Open Access Article
Open Access ArticleA review of sustainable and intensified techniques for extraction of food and natural products
Farid
Chemat
 *a,
Maryline
Abert Vian
a,
Anne-Sylvie
Fabiano-Tixier
a,
Marinela
Nutrizio
b,
Anet
Režek Jambrak
*a,
Maryline
Abert Vian
a,
Anne-Sylvie
Fabiano-Tixier
a,
Marinela
Nutrizio
b,
Anet
Režek Jambrak
 b,
Paulo E. S.
Munekata
c,
Jose M.
Lorenzo
b,
Paulo E. S.
Munekata
c,
Jose M.
Lorenzo
 c,
Francisco J.
Barba
c,
Francisco J.
Barba
 d,
Arianna
Binello
e and
Giancarlo
Cravotto
d,
Arianna
Binello
e and
Giancarlo
Cravotto
 e
e
aAvignon University, INRAE, UMR 408, GREEN Extraction Team, F-84000 Avignon, France. E-mail: Farid.Chemat@univ-avignon.fr
bUniversity of Zagreb, Faculty of Food Technology and Biotechnology, Zagreb, Croatia
cCentro Tecnológico de la Carne de Galicia, rúa Galicia No. 4, Parque Tecnológico de Galicia, San Cibrao das Viñas, Ourense, Spain
dNutrition and Food Science Area, Preventive Medicine and Public Health, Food Science, Toxicology and Forensic Medicine Department, Faculty of Pharmacy, Universitat de València, Avda.Vicent Andrés Estellés, s/n, 46100 Burjassot, València, Spain
eDipartimento di Scienza e Tecnologia del Farmaco, University of Turin, Via P. Giuria 9, 10235 Turin, Italy
First published on 2nd April 2020
Abstract
This review presents innovative extraction techniques and their role in promoting sustainable ingredients for the food, cosmetic and pharmaceutical industries. These techniques (such as microwave, ultrasound, pulse electric field, instant controlled pressure drop, sub- and super-critical fluid processing, extrusion, mechanochemistry, high pressure, and ohmic, UV and IR heating) use or produce less solvent, energy, and hazards. This review will provide the necessary theoretical background and some details about green extraction techniques, their mechanisms, some applications, and environmental impacts. We will pay special attention to the strategies and present them as success stories for research and education and at the industrial scale.
Introduction
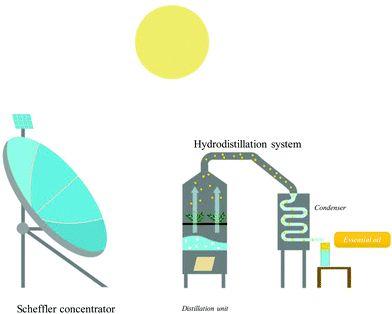
Extraction of food and natural products dates back to ancient civilizations as an important source of ingredients for nutritional, aesthetic and spiritual applications. It seems that Pharaonic civilisation, whose history goes back more than 4000 years, was the first to practice solid–liquid extraction and to take advantage of the plant kingdom to obtain colours, aromas, and materials. Specific sesquiterpenes present in incense extracts, widely used for religious rituals, have been discovered in mummy bandages. Greek and Roman civilizations also brought great discoveries in this field, and they set up the basis of distillation as the discovery of the “ambix” used both for extraction and distillation. Later, Islamic civilization gave great importance to the art of distillation and extraction. It was Geber (721–815) who transformed “ambix” to “al-ambix”, but the name of the “Alembic” process remained undeniably associated with Avicenna (930–1037) who invented the cooling system and distilled ethanol for the first time, whose name is taken from arabic “al-kohol”. Ethanol revolutionized medicine, cooking and extraction to obtain ingredients used as colours, antioxidants and flavours. This official history completely overshadows the role of women in the discovery of extraction techniques. Archaeological excavations in Iraq found specific pots from about 3500 BC. The pot vase resembled a “tajine” for cooking, but its form was examined by scientists and they definitively agreed that it was not only used as a Soxhlet apparatus for the preparation and extraction of perfumes but also for medicinal and religious preparations.1 In all Mediterranean countries, the tradition of preparing these extracts is still passed down from mother to daughter, keeping secrets of bewitching perfumes or ancestral remedies. The most famous chemist remains Maria, known as “Maria the Jewess”, who gave her name to the famous “bain-marie” (Mary's bath). She was recognized as the chemist of Alexandria in 100 BC. The rare treatises saved show that her extraction technique was advanced with the use of solar energy (Fig. 1).2,3Nowadays, we cannot find a production process in the perfume, cosmetic, pharmaceutical, food, bio-fuel, materials, or fine chemical industries, which does not use extraction processes. Extraction of natural products was considered “clean” when compared with the heavy chemical industries, but researchers and professional specialists know that its environmental impact is far greater than it first