Open Access Article
Open Access ArticleAn overview of the biphasic dehydration of sugars to 5-hydroxymethylfurfural and furfural: a rational selection of solvents using COSMO-RS and selection guides†
Jesús
Esteban
 a,
Andreas J.
Vorholt
a,
Andreas J.
Vorholt
 *a and
Walter
Leitner
*a and
Walter
Leitner
 ab
ab
aMax-Planck-Institut für Chemische Energiekonversion, Stiftstraße 34-36, 45470 Mülheim an der Ruhr, Germany. E-mail: andreas-j.vorholt@cec.mpg.de
bInstitut für Technische und Makromolekulare Chemie, RWTH Aachen University, Worringerweg 2, 52074 Aachen, Germany
First published on 14th February 2020
Abstract
The valorization of sugars from lignocellulosic biomass has attracted much interest for the production of chemicals and fuels. From the dehydration of glucose or fructose and xylose, 5-hydroxymethylfurfural (5-HMF) and furfural can be obtained, respectively, which are highly praised chemicals used as building blocks. To increase the productivity of the process avoiding undesired side reactions that furans may undergo in the reaction phase, many authors follow a liquid–liquid approach. This way, an in situ extraction of the dehydration products occurs from the reaction phase (usually aqueous) to an organic solvent phase. Solvent selection is a matter of interest in Green Chemistry; therefore, careful consideration must be given to select the most appropriate alternatives in terms of performance, environmental, health and safety (EHS) impacts and subsequent downstream processing. For performance, the COnductor-like Screening MOdel for Real Solvents (COSMO-RS) has emerged as a tool to screen among different candidates based on structural information of the molecules. For EHS considerations, different solvent guides assist in the assessment of the most favourable alternatives. This review provides a comprehensive survey of the solvents and operational conditions employed in the biphasic dehydration of sugars to 5-HMF and furfural, followed by an account of the selection guides and methods for the evaluation of solvents, including COSMO-RS. Finally, to contrast with the most commonly selected solvents, such as methyl isobutyl ketone, a rational screening is presented here for the biphasic production of furans based on COSMO-RS predictions and the assessment of the selection guides. Predictions and further validation of the distribution coefficients show that ethyl acetate and methyl propionate are promising solvents for the in situ extraction of 5-HMF and furfural from aqueous media whilst being ranked as recommended green solvents by most solvent selection guides available in literature.
Introduction
The current global scenario for energy and chemicals consumption features the impending exhaustion of fossil resources and the undeniable threat of global warming as major challenges to be tackled by mankind. In fact, it has been reported that in 2018 primary energy consumption grew at a rate of 2.9% with respect to the previous year, which represents the fastest since 2010 and practically doubles the 1.5% 10-year average rate.1 Simultaneously, carbon emissions grew 2% in the same year1 and projections made by the US Energy Information Administration in a 2018 report until 2050 foresee a marked increase in the following decades2 if no measures are taken. Therefore, current pledges to achieve carbon neutrality by 2050 reinforce the need to develop strategies to discontinue the use of fossil sources of energy and steer towards an economy based on the use of renewable resources.In the current situation where escalating demands for energy, bulk chemicals and materials come into play, alternative sources of feedstock are restlessly sought after. In the last decades, the biorefinery concept has emerged as an alternative for the generation of these goods via the sustainable processing of biomass of diverse nature following chemical, thermochemical, enzymatic or fermentative pathways.3,4 The most abundant type of biomass is lignocellulosic biomass, which can be obtained from dedicated crops or residues or waste from agricultural or forestry activities as well as food waste,5 the latter being far more preferable to avoid competition with food products.6–8 In addition, the exploitation of such lignocellulosic waste could represent an opportunity for the development of economies in rural economies where agricultural activities prevail.
Lignocellulosic biomass commonly consists of cellulose (35–50%), hemicellulose (20–35%) and lignin (10–25%) as major components, with proteins, oils, and ash completing the remaining fraction.9,10 As summarized in Fig. 1, after the corresponding chemical pretreatment of biomass, the cellulosic and hemicellulosic fractions give rise to the so-called C5 and C6 platforms since they are polymers from whose hydrolysis hexoses like glucose or fructose and pentoses like xylose, most abundantly, can be obtained. Such sugar units are very attractive platform chemicals from which a number of routes can originate by direct oxidation,11 hydrothermal processing,12 fermentative pathways4 or by one-pot reactions through furans,13 to name a few. Furans originate as a consequence of the catalytic dehydration of glucose (via fructose) to 5-hydroxymethylfurfural (5-HMF) and xylose to furfural·5-HMF occurs in human diet being formed from the thermal decomposition of carbohydrates, hence being a good indicator of non-enzymatic browning and used as a reference for deterioration of food that has undergone excessive heating or been stored for too long.14 For its part, furfural is a highly valued solvent that has been used in the extraction of aromatics from lubricants15,16 and also as part of the preparation of phenolic resins,17 to name a couple of examples. Industrially, Swiss-based company AVA Biochem implemented a facility for the production of 20 ton per year of 5-HMF in 2014 by a hydrothermal carbonization process.18 Also, more recently GF Biochemicals has developed a process to produce massively levulinic acid using 5-HMF as an intermediate.19 As for the production of furfural, Zeitsch describes a number of processes in different states of exploitation in his comprehensive book. These include batch processes like the one by the Quaker Oats Company, reportedly the oldest one starting in the 1920′s, or the Agrifurane (Petrol Chemie) process. The Quaker Oats Company would later develop a more modern continuous process, to which other continuously operated technologies would add, such as the Escher Wyss, the Rosenlew, the Supratherm (Krupp), the Stake (Stake Technologies), the Suprayield (Bosch Projects) or the Voest Alpine processes.20
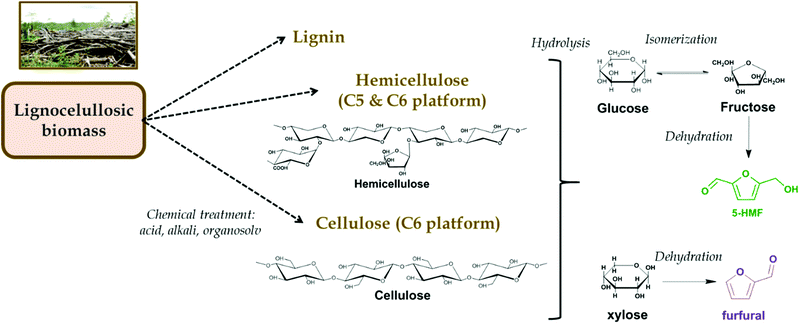 | ||
| Fig. 1 Chemical valorization of lignocellulosic biomass to furans through hydrolysis and dehydration of the hemicellulosic and cellulosic fractions. | ||
Owing to their relevance in synthesis, both of this dehydration products have been added as outstanding building blocks for chemicals and fuels to the US Department of Energy's list of top value-added chemicals from biomass.21 Indeed, both of these compounds offer great possibilities considering their chemical functionality and allow the production of a wide array of chemicals with very diverse applications. De Vries et al. reviewed thoroughly the synthetic pathways starting from 5-HMF leading to products with applications as: monomers for subsequent polymerization, highlighting diols from 5-HMF, 2,5-diformylfuran, 2,5-furandicarboxylic acid or 5-hydroxymethyl-2-furan carboxylic acid; fine chemicals, including products of interest as pharmaceuticals, agrochemicals, flavors and fragrances; and fuel components, such as dimethylfuran, levulinic acid or methyl tetrahydrofuran.14 Precisely the latter application constitutes the main interest of the products deriving from furfural, as several studies have focused on transformations to products like 2-methyl tetrahydrofuran, 2-methylfuran or ethyl levulinate, seen as enhancers properties of interest to fuels, such as research octane numbers, energy density or specific CO2 emissions among others.22,23
After several years of study, the production of both furans through the dehydration of sugars or other carbohydrates has undergone a great degree of development using different solvents for the purpose. The production of 5-HMF has firstly been attempted using different reaction media and Lewis and Brønsted catalysts alike. For example, the isomerization followed by dehydration of glucose employing treated TiO2–Cl catalysts has been reported in an aqueous medium.24 Polar organic solvents like DMSO have also been reported, as was the case for the same reaction with tin porous coordination polymers synthesized on polydopamine coated MnO2.25 Finally, more novel solvents have also acted as reaction media, including ionic liquids (ILs) like [Emim][Br] using SnPO as catalyst26 and different deep eutectic solvents (DESs) based on different carbohydrates as hydrogen bond donor (HBD) and choline chloride as hydrogen bond acceptor (HBA).27 In the latter case, the DESs acted both as solvent and substrate.
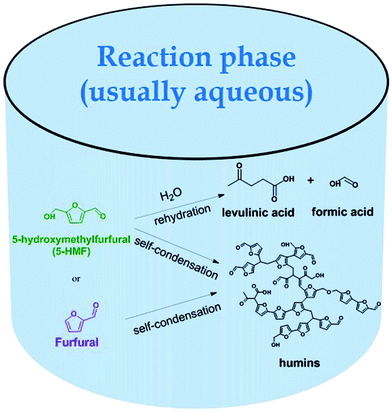
Likewise, the production of furfural by dehydration of xylose and other pentoses has seen similar possibilities. Representatively, water has been studied as solvent using AlCl3 as catalyst with formic acid as enhancer,28 γ-butyrolactone with HY zeolite as dehydration agent29 and IL [Bmim][PF6] with polymer bound sulfonic acid.30 Nevertheless, maintaining the corresponding furan in the reaction medium can affect the yields and selectivities. When dehydration occurs in aqueous medium, 5-HMF can undergo the undesired ring-opening rehydration to levulinic and formic acids in addition to self-condensation in the presence of the catalyst, leading to also undesired polymerization to humins. The latter can also take place for furfural, as shown in Fig. 2.14 Also, owing to their sensitivity to water, its generation during the dehydration of carbohydrates could affect ILs if these are used as solvents. Moreover, using ILs and DESs as solvents in single-liquid phase systems would make the eventual separation of 5-HMF and furfural very energy intensive.
For these reasons, a smart strategy to overcome the low conversion and selectivity values reported in monophasic systems is the use of multiphasic alternatives. Particularly, the use of biphasic systems appears as an attractive option to perform an in situ extraction as the furans generate. The use of such systems would have further advantages, such as thermodynamically shifting the reaction towards the products and concentrating it if the volume of the extracting solvent is kept low.31,32 This approach has also been used to recover N-acetylneuraminic acid from the reacting medium in its enzymatic production from N-acetyl-D-mannosamine and sodium pyruvate33,34 or for the separation of phenyl acetic acid by Alamine 336, a mixture of amines, from the aqueous enzymatic hydrolysis of penillicin G,35,36 in both of cases to prevent enzymatic deactivation.
The selection of solvents is a topic of concern in research and industrial practice as environmental policies lead to more and more restrictive regulations, such as REACH in the EU. This is indeed a driving force for a thoughtful selection for process-efficient and sustainable solvents in industrial activities, which has attracted a great deal of attention from chemists and chemical engineers alike.37,38 Such choice is a multidimensional problem that depends on the expected performance specific to the process subject of study (i.e., “it does the job”), considerations on how to reuse the solvents and considerations on their greenness in many aspects.39 The latter comprehends environmental, health and safety (EHS) issues and life cycle analysis.40,41
For the selection of solvents on the basis of their molecular affinity and dissolution capacity, a series of methods have been developed. Starting by models relying on a number of solubility parameters estimated experimentally like those proposed by Hildebrand,42 Kamlet–Taft43 or Hansen,44 these techniques have evolved to more advanced approaches. Such is the case of the COnductor-like Screening MOdel for Real Solvents (COSMO-RS), which constitutes a method based on quantum chemical calculations to predict chemical potentials of molecules in liquids and therefrom related equilibrium thermodynamic properties of pure and fluid mixtures can be estimated.45
For the assessment of the greenness of the solvents, throughout the years different institutions have elaborated their own selection guides, which they have made available to the public in different publications.46,47 Pharmaceutical companies like Pfizer,48 Astra Zeneca,49 Sanofi50 and GlaxoSmithKline51–53 have released their own guides, which have been updated over the years. In addition, the ACS Green Chemistry Institute Pharmaceutical Roundtable (GCI-PR) and the Innovative Medicines Initiative (IMI)-CHEM2154 have proposed new tools. These guides undoubtedly represent a good starting point for the evaluation of solvents in other applications.
A number of other reviews have covered the topic of the production of furans from sugars and other biomass derived substrates,11,13,14,55–59 in a couple of cases addressing specifically the use of biphasic systems for this purpose.31,32 However, the emphasis of such studies has been put mostly on catalytic and mechanistic considerations. In an effort to make a process to obtain green chemicals even greener and better understand how to achieve this, here we wish to shift the focus towards the rational selection of solvents to perform the biphasic production both of 5-HMF and furfural. For this reason, this critical review will give a survey on the solvents employed for the reaction followed by in situ extraction of these two furans in liquid–liquid systems. This will be followed by a screening of solvents based on the COSMO-RS method taking also into account the EHS criteria to comply with the principles of Green Chemistry.60 This methodology can eventually assist in the selection of solvents in reaction systems approached in multiphasic environments. Finally, an experimental evaluation of the partition of 5-HMF and furfural in the most promising alternatives is presented.
Production of furans in biphasic liquid media
The use of biphasic systems has become a strategy to overcome the problems that the use of single liquid phase systems pose by extracting the dehydration product and isolating it into a second liquid phase. Mainly, these issues regard the occurrence of the undesired rehydration and polymerization reactions to humins, although the use of biphasic systems could also favor the reusability of homogeneous catalysts if these are contained in the reaction medium, to which more substrate could be added. Moreover, subsequent separation of the desired product from monophasic systems of high boiling points, such as DESs and ILs, may become uneconomic. Fig. 3 depicts the general concept of the biphasic operation for the production of furans. Indeed, the number of publications on the use of biphasic systems has seen a marked increase in the last couple of decades, which indicates the trend to shift to this operation mode.31 The subsequent subsections will cover the production of 5-HMF and furfural in biphasic systems from different feedstock making use of assorted catalysts with particular focus on different solvents used.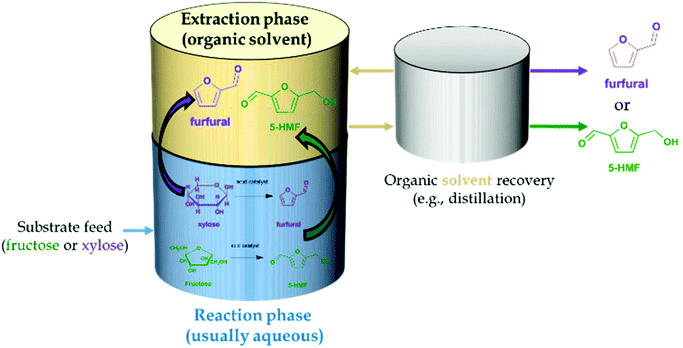 | ||
| Fig. 3 Concept of a process for the biphasic dehydration featuring the in situ extraction of 5-hydroxymethylfurfural and furfural. | ||
Production of 5-hydroxymethylfurfural
The conversion to 5-HMF from hexoses or substrates thereof composed has been widely studied in the literature. Whilst there are diverse mechanistic models that describe the reaction,14 in most cases the production of 5-HMF occurs via dehydration of fructose, which releases three water molecules along the process. This in turn requires prior isomerization when the selected starting substrate is glucose. In addition, in the case of polymers like inulin or dimers like cellobiose or sucrose, hydrolysis to release the hexoses is needed. The tables in this section present compilations of reports on the conversion to 5-HMF from glucose, fructose and other substrates, featuring the reaction conditions and main results. Although there is a plethora of works in the literature reporting biphasic systems, these compilations intend to cover studies performed with different solvent systems, mentioning more than one work in cases where the corresponding pair of solvents has been employed on multiple occasions.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, being 1
4, being 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 the most representative value. Finally, the concentration of substrates in the reaction phase spans widely from as little as 1 or 2% up to 50% and on some occasions even beyond. It is relevant to also mention that in many studies the authors have used salts, mainly NaCl, to promote a salting-out effect in the aqueous phase, whereby these electrolytes dissociate and interact with the water molecules hence weakening the solubility of 5-HMF in water. This will result in a higher amount of 5-HMF leaving the aqueous RP, thus migrating to the EP, which will the distribution coefficient to increase if such magnitude is defined as the ratio of the mass fraction of the solute in the EP to that in the RP. Nevertheless, the presence of such components in the reaction comes with a price to pay, as it can affect the activity of the catalysts, particularly in the case of heterogeneous catalysts like zeolites. Also, these modifiers would require separation if the reaction medium is to be recycled, which in principle is desirable, adding an additional step that could be detrimental to the economy of the process.31
3 the most representative value. Finally, the concentration of substrates in the reaction phase spans widely from as little as 1 or 2% up to 50% and on some occasions even beyond. It is relevant to also mention that in many studies the authors have used salts, mainly NaCl, to promote a salting-out effect in the aqueous phase, whereby these electrolytes dissociate and interact with the water molecules hence weakening the solubility of 5-HMF in water. This will result in a higher amount of 5-HMF leaving the aqueous RP, thus migrating to the EP, which will the distribution coefficient to increase if such magnitude is defined as the ratio of the mass fraction of the solute in the EP to that in the RP. Nevertheless, the presence of such components in the reaction comes with a price to pay, as it can affect the activity of the catalysts, particularly in the case of heterogeneous catalysts like zeolites. Also, these modifiers would require separation if the reaction medium is to be recycled, which in principle is desirable, adding an additional step that could be detrimental to the economy of the process.31
| Reaction phase (RP) | Extractive phase (EP) | Modifiers | Volume ratio RP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EP EP |
Catalyst |
C
cat![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (wt%) a (wt%) |
C
subs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (wt%) a (wt%) |
T (°C) | Time (min) | X substrate (%) | Y 5-HMF (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
a On the basis of reacting phase. Calculated with the data in the references where needed.
b Calculated from a molar ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of glucose to Sn as indicated in ref. 71. 1 of glucose to Sn as indicated in ref. 71.
|
|||||||||||
| H2O | MIBK | Not stated | Lewatit SPC-108 | WHSV = 8.06 g gcat−1 h−1 | Not stated | 78 | 1320 | Not stated | 9 | 61 | |
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.25 2.25 |
Ag3PW12O40 | 1 | 30 | 120 | 240 | 89.6 | 76.3 | 62 | |
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33 2.33 |
Mesoporous Ta2O5 | 3.3 | 10 | 175 | 150 | 69 | 23 | 63 | |
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33 2.33 |
ZrO2-MCM-41 | 3.3 | 10 | 175 | 150 | 82 | 23 | 64 | |
| H2O | MIBK | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
KAl(SO4)2 | 15.5 | 90 | 140 | 360 | Not stated | 49 | 65 |
| H2O | MIBK | CaCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33 2.33 |
γ-Al2O3 | 3.3 | 10 | 175 | 15 | 96 | 52 | 66 |
| H2O | THF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
CrPO4 | 1.25 | 10 | 140 | 30 | 99 | 63 | 67 |
| H2O | THF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
FePO4 | 2 | 10 | 140 | 15 | 97.8 | 23.1 | 68 |
| H2O | THF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cr-β zeolite | 2 | 10 | 150 | 90 | 87 | 72 | 69 |
| H2O | THF | NaCl (4 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HfO(PO4)x | 3 | 2 | 175 | 150 | 99.7 | 90.5 | 70 |
| H2O | 1-BuOH | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Sn-β zeolite, HCl (pH = 1) | 0.03b | 10 | 160 | 90 | 77 | 26 | 71 |
| H2O | 1-BuOH | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Sn-β zeolite, HCl (pH = 1) | 0.03b | 10 | 180 | 70 | 79 | 72 | 71 |
| H2O | sec-Butyl phenol | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Phosphate buffer saline (pH = 2) (10 wt% NaH2PO4·2H2O; 2.2 wt% H3PO4) | Flow rate not given | 1 | 180 | 47 | 99 | 75.7 | 72 | |
| H2O | sec-Butyl phenol | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Nb/CB-2-DP | 2 | 5 | 170 | 120 | 78 | 20 | 73 |
| H2O | MeTHF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33 2.33 |
Phosphated TiO2 | 4.16 | 33.3 | 175 | 80 | 89.4 | 58.8 | 74 | |
| H2O | MeTHF–NMP (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33 2.33 |
Phosphated TiO2 | 4.16 | 33.3 | 175 | 80 | 97.9 | 90.7 | 74 | |
| H2O | Dioxane | NaCl (20 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
TiO2–ZrO2 (0.4 g) + Amberlyst 70 (0.4 g) | 4 | 5 | 175 | 180 | 100 | 86 | 75 |
| H2O | Toluene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33 2.33 |
Al-TUD-1 | 3.33 | 9 | 170 | 360 | 76 | 18 | 76 | |
| H2O | γ-Valerolactone | KBr (saturated) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
CrCl3·6H2O (isomerization) Amberlyst 38 wet (dehydration) | 0.5 | 10 | 160 | 1.48 (CrCl3·6H2O) | 98 | 74 | 77 |
| 1.4 (Amberlyst 38) | |||||||||||
H2O–DMSO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w) 7 w/w) |
Dichloromethane | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
HCl (pH = 1.5) | 0.1 | 10 | 140 | 270 | 62 | 48 | 78 |
H2O–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
β-(OF)-Cal500 | 1 | 2 | 180 | 30 | 80 | 60 | 79 | |
H2O–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w) 5 w/w) |
MIBK–2-butanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
— | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w) 2 (w/w) |
HCl (pH = 1.5) | 0.1 | 10 | 170 | 17 | 50 | 47 | 78 |
H2O–DMSO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
MIBK–1-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
Cr(III)-PDVB-0.3-SSFBI | 0.32 (of Cr(III)) | 5 | 140 | 240 | 95 | 59 | 80 | |
H2O–acetone (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w 2 w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) w) |
EtOAc | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Modified mordenites with NH4Cl Si/Al = 11.2 | 0.63 | 6.25 | 65 | 720 | 98 | 50 | 81 | |
| [Bmim][Cl] | Glycol dimethyl ether | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
CrCl3·6H2O | 7 | 80 | 108 | 60 | 97 | 64 | 82 | |
[Bmim][Cl]–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) 10 v/v) |
Glycol dimethyl ether | 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
CrCl3·6H2O | 5 | 80 | 108 | 60 | 95 | 62 | 82 | |
| [Bmim][Cl] | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
CrCl3·6H2O | 7 | 80 | 108 | 60 | 99 | 54 | 82 | |
| [Bmim][Cl] | MTBE | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
CrCl3·6H2O | 7 | 80 | 108 | 60 | 98 | 50 | 82 | |
| [Bmim][Cl] | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
CrCl3·6H2O | 7 | 80 | 108 | 60 | 98 | 52 | 82 | |
The use of methyl isobutyl ketone (MIBK) has been recurrent in literature, starting with one of the first reports, which used Lewatit SPC-108 in a continuous flow setup operating at 78 °C, a relatively low temperature for this reaction, reaching yields of 5-HMF of only 9%.61 These yields would much more recently be improved to 76% with selectivities of 85% using a heteropolyacid like Ag3PW12O40 as catalyst at 120 °C with loadings of 1 wt%.62 Jiménez-Morales et al. continued with the use of MIBK as EP obtaining yields of 23% after 150 min operating at 175 °C using mesoporous materials as catalysts, such as Ta2O5 or ZrO2–MCM-41.63,64 Also, further studies have been made using modifiers to enhance the performance of the reaction with respect to the system in their absence. For instance, saturation of the aqueous RP with NaCl (35 wt%) leading to yield increases from 40 to 49% using potash alum as catalyst qt 140 °C. More interestingly, the use of CaCl2 has been described as an enhancer of the activity in this reaction. Its use was compared to NaCl and a lean system without salts in preliminary experiments, obtaining the best results with CaCl2. Then, with optimized reaction conditions and γ-Al2O3 as catalyst, after only 15 minutes of reaction at 175 °C, the conversion reached was 96% with 52% yield. Reportedly, the interaction between Ca2+ ions and glucose favors the formation of α-D-glucopyranose hence avoiding the isomerization of glucose to fructose prior to dehydration, which enhances the conversion to 5-HMF.66 The use of this salt appears to open new possibilities, since this is one of the few pieces of work found where a different salt to NaCl was used.
Tetrahydrofuran (THF) has also extensively been employed as EP. At the same temperature conditions of 140 °C, practically complete conversions of glucose were obtained using phosphates as catalysts, although CrPO4 showed a selectivity to 5-HMF of 63%,67 significantly higher than that of FePO4, slightly higher than 23%.68 On the other hand, the use of Cr-β zeolite at 150 °C showed a somewhat lower conversion of 87%, although the yield reached 72%, resulting in an increased selectivity of 83%.69 In these studies, NaCl has been repeatedly utilized as modifier saturating the RP. Last, hafnyl phosphates have provided even higher selectivities of almost 91% for this reaction operating at 175 °C for 150 min, although in this case the concentration of NaCl in the aqueous phase was only of 4 wt%.70
In a study to test the activity of Sn-β zeolites promoted with HCl, 1-butanol (1-BuOH) was used as the EP. In this study, the performance of the system was tried with and without NaCl in the RP, leading to significant differences in the yields of 5-HMF obtained despite achieving similar glucose conversions. Although the temperature and reaction time varied slightly from one case to another, the use of NaCl (180 °C, 70 min) gave yields of 72% of 5-HMF compared to only 26% obtained without the modifier (160 °C, 90 min).71
Sec-butyl phenol has been the EP in a continuous flow microreactor at 180 °C with phosphate buffer saline catalyzing the operation, where the conversion observed was almost complete and the yield reached 76%. In this case, the aqueous phase was used in volumetric excess of 3 to 1.72 The same EP was used to test nanostructured niobia on carbon prepared by deposition precipitation, where despite using NaCl to saturate the RP only a 20% of yield to the product was observed.73
A study analyzed methyl tetrahydrofuran (MeTHF) to form the biphasic system performing the dehydration of glucose at 175 °C during 80 minutes with phosphate TiO2 as catalyst. This work compared the performance using MeTHF by itself and modifying the EP using a volume ratio of 6 to 1 of MeTHF to N-methyl pyrrolidone (NMP), observing a remarkable increase of Xglu from 89.4% to 97.9% and Y5-HMF from 58.8% to 90.7%.74
Among other solvents using as EP, dioxane has been proposed as an alternative after comparing the performance of other solvents including THF, 1-BuOH, MIBK and 1-propanol, all of which with NaCl (20 wt%). For the reaction, a mixed set of catalysts consisting of TiO2–ZrO2 and Amberlyst 70 were employed for 3 h at 175 °C to reach complete conversion of glucose and 86% yield to the product.75 The use of toluene has also been described in this biphasic reaction using aluminium-containing mesoporous TUD-1 as catalyst at 170 °C, with poor yields of 18% being reported after 6 h.76 Finally, the microwave-assisted isomerization-dehydration of glucose was undertaken using an aqueous biphasic system with γ-valerolactone while saturating the aqueous phase with KBr. For this reaction at 160 °C, after comparison with other metal halides, CrCl3 was used as the isomerization catalyst, while Amberlyst 38 wet conducted the dehydration step, reaching almost complete conversion and 74% yield.77
All of the biphasic systems mentioned above used solely water, whether or not including salts, as the RP. There are some examples in literature in which the RP is either modified with a second solvent or else alternative media like ILs are used as polar phase. Addition of different proportions of dimethyl sulfoxide (DMSO) to water is relatively common as has been reported for several cases. In a very comprehensive work, Dumesic et al. used different proportions of H2O–DMSO with different EPs to enhance the dehydration of different carbohydrates using simply HCl as catalyst. The best results were obtained using H2O–DMSO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w)/DCM, reaching yields of 48% to 5-HMF and H2O–DMSO (5
7 w/w)/DCM, reaching yields of 48% to 5-HMF and H2O–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w)/MIBK–2-BuOH (7
5 w/w)/MIBK–2-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) obtaining 47%.78 Similarly, a pair of phases of H2O–DMSO (3
3 w/w) obtaining 47%.78 Similarly, a pair of phases of H2O–DMSO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w)/MIBK–1-BuOH (7
1 w/w)/MIBK–1-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) was used in a reaction where a modified Cr(III)-containing bifunctional Brønsted–Lewis solid acid performed catalysis obtaining 95% of conversion and 59% yield.80 Additionally, using an organic structure directing agent free zeolite β as catalyst at 180 °C for 30 min in a biphasic system composed of H2O–DMSO (9
3 w/w) was used in a reaction where a modified Cr(III)-containing bifunctional Brønsted–Lewis solid acid performed catalysis obtaining 95% of conversion and 59% yield.80 Additionally, using an organic structure directing agent free zeolite β as catalyst at 180 °C for 30 min in a biphasic system composed of H2O–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v)/THF, conversion of 80% and selectivity of 75% were observed.79 A biphasic system consisting of a modified aqueous phase with acetone as RP and ethyl acetate (EtOAc) as EP was employed for the synthesis of 5-HMF reaching 50% yield using mordenites modified with NH4Cl.81
1 v/v)/THF, conversion of 80% and selectivity of 75% were observed.79 A biphasic system consisting of a modified aqueous phase with acetone as RP and ethyl acetate (EtOAc) as EP was employed for the synthesis of 5-HMF reaching 50% yield using mordenites modified with NH4Cl.81
Last, one work focuses on the use of [Bmim][Cl] as RP using different organic solvents as EPs, including glycol dimethyl ether, THF, methyl tert-butyl ether (MTBE) and MIBK. Other solvents like cyclohexanone, EtOAc or 1-BuOH were also unsuccessfully tested for biphasic behavior. In all cases CrCl3 was the catalyst employed and the performance was similar reaching quantitative conversions of glucose and yields ranging from 64 to 50% for the mentioned solvents.82
| Reactive phase (RP) | Extractive phase (EP) | Modifiers | Volume ratio RP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EP EP |
Catalyst | C cat (wt%) | C substrate (wt%) | T (°C) | Time (min) | X substrate (%) | Y 5-HMF (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
a Expressed as gH+/g reacting phase calculated on the basis of the ratio of equivalents of H+ introduced relative to the initial moles of D-fructose as specified in ref. 83.
b Originally stated as organic/catalyst ratio of 1 without further details.84
c For DESs, the ratio in brackets corresponds to the molar ratio of HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD.
d Not possible to calculate volumetric ratio since the density of the DES has not been reported in literature.
e Calculated as the wt% of the acid (HBD) with respect to the overall DES. HBD.
d Not possible to calculate volumetric ratio since the density of the DES has not been reported in literature.
e Calculated as the wt% of the acid (HBD) with respect to the overall DES.
|
|||||||||||
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 88 | 300 | Not stated | 36 | 83 | |
| H2O | MIBK | Not stated | Lewatit SPC-108 | WHSV = 8.06 g gcat−1 h−1 | Not stated | 78 | 420 | Not stated | 74 | 61 | |
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 1.25 |
Diaion PK216 | 100b | 10 | 90 | 1260 | 60 | 28 | 84 | |
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.25 2.25 |
Ag3PW12O40 | 1 | 30 | 120 | 60 | 82 | 78 | 62 | |
| H2O | MIBK | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
KAl(SO4)2 | 15.5 | 90 | 140 | 360 | 66 | 64 | 65 |
| H2O | MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HCl | 0.9 | 30 | 180 | 3 | 68 | 47.6 | 85 | |
| H2O | MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HCl | 0.9 | 50 | 180 | 3 | 71 | 41.9 | 85 | |
| H2O | MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Amberlyst-70 | 3.33 | 30 | 180 | 10 | 86 | 57.6 | 86 | |
| H2O | MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
TESAS-SBA-15 | 3.33 | 30 | 130 | 140 | 84 | 71 | 87 | |
| H2O | THF | NaCl | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
FePO4 | 2 | 2 | 140 | 15 | 99.9 | 71.5 | 68 |
| H2O | THF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cr/β zeolite | 2 | 10 | 150 | 90 | 90 | 74 | 69 |
| H2O | THF | NaCl (4 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HfO(PO4)x | 3 | 2 | 160 | 120 | 99.5 | 94.8 | 70 |
| H2O | γ-Valerolactone | KBr (saturated) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
HCl | 0.5 | 10 | 160 | 1 | >99 | 84 | 77 |
| H2O | 1-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
TiO2 | WHSV not stated | 23 | 200 | 3 | — | 18 | 88 | |
| H2O | 1-BuOH | NaCl (13.3 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6 3.6 |
HCl | 1.3 | 40 | 170 | 25 | 92 | 81.7 | 89 |
| H2O | 2-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Nb2O5·nH2O | 0.5 | 6 | 160 | 60 | 93 | 89 | 90 | |
| H2O | sec-Butyl phenol | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Phosphate buffer saline (pH = 2) (10 wt% NaH2PO4·2H2O; 2.2 wt% H3PO4) | Flow rate not given | 1 | 180 | 12 | 99 | 80.9 | 72 | |
| H2O | Toluene | 2.3 | Al-TUD-1 | 3.33 | 9 | 170 | 120 | 76 | 20 | 76 | |
| H2O | Butyronitrile | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 89 | 300 | Not stated | 24 | 83 | |
| H2O | Diethylketone | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 83 | 300 | Not stated | 17 | 83 | |
| H2O | Heptane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 84 | 300 | Not stated | 6 | 83 | |
| H2O | 2-Nitropropane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 87 | 300 | Not stated | 36 | 83 | |
| H2O | Benzonitrile | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 88 | 300 | Not stated | 40 | 83 | |
| H2O | 2,2′-Dichloroethylether | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 84 | 300 | Not stated | 42 | 83 | |
| H2O | Isopar E | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 85 | 300 | Not stated | 8 | 83 | |
| H2O | Isopar G | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Lewatit SPC-108 | 0.75a | 22.2 | 87 | 300 | Not stated | 9 | 83 | |
H2O–DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
MIBK-2–butanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/w) 3 (w/w) |
MeSAPO-5 | 1 | 10 | 170 | 120 | Not stated | 73.9 | 91 | |
H2O–DMSO (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
Diaion PK216 | 100b | 10 | 90 | 1260 | 68 | 41 | 84 | |
H2O–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
Diaion PK216 | 100b | 10 | 90 | 720 | 90 | 73 | 84 | |
H2O–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
(No catalyst) | 10 | 120 | 1440 | 67 | 52 | 84 | ||
H2O–NMP (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
Diaion PK216 | 100b | 10 | 90 | 1080 | 77 | 52 | 84 | |
H2O–NMP (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
Diaion PK216 | 100b | 10 | 90 | 1080 | 98 | 83 | 84 | |
H2O–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
DCM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
No catalyst | 100b | 10 | 120 | 330 | 92 | 74 | 78 | |
H2O–DMSO–PVP (5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HCl | 0.9 | 30 | 180 | 3 | 89 | 76 | 85 | |
H2O–DMSO–PVP (5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HCl | 0.9 | 50 | 180 | 3 | 92 | 71 | 85 | |
| [Bmim][Cl] | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/w) 20 (w/w) |
WCl6 | 2.2 | 16.7 | 50 | 240 | — | 72 | 93 | |
TEAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fructose (1 fructose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.92)c 0.92)c |
THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
TEAC (catalyst)/NaHSO4 (co-catalyst) | TEAC: 50 | 50 | 120 | 70 | 100 | 82.7 | 94 | |
NaHSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
|||||||||||
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fructose (1 fructose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2)c 0.2)c |
Acetonitrile | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
HCl | 0.3 | 20.1 | 100 | 240 | 100 | 90.3 | 95 | |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (1 citric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33)c 2.33)c |
EtOAc | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w)d 10 (w/w)d |
Citric acide | 77.8 | 1 | 70 | 1440 | Not stated | 92 | 96 | |
Again, MIBK appears to be the solvent of choice in many references, starting by what chronologically seems to be the first work published on the biphasic product of 5-HMF. In this work, the ion exchange resin Lewatit SPC-108 was the catalyst, taking the reaction place for 5 h at the relatively low temperature of 88 °C reaching 36% of yield; other resins tested showing poorer performance where Lewatit SPC-118, Nafion-H and Spherosil S.83 In the same study, the authors used volumetric ratios of 9 to 1 of other EPs to water, including toluene, butyronitrile, diethylketone, heptane, 2-nitropropane, isopar E, isopar G, benzonitrile and 2,2′-dichloroethylether. Benzonitrile and 2,2′-dichloroethylether outperformed the rest at yields of 40 and 42% of 5-HMF, respectively. Nevertheless, the nature of these two solvents is clearly less green.83 Lewatit SPC-108 was also used in continuous flow experiments with MIBK as EP reaching up to 74% yield of 5-HMF, much higher than with the same setup and conditions using glucose as substrate, as addressed in Table 1.61 In more recent work, the strongly acidic resin Diaion PK216 was used in biphasic systems of MIBK where the RP was either H2O or mixtures thereof with DMSO or NMP. When only H2O was used, operation at 90 °C after 21 h achieved a 60% conversion of glucose with 47% selectivity to the desired product. It appears that these results could be improved using H2O–DSMO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w) as RP, reaching yields of 73% with 90% conversion, and even somewhat further to 83% yield and 98% conversion with H2O–NMP (7
