Open Access Article
Open Access ArticleRecycling of bonded NdFeB permanent magnets using ionic liquids†
Mehmet Ali Recai
Önal
 a,
Sven
Dewilde
a,
Sven
Dewilde
 a,
Malik
Degri
a,
Malik
Degri
 b,
Lydia
Pickering
b,
Lydia
Pickering
 b,
Boris
Saje
b,
Boris
Saje
 c,
Sofía
Riaño
c,
Sofía
Riaño
 a,
Allan
Walton
a,
Allan
Walton
 b and
Koen
Binnemans
b and
Koen
Binnemans
 *a
*a
aKU Leuven, Department of Chemistry, Celestijnenlaan 200F, P. O. Box 2404, B-3001 Leuven, Belgium. E-mail: koen.binnemans@kuleuven.be
bMagnetic Materials Group, School of Metallurgy and Materials, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
cApplication Engineering, Kolektor Magnet Technology GmbH, Zur Halbinsel 6, 45356 Essen, Germany
First published on 15th April 2020
Abstract
NdFeB permanent magnets are essential for modern day technology thanks to their excellent magnetic properties. Recycling of critical metals from sintered NdFeB permanent magnets has attracted a lot of attention over the last decade, whereas the recycling of (polymer or resin) bonded NdFeB magnets has been largely neglected. In this paper, an overview of the polymer or resin binders used in commercial bonded magnets is presented and different routes for the recycling of these magnets are explored. Three main types of polymers were found in commercial bonded NdFeB magnets: polyamides (PA6 and PA12), poly-p-phenylene sulfide (PPS) and epoxy. Both types of polyamide resins were easily dissolved by ionic liquids with coordinating anions (chloride, acetate or dialkylphosphate). Removal of the PPS resin was not possible by ionic liquid solvents, but only by using 1-chloronaphthalene and 1,3,5-triphenylbenzene at high temperatures. Although epoxy could be removed by several ionic liquids, reaction between the NdFeB powder and the ionic liquids was observed. A batch of PA6-bonded magnets was treated with an ionic liquid tributylethylphosphonium diethylphosphate, [P4442][Et2PO4], to selectively remove the polymeric portion. The PA-free magnet powder was found to retain >90% of its original magnetic properties. Two epoxy-bonded magnets produced with this recycled magnet powder showed magnetic properties that were close to those of commercial counterparts, proving the versatility of the process and that the materials loop could be successfully closed.
Introduction
Neodymium–iron–boron (NdFeB) permanent magnets are the most important rare-earth permanent magnets with highest energy density. Due to their versatility, they are widely used in wind turbines, hybrid and electric vehicles (HEVs and EVs), household electrical appliances, computer hard disk drives (HDDs), and many small consumer electronic devices.1 Sintered NdFeB magnets are typically composed of about 31–32 wt% rare earth elements (REEs), 1 wt% boron and the rest is mainly iron and other minor additives such as cobalt, gallium, copper, etc. While the neodymium content is typically more than 15 wt%, other REEs such as praseodymium, dysprosium, gadolinium and terbium can be used but comparatively in much lower quantities.1,2 The main phase is Nd2Fe14B.There are two families of permanent NdFeB magnets on the market: sintered and (polymer or resin) bonded magnets. Both magnet families are (partly) built up by magnet alloy powders that can exhibit flow characteristics when very fine. This enables their molding and forging into permanent magnets with desired shapes. In the case of sintered magnets, the magnet powder is pressed into a shape and punched out with the help of lubricants. The obtained product remains very brittle but, by heating in a vacuum furnace, its density and mechanical strength are greatly improved.3,4 A disadvantage of sintered magnets is that expensive machining is required to shape the materials.5 This leads to a significant yield loss particularly when complex shapes are required.
Bonded NdFeB magnets are typically obtained by mixing NdFeB magnet powder with a polymeric binder at an appropriate mass ratio in a mixer or extruder and subjecting the pellet-shaped extrudate to injection or compression molding.6,7 The amount of polymeric material used can vary from 2.5 to 12 wt%.8–10 The main types of polymeric binders that are currently found on the market are the polyamides nylon 6 (PA), nylon 11 (PA11) and nylon 12 (PA12), poly-p-phenylene sulfide (PPS) and epoxy resins. Because of the great difference in their densities, this means that up to 70 vol% of the final magnet can be polymer with only 30 vol% of magnet powder. The magnet powder used in bonded magnets can be produced in two ways that determines its isotropic behavior. If the material is supplied from melt spinning, the powder will be isotropic and nanocrystalline. If the powder is produced via hydrogenation–disproportionation–desorption–recombination (HDDR) process then it will be anisotropic with “easy” and “hard” directions for magnetization.11
Imbedding the NdFeB magnet powder within a polymer matrix facilitates the manufacturing of complex shaped magnets and processing conditions in general. Furthermore, the use of polymers improves electrical and mechanical characteristics and improves the corrosion resistance of these permanent magnets.12,13 However, the overall magnetic strength per volume unit of bonded NdFeB magnet is significantly lower than that of their sintered counterparts since the magnet powder is diluted by a non-magnetic material. Nevertheless, bonded NdFeB magnets are popular because they can be made in complex shapes and at relatively lower costs without extensive loss of material or magnetic performance.7
During the last decade, a lot of attention has been devoted to the recycling of sintered NdFeB magnets and these efforts have been reviewed elsewhere.1,2,14 In general, these routes include pyrometallurgical,15,16 hydrometallurgical17–20 and solvometallurgical21 approaches, or a combination of them.22–24 In contrast, very limited attention has been given to the recycling of bonded NdFeB magnets. This lack of attention can be explained by the much lower rare-earth content of the bonded magnets compared to that of sintered magnets, as well as by the fact that the presence of the polymeric binders or resins makes direct recycling routes (such as hydrogen decrepitation) difficult.25,26 Recycling of resin-bonded magnets requires a twofold approach: (i) successful separation of polymer from the metallic fraction and (ii) preserving the magnet powder by preventing its undesired interaction with the polymer (e.g. carbon diffusion) or, if any, solvent(s) for separation (e.g. dissolution/corrosion). Most approaches are based on the decomposition of the polymeric binders by organic solvents, often at high temperatures (solvolysis).27,28 CO2-expanded water could provide a cleaner approach to solvolysis of epoxy resins.29 In none of these studies, production of a new magnet from the recycled magnet powder was studied. If during the processing the recycled powder becomes enriched in carbon or oxygen due to organic material or corrosion, this can drastically deteriorate the magnetic properties of the powder.
