Editorial: Evidence of a significant advance in green chemistry
Philip
Jessop
Department of Chemistry, Queen’s University, 90 Bader Lane, Kingston, ON, Canada K7L 3N6. E-mail: philip.jessop@chem.queensu.ca
To be published, work must present excellent science and a significant advance in green chemistry. Papers must contain a comparison with existing methods and demonstrate advantages over those methods before publication can be considered.
Comparison of new discoveries to the prior art is already standard practice in the chemical literature. For example, authors of scientific papers in the homogeneous catalysis literature routinely claim that their catalyst is more active or selective than those that are already known and they support those claims, whenever feasible, by benchmarking the new technology in order to prove superiority over the past technology. For example, a new catalyst and the previous-best catalyst could be both tested under identical conditions, demonstrating that the new catalyst is better. Although such benchmark tests aren't always possible, when they can be done they are valuable for proving to the referees, the editor, and the reader that the new technology is an improvement upon the prior art.
Comparison to the prior art is just as important in green chemistry. Without such comparison, the referees, editor and readers will be unconvinced that the new chemistry is greener than the old. The journal policy was implemented not merely to ensure quality of publications but also to encourage researchers to evaluate their own work. Authors who are reporting a new technology (a chemical, reaction, process or method) should ask themselves “Is my new chemistry greener than what has been done before? What environmental impacts will it likely cause, if implemented? What adjustments can be made to my chemistry to make it even greener?” (ASIDE: Authors who are reporting a new understanding, rather than a new chemistry, should address slightly different questions: “Is the new understanding likely to lead to environmental benefit? Why?” Such analysis is necessarily a bit more indirect and uncertain, but arguments for a green advance are still required.)
How can an author of a paper compare their new chemistry to the prior art? Green-ness is much more difficult to measure than the rate of a catalyzed reaction, and is a task that most chemists have never been trained to do. The first step is to specifically identify what prior art your new chemistry is being compared to. Be specific rather than vague. The second and more difficult step is to make the comparison; for that task I offer the following (non-comprehensive) list of suggestions for researchers and authors.
Option #1. Comparing chemistries by a single factor. This option is not preferred, but it is the easiest. Pick one parameter, such as mass of waste or energy consumption, and quantify it for the new chemistry and the previous best chemistry. If you want to quantify mass of waste, you can use E factor, atom economy, process mass intensity, or any of the multitude of similar mass-based metrics.1 Other single parameters you could choose include energy consumption, flammability, acute toxicity, ecotoxicity to fish, etc. (see Table 1).
| Risk type | Metric |
|---|---|
a If experimental data is unavailable, data can be predicted using readily available software tools (e.g. EPA TEST, EPI Suite, ECOSAR, OECD QSAR Toolbox, CEFIC Ambit). Don't expect the predicted values to be highly accurate (although log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow predictions are normally quite good), just use the predicted values as indicators of order of magnitude.
b For more information, see ref. 4.
c Search the EPA IRIS database for experimental determinations or use software packages to obtain predictions. Kow predictions are normally quite good), just use the predicted values as indicators of order of magnitude.
b For more information, see ref. 4.
c Search the EPA IRIS database for experimental determinations or use software packages to obtain predictions.
|
|
| Acidification | A risk if a gas contains N, S, Cl, Br, or F. |
| Bio-accumulation | High risk if any persistent organic compound has a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow > 4.3.a Moderate risk if 3.5 to 4.3. Low risk if log Kow > 4.3.a Moderate risk if 3.5 to 4.3. Low risk if log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow < 3.5 or the molecule is non-persistent. Kow < 3.5 or the molecule is non-persistent. |
| Ecotoxicity | Compare LC50 (daphnia magna) and LC50 (minnow) valuesa for compounds required for the two technologies, especially for water-soluble compounds required in large amounts. |
| Energy usage/global warming | Does the process use a lot of energy? Does it require the distillation of water or high-boiling solvents? Does it emit powerful greenhouse gases such as N2O, SF6, or halogenated C1–C4 organics? |
| Eutrophication | High risk if a compound contains P, medium risk if it contains N, low risk if neither N nor P. Decreased risk for very volatile compounds. |
| Flammability | Do any compounds have flash pointsa close to or below the operating temperature? |
| Human toxicity | Compare LD50 (rat, oral) and LC50 (rat, inhalation) valuesa for compounds required for the two technologies, especially those compounds required in large amounts. |
| Human carcinogenicity | Are any of the substances known or suspected carcinogens?c |
| Mass of waste | For reactions or processes, compare the E factor or process mass intensity for the new and incumbent technologies. AE is less useful because it disregards solvents and other compounds outside the reaction equation. |
| Ozone depletion | A risk if any C1 or C2 compound is volatile and has any of the following elements: Br > Cl ≫ F (exceptions: CH2Cl2 and CHCl3 are not problematic). |
| Persistence | A riskb if any organic compound (a) has a very high MW (e.g. polymers), (b) is highly halogenated, or (c) is a polycyclic aromatic (this is not assessed for inorganics). |
| Resource depletion | A risk if large amounts of the following elements used: Xe ≫ He > Au > Ir, Rh, Ru > Kr, Os > Pt, Sb, Re, Hg, Se, Cd, Ne, Pd, Sn. |
| Smog formation | A risk if any compound is volatile, organic, and may be released in significant quantities. |
| Water consumption | Compare the two technologies in terms of mass of water used. |
Why is this option not preferred? By picking one parameter, you risk missing key disadvantages of the new technology. For example, while the new chemistry might create less waste, the waste might be more toxic. You may therefore be contributing to risk migration,2 where replacements for harmful technology are later found to cause another kind of harm. For that reason, authors should avoid using only a single factor in their evaluation.