5 w/w) as RP, reaching yields of 73% with 90% conversion, and even somewhat further to 83% yield and 98% conversion with H2O–NMP (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w).84
3 w/w).84
The use of the heteropolyacid Ag3PW12O40 catalyst in the biphasic pair of solvents H2O/MIBK using fructose as substrate gave similar results to those described above when glucose was the starting material with yields of 78% after 1 h at 120 °C.62 In a NaCl saturated biphasic H2O/MIBK system, the use of potash alum as catalyst gave relatively low conversions of fructose of 66% at 140 °C and 6 h, although the selectivity to 5-HMF proved excellent being almost complete.65
Dumesic et al. had also tried in a previous study a large set of different modifications of the RP and EP in experiments performed with HCl as catalyst. Using the system H2O/MIBK–2-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w), a conversion of 71% and yield of 42% were obtained with an initial loading of fructose of 50 wt%. Most relevantly, when the RP was H2O–DMSO-polyvinylpyrrolidone (PVP) (5.6
3 w/w), a conversion of 71% and yield of 42% were obtained with an initial loading of fructose of 50 wt%. Most relevantly, when the RP was H2O–DMSO-polyvinylpyrrolidone (PVP) (5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) with MIBK–2-BuOH as EP, the results were improved to 92% conversion and 71% yield.85 Finally, a similar H2O/MIBK–2-BuOH (7
3 w/w) with MIBK–2-BuOH as EP, the results were improved to 92% conversion and 71% yield.85 Finally, a similar H2O/MIBK–2-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) biphasic medium was used by the same authors, for which the performance of resin Amberlyst-7086 and catalysts based on SBA-15 silica87 can be compared. Similar conversion of about 85% was achieved by both catalysts, although the yields were better in the case of the modified SBA-15 at 71%.87
3 w/w) biphasic medium was used by the same authors, for which the performance of resin Amberlyst-7086 and catalysts based on SBA-15 silica87 can be compared. Similar conversion of about 85% was achieved by both catalysts, although the yields were better in the case of the modified SBA-15 at 71%.87
The use of THF as EP was reported in previous studies for glucose, which have also extended their efforts to using fructose as substrate. The performance of the reactive and catalytic systems was quite similar in the cases of Cr-β zeolite and hafnyl phosphates,69,70 although changing the substrate to fructose gave much higher yields of 72% compared to only 23% with glucose using FePO4 as catalyst under the same conditions.68
This comment also applies to the comparison of the microwave-assisted production of 5-HMF in H2O (saturated with KBr)/γ-valerolactone with HCl, where changing to fructose as starting material improved yields to 84%.77
1-BuOH and 2-BuOH have also been used as EPs. In a first attempt, a continuous flow experiment was developed pumping the biphasic systems through TiO2 with a residence time of only 3 min at temperatures as high as 200 °C; however, the yield to 5-HMF only reached 18%.88 In a scaled-up experiment in a volume of 230 mL at 170 °C with HCl as catalyst, the authors optimized variables like the stirring speed in the biphasic system as well as the development of a macrokinetic model. The results reached a conversion of 92% with 81.7% of yield after only 25 min, which relates to finding operation conditions were mass transfer limitations are diminished.89 For its part, 2-BuOH has been the organic medium for the dehydration of fructose with niobia, for which high conversions of 93% were observed with almost complete selectivity after reaction at 160 °C for an hour at low loadings of only 0.5%.90 In addition, biphasic media where the EP included 2-BuOH have been used. A medium H2O–DSMO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 w/w)/MIBK–2-BuOH (7
6 w/w)/MIBK–2-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) was used with metal containing silicoaluminophosphate catalysts prepared from attapulgite reaching 74% of yield to 5-HMF.91 A similar H2O–PVP (10
3 w/w) was used with metal containing silicoaluminophosphate catalysts prepared from attapulgite reaching 74% of yield to 5-HMF.91 A similar H2O–PVP (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w)/MIBK–2-BuOH (7
1 w/w)/MIBK–2-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) biphasic system was put to use with EDTA as catalyst, which showed thermoresponsive behaviour being soluble in the RP at 160 °C, allowing to obtain yields of up to 89%. In this work, the composition of the loads of the RP and EP was optimized to the values mentioned before, although no great difference in performance was observed in any case. This work shows a very interesting approach as it allows to precipitate the catalyst at room temperature and further separate it from the RP.92
3 w/w) biphasic system was put to use with EDTA as catalyst, which showed thermoresponsive behaviour being soluble in the RP at 160 °C, allowing to obtain yields of up to 89%. In this work, the composition of the loads of the RP and EP was optimized to the values mentioned before, although no great difference in performance was observed in any case. This work shows a very interesting approach as it allows to precipitate the catalyst at room temperature and further separate it from the RP.92
Last, in some scattered studies, alternative media have been used as RP, in which one common aspect is that the organic phases used as EP are in larger excess than the typical ones observed for aqueous biphasic systems. Fructose conversion in IL [Bmim][Cl] was made using THF as EP in a mass excess of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, for which WCl6 catalyzed the reaction at only 50 °C, the lowest found for 5-HMF production, obtaining 72% of yield.93 In addition, some works have focused on the use of deep eutectic solvents (DESs) as reaction media. One interesting case is the use of eutectic mixtures formed by tetraalkyl ammonium chloride and alkylamine hydrochloride salts as well as fructose. In these mixtures, the former salts act as catalyst aided by NaHSO4 as co-catalyst, whereas the latter is the substrate of the reaction. Among the many salts tested, the system tetraethyl ammonium chloride with fructose as RP and THF as EP gave the best yield at 83% operating at 120 °C.94 Comparably, a DES system consisting in choline chloride (ChCl) as HBA and the substrate fructose as HBD in a 5 to 1 molar ratio was tested with acetonitrile as EP. In this case, complete conversion of fructose was observed with 90% selectivity with HCl as catalyst after 4 h at 100 °C.95 Finally, a mixture of ChCl with citric acid as HBD acted simultaneously as RP and catalyst with EtOAc forming the biphasic system where the activity was the highest compared to THF, 2-BuOH and MIBK, reaching yields of 92%.96
1, for which WCl6 catalyzed the reaction at only 50 °C, the lowest found for 5-HMF production, obtaining 72% of yield.93 In addition, some works have focused on the use of deep eutectic solvents (DESs) as reaction media. One interesting case is the use of eutectic mixtures formed by tetraalkyl ammonium chloride and alkylamine hydrochloride salts as well as fructose. In these mixtures, the former salts act as catalyst aided by NaHSO4 as co-catalyst, whereas the latter is the substrate of the reaction. Among the many salts tested, the system tetraethyl ammonium chloride with fructose as RP and THF as EP gave the best yield at 83% operating at 120 °C.94 Comparably, a DES system consisting in choline chloride (ChCl) as HBA and the substrate fructose as HBD in a 5 to 1 molar ratio was tested with acetonitrile as EP. In this case, complete conversion of fructose was observed with 90% selectivity with HCl as catalyst after 4 h at 100 °C.95 Finally, a mixture of ChCl with citric acid as HBD acted simultaneously as RP and catalyst with EtOAc forming the biphasic system where the activity was the highest compared to THF, 2-BuOH and MIBK, reaching yields of 92%.96
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid deep eutectic solvent was used as RP with EtOAc as EP to obtain 64% yield of 5-HMF at only 80 °C.97 Also, ethyl butyrate was the extracting agent in one system with the IL [Bmim][Cl] as RP. For this case, with a 2 wt% loading of CrCl3 at 130 °C for 3 h, a conversion of 83% of microcrystalline cellulose was attained, with almost equal yields to 5-HMF and glucose.98
oxalic acid deep eutectic solvent was used as RP with EtOAc as EP to obtain 64% yield of 5-HMF at only 80 °C.97 Also, ethyl butyrate was the extracting agent in one system with the IL [Bmim][Cl] as RP. For this case, with a 2 wt% loading of CrCl3 at 130 °C for 3 h, a conversion of 83% of microcrystalline cellulose was attained, with almost equal yields to 5-HMF and glucose.98
| Substrate | Reactive phase (RP) | Extractive phase (EP) | Modifiers | Volume ratio RP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EP EP |
Catalyst | C cat (wt%) | C substrate (wt%) | T (°C) | Time (min) | X substrate (%) | Y 5-HMF (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
a Originally stated as organic/catalyst ratio of 1 without further details.84
b Ratio in brackets corresponds to the molar ratio of HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD.
c Calculated as the wt% of the acid (HBD) with respect to the overall DES. HBD.
c Calculated as the wt% of the acid (HBD) with respect to the overall DES.
|
||||||||||||
| Inulin | H2O | MIBK | Not stated | Lewatit SPC-108 | WHSV = 8.06 g gcat−1 h−1 | Not stated | 78 | 720 | Not stated | 67 | 61 | |
| Inulin | H2O | 2-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Nb2O5 (NA-p) | 0.5 | 6 | 160 | 140 | 86 | 54 | 90 | |
| Inulin | Water–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
Diaion PK216 | 100a | 10 | 90 | 1260 | 100 | 62 | 84 | |
| Inulin | Water–NMP (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
Diaion PK216 | 100a | 10 | 90 | 1260 | 100 | 69 | 84 | |
| Inulin | Water–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
DCM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
No catalyst | N/A | 10 | 120 | 210 | 100 | 61 | 84 | |
| Inulin | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid (1 oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)b 1)b |
EtOAc | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Oxalic acid | 78.4c | 47 | 80 | 120 | 64 | 64 | 97 | |
| Sucrose | H2O | MIBK | Not stated | Lewatit SPC-108 | WHSV = 8.06 g gcat−1 h−1 | 78 | 720 | Not stated | 82 | 61 | ||
| Sucrose | H2O | THF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cr/β zeolite | 2 | 10 | 150 | 90 | Not stated | 70 | 69 |
| Sucrose | H2O–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
Diaion PK216 | 100a | 10 | 90 | 1260 | 55 | 38 | 84 | |
| Sucrose | H2O–NMP (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
Diaion PK216 | 100a | 10 | 90 | 1260 | 58 | 43 | 84 | |
| Sucrose | H2O–DMSO (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
DCM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) 1 (w/w) |
No catalyst | N/A | 10 | 120 | 210 | 60 | 41 | 84 | |
| Cellobiose | H2O | THF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cr/β zeolite | 2 | 10 | 150 | 90 | Not stated | 56 | 69 |
| D-Galactose | H2O | MIBK | Not stated | Lewatit SPC-108 | WHSV = 8.06 g gcat−1 h−1 | Not stated | 78 | 960 | Not stated | 5 | 61 | |
| D-Mannose | H2O | MIBK | Not stated | Lewatit SPC-108 | WHSV = 8.06 g gcat−1 h−1 | Not stated | 78 | 960 | Not stated | 7 | 61 | |
| L-Sorbose | H2O | MIBK | Not stated | Lewatit SPC-108 | WHSV = 8.06 g gcat−1 h−1 | Not stated | 78 | 720 | Not stated | 47 | 61 | |
| Raffinose | H2O | MIBK | Lewatit SPC-108 | Not stated | 78 | 600 | Not stated | 80 | 61 | |||
| Jerusalem artichoke | H2O | MIBK | Lewatit SPC-108 | Not stated | 78 | 900 | 73 | 61 | ||||
| Microcrystalline cellulose | H2O | THF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cr/β zeolite | 2 | 10 | 150 | 90 | Not stated | 16 | 69 |
| Microcrystalline cellulose | H2O | THF | NaCl (4 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HfO(PO4)x | 3 | 2 | 190 | 240 | 89.7 | 69.8 | 70 |
| Microcrystalline cellulose | H2O | MeTHF | NaCl (4 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HfO(PO4)x | 3 | 2 | 190 | 240 | 90.9 | 71.9 | 70 |
| Microcrystalline cellulose | H2O | Dioxane | NaCl (4 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HfO(PO4)x | 3 | 2 | 190 | 240 | 90.3 | 70.2 | 70 |
| Microcrystalline cellulose | H2O | MIBK | NaCl (4 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HfO(PO4)x | 3 | 2 | 190 | 240 | 89.7 | 69.8 | 70 |
| Microcrystalline cellulose | H2O | 1-BuOH | NaCl (4 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HfO(PO4)x | 3 | 2 | 190 | 240 | 88.3 | 59.6 | 70 |
| Microcrystalline cellulose | H2O | 2-BuOH | NaCl (4 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
HfO(PO4)x | 3 | 2 | 190 | 240 | 87.4 | 54.5 | 70 |
| Microcrystalline cellulose | [Bmim][Cl] | Ethyl butyrate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
CrCl3 | 2 | 10 | 130 | 180 | 82.7 | 41 | 98 | |
Focusing on aqueous biphasic systems, in their work from 1983, apart from fructose and glucose, Rigal and Gaset tried different carbohydrates as feedstock for dehydration reactions in their continuous setup with Lewatit SPC-108 using MIBK as EP. Examples are inulin (with a yield of 67%), Jerusalem artichoke (73%), monosaccharides like D-mannose (7%), D-galactose (5%), D-mannose (7%) or L-sorbose (47%) and oligosaccharaides like sucrose (82%), which features a glucose and a fructose unit, and raffinose (80%), the trisaccharide containing also galactose.61
Inulin has been converted to 5-HMF in further works. For instance, the solvent pair H2O/2-BuOH was utilized for the reaction followed by in situ extraction with loadings of 0.5 wt% of pretreated niobium pentoxide as catalyst in the RP reaching 54% yield in 140 min90 This substrate has also used in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid DES as reaction medium and catalyst simultaneously with EtOAc as EP in a 1
oxalic acid DES as reaction medium and catalyst simultaneously with EtOAc as EP in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 volumetric ratio, reaching 64% of conversion of inulin with full selectivity towards 5-HMF.97 Also, following up on a work previously described, Dumesic et al. tested variations of the RP and EP for this reaction with inulin as substrate, obtaining the best outcome with H2O–NMP (4
10 volumetric ratio, reaching 64% of conversion of inulin with full selectivity towards 5-HMF.97 Also, following up on a work previously described, Dumesic et al. tested variations of the RP and EP for this reaction with inulin as substrate, obtaining the best outcome with H2O–NMP (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 w/w)/MIBK, which gave yields of 69%. In addition, sucrose was converted to 5-HMF showing this solvent system the best results at 43% yield, although the conversion of the substrate was significantly lower, reaching only 58%.84
6 w/w)/MIBK, which gave yields of 69%. In addition, sucrose was converted to 5-HMF showing this solvent system the best results at 43% yield, although the conversion of the substrate was significantly lower, reaching only 58%.84
Cellobiose is the disaccharide consisting of two β-glucose units linked by a β bond, whereas sucrose is composed of one unit of glucose and another of fructose, and as such both can yield 5-HMF from dehydration after hydrolysis. Xu et al. approached their conversion by saturating the aqueous phase with NaCl as reported in many other works and THF as EP. Using Cr/β zeolite during 1.5 h at 150 °C, yields of 56 and 70% were achieved for cellobiose and sucrose, respectively.69
Finally, special mention deserves the work by Cao et al., where they approached the biphasic dehydration of microcrystalline cellulose testing different solvents as EP with water with NaCl (4 wt%) as RP. The reaction conditions included the use of hafnyl phosphates at loadings of 3 wt% operating at 190 °C during 4 h. Regardless of the organic solvent used, conversions of the substrate reached systematically figures around 90%, whereas the yields were of about 70% for MeTHF, dioxane, THF and MIBK, slightly higher than those attained with 1-BuOH and 2-BuOH, below 60%.70
In addition to these studies, it is relevant to mention that the use of biphasic systems for the combined production of 5-HMF and furfural starting from alternative carbohydrate substrates, including actual biomass sources, is gaining increasing attention.5 Moreover, an interesting alternative for the production of furans in biphasic systems could be the synthesis of 5-chloromethylfurfural (CMF). This compound represents an alternative to 5-HMF that could be more practical for subsequent synthesis as a precursor of biofuel 5-ethoxymethylfurfural and whose separation with organic solvents could be favourable considering the fact that it is less polar than 5-HMF.32,99 A case of study of the production of CMF is its biphasic generation from microcrystalline cellulose, corn stover, straw or birch wood using a H2O/dichloroethane solvent pair modified with LiCl.100,101
Production of furfural
The molecular structure of furfural comprises a furan ring and an aldehyde functional group and its production mainly takes place by the dehydration of pentoses. Although the feedstock for the reaction can be sugars like arabinose or ribose,30 xylose is by far the most studied substrate for the production of furfural. The reason for this is that xylose constitutes the backbone of xylan, a biopolymer of great abundance in nature from whose hydrolysis such pentose can be obtained.102 Once the xylose units have been released, the conversion to furfural involves the overall release of three water molecules, which may occur through different mechanisms including enolization, β-elimination or via cyclic intermediates, as covered extensively in a review on the mechanistic aspects of homogenous catalysts for this reaction.103Fig. 4 schematizes the overall conversion starting from xylan as feedstock.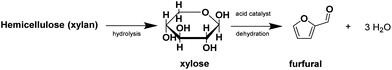 | ||
| Fig. 4 Scheme of the conversion to furfural starting from xylan through its hydrolysis to xylose and further dehydration. | ||
As in the case of 5-HMF, some studies have put their efforts on the exploitation of different biomass sources for the production of furfural following a biphasic approach. However, the number of studies is not as numerous as for 5-HMF. Some examples of sources for the process would be bamboo, for which a H2O/MIBK system was employed,104 wheat straw, performing the in situ extraction with THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 or rice straw, which used 2-PrOH, 1-BuOH, MIBK and THF as EPs.106 Special mention deserves the comprehensive work performed by Yang et al., where they assessed the use of many different biomass feedstock in a H2O/THF biphasic system, including pinewood, switchgrass, corn stover or poplar among others.107 Although the production of furfural prevailed in these works, the nature of the biomass and the carbohydrate polymers within their structure also allowed the generation of 5-HMF. Despite all the carbohydrate sources that can be used as starting material for the process, Table 4 focuses on references where the transformation originates from xylose. As in the case of hexoses, in general the RPs used consisted water, which in some cases were modified with NaCl to promote the migration of furfural to the organic solvent. Concerning the rest of the operating conditions, both the volume ratio of both solvents and the temperature range appear to be similar to the case of hexoses and the catalyst also have acidic properties as needed for the dehydration.
