TFA in either anisole or 1,3-dimethoxybenzene as optimal for the cleavage step. The higher boiling point of these solvents compared with the DCM allows the preparation of protected peptides with less risk of premature removal of the most labile protecting groups, such as the Trt of His. Our findings once again evidence the value/versatility of green solvents in strict chemical terms.


 Please wait while we load your content...
Please wait while we load your content...