Alternatively, if the epoxy is cured with the formation of ester bonds, then saponification with the help of a strong base can also be performed.30 This way, the resin is degraded and potentially recycled. In a recent work, a combined hydro- and solvometallurgical flow sheet was developed to recover REEs from epoxy-bonded NdFeB magnets.31 Here, a high concentration NaOH solution was used to crack the epoxy resin but the faith of the polymer was not considered thoroughly. In another study, nitric acid was applied for recycling of epoxy resin as well.32 However, after these treatments, the magnet powder either corrodes during the removal of the polymer or disintegrates to its constituents by dissolution thereby eliminating a more direct and greener recycling route.
Ionic liquids (ILs) are known to be excellent green solvents for many types of synthetic polymers and biopolymers.33–41 It has also been reported that Lewis-acidic chloroaluminate ionic liquids can be used to dissolve epoxy resins of tantalum capacitors. For these reasons, we decided to explore the use of ionic liquids for the recycling of bonded NdFeB magnets for the first time. This study is also the first one to collect different types of bonded magnets from the global magnet market to simulate a real life-like situation. The dissolution behavior of different types of polymeric binders in ionic liquids was then tested on a selection of bonded magnets. The most promising solvent system was tested on larger scale to prepare a batch of magnet powder that was used to produce new anisotropic epoxy bonded magnets. The magnetic properties of these new magnets were measured and compared to those of commercial counterparts to assess the overall efficiency of the recycling process.
Important to note that the primary goal of this study was to liberate the magnet powder from its organic surrounding using ionic liquids while maintaining the magnetic properties of the recycled powder to produce new magnets. For that reason, quantitative dissolution behavior of organic material or its recyclability were not considered or studied in detail. However, recycling of polymers from resin-bonded NdFeB magnets can clearly be considered as the primary objective of a future study.
Materials and methods
Chemicals
Trihexyl(tetradecyl)phosphonium chloride (Cyphos IL 101, [C101][Cl], 97.7%) was obtained from Cytec Industries (Ontario, Canada). Tricaprylmethylammonium chloride (Aliquat 336, [A336][Cl], 88.2–90.6%), N-methylpyrrolidone extra dry (99.5%), 1,3,5-triphenylbenzene (97%), nitric acid (≥65%, p.a.) and dichloromethane (99.8%) were purchased from Sigma-Aldrich (Diegem, Belgium). Tetracosane (99%), acetone (≥99%) and hydrochloric acid (37%) were obtained from Fisher Chemical (Loughborough, United Kingdom). Hydrogen peroxide (35 wt%) solution was obtained from Chem-Lab Analytical (Zedelgem, Belgium). Diphenyl sulfone (97%), 1-chloronaphthalene (ca. 90%) and 1,4-diphenyl benzene (99+%) were purchased from Acros Organics (Geel, Belgium). 1-Methyl-3-octylimidazolium chloride ([C8MIM][Cl], 98%), 1-butylpyridinium chloride ([C4Pyr][Cl], 99%), 1-butyl-3-methylimidazolium trifluoroacetate, ([C4MIM][CF3COO], >97%), 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C4MIM][NTf2], 99%), 1-ethyl-3-methylimidazolium acetate ([C2MIM][CH3COO], 95%), 1-ethyl-3-methylimidazolium chloride ([C2MIM][Cl], >98%) and tributylethylphosphonium diethylphosphate ([P4442][Et2PO4], >95%) were purchased from Iolitec Ionic Liquids Technologies GmbH (Heilbronn, Germany). Standard solutions of individual elements (1000 mg L−1) for inductively coupled plasma optical emission spectrometer (ICP-OES) were obtained from Merck (Overijse, Belgium). Pure water (MilliQ, Millipore, ≥18 MΩ cm−1) was employed to make all the dilutions. All chemicals were used as received without any further purification.Materials characterization
For the experimental work, 25 different samples of isotropic or anisotropic bonded NdFeB magnets were received or purchased from different suppliers worldwide. The names of the providers and the magnet grades cannot be disclosed for confidentiality reasons. The samples were used as received if they were already demagnetized; otherwise, demagnetization was carried out by heating the samples to 320 °C in an oven.Fully quantitative chemical analysis of the NdFeB magnets was performed using a dual view PerkinElmer Optima 8300 inductively coupled plasma optical emission spectrometer (ICP-OES) equipped with a GemTip CrossFlow II nebulizer, a Scott Spray Chamber Assembly, a sapphire injector and a Hybrid XLT ceramic torch. 200 mg of the PA12 and epoxy samples were digested in concentrated HNO3 at 60 °C while stirring for 4 h. For the poly-p-phenylene sulfide (PPS) magnets, 200 mg of sample was digested in concentrated HNO3 at 80 °C while stirring for 24 h. The obtained solutions were then cooled, filtered and further diluted with 1 M HNO3 for analysis with ICP-OES. All measurements were performed in duplicate. Fourier transform infrared spectroscopy (FTIR) measurements were performed on a Bruker Vertex 70 spectrometer with the attenuated total reflectance module (platinum ATR) equipped with diamond crystal. The FTIR data were processed using OPUS 7.5 software. An infrared spectrum was recorded from every bonded magnet sample that was received. Since only a low amount of polymeric material is used to bind the magnet powder (2.5 wt% for compression and 5 to 12 wt% for injection molded magnets) compared to the total mass of the sample, the IR signals had a very low intensity. The measurements were correlated with known polymers using the ATR-FTIR Polymer Library, Bruker ATR polymer library and Bruker polymer flame retardants library (in total over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 spectra). The best way to correlate the data was via a minima–maxima comparison since the obtained spectra from the bonded magnets were much lower in intensity than those of the existing spectra in the databases. A TA Instruments T500 and an alumina crucible were used for thermogravimetric analysis (TGA) of the NdFeB powder. Each sample was heated at 20 °C min−1 up to 750 °C under a nitrogen atmosphere.
000 spectra). The best way to correlate the data was via a minima–maxima comparison since the obtained spectra from the bonded magnets were much lower in intensity than those of the existing spectra in the databases. A TA Instruments T500 and an alumina crucible were used for thermogravimetric analysis (TGA) of the NdFeB powder. Each sample was heated at 20 °C min−1 up to 750 °C under a nitrogen atmosphere.
Dissolution experiments
Once the magnets were classified based on their resin type, different solvents were selected and tested on each type. The primary aim of these dissolution tests was to find solvents that can be used for the removal of each, and if possible all, of the three polymeric materials without damaging the magnet powder.Screening of the solvents was performed in a qualitative way. The magnet samples were crushed with a pestle and mortar in case of brittle magnets (e.g. epoxy). In case of ductile samples (e.g. PPS), small pieces of magnet sample were cut with scissors. Prepared samples were then added to the respective solvent at a pre-set temperature. At the end of the dissolution experiment, the sludge was filtered under vacuum through a funnel with Whatman-grade 542 filter paper. The filtrate was separated, and the residue was washed with acetone or dichloromethane for organic solvents and with demineralized water for inorganic solvents and dried overnight at 80 °C in a vacuum oven. The dried samples were finally analyzed with FTIR to compare their polymer amount with that of the original samples.
In order to prepare resin-free samples to produce recycled magnets, a batch of about 25 g of PA6 bonded magnet powder was used as-received. The sample was already demagnetized by the supplier under a reverse magnetic field. For the dissolution tests of the polymeric portion, 2.5 or 9 g of magnet powder was transferred to a round bottom flask (50 or 100 mL) and mixed with the ionic liquid tributylethylphosphonium diethylphosphate, [P4442][Et2PO4], in a solid-to-liquid mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The flask was transferred to a pre-heated sand bath at 165 °C and stirred for 12 h. After the dissolution period, the hot dark-colored mixture was filtered on a sintered glass filter connected to a vacuum pump. Hot filtering was done to avoid the increase in the viscosity of the mixture with cooling. Once the glass filter was cooled, the solid was further washed with acetone until the filtrate became transparent. The washed solid sample was then dried in a muffle furnace at 80 °C overnight before being analyzed by FTIR.
5. The flask was transferred to a pre-heated sand bath at 165 °C and stirred for 12 h. After the dissolution period, the hot dark-colored mixture was filtered on a sintered glass filter connected to a vacuum pump. Hot filtering was done to avoid the increase in the viscosity of the mixture with cooling. Once the glass filter was cooled, the solid was further washed with acetone until the filtrate became transparent. The washed solid sample was then dried in a muffle furnace at 80 °C overnight before being analyzed by FTIR.
Preparation of recycled magnets and measurement of magnetic properties
Resin-free magnet powder samples of approximately 150 mg were mixed with wax and sealed in a cylindrical sample holder before being placed in boiling water to melt the wax. The particles were aligned with their c-axis in one direction using an electromagnet with a field of 1.5 T while the wax solidified. Once set, the samples were then pulse-magnetized in the c-axis direction using a field of 4 T from a capacitor discharge pulse magnetizer. The samples were subsequently measured using a Lakeshore 7300 Vibrating Sample Magnetometer (VSM) in both the “easy” direction (parallel to the c-axis) and the “hard” direction (perpendicular to the c-axis) to determine the degree of anisotropy of the powder. The magnetization values determined by the VSM were converted to polarization values using a theoretical density of 7.64 g cm−3 and a self-demagnetization factor of 0.21, which are the values used by the supplier, to allow a direct comparison of recycled magnet powder with the starting powder. To produce compression bonded magnets, 6 g of the recycled powder was mixed with approximately 0.2 g of Araldite rapid two-part epoxy resin. The powder and resin were intimately mixed before loading into a 13 mm diameter Specac die set, the resin powder mix was pressed in a Specac Atlas 25 T press to a load of 8 tons. The magnet was released from the die set and allowed to cure at room temperature for 24 h. The compression bonded magnets were pulse-magnetized using a capacitor discharge magnetizer with a pulsed field of greater than 5 T. The samples were tested on a Magnet Physik EP5 permagraph at room temperature (ca. 22 °C).Results and discussion
Characterization of the bonded magnets
A total of 25 different bonded NdFeB magnets were received or purchased from different companies. As different grades of bonded NdFeB magnets were received, these had different chemical compositions. Therefore, elemental analysis with ICP-OES was performed on every bonded sample available (Table 1).| Sample | Polymer | B | Co | Cu | Dy | Nb | Pr | Fe | Nd | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PA12 | 0.9 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 68.2 | 25.1 | 94.6 |
| 2 | PPS | 0.9 | 2.3 | 0.1 | 0.1 | 0.0 | 0.0 | 60.4 | 24.1 | 88.0 |
| 3 | PA6 + Trilene XL | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 1.6 | 73.2 | 15.3 | 91.1 |
| 4 | PA6 + Trilene XL | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 74.4 | 17.8 | 92.0 |
| 5 | Epoxy | 0.7 | 0.8 | 0.0 | 0.1 | 0.0 | 0.0 | 67.9 | 24.1 | 93.7 |
| 6 | Epoxy | 0.8 | 0.8 | 0.0 | 0.1 | 0.0 | 2.6 | 68.2 | 21.1 | 93.8 |
| 7 | Epoxy | 0.8 | 0.9 | 0.0 | 0.1 | 0.0 | 1.8 | 67.8 | 21.8 | 93.3 |
| 8 | PA12 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 68.7 | 23.5 | 93.1 |
| 9 | PPS | 0.6 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 69.5 | 21.1 | 93.3 |
| 10 | PPS | 1.0 | 4.6 | 0.1 | 0.1 | 0.0 | 0.0 | 62.2 | 25.2 | 93.3 |
| 11 | Epoxy | 1.0 | 0.0 | 0.1 | 0.1 | 0.7 | 0.0 | 66.8 | 26.5 | 95.3 |
| 12 | Epoxy | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 71.4 | 23.4 | 96.0 |
| 13 | Epoxy | 1.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 66.0 | 24.5 | 92.0 |
| 14 | Epoxy | 1.1 | 0.5 | 0.1 | 0.1 | 0.0 | 0.0 | 67.9 | 24.2 | 94.0 |
| 15 | Epoxy | 0.67 | 0.73 | 0.39 | 0.43 | 0.0 | 3.65 | 66.4 | 20.5 | 93.3 |
| 16 | PA12 | 0.83 | 0.49 | 0.25 | 0.28 | 0.0 | 2.55 | 69.4 | 17.4 | 91.7 |
| 17 | PPS | 0.7 | 0.0 | 0.2 | 0.0 | 0.0 | 8.9 | 67.8 | 16.7 | 94.7 |
| 18 | Epoxy | 0.6 | 1.1 | 0.1 | 0.0 | 0.0 | 0.8 | 69.3 | 24.1 | 96.8 |
| 19 | Epoxy | 0.6 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 70.1 | 23.1 | 94.3 |
| 20 | PA6 + Trilene XL | 0.7 | 0.4 | 0.1 | 0.0 | 0.0 | 7.0 | 69.3 | 17.6 | 95.4 |
| 21 | Epoxy | 0.7 | 0.0 | 0.1 | 0.0 | 0.0 | 4.8 | 69.9 | 18.7 | 94.6 |
| 22 | Epoxy | 0.6 | 1.5 | 0.1 | 0.0 | 0.0 | 4.7 | 67.7 | 22.9 | 97.8 |
| 23 | Epoxy | 0.6 | 1.2 | 0.1 | 0.0 | 0.0 | 0.2 | 66.8 | 24.3 | 93.3 |
| 24 | Epoxy | 0.6 | 1.5 | 0.1 | 0.0 | 0.0 | 2.8 | 69.0 | 24.1 | 98.4 |
| 25 | Epoxy | 0.9 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 68.2 | 25.1 | 94.6 |
The total rare-earth content of the magnet powder in the samples varied between 16.9 and 26.5 wt%. Only samples numbered as 15 and 16 contained considerable amounts dysprosium. In contrast, praseodymium was more commonly found with concentrations up to 8.9 wt%. The cobalt content varied between 0 and 4.6 wt%, the iron content between 60.4 and 74.4 wt% and the boron content between 0.6 and 1.1 wt%. For all magnet samples, the sum of all concentrations is less than the theoretical value of 100 wt% since the associated organic portion of the samples (from the polymeric binders) was not quantified. It is also noteworthy that the compositional and elemental diversity that is often found in sintered NdFeB magnets seems to be absent for bonded magnets. Since the sintered magnets are prone to oxidation and corrosion and harder to manufacture/machine, commonly found minor elements (e.g. gallium, copper, niobium) in such magnets are used to reduce these drawbacks and to improve the sintering of the magnet particles. However, since bonded magnets already contain a polymeric portion for the same purpose, there is much less need for such additives.