105 or rice straw, which used 2-PrOH, 1-BuOH, MIBK and THF as EPs.106 Special mention deserves the comprehensive work performed by Yang et al., where they assessed the use of many different biomass feedstock in a H2O/THF biphasic system, including pinewood, switchgrass, corn stover or poplar among others.107 Although the production of furfural prevailed in these works, the nature of the biomass and the carbohydrate polymers within their structure also allowed the generation of 5-HMF. Despite all the carbohydrate sources that can be used as starting material for the process, Table 4 focuses on references where the transformation originates from xylose. As in the case of hexoses, in general the RPs used consisted water, which in some cases were modified with NaCl to promote the migration of furfural to the organic solvent. Concerning the rest of the operating conditions, both the volume ratio of both solvents and the temperature range appear to be similar to the case of hexoses and the catalyst also have acidic properties as needed for the dehydration.
| Reactive phase (RP) | Extractive phase (EP) | Modifier | Volume ratio RP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EP EP |
Catalyst | C cat (wt%) | C subs (wt%) | T (°C) | Time (min) | X subs (%) | Y fur (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
KAl(SO4)2 | 15 | 75 | 190 | 360 | Not stated | 55 | 65 | |
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33 2.33 |
Lignin-derived sulphated carbon | 1.67 | 6.67 | 175 | 180 | 98 | 68 | 108 | |
| H2O | MIBK | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
SO42−/TiO2–ZrO2/La3+ | 0.66 | 1.33 | 120 | 240 | 49.2 | 0.9 | 109 | |
| H2O | MIBK | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
CrPO4 | 1.5 | 10 | 160 | 60 | 98 | 86 | 110 |
| H2O | CPME | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.71 2.71 |
Nb2O5 | 3 | 4.5 | 130 | 360 | 99 | 58 | 111 | |
| H2O | CPME | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Sulfonated carbon-based (from Miscanthus × giganteus) | 1.5 | 15 | 190 | 60 | 97 | 62 | 112 | |
| H2O | CPME | NaCl (8.8 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Sulfonated sporollenin | 10 | 15 | 190 | 40 | 98 | 69 | 113 |
| H2O | CPME | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
No catalyst | N/A | 2.79 | 190 | 180 | 100 | 78 | 114 | |
| H2O | 1-BuOH | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
CrPO4 | 1.5 | 10 | 160 | 60 | 96 | 48 | 110 |
| H2O | 2-BuOH | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
CrPO4 | 1.5 | 10 | 160 | 60 | 82 | 19 | 110 |
| H2O | 1-PrOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Nb2O5 | 3 | 4.5 | 130 | 360 | 90 | 50 | 111 | |
| H2O | γ-Valerolactone | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.57 8.57 |
Nb2O5 | 3 | 4.5 | 130 | 360 | 97 | 48 | 111 | |
| H2O | Toluene | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
CrPO4 | 1.5 | 10 | 160 | 60 | 100 | 71 | 110 |
| H2O | Toluene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
M-20 MOR zeolite | 10 | 10 | 150 | 960 | 78.6 | 42.1 | 115 | |
| H2O | Toluene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Terephthalic acid | 2.5 | 8.9 | 190 | 180 | 91.8 | 70.9 | 116 | |
| H2O | MeTHF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
CrPO4 | 1.5 | 10 | 160 | 60 | 96 | 82 | 110 |
| H2O | MeTHF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
No catalyst | N/A | 2.79 | 170 | 180 | 92 | 71 | 114 | |
| H2O | MeTHF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
SO42−/Sn-MMT | 0.5 | 2 | 160 | 120 | Not stated | 79.7 | 117 |
| H2O | THF | NaCl | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
AlCl3·6H2O | 2.4 | 3.75 | 140 | 45 | 99 | 80 | 107 |
| H2O | THF | NaCl (35 wt%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
CrPO4 | 1.5 | 10 | 160 | 60 | 99 | 88 | 110 |
| H2O | Isophorone | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
No catalyst | N/A | 2.79 | 170 | 180 | 92 | 49 | 114 | |
| H2O | Butyl acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Terephthalic acid | 1.75 | 10 | 180 | 180 | Not stated | 60.1 | 116 | |
H2O–1,3-dimethyl-2-imidazolidinone (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
SO42−/TiO2–ZrO2/La3 | 0.66 | 1.33 | 180 | 720 | 97.9 | 53.5 | 109 | |
H2O–DMSO (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
SO42−/TiO2–ZrO2/La3 | 0.66 | 1.33 | 120 | 240 | 60.8 | 10.8 | 109 | |
H2O–DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
MIBK-2–BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
SO42−/TiO2–ZrO2/La3 | 0.66 | 1.33 | 120 | 240 | 63.4 | 8.1 | 109 | |
| H2O | sc-CO2 | CO2 flow of 3.77 g min−1. P = 20 MPa | Amberlyst 70 | 10 | 10 | 150 | 960 | 87.8 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
115 |
MIBK has also been extensively used for the biphasic production of furfural. When using potash alum for 6 h at 190 °C, 55% yield to furfural was obtained65 and even higher yields of 68% could be reached at slightly lower temperatures and half of the time with lignin-derived sulphated carbon catalysts.108 However, when a solid acid catalyst generically denoted as SO42−/TiO2–ZrO2/La3+ was put to use, the H2O/MIBK biphasic system gave poor yields below 1%, reason for which the RP and EP were altered further in this study. Using H2O–DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w)/MIBK–2-BuOH (7
2 w/w)/MIBK–2-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) and H2O–DMSO (8
3 w/w) and H2O–DMSO (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w)/MIBK–2-BuOH (7
2 w/w)/MIBK–2-BuOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) led to improving the performance slightly up to 8 and 11% approximately, although a much greater increase was observed when the RP consisted of H2O-1,3-dimethyl-2-imidazolidone (8
3 w/w) led to improving the performance slightly up to 8 and 11% approximately, although a much greater increase was observed when the RP consisted of H2O-1,3-dimethyl-2-imidazolidone (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) with the mentioned EP. With this solvent pair, almost complete conversion of xylose was reached with 53.5% yield to furfural after 12 h at 180 °C, for which a kinetic study was performed.109 Last, on the use of MIBK as organic solvent, saturation of the aqueous phase with NaCl led to an excellent performance of 86% yield with CrPO4 after 1 h at 160 °C. This study compared the performance of THF, which obtained a similar 88%, with linear alcohols 1-BuOH and 2-BuOH, both reaching significantly lower yields in the same conditions.110
2 w/w) with the mentioned EP. With this solvent pair, almost complete conversion of xylose was reached with 53.5% yield to furfural after 12 h at 180 °C, for which a kinetic study was performed.109 Last, on the use of MIBK as organic solvent, saturation of the aqueous phase with NaCl led to an excellent performance of 86% yield with CrPO4 after 1 h at 160 °C. This study compared the performance of THF, which obtained a similar 88%, with linear alcohols 1-BuOH and 2-BuOH, both reaching significantly lower yields in the same conditions.110
Cyclopentyl methyl ether (CPME) is an emerging solvent that can be obtained from the etherification of biobased cyclopentanol, which can be produced from furfural.118 To the best of our knowledge, CPME remains to be reported for the biphasic production of 5-HMF, although it appears recurrently for the transformation of xylose to furfural. For example, different catalysts based on niobia supported on silica-zirconia were tested in an aqueous biphasic system at 130 °C for 6 h, reaching quantitative conversion of xylose with 58% yield to furfural with CPME as solvent as compared to 1-PrOH (50%) and γ-valerolactone (48%).111 These yields were improved by Len et al. operating at higher temperatures of 190 °C during only 1 h in the microwave-assisted reaction with sulfonated carbon-based catalysts prepared from Miscanthus × giganteus.112 Subsequently, performance was even better reaching 69% yields using sulfonated sporollenin implementing also the modification of the RP with 8.8 wt%. NaCl; in this study, xylan was also used as carbohydrate attaining yields to furfural of 37%.113 Finally, a recent study reported on the use of the H2O/CPME system for this reaction in the absence of any catalyst. The authors claim that an autocatalytic effect takes place when operating with microwaves at 190 °C during 3 h, reporting complete conversion of xylose with 78% selectivity; the authors also reported the use of MeTHF and isophorone as EP under the same conditions with lower performance. In addition, birch hydrolysate was employed as substrate with similar conversions and yields of 68% after only 1.5 h.114
The biphasic dehydration of xylose with toluene as EP has been reported with different zeolites, where MOR-type M-20 (SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3 = 20
Al2O3 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) achieved 42% yield to furfural and 79% conversion of xylose after 3 h at 190 °C in a screw-stirred autoclave.115 Recently, a loading of 2.5 wt% of terephthalic acid obtained from waste polyethylene terephthalate has provided good results as catalyst in H2O/toluene medium leading to 71% yields at 190 °C in 3 h, which was higher than when butyl acetate was the extracting agent in comparable reaction conditions, reaching 60%.116 Aqueous biphasic systems of H2O with toluene and MeTHF were used with loadings of 1.5 wt% of CrPO4 at 160 °C in 1 h, obtaining better results with the latter (82% yield with better selectivities) than with toluene (71% yield).110 In an uncommon manner, the latter solvent was used in volumetric defect with respect to water saturated in NaCl in one study, in which xylose was transformed utilizing sulphated tin ion-exchanged montmorillonites as catalysts, reaching yields of almost 80% at 160 °C for 1 h. The use of this systems was extended to the valorization of xylan, obtaining 74% of yield.117 Finally, THF was employed with NaCl-saturated water to perform the microwave-assisted catalytic dehydration of xylose and xylan with AlCl3, attaining at 140 °C and 45 min yields of 80% and 64%, respectively.107
1) achieved 42% yield to furfural and 79% conversion of xylose after 3 h at 190 °C in a screw-stirred autoclave.115 Recently, a loading of 2.5 wt% of terephthalic acid obtained from waste polyethylene terephthalate has provided good results as catalyst in H2O/toluene medium leading to 71% yields at 190 °C in 3 h, which was higher than when butyl acetate was the extracting agent in comparable reaction conditions, reaching 60%.116 Aqueous biphasic systems of H2O with toluene and MeTHF were used with loadings of 1.5 wt% of CrPO4 at 160 °C in 1 h, obtaining better results with the latter (82% yield with better selectivities) than with toluene (71% yield).110 In an uncommon manner, the latter solvent was used in volumetric defect with respect to water saturated in NaCl in one study, in which xylose was transformed utilizing sulphated tin ion-exchanged montmorillonites as catalysts, reaching yields of almost 80% at 160 °C for 1 h. The use of this systems was extended to the valorization of xylan, obtaining 74% of yield.117 Finally, THF was employed with NaCl-saturated water to perform the microwave-assisted catalytic dehydration of xylose and xylan with AlCl3, attaining at 140 °C and 45 min yields of 80% and 64%, respectively.107
Other than solvents that are regularly liquid at ambient conditions, in situ extraction of furfural was attempted with supercritical CO2, for which pressurization at 20 MPa at 150 °C was needed to achieve 88% conversion and 52% of yield with a 10 wt% loading of Amberlyst 70.115 One last note goes to a study where Eucaliptus urophydis as source of biomass was dehydrated to furfural using a DES and MIBK as biphasic system. In this case, ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid (2
oxalic acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was the RP, where AlCl3 was used as catalyst at 140 °C during 90 minutes reaching a 70.3% yield to furfural in addition to 18.7% yield to 5-HMF.119
1) was the RP, where AlCl3 was used as catalyst at 140 °C during 90 minutes reaching a 70.3% yield to furfural in addition to 18.7% yield to 5-HMF.119
Tools and resources for the selection of solvents
Green solvent selection guides
Owing to the unceasing and ever growing pressure of regulations and legislative constraints, the utilization of cleaner and green chemicals is a must in current activities in chemistry both in industry and research. Regulations like that concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH, EC 1907/2006) and the Integrated Pollution Prevention and Control (IPPC, EC 1/2008) have been adopted within the EU frame.120The use of solvents is ubiquitous in chemical applications and, despite the possibility of reutilization by means of separation processes, the volume used during the corresponding operations usually amounts to several times the mass of the actual product to be synthesized or isolated. Subsequently, the use of solvents that comply with the principles of Green Chemistry60,121 is more than desired. The definition of a green solvent can entail many implications, since it can be considered that not only EHS parameters should be taken into account, but also material and energy demands involved in their production, usually accounted for by means of life cycle assessments with software and databases.40,122,123 Merely based on EHS considerations as criteria for the selection, more computer-aided tools have been developed. In 2000 Hungerbühler et al. developed an automated method for the assessment EHS performance in the early design stages by considering up to 11 effect categories; this method resorted to databases and closed data gaps by means of quantitative structure activity relationships (QSAR).124 Other more recent examples are the tool developed by Slater and Savelski, which considered acute toxicity or biodegradation among a total of 12 categories125 or the Paris III software, which implements a EHS evaluation for the comparison and eventual substitution of solvents based on 8 environmental impact parameters, 8 physicochemical properties and 10 chemical family classifications and the use of the Toxicity Estimation Software Tool of the EPA as database.126
In spite of the efforts mentioned above, in the spirit of providing bodies of information of easy use regarding EHS criteria and LCA considerations, a series of guides have appeared based on quick color-coded (green, amber and red) classification. These have been developed by different institutions, mainly pharmaceutical companies, to establish preferences of use according to their own practice and policies. One remarkable aspect of most of these guides is that they present separate scoring systems for EHS and LCA items, as opposed to some of the software tools, which give a lumped scored. This can lead to somewhat misleading averages, such as in the use of dipolar aprotic solvents, safe to use but with considerable toxicity.46 A brief summary of these guides is presented in Table 5 summarizing their most relevant features concerning the amount of solvents tested and EHS and LCA parameters considered as well as the denomination of their conclusion for recommendation.
| a No overall conclusion, but EHS parameters individually considered. |
|---|
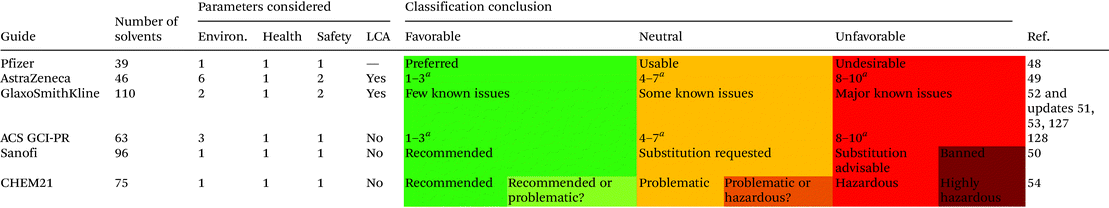
|
Although not chronologically the first, Pfizer published a guide for medicinal chemists dealing with 39 classical solvents, which they classified into three categories (preferred, usable or unpreferred). Their analysis was based on three general areas, namely: worker safety, process safety and environmental and regulatory considerations, which could correspond to health, safety and environment.48 AstraZeneca developed their own guidelines considering originally a set of up to 10 EHS parameters in total, although they would further update their tool with properties relevant to their actual use in practice, including physicochemical and solvent properties.49 GlaxoSmithKline has performed a continuous effort of developing and updating their solvent selection guides, from their first version in 1999 where the four areas waste, impact, health and safety were featured52 to more recent versions, where LCA assessments have been included to an increasing number of chemicals as well as regulatory flags and a more detailed breakdown of the EHS areas.51,53,127 The ACS Green Chemistry Institute created the Pharmaceutical Roundtable (GCI-PR) also published their guide128 along with resources on their website based on the original AstraZeneca tool.129 The company Sanofi made an effort for up to 96 substances, where they included further detail in the scores by integrating the solvent limits for pharmaceuticals following the International Conference on Harmonization (ICH).50 Owing to their recurrent use as reference, in 2014 Prat et al. published a short survey on these solvent selection guides, in which the pool of 51 solvents in the guides evaluated at that time were classified into the categories of recommended, problematic, hazardous and highly hazardous according to the assessment provided in the original guides. Despite not trying to create a universal guide, they tried to systematize the evaluation in AstraZeneca's, GCI-PR's and GSK's guides giving numerical values from the EHS evaluation and hence propose an overall ranking of solvents.47
Finally, Innovative Medicines Initiative (IMI)-CHEM21 originated as a collaborative effort among different companies and public institutions. Even though the previous guides include a good number of common solvents in industrial practice, new chemicals are becoming increasingly available for use, especially those derived from biobased sources or from transformations thereof. In their efforts to develop the application of more sustainable chemical methodologies, in a publication of 2016 the CHEM21 guide provides an outstanding spreadsheet-based tool as ESI† that allows obtaining EHS scores based on H3XX and H4XX statements, which can normally be retrieved from the REACH database of registered substances.130 Despite these statements not being given to chemicals whose toxicological data are not available, which in turn means that this tool will assign a default score for these cases,51 this tool appears to be very comprehensive and user-friendly as only a few pieces of information that are easily available are needed.
In addition to these helpful and convenient guides, there are other tools that allow estimating different magnitudes related to persistence, bioaccumulation and toxicity (PBT). As was addressed in a recent review for the selection of solvents in biphasic enzymatic processes, the generation of aqueous waste streams with low levels of organic solvents should be considered.131 For this reason, these authors decided to include in their compilation of solvents an evaluation of PBT, for which they used the criteria described by the New Chemicals Program of the EPA,132 to which an online assessment tool named PBT profiler is associated. This tool appears to feature a series of predictive methods for the estimation of a number of items, such as bioconcentration factors or toxicity to fish, which had already been implemented in the Estimation Program Interface (EPI Suite TM), also developed by EPA.133 Nevertheless, as of August 2019, the site for the PBT profiler appears to be unavailable to the public, although EPI Suite TM is still online. More recently, the Laboratory of Environmental Chemistry and Toxicology of the Mario Negri Institute of Pharmacology has developed the VEGA application. This application implements models from different previous projects (5 from CAESAR, 2 from EPA's Toxicity Estimation Software Tool, 1 from DEMETRA and 3 from EPI Suite TM) and 18 developed by the Laboratory to estimate different PBT parameters.134 These tools are based on quantitative structure activity relationships, for which the input required is the structure of the molecule, usually given in SMILES format.
Methodologies for solvent selection. The COSMO-RS method
Throughout the years a number of methods have appeared to better understand and predict the solvation capacity of chemical compounds and, subsequently, be able to predict the ability of solvents to perform properly for the desired purpose, including reaction or separation processes.This exploratory work has undergone developments as the models to describe these phenomena became more complex and included more parameters. In 1965, Reichardt reviewed several empirical paramaters to describe the polarity of solvents with associated polarity scales in most cases. These parameters included the Y-values (a quantitative measure of the ionizing power of a solvent), X-values (proportional to the polarity of the solvent taking glacial acetic acid as reference), Ω-values (an empirical parameter of the polarity of the solvent), Z-values (a direct empirical measure of the solvation behavior of a solvent towards 1-ethyl-4-methoxycarbonylpyridinium iodide, a dye used as reference), R and S-values (which relate to the free enthalpy of reactions of substituted benzene derivatives accounting for the kinetic effects taking place when the solvent for the reaction is changed). Finally, Reichard himself developed the E(T)-values, which represents a measure of the ionizing power of a solvent based on the maximum wavenumber of the longest wavelength electronic absorption band of pyridinium N-phenolbetaine, taking as reference substance.135 The latter parameter constitutes a joint measure of polarity and acidity. Other efforts include the Hildebrand solubility parameter (δ), which is an estimate of the degree of interaction between solvents and solutes, in which compounds with similar δ values are more likely to have mutual miscibility. δ is calculated as a function of the cohesive energy density of the solvent, which corresponds to the energy required to remove a unit volume of molecules from the surrounding molecules to infinite separation (i.e., ideal gas state). This parameter gives better results when nonpolar and slightly dipolar interactions occur, but fails to predict the solubility when polar interactions such as hydrogen bonding (HB) prevail.136 Afterwards, Hansen presented further work suggesting that the cohesion of a substance takes places as result of the combination of three intermolecular interactions, explaining each with a different parameter, namely: London dispersion forces (δd), polar interactions related to Keesom forces (δp), and HB (δh). The so-called Hansen solubility parameter (δH) results from the square root of the sum of the squares.44 Later, the Kamlet–Taft solvatochromic scale was put forward, whose parameters are the HBD capacity (α), the HBA ability (β) and last the dipolarity-polarizability of the compound (π*).43,137,138Table 6 summarizes the essential information of these models.
| Model | Parameters | Ref. |
|---|---|---|
| Reichard's parameter | 1 (E(T)): a measure of both polarity and acidity together, although it is not dependent on polarizability | 135 |
| Hildebrandt solubility parameter | 1 (δ): numerical estimate of the degree of interaction between solvents and solutes | 136 |
| Hansen solubility parameters | 3 (δd, δp and δh): for London dispersion forces, polar interactions related to Keesom forces and HB | 44 |
| Kamlet–Taft parameters | 3 (α, β, and π*): for hydrogen bond donor ability (acidity), hydrogen bond acceptor ability (basicity) and polarizability | 43, 137 and 138 |
Despite the importance of the aforementioned empirical models, owing to the outstanding advances in computational chemistry, more advanced predictive methodologies have been put to use more recently. Such is the case of the COnductor-like Screening MOdel for Real Solvents (COSMO-RS), a well-known quantum chemistry-based method to predict the thermodynamic equilibria of fluids and liquid mixtures developed by Klamt.139,140 In this method, first quantum chemical COSMO calculations are performed to obtain the screening charge densities σ on the molecular surface, for which a prior step of geometry optimization of the molecular structure is needed. Then, the chemical potentials of each chemical species is computed using a statistical thermodynamic approach, for which an integration of the so-called σ-profile is performed. This σ-profile, obtained from geometry optimization, represents a histogram of charge density distribution around the molecular surface, which can be envisioned as molecular descriptors, for they also provide relevant information about the interaction energies of the species in solution, such as hydrogen bonding, van der Waals forces and misfit and electrostatic interactions. The computed chemical potentials are then the foundation for the subsequent calculation of key thermodynamic properties, such as activity coefficients, solubilities or gas–liquid and liquid–liquid equilibria (LLE). Remarkably, unlike the empirical models described in Table 6, COSMO-RS does not require experimental data for specific method adjustments, thereby allowing the estimation of properties solely from the structure of the chemicals, which makes it highly versatile for solvent design changing structural information.120,139–141 This method has been successfully applied in a wide array of case studies in process chemistry apart from identifying solvents for 5-HMF extraction.142 One example is the screening of solvents for the extraction of artemisinin in a range of conventional and novel solvents, such as organic carbonates.143 Similarly to the case of the biphasic dehydration of sugars with in situ extraction of furans subject of this review, COSMO-RS has also previously been used for the screening of solvents in aqueous biphasic system in the enzymatic production of (R)-3,3′,5,5′-tetramethoxy-benzoin.144 Another case is the estimation of the partition ratio of Biphephos ligand in different pairs of solvents for the selection of thermomorphic solvent systems in which to recycle molecular catalysts in the hydroformylation of olefins.145 In addition to these LL-based systems with molecular solvents, this method has also proven successful in predictions in alternative media, like the solubility of gases like CO2 in ILs146 or the extraction of linalool from terpenes in ChCl-based DESs.147
Use of COSMO-RS and methodology for the selection of green solvents
Computational details
The COSMO-RS method has been used through its implementation in the COSMOthermX software, version C30, release 18.0.0 at the corresponding parameterization BP_TZVP_C30_1800. As input files, the COSMO files included in COSMObase (v.17) were used, which have undergone geometry optimization and calculation of the screening charge density for all conformers.148Comparison with values reported in literature
In the present work, first a comparison is made between results present in literature and those predicted by the COSMO-RS. This validation is made on the basis of the distribution coefficient (R), defined as the ratio of the mass fraction (w) of the solute (furans in this case) in the EP (organic solvent) to that in the RP (aqueous), as appears in eqn (1).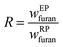 | (1) |
These calculations in the COSMOtherm software were made as LLE of ternary or multinary systems, as appropriate, to which compositions of the overall feed must be entered.