Option #2. Qualitative comparison by several factors. Table 1 shows a list of example metrics or impacts that could be considered. Pick as many as possible, especially those that are most relevant to the technology. Evaluate whether your technology is likely to be better than, comparable to, or worse than the incumbent technology for each of the factors you've chosen. Reasonable arguments showing why your technology is expected to be greener, in terms of several of the factors, are likely to be quite convincing to referees and readers. If you believe that your technology has one or more weaknesses, mention them in the manuscript (honesty is always a good strategy in scientific writing), and discuss future strategies for ameliorating those issues.
Why use qualitative comparison? Qualitative comparisons are acceptable and indeed are sometimes more appropriate than quantitative comparisons, especially in early-stage research. For example, when one tries to compare a new technology to a highly-optimized industrial technology, it is almost impossible to do a fair quantitative comparison. Such an analysis is likely to be difficult due to insufficient data, and biased (in favour of the incumbent technology) due to the different degree of optimization of the two processes.
However, if some factors such as E can be calculated and bias is not anticipated, then by all means make those comparisons quantitatively.
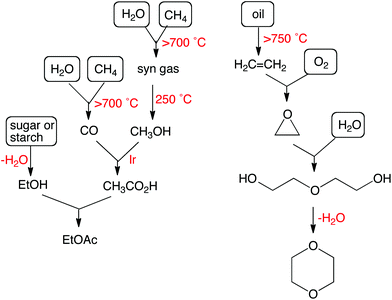
Option #3. Life cycle thinking. Take option #2 further by considering not just your chemical or method but also the life stages before and after the use of your chemistry. For example, if you are proposing a new chemical to replace a harmful chemical, then you would naturally consider the environmental, health, and safety impacts of both chemicals, but don't forget to qualitatively compare the harm caused during the manufacturing of your chemical to the harm caused during the manufacturing of the incumbent chemical. How? Consider drawing a synthesis tree for each chemical and looking for red flags in each route (Fig. 1).3
Option #4. Life cycle assessment. If you or a collaborator can supply an LCA in the paper or in an accompanying paper, then you will have given more evidence for a green advance than 99% of the papers ever published in the field of green chemistry. The LCA would preferably include several kinds of impacts, not just energy consumption. However an LCA is not a trivial undertaking and there is a shortage of trained LCA practitioners. So if you can do it, great! But this is not a requirement for publication.
Note that an LCA is not iron-clad proof of a green advance because (a) no LCA considers all kinds of possible impacts, (b) LCAs, like any other kind of study, can be performed or interpreted poorly, and (c) as mentioned previously, LCAs naturally display a bias towards optimized incumbent technologies over unoptimized new technologies simply because of the difference in degree of optimization. However, a well-executed multi-factor LCA is still excellent evidence for a green advance.
Whichever option you choose, please specify in your paper what prior technology your new technology is being compared to. With a clear comparison of this kind, preferably with several factors, your argument for a green advance should be clear to the referee, editor, and reader alike.
Philip Jessop
Chair, Editorial Board
Green Chemistry
References
- M. G. T. C. Ribeiro and A. A. S. C. Machado, Green Chem. Lett. Rev., 2013, 6, 1–18 CrossRef CAS.
- R. E. Alcock and J. Busby, Risk Anal., 2006, 26, 369–381 CrossRef PubMed.
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
- R. S. Boethling, E. Sommer and D. DiFiore, Chem. Rev., 2007, 107, 2207–2227 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |