Dehydra-decyclization of 2-methyltetrahydrofuran to pentadienes on boron-containing zeolites†
Abstract
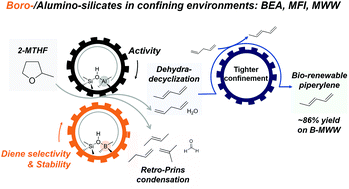
1,3-Pentadiene (piperylene) is an important monomer in the manufacturing of adhesives, plastics, and resins. It can be derived from biomass by the tandem ring-opening and dehydration (dehydra-decyclization) of 2-methyltetrahydrofuran (2-MTHF), but competing reaction pathways and the formation of another isomer (1,4-pentadiene) have limited piperylene yields to <60%. In this report, using detailed kinetic measurements of 2-MTHF dehydra-decyclization on zeolites with disparate acidities (boro-, and alumino-silicates) and micropore environments (MFI, MWW, and BEA), weakly acidic borosilicates were shown to exhibit ca. 10–30% higher selectivity to dienes at about five-to-sixty times lower proton-normalized rates than aluminosilicates (453–573 K). Dehydra-decyclization site time yields (STYs) were invariant for aluminosilicates within the investigated frameworks, indicating the absence of pore-confinement influence. However, individual site-normalized reaction rates varied by almost an order of magnitude on borosilicates in the order MWW > MFI > BEA at a given temperature (523 K), indicating the non-identical nature of active sites in these weak solid acids. The diene distribution remained far from equilibrium and was tuned towards the desirable conjugated diene (1,3-pentadiene) by facile isomerization of 1,4-pentadiene. This tuning capability was facilitated by high bed residence times, as well as the smaller micropore sizes among the zeolite frameworks considered. The suppression of competing pathways, and promotion of 1,4-pentadiene isomerization events lead to a hitherto unreported ∼86% 1,3-pentadiene yield and an overall ∼89% combined linear C5 dienes’ yield at near quantitative (∼98%) 2-MTHF conversion on the borosilicate B-MWW, without a significant reduction in diene selectivities for at least 80 hours time-on-stream under low space velocity (0.85 g reactant per g cat. per h) and high temperature (658 K) conditions. Finally, starting with iso-conversion levels (ca. 21–26%) and using total turnover numbers (TONs) accrued over the entire catalyst lifetime as the stability criterion, borosilicates were demonstrated to be significantly more stable than aluminosilicates under reaction conditions (∼3–6× higher TONs).
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...