appeared. Essential oils are defined as products obtained from raw plant materials which must be isolated by physical means only. The physical methods used are alembic distillation (steam, steam/water and water), expression (also known as cold pressing for citrus peel oils), or dry distillation of natural materials. These processes are time, energy, solvent and water consuming. Lipids, aromas, colours, and antioxidants are obtained by solvent extraction using petroleum solvent. It is frequently done by the Soxhlet extraction method, based on an iterative percolation of a fresh solvent generally hexane, dichloromethane, acetone, and methanol. Nowadays, Soxhlet apparatus is still commonplace in laboratories and in industries and has been the standard and reference method for solid–liquid extraction in most cases. Soxhlet extraction nevertheless has some disadvantages such as requirement of long operation time (several hours), large solvent volumes, evaporation and concentration needed at the end of the extraction, and inadequacy for thermolabile analytes.
For example, obtaining 1 kg of rose absolute requires not only almost 2 tons of fresh roses as the raw material but also a large quantity of petroleum solvents (hexane), energy (mainly fossil) for extraction and evaporation, and water to cool and clean, and generates toxic solid waste with petroleum solvent, wastewater, and COV emissions. With the increasing energy prices and the drive to reduce CO2 emissions, the chemical and food industries are challenged to find new extraction technologies in order to reduce energy, solvent and water consumption, to meet legal requirements on emissions, product/process safety and control, and for cost reduction and increased quality as well as functionality. Extraction is one of the promising innovation themes that could contribute to the sustainable growth of the chemical and food industries. For example, existing extraction technologies have considerable technological and scientific bottlenecks to overcome: often requiring up to 50% of investments in a new plant and more than 70% of total process energy, solvent, and water consumption in the food, fine chemical and pharmaceutical industries.
Therefore, the search for environmentally-friendly alternatives and safer techniques, procedures, and solvents is currently a matter of intense research. Green Chemistry, guided by the twelve principles proposed by Anastas and Warner200 are the bases of this interest. The principles of Green Chemistry should be considered by all scientists when preparing their experiments and designing new compounds or working techniques and procedures in order to eliminate or replace hazardous products and reagents, inefficient processes and unsustainable raw materials.
Keeping in mind these constraints, the field of extraction has entered its “green” revolution. Chemat et al.4 introduced in 2012 the concept of “green extraction of natural products” on the basis of “green chemistry” and “green engineering” further referring to modern sustainable processes: “Green Extraction is based on the discovery and design of extraction processes which will reduce energy consumption, allow the use of alternative solvents and renewable natural products, and ensure a safe and high quality extract/product”. The challenges require innovations that break away from the past rather than simple continuity. Such an extraction method under extreme or non-conventional conditions is currently a dynamically developing area in applied research and industry. Using green extraction techniques, complete processes can now be completed in minutes instead of hours with high reproducibility, reduced consumption of solvent, simplified manipulation, higher purity of the final product, elimination of post-treatment of wastewater and consumption of only a fraction of energy normally needed for a conventional extraction method.
This review will present a complete picture of current knowledge on sustainable techniques for extraction as success stories for research and education and also at the industrial scale. The readers like chemists, biochemists, chemical engineers, physicians, and food technologists even from academia or industry will find the major solutions to problems related to design and demonstrate sustainable extraction processing techniques on the laboratory, classroom and industrial scales to achieve optimal consumption of raw food materials, water and energy in the following ways: (1) improving and optimization of existing processes; (2) using non-dedicated equipment; and (3) innovation in processes and procedures.