The type of polymer in each bonded magnet sample was characterized using infrared spectroscopy (Table 1). Typical examples of the infrared spectra of samples from each magnet type, together with the correlated spectra from the polymer libraries are presented in the ESI (Fig. S1–S4†). The main conclusion of these measurements is that polyamides PA6 and PA12, poly-p-phenylene sulfide (PPS) and epoxy binders are consistently used for commercially available bonded NdFeB magnets, regardless of their supplier.
From 25 collected samples, 6 contained polyamides, 4 contained poly-p-phenylene sulfide and 15 contained epoxy resin. Some magnets contained polyamide 6 instead of polyamide 12 and this polymer was always found to be blended with a fluorocarbon polymer (Trilene XL). The epoxy materials were all based on poly(bisphenol A-co-epichlorohydrin).
A thermogravimetric analysis (TGA) was also performed for all the samples collected in order to determine the amount of polymeric binder material since this is the only part of the magnet that could decompose. Examples of TGA results for each resin type are given in the ESI (Fig. S5–S8†). It was observed that once a fraction of the polymer decomposed, the mass of the sample started to increase. This typical observation can only be attributed to the reactive characteristics of the exposed NdFeB powder. Because only nitrogen was present in the chamber during thermal decomposition, it can be concluded that the mass increase resulted from the formation of metal nitrides. This side effect made it impossible to accurately determine the amount of organic material that was used in the bonded magnet. Regardless of their supplier, the bonded magnets containing the same polymeric material showed a similar amount of mass loss. The highest mass losses prior to a mass increase were observed in polyamide 12 bonded magnets, while the lowest losses were recorded for the epoxy-bonded magnets. This result is in agreement with the literature data.8–10 While the injection-molded magnets (i.e. PA12 and PPS) can contain up to 12 wt% polymer, the compression-molded magnets (i.e. epoxy) can only take up to 5 wt%.
Another trend was that the onset of thermal decomposition of epoxy samples varied in the range of 270 to 320 °C. However, PPS and PA12 type magnets showed, in general, a much higher thermal stability, some exceeding 400 °C. This means that, regardless of the atmosphere, it could be difficult to thermally demagnetize the epoxy-bonded NdFeB magnets without (partly) decomposing the epoxy material since the Curie temperature of bonded NdFeB magnets orbits around 300–350 °C range depending on their grade.42
Dissolution experiments for selective removal of polymers
Poly-p-phenylene sulfide (PPS) is a high-performance aromatic hydrocarbon polymer with highly crystalline linear regions that are very hard to dissolve. Thanks to this high crystallinity, PPS has high chemical resistance, thermal stability and tensile strength. Literature on the solubility of PPS in organic solvents is scarce, but it is commonly accepted that no solvent can dissolve PPS at a temperature below 200 °C. That is because crystalline regions of PPS become more amorphous with increasing temperature thereby increasing the solubility of PPS in solvents like aromatic hydrocarbons.43 Many solvents cannot maintain their liquid state at such relatively high temperatures or, in the case of ionic liquids, they start to degrade.In a study investigating the inherent viscosity of PPS, it was stated that the sulfide groups of PPS can be partly converted to sulfone groups which could make the polymer soluble in methane sulfonic acid.44 It was also reported that 1-chloronapthalene can dissolve PPS above 200 °C. In another study, other high boiling aromatic hydrocarbons were reported to be able to dissolve PPS, but once again, only at high temperatures.45 The dissolution results of PPS-bonded magnets obtained in this study are given in Table 2. Among the tested solvents, only 1-chloronaphthalene and 1,3,5-triphenylbenzene were able to dissolve PPS, but at temperatures higher than 200 °C. That is due to the shared aromatic structure of these solvents with the PPS structure (i.e. like dissolves like). Hydrogen bonds in such systems have no role as there are no hydrogen bond donors or acceptors. An interesting donor–acceptor system was shown between sulfur and pyridine-type nitrogen atom.46–48 Hence, it is quite possible that the sulfur atom in PPS interacts with the aromatic solvents by a similar donor–acceptor mechanism between such as sulfur-chlorine in the case of 1-chloronaphthalene. It should also be noted that although some ionic liquids and a high boiling aliphatic hydrocarbon solvent (tetracosane) were tested, none of these solvents could dissolve PPS, not even at temperatures up to 265 °C.