In non-reactive systems where LLE were measured, to compare COSMO-RS predictions with experimental results in which the literature only provides LLE data corresponding to the composition of the tie-lines but not to that of the feed, the latter was estimated using an image digitizer tool incorporated in OriginPro 9.1.0.149 As a measure of the deviation of the COSMO-RS prediction to the reported values, the residual mean square deviation is computed, as defined in eqn (2) for ternary systems:150,151
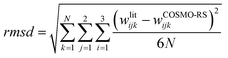 | (2) |
In references where distribution coefficients were measured after performing a reaction, the composition of the feed for was determined from the experimental data supplied in each individual reference and transformed to molar fraction.
Methodology and workflow for the selection of green solvents
Regarding the methodology for the selection of solvents, the COSMO-RS method has been used as the basis for the workflow followed during the evaluation of organic solvents for the separation of 5-HMF and furfural from an aqueous reacting phase, for which a schematic representation is shown in Fig. 5. This workflow has some common points with that described in a previous work142 and consists of the following steps:1. Given the wide pool of chemicals assessed in the solvent selection guides described in section 3.1 and the great interest that they have awakened over the years since their publication, the solvents therein included will serve as a basis for the selection of an EP for the in situ extraction of 5-HMF and furfural from aqueous systems.48,50,51,54,128 In addition, our pool of solvents also includes the compounds featured in a list of green solvents included in a previous work dealing with the usefulness of COSMO-RS as an approach to build a panorama of solvents based on their structural characteristics.152 In this reference, the authors use this method to classify a list of green solvents (mainly biobased or acceptable for pharmaceutical applications) into a series of clusters depending on their polarity rather than molecular functionality as in the other lists. For this classification, they apply principal component analysis to the σ-profiles obtained by COSMO-RS, which can be envisaged as a molecular descriptor of the density charge around the surface of the molecule.152 Table S1in the ESI† includes the pool of 176 solvents classified by functionality.
2. To discard solvents from further evaluation, binary LLE are calculated in COSMO-RS to check for the presence of a miscibility gap of solvents with water. The candidates showing such behavior are able to conform biphasic systems.
3. With the candidates showing limited miscibility with water a solvent screening is performed, whereby the relative solubility between the solute of interest (5-HMF or furfural) and the solvents is calculated. The solubility of a compound can be calculated following eqn (3):
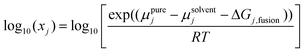 | (3) |
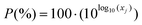 | (4) |
4. To filter among the remaining candidates, a threshold value is selected, that being the predicted probability of solubility for MIBK, the most utilized solvent for the biphasic production of 5-HMF and furfural according to the literature survey presented in previous sections.
5. With the candidates that pass through the previous sieve showing potential for the in situ separation of furans, COSMO-RS predictions of the LLE of the ternary systems consisting of {H2O + furan + solvent} are made.
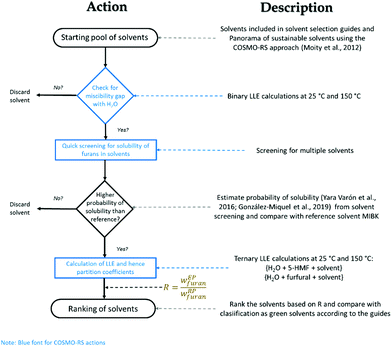 | ||
| Fig. 5 Workflow followed for the evaluation of solvents for the extraction of furans from an aqueous phase with organic solvents using COSMO-RS. | ||
To compute these LLE, different compositions of feeds were given as input based on the amounts of 5-HMF and furfural that could be obtained from different initial concentrations of sugars (1 wt%, 5 wt%, 10 wt%, 25 wt% and 50 wt% referred to the aqueous phase) as substrates assuming complete conversion with 100% selectivity to furans. Additionally, a volume ratio of 3 to 1 between the solvent and aqueous phase was assumed, with the corresponding mass ratios being calculated based on densities. For example, for a biphasic system of water and MIBK with a feed containing 1% of fructose, the composition of the feed would be: wH2O = 0.2930; w5-HMF = 0.0021; wMIBK = 0.7049. The COSMO-RS predictions were made at 298.15 and 423.15 K to have a reference at room temperature and a representative temperature from all the works surveyed.
With these results, partition coefficients are calculated following eqn (1), which will be the basis for the solvent ranking in terms of predicted performance in extracting the products from the RP. In previous work, in search for less computationally expensive calculations given the large size of their pool of solvents, the partition coefficient was predicted with COSMO-RS by calculating at infinite and non-infinite dilutions, avoiding the calculation of more time consuming LLE.142
6. Finally, the top performing solvents in terms of R are presented together with a compilation of the assessments of the different solvent guides existing in literature. The CHEM21 solvent selection tool was employed in cases where this guide had not presented an assessment previously.54
Overview of the distribution of furans in biphasic systems in literature and comparison with COSMO-RS predictions
Most of the works dedicated to the biphasic production of furans from sugars report on the activity of catalysts in terms of conversion of substrate and yields to product (and inherently selectivity) without paying much attention to the distribution of the products between the EP and RP. Considering that the biphasic strategy intends to separate the products to prevent undesired side reactions, evaluating the distribution coefficient could be a key element to enhance the selectivity of a catalyst in the process.As the number of studies on this topic has increased throughout the last years, more investigations have included data on the distribution of the products between the RP and the EP. Some efforts have focused on the evaluation of the LLE present in different ternary systems, whereas others have directly reported the distribution of the furans in reactive systems after a certain time of reaction.
Distribution of furans in reactive systems
Fig. 6 and 7 show parity plots in which predictions of the distribution coefficients obtained with COSMO-RS versus data in literature when the samples were obtained from reacting systems. For the COSMO-RS predictions, the ternary or multinary LLE was computed, whereas from experimental data obtained in literature, calculations were made as appropriate to obtain R as a ratio of mass fractions in each phase.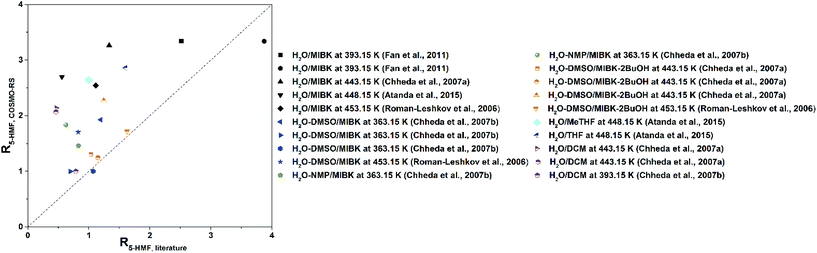 | ||
| Fig. 6 Comparison of distribution coefficients of 5-HMF reported in literature62,75,78,84,85 and COSMO-RS predictions in systems where reaction was performed. Note: When several values are present in the corresponding articles, those obtained from the best conditions in terms of yield were represented. | ||
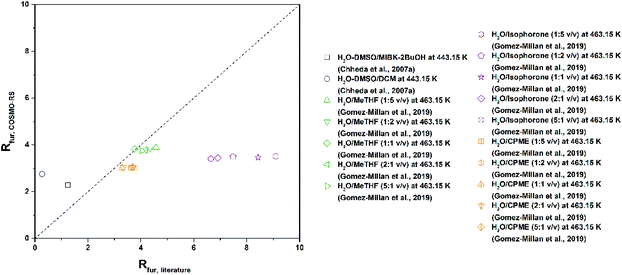 | ||
| Fig. 7 Comparison of partition ratios of furfural reported in literature78,114 and COSMO-RS predictions in systems where reaction was performed. Note: When several values are present in the corresponding articles, those obtained from the best conditions in terms of yield were represented. | ||
Fig. 6 depicts the comparison of distribution coefficients of 5-HMF obtained by different authors under different temperature conditions ranging from 90 to 180 °C,62,75,78,84,85 as summarized in the legend. The X-axis shows the experimental values of R as reported in literature from a number of different works for different multicomponent biphasic systems. For its part, the Y-axis gives the distribution coefficient calculated from COSMO-RS predictions of LLE for the same multicomponent systems at the temperature stated in the corresponding reference. Therefore, in the parity plot presented in Fig. 6, COSMO-RS would most ideally predict the partition of 5-HMF in the cases where the data points lie the closest to the diagonal line. Some values are well predicted, such as those of the H2O–DMSO/MIBK and H2O–DMSO/MIBK–2-BuOH biphasic systems, or even some values of H2O/DCM or H2O/MIBK. Nevertheless, there appears to be a tendency of COSMO-RS to overestimate the presence of 5-HMF in the organic phase, thereby increasing R.
Likewise, figure displays the displays the similarity between COSMO-RS predictions of the distribution coefficient of furfural and that obtained in individual experiments at 170 and 190 °C.78,114 In this case, not as many solvent systems have been studied as in the previous case. Multinary systems of H2O–DMSO/DCM and H2O–DMSO/MIBK–2BuOH were reported, where the COSMO-RS predicted R is overestimated with respect to the experimental value, particularly in the case of the latter system. Another study tried different solvent to water volume ratios and analyzed the distribution of the product. When THF and cyclopentyl methyl ether (CPME) were used as extracting agents, the predictions were quite accurate, although there was an increasing tendency to underestimate R as the organic to volume ratio increased. This is even more clear in the case of isophorone being used as the organic solvent, in whose case R was always very underestimated, being the relative difference using different organic to water volume ratios more remarkable than in the previous two cases.114
Apart from errors either from the experimental side or from the model predictions, it has to be taken into account that in these reacting systems the systems do not only contain 5-HMF as a solute unless complete conversion and selectivity are achieved. This in turn means that the presence of unreacted sugars or undesired side-products like levulinic or formic acid might affect the distribution coefficients. For instance, the sugars in the medium can cause a salting-out effect of the furans, although they do not appear to show a significant effect on the relative ranking of distribution coefficients among solvents.142 Therefore, the presence of these compounds could potentially represent a source of error in the deviation of the predictions although not affecting a relative screening of solvents.
Distribution of furans in systems. Measurements of LLE
It has only been recently that the detailed analysis of LLE of ternary systems {H2O + furan + solvent} has been undertaken. A common element of all of these studies is the fact that the equilibrium studies were performed at temperatures much lower than those of interest for the catalytic chemical reaction (25 to 70 °C), obviously owing to experimental limitations derived from the boiling points of the compounds involved.Figure presents R calculated from the data given in the corresponding reference157,158 and from the COSMO-RS predictions as a function of the mass fraction of 5-HMF in the organic phase. First, much like in the case of reacting systems described above, there appears to be generally an overestimation of the COSMO-RS predicted R with respect to the experimental values. In addition, COSMO-RS does not predict significant changes of this magnitude with respect to wEP5-HMR, which are clearly observed experimentally in the cases of 2-BuOH and 1-pentanol.157,158 The full ternary diagrams comparing the experimental results and COSMO-RS predictions are in Fig. S1 in the ESI.† These diagrams show that the predictions of the LLE fall in relatively good agreement in terms of tendency, although significant deviations can be observed for cases like {H2O + 5-HMF + MIBK}, especially at 298.15 K. The residual mean squared deviations were calculated with eqn (2) and presented by the corresponding ternary diagram, falling these errors between 2.85% and 9.93%, which corresponds to the aforementioned case.
In a study with COSMO-RS predictions, the distribution ratio of 5-HMF has been computed at 25 °C from the partition coefficient calculation functionality in COSMOtherm rather than the computation of the LLE.142 In this case, the COSMO-RS-predicted distribution coefficients were also overestimated with respect to their own experimental measurements with 2-MeTHF, MIBK and 2-BuOH. In the case of 1-BuOH the overestimation of R was less significant, although their experimental values appear to be somewhat lower (ranging from 1.88 to 1.53) than those observed in a LLE study providing the full composition of the system (ranging from 3 to 2, approximately).158
On the other hand, after screening, o-propylphenol and o-isopropylphenol appear to give the best results, achieving distribution coefficients of 9.47 and 9.29, respectively, which turned out to be underestimated after experimental verification (11.47 and 11.97, respectively). These authors also analyzed the effect of adding fructose and its effect on the partition ratios, observing increases due to salting-out effects. This seems to suggest that keeping a continuous feed of substrate could enhance the performance of the process. Nevertheless, the biphasic nature of the system could also be affected in some cases forming stable emulsions in some cases or single liquid phases systems.142
In a similar fashion, Fig. 9 shows the distribution coefficients calculated from experimental159,160 and COSMO-RS stimations. Overall, it can be seen that the values of this magnitude are significantly higher than those of 5-HMF; however, and most importantly, it can be seen that the values predicted by COSMO-RS are clearly underestimated, with a much higher relative difference than was observed in figure, where some values of experimental and predicted values even overlapped. Fig. S2† presents the ternary diagrams of the LLE studied experimentally and the COSMO-RS predictions, where it can be seen that, although the compositions of the water-rich phases are in good agreement with experimental values, generally the mass fraction of furfural in the solvent-rich phase is predicted clearly below the experimental values by COSMO-RS. In the case of furfural predictions, the rmsd values show deviations between 4.74% and 9.45% when 2-MeTHF was the organic solvent and between 3.33% and 4.70% for CPME, which are more or less of the same order of magnitude as the predictions for LLE with 5-HMF. However, the error in the predictions of the equilibria with isophorone as extracting agent is significantly higher and ranges between 20 and 25% approximately, mostly due to an underestimation of the furfural solubility in the isophorone-rich as well as an overestimation of the mass fraction of water therein. Last, in spite of the potential operational issues that the use of salts to promote the salting-out effect of furans to the EP entail, it is also worth mentioning that Sadowski, Smirnova and co-workers have investigated the equilibria considering the use of salts like chlorides, nitrates or methoxides. First, they studied the applicability of COSMO-RS to predict the equilibrium of a H2O/MIBK system in the presence of these salts.161 COSMO-RS does not use system-specific parameters for electrolytes, which led them to extend the model using an extension developed for monoatomic electrolytes.162 On the other hand, the results were described with good agreement with their ePC-SAFT model,161 and was subsequently applied with success to the system {H2O + 5-HMF + 1-BuOH}.163
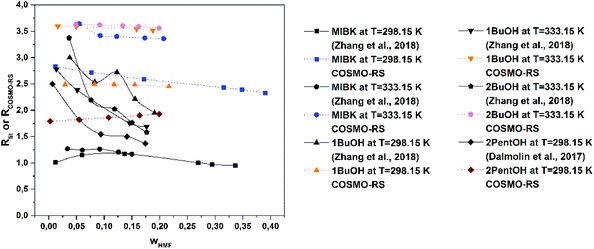 | ||
| Fig. 8 Comparison of distribution coefficients obtained in LLE studies in literature157,158 and COSMO-RS predictions as a function of the mass fraction of 5-HMF in the organic solvent phase. | ||
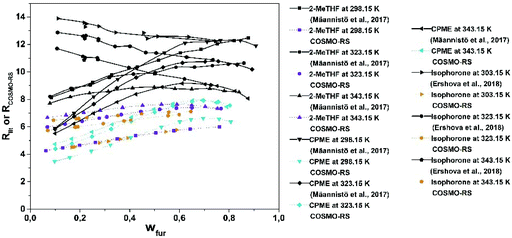 | ||
| Fig. 9 Distribution coefficients obtained in LLE studies in literature159,160 and COSMO-RS predictions as a function of the mass fraction of furfural in the organic solvent phase. | ||
Towards a rational selection of green solvents for the biphasic dehydration of sugars
Following a workflow for the selection of solvents
Given the wide pool of chemicals assessed in the solvent selection guides described above,48,50,51,54,128 the great interest they have awakened since their publication and their ample commercial availability, the solvents therein included will serve as a basis for the selection of an EP for the in situ extraction of 5-HMF and furfural from aqueous systems. In addition, the list of novel bio-based solvents and solvents acceptable for pharmaceutical applications included in the panorama of sustainable solvents devised using the COSMO-RS approach by Aubry et al.152 was added. This includes examples of novel solvents derived from renewable resources like solketal, glycerol carbonate or triacetin from glycerol164,165 or a large number of esters derived from fatty acids of different chain length and saturation degrees to mention a few. The entire combination results in a starting pool of 177 solvents from the merged combination of these lists. The complete list is presented in Table S1† classified by solvent family.As first step, the binary LLE of water with each of these potential candidates was predicted to select solvents that would form biphasic systems in operation. As discussed in section 5.2, some authors have studied the equilibria at 25 °C or at temperatures much lower than those at which the dehydration of sugars usually take place. The importance of the biphasic system does not only lie on the separation of the phases after operation for recycling of the solvent phase, but also during reaction, where the presence of two phases is deliberately desired. Therefore, the prediction of miscibility gaps were originally predicted with COSMO-RS at 25 °C and 150 °C, the latter being a typical reaction temperature. Table S1 in the ESI† compiles the computed compositions of the water and solvent-rich phases where a miscibility gap is predicted. By doing this, thermoresponsive or thermomorphic behaviour of systems can be foreseen, whereby the mutual solubility of the systems changes with temperature allowing the operation in a single liquid phase system. Such behavior helps to overcome possible mass transfer limitations once these upper (or lower in some cases) critical solution temperatures are exceeded and has seen application in other chemical reactions.145,166–168 For the case at hand, miscibility gaps at both temperatures were predicted for a total of 128 solvents. Interestingly, 11 cases showed different miscibility behaviors at the lowest temperature and not at the highest. Propylene carbonate and nitromethane show miscibility with water at 25 °C but not at 150 °C, which could be indicative of an existing upper critical solution temperature; on the other hand, a total of 9 solvents (see Table S1†) show the opposite behavior and could present lower critical solution points. In any case, the existence of these temperatures should always be validated.