Instant controlled pressure drop technique
Process and procedure
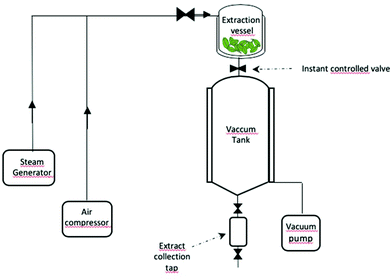
The Instant Controlled Pressure Drop method, called DIC (Détente Instantanée Contrôlée) in French, was first introduced by Allaf5 and since its inception, this technique has been deployed for extracting various kinds of natural substances and chemical constituents of interest. As a matter of fact, DIC has been constantly improvised and mended in the domain of extraction and related applications. It is considered as a high temperature/high pressure short time treatment exhibiting thermo-mechanical effects which are induced by subjecting the raw material for a short period of time to saturated steam followed by an abrupt pressure drop towards vacuum. Typically, the humidified sample, placed in the extraction vessel, is subjected to the initial pressure drop. Subsequently, the sample is subjected to heating under high saturated pressure (up to 1 MPa) and high temperature (up to 180 °C) for a short time (5 to 60 s), followed by an abrupt pressure drop to vacuum (3–5 kPa, Δt = 20–200 ms). The abrupt pressure drop (ΔP/Δt > 25.106 Pa s−1) induces significant mechanical stress wherein an event of auto-vaporization of water, an instantaneous cooling of the sample, and a swelling phenomenon occurs simultaneously, causing the rupture of cells and secretion of metabolites through cell walls.6 The sequence of the aforementioned phenomena aid in the qualification of DIC as a green extraction technique and its utilization of water vapor as a solvent results in reduced water consumption.7 Other perks including low extraction and heating time needed for the process completion, significantly reduced energy usage and preservation of heat-sensitive and thermo-labile components give it an additional edge among the other existing alternative extraction pre-treatment techniques. The DIC system is composed of four main components (Fig. 2), (i) an extraction vessel where the product to be treated is placed is equipped with a heating jacket that acts as an autoclave, (ii) a valve connecting a vacuum pump to control the instant pressure drop and to ensure the control of pressure induced by steam in the vessel, (iii) a vacuum system comprising a vacuum pump and a tank with a cooling jacket and (iv) a collection vessel to recover condensates.Applications in sustainable extraction
DIC pretreatment is considered as an efficient method to enhance the solvent extraction process. This is achieved by improving and expanding the texture of the solvent to attain better porosity in plant tissues. Mkaouar et al.8 illustrated the efficacy of DIC treatment, where post-treatment, a steep enhancement in the ethanol solvent extraction of phenolic compounds from olive leaves (Olea europaea) was witnessed; the extraction yield augmented by 312% and manifested an extract richer in bioactive compounds. DIC pretreatment has shown a positive and significant effect on the extraction of different phenolic compounds from grape stalk powder executed with several types of solvents.9 Another study has proved that DIC promoted enhanced lipid extraction from rapeseed and jatropha seeds without significant modification of fatty acid composition in comparison with conventional Soxhlet extraction.10 Allaf et al.11 in their study showed that lipid extraction could be enhanced by DIC treatment and was aptly evidenced by calculation of effective diffusivity. More recently, Eikani et al.12 reported DIC as an efficient pre-treatment for the intensification of bio-oil extraction from safflower and castor seeds. Applications of DIC technology to green extraction are shown in Table 1.| Name of plants | Compounds to be extracted | Solvent | T (°C)/P (MPa) | Ref. |
|---|---|---|---|---|
| Perilla frutescens | Lipids | n-Propane | 40 °C, 0.8 MPa | 41 |
| Sesame seed Sesamun indicum | Fatty acids + antioxidants + proteins | n-Propane | 60 °C, 12 MPa | 42 |
| Euglena gracilis | Lipids | DME | 20 °C, 0.7 MPa | 43 |
| Botryococcus braunii | Hydrocarbons | DME | 20 °C, 0.7 MPa | 44 |
| Salvia hispanica L. | Fatty acids + antioxidants | n-Propane | 45 °C, 10 MPa | 49 |
| Microalgae | Lipids | DME | 30 °C, 0.7 MPa | 50 |