| Solvent | Dissolution | Temperature (°C) |
|---|---|---|
| 1-Chloronaphthalene | Yes | 240 |
| 1,3,5-Triphenylbenzene | Yes | 255 |
| 1,4-Diphenylbenzene | No | ≤265 |
| Diphenyl sulfone | No | ≤265 |
| Tetracosane | No | ≤265 |
| Cyphos IL 101 | No | ≤265 |
| [P4442][Et2PO4] | No | ≤265 |
| [C4MIM][CF3COO] | No | ≤265 |
| [C8MIM][Cl] | No | ≤265 |
| [C4Pyr][Cl] | No | ≤265 |
Epoxy resins are built up by two components: a pre-polymer and a hardener. The reaction between these components creates a dense network that provides the strength and chemical resistance to epoxy materials. In this study, the epoxy-bonded magnets were found to completely disintegrate in several ionic liquids, such as [C4MIM][Cl], [P4442][Et2PO4] and Cyphos IL 101. NdFeB powder was obtained together with a brown viscous oil and an insoluble black powder that were both recognized as bisphenol A based materials. It is likely that the coordinating anion of the ionic liquid simply broke down the functional groups of the resin. Alternatively, any water entrapped in tested ILs could also be responsible for the breakdown of epoxy resin since these solvents were not dried prior to experiments. However, studied ionic liquids also showed signs of decomposition. The distinctive smell of methylimidazole was detected when [C4MIM][Cl] was used. Similarly, a phosphine smell was noticed after using [P4442][Et2PO4] and Cyphos IL 101. This could indicate that during dissolution of the epoxy, the exposed NdFeB powder reduces the ionic liquid cation. The 1H NMR analysis of recovered [P4442][Et2PO4] after the dissolution experiment of an epoxy-bonded magnet suggests that the IL was not pure anymore since relatively less phosphate anions are present than there are phosphonium cations (ESI, Fig. S9†). These results show that ionic liquids with easily reducible cations are not suitable for recycling of epoxy bonded NdFeB magnets. On the other hand, these magnets did also disintegrate by refluxing overnight in pure water or in an aqueous NaOH solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NaOH-to-water mass ratio). After removing the magnet powder, a white precipitate was found that was identified as N,N-ethylene bis(stearamide), a lubricating agent for molding applications. In the case of NaOH solution, the magnet powder clearly showed signs of oxidation as a rusty colored precipitate was obtained (ESI, Fig. S10†). Additionally, a white powder was obtained that was identified as boric acid after FTIR analysis. Boric acid can be applied as a filler in epoxy materials for its flame-retardant properties. Since it is a trifunctional chemical, it can also be applied as a crosslinking agent.
1 NaOH-to-water mass ratio). After removing the magnet powder, a white precipitate was found that was identified as N,N-ethylene bis(stearamide), a lubricating agent for molding applications. In the case of NaOH solution, the magnet powder clearly showed signs of oxidation as a rusty colored precipitate was obtained (ESI, Fig. S10†). Additionally, a white powder was obtained that was identified as boric acid after FTIR analysis. Boric acid can be applied as a filler in epoxy materials for its flame-retardant properties. Since it is a trifunctional chemical, it can also be applied as a crosslinking agent.
In general, epoxy-bonded magnets seem to easily break down as they were found to be very brittle. More importantly, several ionic liquids, water and a basic solution, were all able to separate the organic materials from the magnet powder. Although not considered in this work, a brine solution with 5 M NaCl concentration was also shown to corrode an epoxy bonded NdFeB magnet. The presence of epoxy resin did not affect nor prevented possible interactions of the magnet powder with the solvent.49 However, it is not fully clear whether this phenomenon occurs via a chemical process (i.e. dissolution of epoxy resin). Alternatively, the organic material may also lose its adhesion to the NdFeB powder through heating and stirring, since the epoxy amount is very low in the bonded magnet. If this is the case, then it is rather a physical separation than a chemical one. In either case, care must be taken with removal of epoxy from the magnet powder because the reactive magnet powder will come into direct contact with the solvent which can result in undesired chemical reaction(s).
Polyamide 12 (PA12) is a chemically resistant polymer. Secondary amide bonds are quite stable and give strength to the material due to formation of intermolecular hydrogen bonds. On the other hand, the long alkyl chain that links the amide bonds gives rise to an almost paraffin-like structure. The combination of polar and apolar groups makes it difficult to find a suitable solvent for this polymer, but ionic liquids are known to dissolve polyamides.35
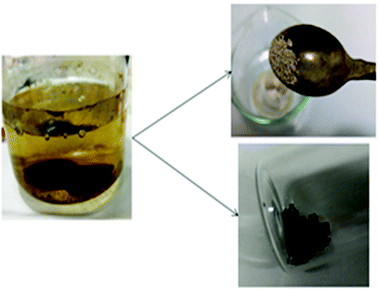
The results of the dissolution test of PA12 with ionic liquids (Table 3) show that dissolution always occurred at elevated temperatures (>80 °C). It is not clear yet whether this is caused by the thermodynamics or the kinetics of the process since the ionic liquids are highly viscous at room temperature. Fig. 1 shows the dissolution of a PA12-bonded NdFeB magnet in [P4442][Et2PO4]. After dissolution of the resin, the free magnet powder settled at the bottom of the flask and could easily be collected with the help of a magnet.
| Solvent | Dissolution |
|---|---|
| [P4442][Et2PO4] | Yes |
| [C2MIM][CH3COO] | Yes |
| [C2MIM][Cl] | Yes |
| [C8MIM][Cl] | Yes |
| Cyphos IL 101 | Yes |
| Aliquat 336 | Yes |
| [C4MIM][NTf2] | No |
| N-methylpyrrolidone | Yes |
The dissolved resin could be precipitated as a white powder by the addition of water to the ionic liquid and collected by filtration. The collected very fine magnet powder did not spontaneously oxidize in the presence of air because the magnet powder remained coated by a polysiloxane-polyether layer that could not be removed by the ionic liquid. Based on these dissolution experiments, it is evident that none of the tested ionic liquids could selectively remove all three of the polymers, at least without damaging the magnet powder. On the other hand, a three-step methodology can be used when treating a mixture of waste resin-bonded NdFeB magnets. In the first step, epoxy-bonded magnets can be selectively targeted by refluxing and washing with water. In a second step, PA6 and PA12 can be dissolved at temperatures above 80 °C using an ionic liquid with coordinating anions (e.g. chloride, dialkylphosphate or acetate). Finally, the PPS-bonded magnets can be treated by a high boiling point molecular solvent such as 1-chloronaphtalene to selectively remove the PPS.
After each treatment the solid residue can be collected by decantation and forwarded to the next process step. However, in between the first two steps, there is still a need to separate the exposed magnet powder. After removal of the epoxy resin, the magnet powder has to be isolated from the rest to prevent its undesired reaction with the IL needed in the second step (PA-removal). One such treatment could be flotation. Since the rest of the bonded magnets are injection molded (i.e. high plastic fractions), the high-density difference between the resin-free and resin-bonded magnet could be used for their physical separation. If need be, froth flotation can also be tested as a better option.50,51 It is not clear if there will be such a need in between the second and third steps. Although solvents that were able to dissolve PPS (e.g. 1-chloronapthalene) did not react further with the magnet powder, this has to be tested in presence of PA-free magnet powder too.
