Eutectic hardener from food-based chemicals to obtain fully bio-based and durable thermosets†
Abstract
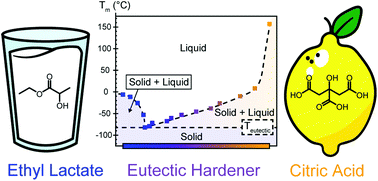
This paper presents a facile strategy to activate citric acid (CA) for room temperature curing. The high melting point of CA is reduced by preparing a eutectic mixture of CA and ethyl lactate (EL). By using CA and EL in the molar ratio of 1 : 1, the melting point of CA can be reduced from 200 °C to −50 °C. This eutectic mixture is then used to cure epoxidized linseed oil (ELO) at room temperature and higher temperatures. Room temperature curing was demonstrated by studying the evolution of the storage and loss modulus as a function of time, as well as by monitoring the changes in the glass transition temperature (Tg) with time. By isothermal curing experiments, the activation energy was determined to be 73 kJ mol−1. A comparison between the performance of the samples prepared at room temperature and that of samples prepared at higher temperatures was made, and it was found that full curing cannot be achieved at lower temperatures. By using only bio-based molecules, a fully bio-based thermoset with applicability in everyday life was obtained.


 Please wait while we load your content...
Please wait while we load your content...