Second, a solvent screening was conducted, in which the relative solubilities were estimated and the probability of solubility subsequently calculated according to eqn (4) following the explanations given elsewhere.154–156 Table S2† gives a detailed account of both magnitudes for 5-HMF and furfural at 25 and 150 °C. As a reference for further filtering, the probability of solubility for MIBK at 150 °C was selected due to the fact that this is the most recurrent solvent for these two biphasic systems.
For the screening of 5-HMF, Fig. 10 presents a summarized version of the complete screening shown in Table S2† and Fig. S3† highlighting only the best and the worst candidates for each family of compounds for the probability of solubility. With a set reference value of 51.4% for MIBK, 50 solvents (including MIBK) show at least as good prospects as the reference, being all the aromatic and aliphatic hydrocarbons dismissed as well as most of the halogenated. For the rest of the families of compounds, some esters, alcohols, ketones and ethers, organic carbonates and bases could be promising. From Fig. 10, it can be seen that in principle an alcohol like furfuryl alcohol, a dipolar aprotic compound like hexamethylphosphoramide or a base like pyridine show a very good chance to dissolve 5-HMF and therefore make it to the following step of the decision-making process. On the other hand, halogenated compounds like perfluorooctane or an aliphatic hydrocarbon like pentane show the worst probabilities, hence being dismissed for further consideration together with others showing worse prospects than reference MIBK.
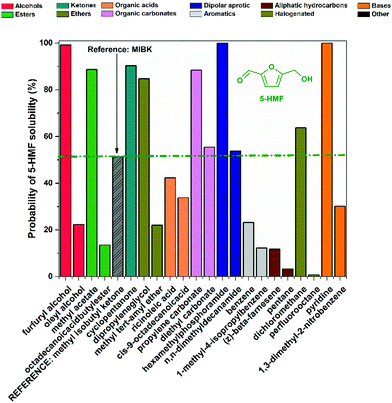 | ||
| Fig. 10 Probability of solubility of 5-HMF in different solvents at 150 °C as predicted from the relative solvent screening by COSMO-RS: selection of solvents showing the best and worst probability of solubility for each solvent family. Refer to Fig. S4 and Table S2† for the complete screening. | ||
An analogous analysis has been made for furfural. Considering the entire set of data contained in Table S2 and Fig. S4,† in this case, only 57 candidates showed a probability of dissolving furfural of 69.7%, which corresponds to MIBK. As in the case of 5-HMF, the solvents that made this cut showed similar trends in behaviour as a function of the family to which they belong, which is understandable considering the similarity between 5-HMF and furfural. As an exception, halogenated compounds showed generally better predicted results for furfural. For the case of furfural, Fig. 11 shows a summarized set of the best and worst solvents per family of compounds scrutinized in this step. Among the alcohols, benzyl alcohol appears to show a very high probability of solubility as well as methyl acetate do for esters, hexamethylphosphoramide for dipolar aprotics, dichloromethane for halogenated or tetramethylurea for bases.
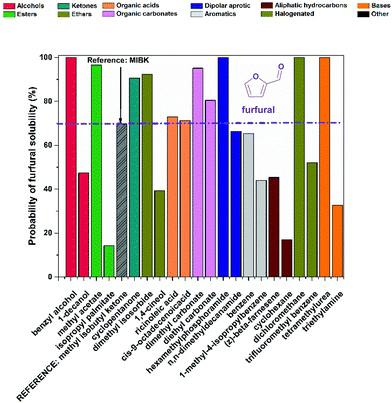 | ||
| Fig. 11 Probability of solubility of furfural in different solvents at 150 °C as predicted from the relative solvent screening by COSMO-RS: selection of solvents showing the best and worst probability of solubility for each family. Refer to Fig. S4 and Table S2† for the complete screening. | ||
The remaining solvents were individually evaluated in COSMO-RS for predictions of the LLE {H2O + furan + solvent} and from the compositions, the distribution coefficients were computed. The equilibria were calculated at 25 and 150 °C for feed compositions corresponding to different concentrations of the substrates, as detailed in section 4.3. The results are available in Tables S3 and S4 (see ESI†) for 5-HMF and furfural, respectively.
In these tables, it can be observed that an increase of temperature does not always promote higher distribution coefficients neither in 5-HMF or furfural, as the trends can vary. For instance, the predicted distribution coefficient of 5-HMF when 2,4,6-collidine is employed as solvent to extract from an aqueous phase declines from 3.31 at 25 °C to 2.51 at 150 °C. However, when ethyl succinate is used, R acquires a value of 1.08 at 25 °C and almost double it to 2.07 at 150 °C. There also examples for the case of furfural as solute. When furfuryl alcohol is used as organic phase to separate furfural, R decreases from 8.37 at 25 °C to 2.53 at 150 °C. On the other hand, when furfural is separated with 3-methoxy-3-methylbutanol from water, R increases from 2.68 at 25 °C to 4.39 at 150 °C. This changes in the tendency are caused by the different relative effects of temperature on the solubility (or more precisely said, the mass fraction) of the solutes 5-HMF or furfural in each of the solvents (water and organic solvent). The overall effect of temperature on the partition will be determined by whether the mass fraction of the solute increases more in the aqueous or in the solvent phase as the temperature increases or decreases.
These results highlight the need for predictions at several temperature conditions and subsequent validation. Other results that remark the need for validation owing to the effect of variables would be the prediction of equilibria with different mass fractions of 5-HMF or furfural. For instance, water and methyl acetate were predicted to show a miscibility gap at 25 °C; however, even little additions of 5-HMF or furfural to the system could lead to the presence of a single liquid phase, as seen in the predictions of Tables S3 and S4.† This is in agreement with data in literature, where the existence of two phases in water and methyl acetate could disappear with concentrations of methanol of about 5 wt%.169 However, at 150 C the distribution of 5-HMF in two phases occurs until high concentrations of 5-HMF are present.
To finally establish a ranking based on the extraction ability of these solvents for application in the biphasic dehydration of sugars, the compositions corresponding to feeds of 1 wt% of fructose (or glucose) and xylose were selected, which can be seen highlighted in Tables S3 and S4.†
The top 15 solvent candidates for the in situ extraction of 5-HMF are listed in Table 7, where it can be seen that COSMO-RS predicts the highest distribution coefficient using EtOAc, followed closely by benzyl alcohol and then slightly below by dichloromethane and methyl propionate in the 4th position. These four solvents are alternatives classified differently in the CHEM21 guide:54 EtOAc and methyl propionate are recommended, benzyl alcohol is problematic and dichloromethane is hazardous, all of which agrees with classification following other guides. In the case of methyl propionate, only the GSK guide51 provided an evaluation of this compound rating it as with “some known issues”, which is the reason why the CHEM21 spreadsheet54 was used with the aid of data from the REACH database,130 for which a full registration is available. From the CHEM21 evaluation, methyl propionate was ranked as recommended only giving a score of 5 in terms of safety. In this list also other solvents used commonly for this process can be found, such as the isomers of butanol, which obviously show a similar predicted performance. Additionally, some ethers are shown, which are classified as hazardous and do not show a miscibility gap with water at the two reference temperatures, subsequently not making them entirely useful for separation after reaction at room temperature. While THF is known to be largely miscible with water, COSMO-RS predicted a miscibility gap both at 25 and 150 °C. Indeed, as reported in Tables 1–4, this compound has been repeatedly used for the process under scrutiny, whereby large concentrations of NaCl were present in the reacting aqueous phase supporting phase separation. The LLE between H2O and THF has been assessed in the presence of 5-HMF and LiCl, NaCl and KCl most recently.170 Finally, MIBK is also included in this list for reference, although its predicted value of R of 3.01 is somewhat lower than the top candidates, which ranks it the 28th among the pool of solvents.
| a Calculated from the liquid–liquid equilibria at T = 423.15 K predicted by COSMO-RS from a feed consisting of wH2O = 0.2930; w5-HMF = 0.0021; wsolvent = 0.7049. b Computed as the arithmetic mean of the scores for environmental air, water and waste. c Calculated with the CHEM21 spreadsheet tool54 with data from the REACH database130 when present; otherwise, directly from MSDS of each chemical. d Based only on the environmental scores for water and waste. e Strong divergence between the recommendation by default (recommended) and after discussion (hazardous).54 f Based only on the environmental scores for waste. |
|---|
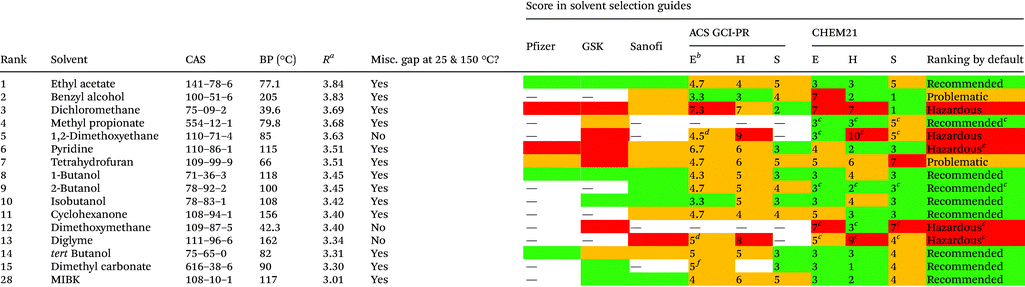
|
A large starting set of solvents was used for a screening for the extraction of 5-HMF at 25 °C, which gave 2-phenyl-1,1,1,3,3,3-hexafluoro-2-propanol as compound showing the best predicted performance. Manual selection up to 110 solvents was made using their availability in commercial suppliers as criterion, many of which were based on phenol, amine derivatives or N-based heterocycles.142 The authors proposed o-propylphenol and o-isopropylphenol from their crafted list, subsequently validating the predicted distribution coefficients with acceptable agreement, as mentioned in section 5.2.142 However, these compounds do not comply greatly with EHS criteria and, furthermore, show boiling points of 220 and 230 °C, respectively, which would make subsequent downstream separation energy intensive. For all these reasons, the likely identification of uncommon but still potentially hazardous solvents makes it reasonable to start the screening process from a pool of well-known and commonly used alternatives incorporating solvents with prospects of being benign.152
Likewise, Table 8 presents the 15 top candidates for the separation of furfural at the representative temperature of reaction of 150 °C. In this case, the predictions for DCM make it the best prospect, but up to the top 4 solvents the candidates are halogenated or aromatic, which make them unfavourable for use according to their classification in the solvent guides either being hazardous or problematic. In the 5th and 7th position appear methyl propionate and EtOAc, respectively, which makes them promising green solvents for the separation of furfural as in the case of 5-HMF. With similar predicted values of R, n-propyl acetate and isopropyl acetate qualify as recommended and slightly below is MIBK, the reference solvent for this process. The rest of this shortlisted candidates show either problematic or hazardous behaviour.
| a Calculated from the liquid–liquid equilibria at T = 423.15 K predicted by COSMO-RS from a feed consisting of wH2O = 0.2929; wfur = 0.0019; wsolvent = 0.7052. b Computed as the arithmetic mean of the scores for Environmental air, water and waste. c After discussion it was ranked as highly hazardous.54 d Calculated with the CHEM21 spreadsheet tool54 with data from the REACH database130 when present; otherwise, directly from MSDS of each chemical. e Based only on the environmental scores for water and waste. f After discussion it was ranked as recommended.54 g Strong divergence between the recommendation by default (problematic) and after discussion (highly hazardous).54 h Based only on the environmental scores for waste. |
|---|
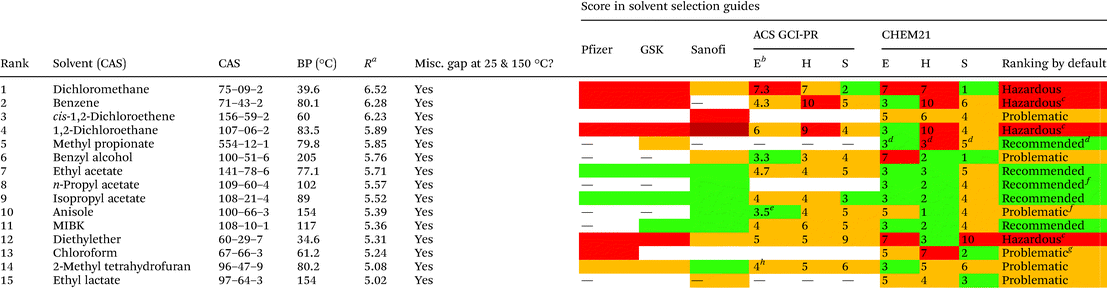
|
Fig. 12 shows a radar plot featuring a selection of the top 10 solvents based on the distribution coefficients predicted by COSMO-RS from Tables 7 and 8. The axes show EHS scores (the higher the score the worse for its selection) as well as the recommendation by CHEM21. In addition, variables of operational relevance are included, such as whether there is a miscibility gap at 25 and 150 °C, the boiling point as a measure of the potential for recycling of the solvent (the higher the boiling point the higher the energy expense for distillation) and the distribution coefficients of 5-HMF and furfural in the aqueous biphasic systems. In the radar plot it can be observed that EtOAc and methyl propionate are in the outermost part of the graphs for most of the variables considered. Thus, from the screening shown, it appears that these two solvents could be promising alternatives in this multiparameter problem.
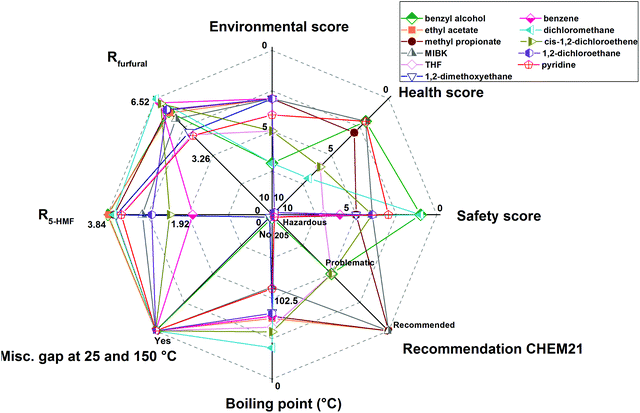 | ||
| Fig. 12 Radar plot of the EHS, COSMO-RS predicted partition coefficients and operational considerations of selected top candidates. | ||
The use of EtOAc has previously been acknowledged, although not to extract the products from a strictly aqueous phase: it has been described its use to separate 5-HMF from reacting media consisting of H2O–acetone81 or DESs like ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid96 or ChCl
citric acid96 or ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid.97 In this cases, the conversion of the corresponding substrates attained were high and the selectivities ranged from 50 to 98%. On the other hand, after extensive literature research, to the best of our knowledge, methyl propionate remains to be reported for the biphasic production of 5-HMF and furfural. The purpose of using solvents that may enhance separation of the products while the reaction takes place is to increase the overall productivity of the process, in terms of conversion and selectivity. Whilst their magnitudes are obviously dependent on the catalysts used in each case, the use of this solvent may open new alternatives from aqueous phases. Regarding engineering aspects for scale-up, for EtOAc, at room temperature, the density is 0.894 g cm−3 and the viscosity is 0.426 cP171 and for methyl propionate these values are 0.915 g cm−3 and 0.456 cP,172 which make them favourable for operations like stirring of the biphasic dispersion upon reaction or pumping. Also, the boiling point of EtOAc is only 77.1 °C and that of methyl propionate is 79.8 °C, which undoubtedly help downstream processing in terms of energy expense when separating the products from the solvent and recycling the latter to the process.
oxalic acid.97 In this cases, the conversion of the corresponding substrates attained were high and the selectivities ranged from 50 to 98%. On the other hand, after extensive literature research, to the best of our knowledge, methyl propionate remains to be reported for the biphasic production of 5-HMF and furfural. The purpose of using solvents that may enhance separation of the products while the reaction takes place is to increase the overall productivity of the process, in terms of conversion and selectivity. Whilst their magnitudes are obviously dependent on the catalysts used in each case, the use of this solvent may open new alternatives from aqueous phases. Regarding engineering aspects for scale-up, for EtOAc, at room temperature, the density is 0.894 g cm−3 and the viscosity is 0.426 cP171 and for methyl propionate these values are 0.915 g cm−3 and 0.456 cP,172 which make them favourable for operations like stirring of the biphasic dispersion upon reaction or pumping. Also, the boiling point of EtOAc is only 77.1 °C and that of methyl propionate is 79.8 °C, which undoubtedly help downstream processing in terms of energy expense when separating the products from the solvent and recycling the latter to the process.
Experimental comparison with COSMO-RS predictions and other reported values
Considering the predictions shown by COSMO-RS for EtOAc and methyl propionate, an experimental assessment of the distribution coefficients of 5-HMF and furfural was made at 25 and 150 °C.The selected concentrations of 5-HMF were 0.7 wt% and 7 wt% in the aqueous phase, which correspond to the maximum theoretical concentrations that could be obtained from a solution of 1 wt% and 10 wt% of fructose, respectively. Similarly, the concentrations of furfural were 0.64 wt% and 6.4 wt%, which relate to the maximum concentrations that could be reached from starting aqueous solutions of 1 wt% and 10 wt% of xylose. The details of the experimental procedure for the determination of the distribution coefficient can be found in section S1 of the ESI.†Table 9 shows the values of the distribution coefficients obtained experimentally as well as COSMO-RS predictions.
| Distribution coefficient, R solvent | T (°C) | 5-HMF | Furfural | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.7 wt%a | 7 wt%b | 0.64 wt%c | 6.4 wt%d | ||||||
| Experimental | COSMO-RS | Experimental | COSMO-RS | Experimental | COSMO-RS | Experimental | COSMO-RS | ||
| Overall compositions of the feeds:a wH2O = 0.2930; w5-HMF = 0.0021; wsolvent = 0.7049.b wH2O = 0.2873; w5-HMF = 0.0216; wsolvent = 0.6911.c wH2O = 0.2930; wfurfural = 0.0019; wsolvent = 0.7051.d wH2O = 0.2930; wfurfural = 0.0197; wsolvent = 0.6925. | |||||||||
| Methyl propionate | 25 | 1.34 | 3.11 | 1.38 | 3.10 | 8.47 | 7.40 | 9.08 | 7.39 |
| 150 | 1.41 | 3.68 | 1.42 | 3.65 | 8.05 | 5.85 | 6.92 | 5.80 | |
| Ethyl acetate | 25 | 1.64 | 3.61 | 1.56 | 3.55 | 8.07 | 6.64 | 8.50 | 6.63 |
| 150 | 1.80 | 3.84 | 1.60 | 3.78 | 7.41 | 5.71 | 8.36 | 5.65 | |
First, it can be seen that COSMO-RS clearly overestimates the values of R for the case of 5-HMF at both temperatures and concentrations. This is in agreement with the data compiled in Fig. 6 and 8, where it was shown that this predictive method overall also calculated distribution coefficients in excess of the actual experimental values for a number of different ternary and multinary systems both in reacting and non-reacting conditions. The distribution coefficients of methyl propionate at 150 °C at concentrations of 5-HMF of 0.7 wt% and 7 wt% were 1.41 and 1.42, respectively, whereas for EtOAc these values were 1.80 and 1.60. Comparing with other solvent systems in reacting conditions shown in Fig. 6, methyl propionate appears to have similar values of R compared to H2O/THF at 175 °C75 or H2O–DMSO/MIBK–2-BuOH at 180 °C.85 For its part, EtOAc could perform better than these two, although the binary system H2O/MIBK at 120 °C has shown values of this coefficient exceeding 2.5 in the past.62 The comparison was made at similar temperatures to those reported in Fig. 6, and it appears that methyl propionate and EtOAc could perform better than many alternatives in reacting conditions. As for non-reacting binary systems (i.e., LLE experiments), the distribution coefficients of 5-HMF at 0.7 wt% and 7 wt% predicted at 25 °C were of 1.34 and 1.38 using methyl propionate, respectively, and 1.64 and 1.56 with EtOAc. The experimental values of w5-HMF in the organic solvent phase ranged between 0.002 and 0.020 approximately for both solvents. In comparison with the values reported in literature at such low values of w5-HMF at the same temperatures (see Fig. 8), methyl propionate and EtOAc show higher distribution coefficients than MIBK, which contrasts the observations in reacting systems. On the other hand, 2-pentanol and 1-BuOH appear to show higher values of R.