Dissolution experiments for selective removal of NdFeB magnet powder
In the second part of the dissolution experiments, a different approach was tested. Here, the preservation of the magnet powder was ignored during removal of the polymeric material. Instead, by oxidizing or selectively dissolving the magnet powder, the aim was to physically isolate the polymer.In concentrated H2SO4 (96%) at 80 °C, epoxy and PPS did not show any chemical reaction. This means that the highly acidic environment could not hydrolyze any bond or enable a physical separation. On the other hand, PA12 dissolved and eventually accumulated on top of the liquid as a separate phase. Meanwhile a white precipitate (metal sulfates) was found at the bottom of the flask (ESI, Fig. S11†).
All bonded magnets reacted with concentrated HNO3 (65%) and concentrated HCl (37%) at 60 °C. The reaction with epoxy was violent, and a bisphenol A based precipitate was formed. PA12 reacted slowly with these acids and could be recovered after re-precipitation. The metals completely dissolved. In literature it is know that the sulfide groups of PPS can be oxidized into sulfone groups when reacted with HNO3.44 In this study, reaction of PPS with concentrated HCl and HNO3 was slow and the polymer could be partly recovered. Again, the metals did dissolve quantitatively. The reaction of the magnets with hydrogen peroxide (H2O2) was also tested. The three bonded magnets were stirred in a H2O2 solution (35 wt%) at 60 °C for more than a day. In all cases, the bonded magnets eventually fell apart into a fine powder. By addition of a diluted acid solution (e.g. HCl), it was later possible to dissolve the oxidized magnet powder and to isolate the polymers by filtration (ESI, Fig. S12†).
Removal of PA6 and magnetic properties of resin-free magnet powder
To test whether a magnet powder released from its polymeric binder can maintain its magnetic properties, and thus qualify for manufacturing of new bonded magnets, a batch of PA6-bonded magnet powder was used in a larger scale experiment. The demagnetized bonded magnet was first treated with the ionic liquid tributylethylphosphonium diethylphosphate, [P4442][Et2PO4] (Table 3 and Fig. 1). The FTIR spectra of the original and IL-treated magnet powder are given in Fig. 2. The result obtained from the untreated bonded magnet (black pattern) matched with PA6 (or nylon 6). After dissolution treatment with the ionic liquids, all recognizable IR bands disappeared (red pattern) meaning that the polyamide matrix has been successfully removed.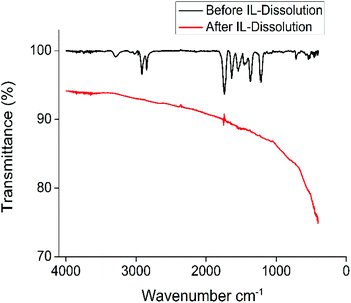 | ||
| Fig. 2 FTIR spectra of a PA6-bonded NdFeB magnet batch before (black) and after (red) dissolution of the polymer in the ionic liquid tributylethylphosphonium diethylphosphate, [P4442][Et2PO4]. | ||
In total five powder samples taken from the polyamide-free magnet batch were then measured in both the “easy” and “hard” magnetization directions on a Vibrating Sample Magnetometer (VSM). The “easy” and “hard” direction curves are very similar for all five of the measured samples which indicates that the magnetic properties of the powder are uniform across the produced batch by IL-dissolution. The demagnetization quadrant of one of the measured samples is shown in Fig. 3 where the sample has a remanence value of 0.91 T and coercivity of 701 kA m−1 in the “easy” direction. It should be noted that the small difference in both remanence and curve shape between the “easy” and “hard” directions indicates the slightly anisotropic nature of the powder.
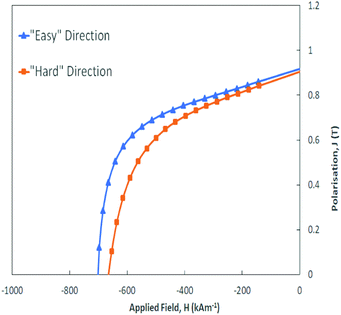 | ||
| Fig. 3 Demagnetization curve in the “easy” direction (blue, triangle markers) and “hard” direction (orange, square markers) for the resin-free magnet powder sample measured on the VSM. | ||
Table 4 presents the average magnetic properties of the recycled powder in the “easy” direction, taken across the five measured samples. Using the same theoretical density and self-demagnetization factors as the supplier, the remanence (Br) in the recycled powder is only slightly lower than the powder data values quoted by the magnet manufacturer: 0.89 vs. 0.90 T, which may be due to a reduction in the degree of alignment of the Nd2Fe14B grains. Similar values to this quotation of the supplier can be found elsewhere.52 In addition, the presence of a self-demagnetizing field in the VSM leads to a lower perceived remanence and hysteresis loop squareness. The intrinsic coercivity and maximum energy product values are also only slightly below the values quoted by the magnet manufacturer: Hci = 695 vs. 759 kA m−1 and BHmax = 120 vs. 125 kJ m−3. Overall, this work has shown that more than 90% of the magnetic properties of the starting material can be recovered using ionic liquid processing and, therefore, this method can be considered as an effective recycling method for bonded NdFeB magnets.
| Property | Value | Standard deviation |
|---|---|---|
| Coercivity Hci (kA m−1) | 695 | 8.25 |
| Remanence Br (T) | 0.89 | 0.05 |
| Maximum energy product BHmax (kJ m−3) | 120.08 | 12.59 |
After observing that the magnet powder retained more than 90% of it magnetic properties during polyamide removal, three compression molded magnets were produced from the batch (Fig. 4). The volume ratio between the magnet powder and epoxy was 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. Unfortunately, the third sample broke during magnetic property measurements which is why the remanence (Br) and intrinsic coercivity (Hcj) values for the third sample are not given in Fig. 5 or Table 5. The data in Table 5 shows the magnetic properties of the injection-molded magnet powder before recycling, the quoted values for compression bonded magnets from the powder manufacturer and two compression bonded magnets that were produced at lab-scale using the recycled magnet powder.
20. Unfortunately, the third sample broke during magnetic property measurements which is why the remanence (Br) and intrinsic coercivity (Hcj) values for the third sample are not given in Fig. 5 or Table 5. The data in Table 5 shows the magnetic properties of the injection-molded magnet powder before recycling, the quoted values for compression bonded magnets from the powder manufacturer and two compression bonded magnets that were produced at lab-scale using the recycled magnet powder.
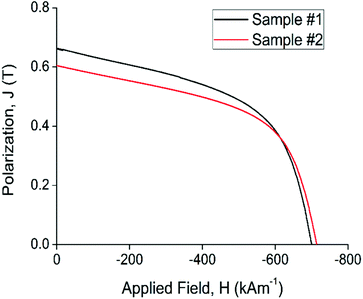 | ||
| Fig. 5 Demagnetization traces for sample #1 and sample #2, compression-bonded magnets produced from the recycled powder (80 vol%). | ||
Comparison of the recycled bonded magnets with the starting material shows higher Br values for both sample #1 and #2. This is due to the increased volume of magnet powder for the compression-bonded samples (ca. 80%) compared to the injection-molded samples (60%). The coercivity of the recycled magnets was between 96% and 98% of the values from the starting magnet prior to recycling. Comparing the recycled bonded magnets with the quoted values for a 77.5% compression bonded magnet from the powder manufacturer,53 there is a 100% recovery in Br for the recycled magnet sample #1 and a 14% reduction for sample #2. The intrinsic coercivity (Hci) of the recycled magnets on the other hand pairs with that of the commercial magnet with a 1% reduction for sample #1 and a 2% increase for sample #2.
The magnetic strength or the maximum energy product (BHmax) values measured for the recycled magnets are also similar to the value of the commercial counterpart which was calculated from the manufacturer data. The lower calculated (BH)max values for the recycled magnets is likely to be due to a reduction in coercivity (Hci) of sample #1 and the lower remenance (Br) value of sample #2. The variability of the magnetic properties of the recycled magnets is most likely due to the lab-scale processing of the epoxy-bonded magnets. Processing was performed in small batch sizes which means the measurement error and consistency of mixing could have led to a volume percent error in the final bonded magnet (estimated as ±5%).
The VSM data has shown that when measuring the recycled powder more than 90% of the magnetic properties of the starting material can be recovered using an ionic liquid processing method. The production of bonded magnets using the recycled powder also shows good recovery of magnetic properties when recycled. For the first time, it is being reported by this study that a resin bonded NdFeB magnet can be successfully recycled using a commercial ionic liquid. The method is not only environmentally friendly but also versatile since it allows the production of new recycled magnets in different shapes and with different polymers.
Conclusions
Ionic liquids have been used for the first time to recycle bonded NdFeB magnets. A collection of 25 different NdFeB magnets from different suppliers were investigated. Polyamides PA6 and PA12, poly-p-phenylenesulfide (PPS) and epoxy have been found as the polymeric binders. Chemical analysis of the magnet samples showed that besides neodymium, only praseodymium was present in significant amounts. Minor elements that are typically found in sintered magnets (e.g. copper, gallium, niobium, zirconium) were absent in the bonded magnets, showing that bonded NdFeB magnets have a simpler chemical composition than sintered NdFeB magnets.Of all the polymer binders, PA6 and PA12 were found to be well soluble in ionic liquids with coordinating anions, such as chloride, acetate or dialkylphosphate. PPS could not be dissolved in ionic liquids, whereas epoxy could be removed by refluxing in water or in a sodium hydroxide solution. The dissolution experiments were performed in a larger batch scale for the ionic liquid tributylethylphosphonium diethylphosphate, [P4442][Et2PO4], and PA6-bonded NdFeB magnet powder. The magnet powder could be separated from the polymer. The free magnet powder retained more than 90% of its original magnetic properties. New compression-bonded magnets were produced from the polymer-free magnet powders and their magnetic properties were found to be very similar to those of a commercial counterpart. This work shows that ionic liquid processing of end-of-life bonded NdFeB magnets is a promising method for direct recycling of these magnets. In addition to being environmentally friendly, the process is also a versatile route that allows production of secondary bonded magnets with new design and polymeric material.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 720838 (NEOHIRE). The authors would like to extend their gratitude to Dr. Dženita Avdibegović for her help in some of the FTIR measurements and Bart Van Huffel for his assistance in ICP-OES measurements.References
- K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton and M. Buchert, J. Cleaner Prod., 2013, 51, 1–22 CrossRef CAS.
- Y. Yang, A. Walton, R. Sheridan, K. Guth, R. Gauß, O. Gutfleisch, M. Buchert, B.-M. Steenari, T. Van Gerven, P. T. Jones and K. Binnemans, J. Sustainable Metall., 2017, 3, 122–149 CrossRef.
- M. Sagawa, T. Mizoguchi and Y. Une, United States patent, US9837207B2, 2017 Search PubMed.
- A. G. Popov, E. G. Gerasimov, P. B. Terent'Ev, V. S. Gaviko, K. Y. Shunyaev, T. L. Mikhailova, V. O. Vas'Kovskii and N. A. Kulesh, Phys. Met. Metallogr., 2013, 114, 285–294 CrossRef.
- T. Maeda, A. Watanabe and K. Yamada, SEI Tech. Rev., 2016, 66–71 Search PubMed.
- J. Li, Y. Liu, S. J. Gao, M. Li, Y. Q. Wang and M. J. Tu, J. Magn. Magn. Mater., 2006, 299, 195–204 CrossRef CAS.
- X. Zhang and W. Xiong, Rare Met., 2009, 28, 248–252 CrossRef CAS.
- J. Liu and M. Walmer, in Handbook of Advanced Magnetic Materials, ed. Y. Liu, D. J. Sellmyer and D. Shindo, Springer US, Boston, MA, 2006, pp. 1008–1044 Search PubMed.
- L. Li, A. Tirado, I. C. Nlebedim, O. Rios, B. Post, V. Kunc, R. R. Lowden, E. Lara-Curzio, R. Fredette, J. Ormerod, T. A. Lograsso and M. P. Paranthaman, Sci. Rep., 2016, 6, 36212 CrossRef CAS PubMed.
- C. Huber, C. Abert, F. Bruckner, M. Groenefeld, S. Schuschnigg, I. Teliban, C. Vogler, G. Wautischer, R. Windl and D. Suess, Sci. Rep., 2017, 7, 9419 CrossRef PubMed.
- J. A. Engerroff, L. H. Justo, L. U. Lopes, P. A. Wendhausen, F. O. Keller and N. V. Junior, 2015 IEEE Int. Magn. Conf. INTERMAG 2015, 2015, 2007.
- S. Suprapedi, P. Sardjono and M. Muljadi, J. Phys.: Conf. Ser., 2016, 776, 012015 CrossRef.
- Muljadi, P. Sardjono and Suprapedi, Energy Procedia, 2015, 68, 282–287 CrossRef CAS.
- M. Firdaus, M. A. Rhamdhani, Y. Durandet, W. J. Rankin and K. McGregor, J. Sustainable Metall., 2016, 2, 276–295 CrossRef.