In the case of furfural, from the data in Table 9 it is inferred that COSMO-RS underestimates the partition between water and organic solvents as was also seen in Fig. 7 and 9 for other biphasic systems. At 150 °C and initial concentrations of furfural in the aqueous phase of 0.64 wt% and 6.4 wt%, the values of R obtained in our assessment were of 8.05 and 6.92 for methyl propionate and 7.41 and 8.36 in the case of EtOAc.
Contrasting with the values in Fig. 7 for reacting batches, it can be seen that both methyl propionate and EtOAc show better values of R than H2O–DMSO/MIBK–2-BuOH and H2O–DMSO/DCM at 170 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 or H2O/MeTHF and H2O/CPME at 190 °C.114 On the contrary, the use of isophorone at 190 °C as EP gives similar distribution coefficients ranging between 7 and 9,114 thus being comparable to the two proposed solvents.
78 or H2O/MeTHF and H2O/CPME at 190 °C.114 On the contrary, the use of isophorone at 190 °C as EP gives similar distribution coefficients ranging between 7 and 9,114 thus being comparable to the two proposed solvents.
Similar to the comment with 5-HMF, a comparison is established with values obtained in literature in inert conditions (Fig. 9). At low mass fractions of furfural in the organic phase (ours ranged between 0.002 and 0.025) and temperatures of 25 °C methyl propionate showed values of R of 8.47 (at 0.64 wt%) and 9.08 (at 6.4 wt%), whereas EtOAc reached 8.07 (at 0.64 wt%) and 8.50 (at 6.4 wt%). These values are higher than those reached with CPME (5.88 at wfur = 0.099 in the organic-rich phase) or 2-MeTHF at the same temperature (8.04 at wfur = 0.084 in the organic-rich phase).160 On the other hand, the use of isophorone as EP at a close temperature of 30 °C gave distribution coefficients of 13.89 at wfur = 0.111 in the solvent phase.159
As for the effect of temperature in the COSMO-RS predictions and the experimental values, it seems that for 5-HMF an increase in temperature has a slight positive effect on the distribution coefficient with both solvents. For furfural, the increase from 25 to 150 °C shows declining values of R in both cases. This means that in the case of 5-HMF its mass fraction increases more in the extracting phase in comparison to the aqueous phase as temperature increases. Conversely, a rise in temperature causes the opposite effect for furfural.
Anyhow, it appears that the screening using COSMO-RS predictions gives the absolute best solvents neither for the distribution of 5-HMF nor furfural in aqueous biphasic systems. The predictions, however, seem to behave following the same trends in terms of relative values of the predictions vs. the actual values of the distribution coefficient in both cases, which makes this method a useful tool.
Insights into the interaction of 5-HMF and furfural with the extracting solvents
Last, as a way to give further insights into the relative affinity of the solutes with each of the solvent phases, COSMO-RS can supply with relevant qualitative information. Fig. 13 depicts the σ-profiles and the σ-surfaces of H2O, as the phase originally containing 5-HMF and furfural, as well as EtOAc and methyl propionate as the most promising extraction solvents. Whilst the σ-surface is a visual representation of the screening charge around a molecule, the σ-profiles can be envisaged as a histogram that describes the 3D polarized charge distribution, hence showing the amount of surface segment type of a given polarity.145 The σ-profile histograms can be split into three regions: HBD (σ < −0.0082 e Å−2), non-polar region (−0.0082 e Å−2 ≤ σ ≤ 0.0082 e Å−2) and HBA region (σ > 0.0082 e Å−2).147,173–175 The nature of the interaction among the components can be described qualitatively according to these plots.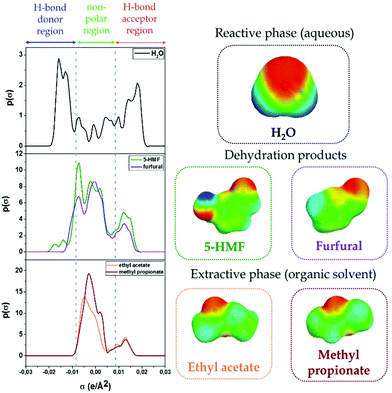 | ||
| Fig. 13 σ-Profiles and COSMO surfaces of the most stable conformers of water, 5-hydroxymethylfurfural, furfural, ethyl acetate and methyl propionate. | ||
First, it can be seen that the σ-profile of H2O is approximately bimodal, showing maxima in the regions close to σ ∼ −0.015 e Å−2 and σ ∼ +0.015 e Å−2, which relate strongly to its HBD and HBA capacities, respectively. At the same time, there is a decline of the charge density for the non-polar region, as expected from this compound. On the other hand, the two candidates EtOAc and methyl propionate exhibit prominent peaks in the non-polar region and another smaller peak at σ ∼ +0.012 e Å−2, which corresponds to hydrogen bond accepting capacity of the carboxyl oxygen of the esters. For their part, the solutes 5-HMF and furfural both show quite wide non-polar regions of similar intensities similar to those of EtOAc and methyl propionate. Additionally, the two furans also show peaks at σ ∼ +0.012 e Å−2, ascribable to the carbonyl moiety present. The higher resemblance of the σ-profiles of 5-HMF and furfural to those of methyl propionate and EtOAc than to that of water can well explain the tendency of these solutes to migrate from water to the organic extracting agents. This in turn showcases the fact that the distribution coefficients are greater than 1.
Additionally, unlike furfural, 5-HMF presents charge density in the HBD region, which is caused by the hydroxyl moiety that 5-HMF possesses. The existence of this region causes 5-HMF to exhibit a higher degree of interaction with H2O than furfural. This explains why in general the distribution coefficients for 5-HMF are lower than those for furfural as seen in Tables 7 and 8.
Conclusions and outlook
5-HMF and furfural can be produced following a biphasic approach to prevent undesired reactions in the reacting phase, usually aqueous, where rehydration of the products or self-polymerization to humins may occur. The catalysts used are Brønsted or Lewis acids that will promote dehydration. Mostly, the temperatures applied lie between 120 and 180 °C and the volume ratio of extraction to reaction phase between 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reaction phase consists of water (or less commonly, mixtures of water with compounds like DMSO), sometimes saturated with salts like NaCl to promote the migration of 5-HMF and furfural to the extraction phase. Among the organic solvents used as extractive phases, MIBK and 1-butanol can be highlighted as common solvents that have been reported to extract both furans, with the addition of CPME in the case of furfural.
1. The reaction phase consists of water (or less commonly, mixtures of water with compounds like DMSO), sometimes saturated with salts like NaCl to promote the migration of 5-HMF and furfural to the extraction phase. Among the organic solvents used as extractive phases, MIBK and 1-butanol can be highlighted as common solvents that have been reported to extract both furans, with the addition of CPME in the case of furfural.
Motivated by the importance of solvent selection in chemical reactions and multiphase operations, scientific and industrial efforts resort to selection guides (Pfizer, GlaxoSmithKline, Sanofi, ACS Green Chemistry-Pharmaceutical Roundtable and CHEM21) and different tools. The COnductor-like Screening MOdel for Realistic Solvents (COSMO-RS) is based on quantum chemistry and allows making computational predictions of thermodynamic properties from information of the molecular structure. This method is used here to screen solvents that offer a high distribution coefficient of 5-HMF and furfural with respect to water as reaction medium. To understand its accuracy, a comparison between experimental and predicted distribution coefficients was made. For 5-HMF, the distribution coefficient predictions are generally higher than experimental values in literature both when the reaction took place and in inert systems. The errors in the predictions of ternary equilibria {H2O + 5-HMF + solvent} were between 3 and 10% with solvents like MIBK, 1-BuOH or 1-pentanol. On the contrary, for furfural the overall tendency was for COSMO-RS to overestimate the distribution coefficient. In this case, the errors in LLE predictions were of the same order of magnitude with 2-MeTHF and CPME as solvents, although they reached values up to 20 to 25% with isophorone.
A workflow based on the use of COSMO-RS is proposed to screen solvents. It starts by compiling a pool of 177 solvents obtained from the solvent selection guides and a list of biobased and other adequate solvents for pharmaceutical applications proposed for evaluation with the COSMO-RS method.152 After filtering predicting miscibility gaps with water (139 candidates), multiple screening assessment taking MIBK as reference (50 solvents for 5-HMF and 57 for furfural) and computation of LLE, a ranking of the top 15 solvent candidates for 5-HMF and furfural is proposed along with the recommendation of the selection guides. It can be said that overall short chain esters can be viewed as promising solvents for this operation with a marked green profile, followed by alcohols like benzyl alcohol or the isomers of butanol. On the other hand, although the predictions for the separation of halogenated solvents are favourable, their EHS profiles are not ideal. Thus, taking into consideration these factors as well as the boiling points as a measure of their recyclability by distillation, EtOAc and methyl propionate appear to give the best prospects.
Validation of the distribution coefficients of 5-HMF and furfural were at 25 and 150 °C confirmed that the distribution coefficients of the latter are significantly higher than those of the former. In addition, it corroborated the trends explored in the survey relating to COSMO-RS overpredicting the values of R in the case of 5-HMF and underpredicting in the case of furfural. Comparing with other solvent systems in literature, in many cases methyl propionate and EtOAc showed better partitioning of 5-HMF and furfural from aqueous media. However, in some cases other alternatives have shown better results, such as the use of 2-pentanol or 1-BuOH for 5-HMF or isophorone for furfural. This makes it necessary to continue further validation work of more COSMO-RS predictions shown in this critical review prior to scaled-up efforts. In addition, predictions and validation of more complex systems should be given to approach realistic conditions, such as those featuring sugars and/or by-products of the reaction like levulinic or formic acid. Subsequently, understanding of the engineering aspects will be needed for eventual reactor and mixing designs associated with multiphase systems,176 including the study of reaction kinetics, mass transfer coefficients and behaviour of the dispersion formed upon agitation.
This critical review has mostly focused on the use of molecular solvents, although this methodology provides a tool for screening alternative media as extracting phases. For example, work has recently been reported on the use of hydrophobic (deep) eutectic solvents177,178 to extract furans from reacting media. The use of COSMO-RS could be beneficial to predict the effect of changing hydrogen bond donors and acceptors, thereby allowing tuning the composition of the solvent for improved extraction performance.
Finally, the use of COSMO-RS here evidences the fact that this tool has opened many doors for the selection of solvents and could open more for the screening of entrainers in combined operations for process intensification, including reactions followed by in situ extractions,33–36 reactive extractions or reactive distillations, to name a few.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The Max Planck Society is gratefully acknowledged for financial support. The studies were carried out as part of our activities in the “The Fuel Science Center” funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germanýs Excellence Strategy – Exzellenzcluster 2186, ID: 390919832. JE would like to thank Dr María González-Miquel for countless hours of discussion on COSMO-RS and training in the use of the COSMOTherm software. Open Access funding provided by the Max Planck Society.Notes and references
- BP, BP Statistical Review of World Energy, 2019. Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html (accessed on 11 July 2019) Search PubMed.
- US Energy Information Administration, Annual Energy Outlook 2018: With Projections to 2050, 2018. Available online: https://www.eia.gov/outlooks/aeo/pdf/AEO2018.pdf Search PubMed (accessed on 11 July 2019).
- M. Aresta, A. Dibenedetto and F. Dumeignil, Biorefinery: from Biomass to Chemicals and Fuels, Walter de Gruyter & Co., Berlin, 2012 Search PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- F. Menegazzo, E. Ghedini and M. Signoretto, Molecules, 2018, 23, 2201 CrossRef PubMed.
- H. Zabed, J. N. Sahu, A. N. Boyce and G. Faruq, Renewable Sustainable Energy Rev., 2016, 66, 751–774 CrossRef CAS.
- J. Esteban and M. Ladero, Int. J. Food Sci. Technol., 2018, 53, 1095–1108 CrossRef CAS.
- H. S. Al-Battashi, N. Annamalai, N. Sivakumar, S. Al-Bahry, B. N. Tripathi, Q. D. Nguyen and V. K. Gupta, Rev. Environ. Sci. Biotechnol., 2019, 18, 183–205 CrossRef CAS.
- L. Dai, Y. Wang, Y. Liu, R. Ruan, C. He, Z. Yu, L. Jiang, Z. Zeng and X. Tian, Renewable Sustainable Energy Rev., 2019, 107, 20–36 CrossRef CAS.
- V. Menon and M. Rao, Prog. Energy Combust. Sci., 2012, 38, 522–550 CrossRef CAS.
- Z. Zhang and G. W. Huber, Chem. Soc. Rev., 2018, 47, 1351–1390 RSC.
- X. Zhang, K. Wilson and A. F. Lee, Chem. Rev., 2016, 116, 12328–12368 CrossRef CAS PubMed.
- J. Esteban, P. Yustos and M. Ladero, Catalysts, 2018, 8, 39 CrossRef.
- R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- B. Coto, R. van Grieken, J. L. Peña and J. J. Espada, Chem. Eng. Sci., 2006, 61, 4381–4392 CrossRef CAS.
- S. M. Fakhr Hoseini, T. Tavakkoli and M. S. Hatamipour, Sep. Purif. Technol., 2009, 66, 167–170 CrossRef CAS.
- G. H. Sui, Y. Y. Cheng, X. M. Yang, X. F. Wang and Z. C. Wang, J. Appl. Polym. Sci., 2019, 136 Search PubMed.
- T. Klausli, Green Process. Synth., 2014, 3, 235–236 Search PubMed.
- B. D. Mullen, D. J. Yontz and C. M. Leibig, US9598341B2, 2017.
- K. J. Zeitsch, The Chemistry and Technology of Furfural and its Many By-Products, Elsevier Science BV, Amsterdam, The Netherlands, 2000 Search PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- M. A. Ershov, E. V. Grigor'eva, A. I. Guseva, N. Y. Vinogradova, D. A. Potanin, V. S. Dorokhov, P. A. Nikul'shin and K. A. Ovchinnikov, Russ. J. Appl. Chem., 2017, 90, 1402–1411 CrossRef CAS.
- J. P. Lange, E. Van Der Heide, J. Van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150–166 CrossRef CAS PubMed.
- A. Chareonlimkun, V. Champreda, A. Shotipruk and N. Laosiripojana, Fuel, 2010, 89, 2873–2880 CrossRef CAS.
- K. Li, M. Du and P. Ji, ACS Sustainable Chem. Eng., 2018, 6, 5636–5644 CrossRef CAS.
- Q. Hou, M. Zhen, L. Liu, Y. Chen, F. Huang, S. Zhang, W. Li and M. Ju, Appl. Catal., B, 2018, 224, 183–193 CrossRef CAS.
- F. Ilgen, D. Ott, D. Kralisch, C. Reil, A. Palmberger and B. König, Green Chem., 2009, 11, 1948–1954 RSC.
- M. Lopes, K. Dussan and J. J. Leahy, Chem. Eng. J., 2017, 323, 278–286 CrossRef CAS.
- L. Wang, H. Guo, Q. Xie, J. Wang, B. Hou, L. Jia, J. Cui and D. Li, Appl. Catal., A, 2019, 572, 51–60 CrossRef CAS.
- Z. Zhang, B. Du, Z.-J. Quan, Y.-X. Da and X.-C. Wang, Catal. Sci. Technol., 2014, 4, 633–638 RSC.
- J. E. Romo, N. V. Bollar, C. J. Zimmermann and S. G. Wettstein, ChemCatChem, 2018, 10, 4805–4816 CrossRef PubMed.
- B. Saha and M. M. Abu-Omar, Green Chem., 2014, 16, 24–38 RSC.
- V. Zimmermann and U. Kragl, Sep. Purif. Technol., 2008, 61, 60–67 CrossRef CAS.
- V. Zimmermann, I. Masuck and U. Kragl, Sep. Purif. Technol., 2008, 63, 129–137 CrossRef CAS.
- H. K. Gaidhani, V. L. Tolani, K. V. Pangarkar and V. G. Pangarkar, Chem. Eng. Sci., 2002, 57, 1985–1992 CrossRef CAS.
- H. K. Gaidhani, K. L. Wasewar and V. G. Pangarkar, Chem. Eng. Sci., 2002, 57, 1979–1984 CrossRef CAS.
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
- S. Abbott and S. Shimizu, in Renewable Resources for Surface Coatings, Inks and Adhesives, ed. R. Höfer, A. S. Matharu and Z. Zhang, RSC Publishing, Cambridge, 2019, pp. 18–48 Search PubMed.
- J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Science, 2020, 367, 397–400 CrossRef CAS PubMed.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC.
- C. Estévez, Sustainable Solutions – Green Solvents for Chemistry in Sustainable Solutions for Modern Economies, The Royal Society of Chemistry, 2009, pp. 407–424 Search PubMed.
- J. H. Hildebrand and R. L. Scott, The Solubility of Non-electrolytes, Reinhold, New York, 3rd edn, 1950 Search PubMed.
- R. W. Taft and M. J. Kamlet, J. Am. Chem. Soc., 1976, 98, 2886–2894 CrossRef CAS.
- C. M. Hansen, J. Paint Technol., 1967, 39, 505 CAS.
- F. Eckert and A. Klamt, AIChE J., 2002, 48, 369–385 CrossRef CAS.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- L. J. Diorazio, D. R. J. Hose and N. K. Adlington, Org. Process Res. Dev., 2016, 20, 760–773 CrossRef CAS.
- D. Prat, O. Pardigon, H.-W. Flemming, S. Letestu, V. Ducandas, P. Isnard, E. Guntrum, T. Senac, S. Ruisseau, P. Cruciani and P. Hosek, Org. Process Res. Dev., 2013, 17, 1517–1525 CrossRef CAS.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC.
- A. D. Curzons, D. C. Constable and V. L. Cunningham, Clean Prod. Processes, 1999, 1, 82–90 Search PubMed.
- R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- F. Delbecq and C. Len, Molecules, 2018, 23, 1973 CrossRef PubMed.
- F. Delbecq, Y. T. Wang, A. Muralidhara, K. El Ouardi, G. Marlair and C. Len, Front. Chem., 2018, 6, 29 CrossRef PubMed.
- S. Dutta, S. De, B. Saha and M. I. Alam, Catal. Sci. Technol., 2012, 2, 2025–2036 RSC.
- Z. M. Xue, M. G. Ma, Z. H. Li and T. C. Mu, RSC Adv., 2016, 6, 98874–98892 RSC.
- A. Bayu, A. Abudula and G. Q. Guan, Fuel Process. Technol., 2019, 196, 106162 CrossRef CAS.
- P. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 2008 Search PubMed.
- L. Rigal and A. Gaset, Biomass, 1983, 3, 151–163 CrossRef CAS.
- C. Fan, H. Guan, H. Zhang, J. Wang, S. Wang and X. Wang, Biomass Bioenergy, 2011, 35, 2659–2665 CrossRef CAS.
- I. Jiménez-Morales, M. Moreno-Recio, J. Santamaría-González, P. Maireles-Torres and A. Jiménez-López, Appl. Catal., B, 2014, 154–155, 190–196 CrossRef.