- Y. Bian, S. Guo, L. Jiang, J. Liu, K. Tang and W. Ding, ACS Sustainable Chem. Eng., 2016, 4, 810–818 CrossRef CAS.
- Z. Hua, J. Wang, L. Wang, Z. Zhao, X. Li, Y. Xiao and Y. Yang, ACS Sustainable Chem. Eng., 2014, 2, 2536–2543 CrossRef CAS.
- H. M. D. Bandara, K. D. Field and M. H. Emmert, Green Chem., 2015, 753–759 Search PubMed.
- P. Venkatesan, T. Vander Hoogerstraete, T. Hennebel, K. Binnemans, J. Sietsma and Y. Yang, Green Chem., 2018, 20, 1065–1073 RSC.
- P. Venkatesan, T. Vander Hoogerstraete, K. Binnemans, Z. Sun, J. Sietsma and Y. Yang, ACS Sustainable Chem. Eng., 2018, 6, 9375–9382 CrossRef CAS.
- M. A. R. Önal, C. R. Borra, M. Guo, B. Blanpain and T. Van Gerven, J. Rare Earths, 2017, 35, 574–584 CrossRef.
- D. Dupont and K. Binnemans, Green Chem., 2015, 17, 2150–2163 RSC.
- T. Vander Hoogerstraete, B. Blanpain, T. Van Gerven and K. Binnemans, RSC Adv., 2014, 4, 64099–64111 RSC.
- M. A. R. Önal, E. Aktan, C. R. Borra, B. Blanpain, T. Van Gerven and M. Guo, Hydrometallurgy, 2017, 167, 115–123 CrossRef.
- M. Orefice, K. Binnemans and T. Vander Hoogerstraete, RSC Adv., 2018, 8, 9299–9310 RSC.
- A. Walton, H. Yi, N. A. Rowson, J. D. Speight, V. S. J. Mann, R. S. Sheridan, A. Bradshaw, I. R. Harris and A. J. Williams, J. Cleaner Prod., 2015, 104, 236–241 CrossRef CAS.
- O. Gutfleisch, K. Güth, T. G. Woodcock and L. Schultz, Adv. Energy Mater., 2013, 3, 151–155 CrossRef CAS.
- Y. Zhang, M. Liu, S. Sun, X. Yin, Y. Yin, J. Guo, W. Liu, D. Zhang and M. Yue, J. Magn. Magn. Mater., 2019, 475, 465–469 CrossRef CAS.
- T. Terada, H. Onishi and T. Kawakami, J. Jpn. Inst. Met., 2001, 65, 627–634 CrossRef CAS.
- G. Oliveux, L. O. Dandy and G. A. Leeke, Polym. Degrad. Stab., 2015, 118, 96–103 CrossRef CAS.
- P. Yang, Q. Zhou, X.-X. Yuan, J. M. N. van Kasteren and Y.-Z. Wang, Polym. Degrad. Stab., 2012, 97, 1101–1106 CrossRef CAS.
- M. A. R. Önal, S. Riaño and K. Binnemans, Hydrometallurgy, 2020, 191, 105213 CrossRef.
- W. Dang, M. Kubouchi, S. Yamamoto, H. Sembokuya and K. Tsuda, Polymer, 2002, 43, 2953–2958 CrossRef CAS.
- P. Kubisa, Prog. Polym. Sci., 2004, 29, 3–12 CrossRef CAS.
- N. Winterton, J. Mater. Chem., 2006, 16, 4281–4293 RSC.
- S. Dewilde, W. Dehaen and K. Binnemans, Green Chem., 2016, 18, 1639–1652 RSC.
- J. Winters, W. Dehaen and K. Binnemans, Phys. Chem. Chem. Phys., 2019, 21, 4053–4062 RSC.
- S. Dewilde, T. Vander Hoogerstraete, W. Dehaen and K. Binnemans, ACS Sustainable Chem. Eng., 2018, 6, 1362–1369 CrossRef CAS.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
- S. Mallakpour and M. Dinari, Iran. Polym. J., 2010, 19, 983–1004 CAS.
- D. M. Phillips, L. F. Drummy, D. G. Conrady, D. M. Fox, R. R. Naik, M. O. Stone, P. C. Trulove, H. C. De Long and R. A. Mantz, J. Am. Chem. Soc., 2004, 126, 14350–14351 CrossRef CAS PubMed.
- H. Xie, S. Li and S. Zhang, Green Chem., 2005, 7, 606–608 RSC.
- B. M. Ma, J. W. Herchenroeder, B. Smith, M. Suda, D. Brown and Z. Chen, J. Magn. Magn. Mater., 2002, 239, 418–423 CrossRef CAS.
- A. Mellace, J. E. Hanson and J. Griepenburg, Chem. Mater., 2005, 17, 1812–1817 CrossRef CAS.
- D. Daoust, S. Bebelman, P. Godard, J. M. Coisne and C. Strazielle, Polymer, 1996, 37, 3879–3888 CrossRef CAS.
- H. N. Beck, J. Appl. Polym. Sci., 1992, 45, 1361–1366 CrossRef CAS.
- A. Rogoza, G. G. Furin, Y. V. Gatilov and B. Y. I, Russ. Chem. Bull., 2001, 50, 1072–1077 CrossRef CAS.
- V. Y. Senichev and V. V. Tereshatov, ChemInform, 2003, 34, 133–275 Search PubMed.
- A. V. Rogoza, G. G. Furin, I. Y. Bagryanskaya and V. Y. Gatilov, Russ. Chem. Bull. Int. Ed., 2001, 50, 1446–1448 CrossRef CAS.
- T. Hosoya, K. Nishimura, T. Namiki, S. Teraoka and H. Komura, Microscopy, 2018, 67, i45 CrossRef.
- N. Fraunholcz, Miner. Eng., 2004, 17, 261–268 CrossRef CAS.
- F. Pita and A. Castilho, Waste Manag., 2017, 60, 91–99 CrossRef CAS PubMed.
- Technical data sheet for MQP-B+-20056-070 isotropic magnet powder, https://mqitechnology.com/wp-content/uploads/2017/09/mqp-b-20056-070.pdf, (accessed 13 November 2019).
- Technical data sheet for MQP-B+-20441 (B4+) bonded NdFeB magnets, https://mqitechnology.com/product/mqp-b-20441-b4/, (accessed 13 November 2019).
Footnote |
| † Electronic supplementary information (ESI) available: Selected infrared spectra, TGA curves and pictures of dissolution experiments. See DOI: 10.1039/d0gc00647e |
| This journal is © The Royal Society of Chemistry 2020 |