- I. Jiménez-Morales, J. Santamaría-González, A. Jiménez-López and P. Maireles-Torres, Fuel, 2014, 118, 265–271 CrossRef.
- D. Gupta, E. Ahmad, K. K. Pant and B. Saha, RSC Adv., 2017, 7, 41973–41979 RSC.
- C. García-Sancho, I. Fúnez-Núñez, R. Moreno-Tost, J. Santamaría-González, E. Pérez-Inestrosa, J. L. G. Fierro and P. Maireles-Torres, Appl. Catal., B, 2017, 206, 617–625 CrossRef.
- S. Xu, X. Yan, Q. Bu and H. Xia, RSC Adv., 2016, 6, 8048–8052 RSC.
- L. Yang, X. Yan, S. Xu, H. Chen, H. Xia and S. Zuo, RSC Adv., 2015, 5, 19900–19906 RSC.
- S. Xu, D. Pan, F. Hu, Y. Wu, H. Wang, Y. Chen, H. Yuan, L. Gao and G. Xiao, Fuel Process. Technol., 2019, 190, 38–46 CrossRef CAS.
- Z. Cao, Z. X. Fan, Y. Chen, M. Li, T. Shen, C. J. Zhu and H. J. Ying, Appl. Catal., B, 2019, 244, 170–177 CrossRef CAS.
- E. Nikolla, Y. Román-Leshkov, M. Moliner and M. E. Davis, ACS Catal., 2011, 1, 408–410 CrossRef CAS.
- Y. Muranaka, H. Nakagawa, R. Masaki, T. Maki and K. Mae, Ind. Eng. Chem. Res., 2017, 56, 10998–11005 CrossRef CAS.
- H. Xiong, T. Wang, B. H. Shanks and A. K. Datye, Catal. Lett., 2013, 143, 509–516 CrossRef CAS.
- L. Atanda, M. Konarova, Q. Ma, S. Mukundan, A. Shrotri and J. Beltramini, Catal. Sci. Technol., 2016, 6, 6257–6266 RSC.
- L. Atanda, A. Silahua, S. Mukundan, A. Shrotri, G. Torres-Torres and J. Beltramini, RSC Adv., 2015, 5, 80346–80352 RSC.
- S. Lima, M. M. Antunes, A. Fernandes, M. Pillinger, M. F. Ribeiro and A. A. Valente, Molecules, 2010, 15, 3863–3877 CrossRef CAS PubMed.
- P. Wrigstedt, J. Keskiväli and T. Repo, RSC Adv., 2016, 6, 18973–18979 RSC.
- J. N. Chheda, Y. Román-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342–350 RSC.
- R. Otomo, T. Yokoi and T. Tatsumi, ChemCatChem, 2015, 7, 4180–4187 CrossRef CAS.
- X. Wang, H. Zhang, J. Ma and Z.-H. Ma, RSC Adv., 2016, 6, 43152–43158 RSC.
- W. Mamo, Y. Chebude, C. Márquez-Álvarez, I. Díaz and E. Sastre, Catal. Sci. Technol., 2016, 6, 2766–2774 RSC.
- J. Zhou, Z. Xia, T. Huang, P. Yan, W. Xu, Z. Xu, J. Wang and Z. C. Zhang, Green Chem., 2015, 17, 4206–4216 RSC.
- D. Mercadier, L. Rigal, A. Gaset and J.-P. Gorrichon, J. Chem. Technol. Biotechnol., 1981, 31, 489–496 CrossRef CAS.
- J. N. Chheda and J. A. Dumesic, Catal. Today, 2007, 123, 59–70 CrossRef CAS.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933 CrossRef PubMed.
- A. J. Crisci, M. H. Tucker, J. A. Dumesic and S. L. Scott, Top. Catal., 2010, 53, 1185–1192 CrossRef CAS.
- A. J. Crisci, M. H. Tucker, M.-Y. Lee, S. G. Jang, J. A. Dumesic and S. L. Scott, ACS Catal., 2011, 1, 719–728 CrossRef CAS.
- C. V. McNeff, D. T. Nowlan, L. C. McNeff, B. Yan and R. L. Fedie, Appl. Catal., A, 2010, 384, 65–69 CrossRef CAS.
- N. Jiang, W. Qi, R. Huang, M. Wang, R. Su and Z. He, J. Chem. Technol. Biotechnol., 2014, 89, 56–64 CrossRef CAS.
- F. Yang, Q. Liu, X. Bai and Y. Du, Bioresour. Technol., 2011, 102, 3424–3429 CrossRef CAS PubMed.
- H. Shao, J. Chen, J. Zhong, Y. Leng and J. Wang, Ind. Eng. Chem. Res., 2015, 54, 1470–1477 CrossRef CAS.
- Z. Cao, M. Li, Y. Chen, T. Shen, C. L. Tang, C. J. Zhu and H. J. Ying, Appl. Catal., A, 2019, 569, 93–100 CrossRef CAS.
- J. Y. G. Chan and Y. Zhang, ChemSusChem, 2009, 2, 731–734 CrossRef CAS PubMed.
- Q. Cao, X. Guo, J. Guan, X. Mu and D. Zhang, Appl. Catal., A, 2011, 403, 98–103 CrossRef CAS.
- M. Zuo, K. Le, Z. Li, Y. Jiang, X. Zeng, X. Tang, Y. Sun and L. Lin, Ind. Crops Prod., 2017, 99, 1–6 CrossRef CAS.
- T. Kobayashi, M. Yoshino, Y. Miyagawa and S. Adachi, Sep. Purif. Technol., 2015, 155, 26–31 CrossRef CAS.
- S. Hu, Z. Zhang, Y. Zhou, J. Song, H. Fan and B. Han, Green Chem., 2009, 11, 873–877 RSC.
- H. Zhang, Z. Wang and H. K. Gao, BioResources, 2018, 13, 1189–1201 CAS.
- M. Mascal, ACS Sustainable Chem. Eng., 2019, 7, 5588–5601 CrossRef CAS.
- M. Mascal and E. B. Nikitin, Angew. Chem., Int. Ed., 2008, 47, 7924–7926 CrossRef CAS PubMed.
- M. Mascal and E. B. Nikitin, ChemSusChem, 2009, 2, 423–426 CrossRef CAS PubMed.
- C. Nitsos, U. Rova and P. Christakopoulos, Energies, 2018, 11, 50 CrossRef.
- B. Danon, G. Marcotullio and W. de Jong, Green Chem., 2014, 16, 39–54 RSC.
- N. Sweygers, J. Harrer, R. Dewil and L. Appels, J. Cleaner Prod., 2018, 187, 1014–1024 CrossRef CAS.
- S. Xu, D. Pan, Y. Wu, N. Xu, H. Yang, L. Gao, W. Li and G. Xiao, Ind. Eng. Chem. Res., 2019, 58, 9276–9285 CrossRef CAS.
- H. Amiri, K. Karimi and S. Roodpeyma, Carbohydr. Res., 2010, 345, 2133–2138 CrossRef CAS PubMed.
- Y. Yang, C.-W. Hu and M. M. Abu-Omar, ChemSusChem, 2012, 5, 405–410 CrossRef CAS PubMed.
- C. A. Antonyraj and A. Haridas, Catal. Commun., 2018, 104, 101–105 CrossRef CAS.
- H. Li, A. Deng, J. Ren, C. Liu, W. Wang, F. Peng and R. Sun, Catal. Today, 2014, 234, 251–256 CrossRef CAS.
- S. Xu, D. Pan, Y. Wu, X. Song, L. Gao, W. Li, L. Das and G. Xiao, Fuel Process. Technol., 2018, 175, 90–96 CrossRef CAS.
- M. J. C. Molina, M. L. Granados, A. Gervasini and P. Carniti, Catal. Today, 2015, 254, 90–98 CrossRef CAS.
- Y. Wang, F. Delbecq, W. Kwapinski and C. Len, Mol. Catal., 2017, 438, 167–172 CrossRef CAS.
- Y. Wang, T. Len, Y. Huang, A. Diego Taboada, A. N. Boa, C. Ceballos, F. Delbecq, G. Mackenzie and C. Len, ACS Sustainable Chem. Eng., 2017, 5, 392–398 CrossRef CAS.
- G. Gómez Millán, S. Hellsten, A. W. T. King, J.-P. Pokki, J. Llorca and H. Sixta, J. Ind. Eng. Chem., 2019, 72, 354–363 CrossRef.
- O. Sato, N. Mimura, Y. Masuda, M. Shirai and A. Yamaguchi, J. Supercrit. Fluids, 2019, 144, 14–18 CrossRef CAS.
- M. Hronec and K. Fulajtárová, Catal. Today, 2019, 324, 27–32 CrossRef CAS.
- Q. X. Lin, H. L. Li, X. H. Wang, L. F. Jian, J. L. Ren, C. F. Liu and R. C. Sun, Catalysts, 2017, 7 CrossRef.
- G. de Gonzalo, A. R. Alcántara and P. Domínguez de María, ChemSusChem, 2019, 12, 2083–2097 CrossRef CAS PubMed.
- Z.-K. Wang, X.-J. Shen, J.-J. Chen, Y.-Q. Jiang, Z.-Y. Hu, X. Wang and L. Liu, Int. J. Biol. Macromol., 2018, 117, 721–726 CrossRef CAS PubMed.
- M. González-Miquel and J. Esteban, in Comprehensive Biotechnology, ed. M. Moo-Young, Pergamon, Oxford, 3rd edn, 2019, pp. 790–806 Search PubMed.
- H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929–1961 RSC.
- D. Kralisch, D. Ott and D. Gericke, Green Chem., 2015, 17, 123–145 RSC.
- M. W. Nelson, Green Solvents for Chemistry: Perspectives and Practice, Oxford University Press, Oxford, 2003 Search PubMed.
- G. Koller, U. Fischer and K. Hungerbühler, Ind. Eng. Chem. Res., 2000, 39, 960–972 CrossRef CAS.
- C. S. Slater and M. Savelski, J. Environ. Sci. Health, Part A, 2007, 42, 1595–1605 CrossRef CAS PubMed.
- P. Harten, T. Martin, M. Gonzalez and D. Young, Environ. Prog. Sustainable Energy, 2019, 39, 13331 CrossRef.
- C. Jiménez-González, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Clean Technol. Environ. Policy, 2004, 7, 42–50 CrossRef.
- ACS Green Chemistry Institute® Pharmaceutical Roundtable. Solvent Selection Guide: Version 2.0. March 21, 2011. Accessed on 6th November, 2019 from: http://www.acs.org/content/acs/en/greenchemistry/research-innovation/tools-for-green-chemistry.html.
- ACS Green Chemistry Institute-Pharmaceutical Roundtable. Solvent selection tool. https://www.acs.org/content/acs/en/greenchemistry/research-innovation/tools-for-green-chemistry/solvent-tool.html. Accessed on 06th November 2019.
- European Chemicals Agency . https://echa.europa.eu/information-on-chemicals/registered-substances, (Accessed on 6th November, 2019).
- I. P. Rosinha Grundtvig, S. Heintz, U. Krühne, K. V. Gernaey, P. Adlercreutz, J. D. Hayler, A. S. Wells and J. M. Woodley, Biotechnol. Adv., 2018, 36, 1801–1814 CrossRef CAS PubMed.
- Environmental Protection Agency, Estimating persistence, bioaccumulation, and toxicity using the PBT profiler, in Sustainable Futures/P2 Framework Manual 2012 EPA-748-B12-001, 2012, U.S. Environmental Protection Agency Office of Chemical Safety and Pollution Prevention Search PubMed.
- Environmental Protection Agency, EPI Suite TM. Estimation Program Interface, 2012. https://19january2017snapshot.epa.gov/tsca-screening-tools/download-epi-suitetm-estimation-program-interface-v411_.html, File available at: https://19january2017snapshot.epa.gov/sites/production/files/2015–09/episetup_v411_3.exe, (Accessed on September 17th, 2019) Search PubMed.
- Laboratory of Environmental Chemistry and Toxicology of the Mario Negri Institute of Pharmacology. https://www.vegahub.eu/portfolio-types/in-silico-models/, (Accessed on 17th September, 2019).
- C. Reichardt, Angew. Chem., Int. Ed., 1965, 4, 29–40 CrossRef.
- J. H. Hildebrand and R. L. Scott, The Solubility of Non-electrolytes, Reinhold, New York, 3rd edn, 1950 Search PubMed.
- M. J. Kamlet, J. L. Abboud and R. W. Taft, J. Am. Chem. Soc., 1977, 99, 6027–6038 CrossRef CAS.
- M. J. Kamlet and R. W. Taft, J. Am. Chem. Soc., 1976, 98, 377–383 CrossRef CAS.
- A. Klamt, J. Phys. Chem., 1995, 99, 2224–2235 CrossRef CAS.
- A. Klamt, COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design, Elsevier, Amsterdam, The Netherlands, 2005 Search PubMed.
- M. Diedenhofen and A. Klamt, Fluid Phase Equilib., 2010, 294, 31–38 CrossRef CAS.
- L. C. Blumenthal, C. M. Jens, J. Ulbrich, F. Schwering, V. Langrehr, T. Turek, U. Kunz, K. Leonhard and R. Palkovits, ACS Sustainable Chem. Eng., 2016, 4, 228–235 CrossRef CAS.
- A. A. Lapkin, M. Peters, L. Greiner, S. Chemat, K. Leonhard, M. A. Liauw and W. Leitner, Green Chem., 2010, 12, 241–251 RSC.
- M. Peters, M. Zavrel, J. Kahlen, T. Schmidt, M. Ansorge-Schurnacher, W. Leitner, J. Buchs, L. Greiner and A. C. Spiess, Eng. Life Sci., 2008, 8, 546–552 CrossRef CAS.
- K. McBride, T. Gaide, A. Vorholt, A. Behr and K. Sundmacher, Chem. Eng. Process., 2016, 99, 97–106 CrossRef CAS.
- M. Gonzalez-Miquel, J. Palomar, S. Omar and F. Rodriguez, Ind. Eng. Chem. Res., 2011, 50, 5739–5748 CrossRef CAS.
- B. Ozturk and M. Gonzalez-Miquel, Sep. Purif. Technol., 2019, 227, 115707 CrossRef CAS.
- COSMOtherm, COSMOtherm, C30, Release 18.0.0, COSMOlogic GmbH & Co KG, http://www.cosmologic.de.
- OriginLab Corporation, OriginPro 9.1.0 Sr3.
- J. Esteban, M. Ladero and F. García-Ochoa, Chem. Eng. Res. Des., 2015, 94, 440–448 CrossRef CAS.
- J. Esteban, A. J. Vorholt, A. Behr, M. Ladero and F. Garcia-Ochoa, J. Chem. Eng. Data, 2014, 59, 2850–2855 CrossRef CAS.
- L. Moity, M. Durand, A. Benazzouz, C. Pierlot, V. Molinier and J.-M. Aubry, Green Chem., 2012, 14, 1132–1145 RSC.
- COSMOlogic, COSMOthermX User Guide Version 18.0. Section 6.11 Partition coefficient calculation (log P/log D).
- C. Breil, A. Meullemiestre, M. Vian and F. Chemat, Molecules, 2016, 21, 196 CrossRef PubMed.
- E. Yara-Varón, A. S. Fabiano-Tixier, M. Balcells, R. Canela-Garayoa, A. Bily and F. Chemat, RSC Adv., 2016, 6, 27750–27759 RSC.
- B. Ozturk, J. Winterburn and M. Gonzalez-Miquel, Biochem. Eng. J., 2019, 151, 107298 CrossRef CAS.
- I. Dalmolin, R. R. Pinto, L. H. de Oliveira, L. A. Follegatti-Romero, A. C. Costa and M. Aznar, J. Chem. Thermodyn., 2017, 111, 80–87 CrossRef CAS.
- Y. Zhang, X. Guo, J. Xu, Y. Wu and M. Lu, J. Chem. Eng. Data, 2018, 63, 2775–2782 CrossRef CAS.
- O. Ershova, J.-P. Pokki, A. Zaitseva, V. Alopaeus and H. Sixta, Chem. Eng. Sci., 2018, 176, 19–34 CrossRef CAS.
- M. Männistö, J.-P. Pokki, L. Fournis and V. Alopaeus, J. Chem. Thermodyn., 2017, 110, 127–136 CrossRef.
- S. Mohammad, C. Held, E. Altuntepe, T. Kose, T. Gerlach, I. Smirnova and G. Sadowski, Fluid Phase Equilib., 2016, 416, 83–93 CrossRef CAS.
- T. Ingram, T. Gerlach, T. Mehling and I. Smirnova, Fluid Phase Equilib., 2012, 314, 29–37 CrossRef CAS.
- S. Mohammad, G. Grundl, R. Müller, W. Kunz, G. Sadowski and C. Held, Fluid Phase Equilib., 2016, 428, 102–111 CrossRef CAS.
- J. I. García, H. García-Marín and E. Pires, Green Chem., 2014, 16, 1007–1033 RSC.
- A. Behr, J. Eilting, K. Irawadi, J. Leschinski and F. Lindner, Green Chem., 2008, 10, 13–30 RSC.
- T. Gaide, J. M. Dreimann, A. Behr and A. J. Vorholt, Angew. Chem., Int. Ed., 2016, 55, 2924–2928 CrossRef CAS PubMed.
- A. Behr, L. Johnen and A. J. Vorholt, ChemCatChem, 2010, 2, 1271–1277 CrossRef CAS.
- J. Esteban and A. J. Vorholt, J. Ind. Eng. Chem., 2018, 63, 124–132 CrossRef CAS.
- Y. Zeng, X. Chen, C. Guo, S. Zhao, H. Zheng and L. E. Wang, Fluid Phase Equilib., 2013, 354, 319–325 CrossRef CAS.
- B. González, N. Calvar, E. Gómez and Á. Domínguez, J. Chem. Thermodyn., 2007, 39, 1578–1588 CrossRef.
- Y. J. Xie, F. Y. Yu, Y. Wang, X. L. He, S. R. Zhou and H. Y. Cui, Fluid Phase Equilib., 2019, 493, 137–143 CrossRef CAS.
- M. S. Ding and T. R. Jow, J. Electrochem. Soc., 2005, 152, A1199–A1207 CrossRef CAS.
- M. A. Kassim, N. A. Sairi, R. Yusoff, A. Ramalingam, Y. Alias and M. K. Aroua, Thermochim. Acta, 2016, 639, 130–147 CrossRef CAS.
- U. Domańska, P. Okuniewska, K. Paduszyński, M. Królikowska, M. Zawadzki and M. Więckowski, J. Phys. Chem. B, 2017, 121, 7689–7698 CrossRef PubMed.
- J. Esteban and M. Gonzalez-Miquel, J. Mol. Liq., 2018, 263, 125–138 CrossRef CAS.
- M. Schrimpf, J. Esteban, T. Rösler, A. J. Vorholt and W. Leitner, Chem. Eng. J., 2019, 372, 917–939 CrossRef CAS.
- C. H. J. T. Dietz, F. Gallucci, M. van Sint Annaland, C. Held and M. C. Kroon, Ind. Eng. Chem. Res., 2019, 58, 4240–4247 CrossRef CAS.
- C. H. J. T. Dietz, M. Verra, S. Verberkt, F. Gallucci, M. C. Kroon, M. F. Neira D'Angelo, M. Papaioannou and M. van Sint Annaland, Ind. Eng. Chem. Res., 2019, 58, 16116–16125 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9gc04208c |
| This journal is © The Royal Society of Chemistry 2020 |