| Arthrospira platensis | Lipids | DME | 20 °C, 0.5 MPa | 45 |
| Orange waste | Terpenoids | LPG | 35 °C, 0.45 MPa | 46 |
| Citrus leaves | Essential oil | DME | 35 °C, 0.78 MPa | 48 |
| Carapa guianensis | Fatty acids and phenolic compounds | n-Butane | 25 °C, 0.7 MP | 38 |
| Helianthus annuus L. | Fatty acids | n-Butane | 40 °C, 0.4 MPa | 39 |
The versatility of DIC makes it suitable for the extraction of essential oils as the time of extraction and volume of solvents utilized are considerably lower relative to the traditional extraction methods such as steam and hydro-distillation. Kristiawan et al.13 reported that the extraction yield of essential oil (EO) from Cananga odorata dry flowers using DIC was higher as compared to steam distillation corresponding to 4.34% (4 min) and 2.71% (24 h), respectively. In another study by Allaf et al.,14 the authors found that DIC extraction of EO from orange peel was far more expedient and efficient than the conventional method using hydro-distillation. The latter afforded only 1.97 mg g−1 DW (4 h) of EO while the former yielded an impressive 16.57 mg g−1 DW (2 min). Also, the process of using residual matter to extract other compounds of interest indicates that this method could be used in a biorefinery platform thereby establishing its dynamic nature in the extraction domain. Ideally, the efficiency of DIC extraction could be enhanced if parameters such as temperature, time of contact, and the number of DIC cycles were optimized.15
Success story
The technology of instant controlled pressure drop has been developed and well elucidated by Allaf's group since 1988, and several industrial projects have been developed in this regard. Numerous patents have been filed since 1993![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 and more than twenty PhD research studies have been carried out outlining the subject from different angles. Today, the Allaf group conducts research while participating in the design of machinery, a transition from laboratory to industrial scale-up. The process is operated by ABCAR-DIC Process Company, located in La Rochelle (France).
16 and more than twenty PhD research studies have been carried out outlining the subject from different angles. Today, the Allaf group conducts research while participating in the design of machinery, a transition from laboratory to industrial scale-up. The process is operated by ABCAR-DIC Process Company, located in La Rochelle (France).
Swell-drying is largely used at the industrial scale to produce swell-dried products of more than 200 varieties of fresh produce such as apple, banana, strawberry, onion, tomato etc. These products are available in the form of cubes, slices and powder that are found in functional foods and unique known brands like “greedy snacking”, “fruit snacks” or “vegetable petals” are a few players that have commercialized food products in this category. Other key features of DIC are its applicability for decontamination, dehydration and texturization of various food products. Furthermore, intermediate food products obtained after DIC treatment are used for the development of dehydrated meals and dairy products. DIC technology also plays a vital role in post-harvest management and it has been successfully adapted for rice processing. The USDA reported that Egypt's rice paddy production in the year May 2012 to April 2013 was expected to rise to 6.37 million tons of dried paddy rice, i.e., 4.5 million tons of dried unbroken white grain rice. In China, a novel product of teabags treated using DIC has been commercialized that allows greater diffusion of tea in water and even in cold water.17
Supercritical fluids
Process and procedure
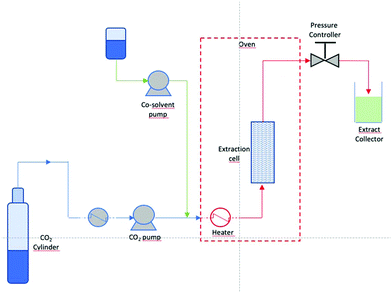
Supercritical fluids (SCF) are a well-documented alternative to traditional organic solvents suitable for various extraction methodologies.18 A fluid is considered to be in its critical state when it is both heated above its critical temperature (Tc) and pressurized above its critical pressure (Pc). The specificity of SCF relies on their physicochemical properties, which can be modulated by an increase of pressure and/or temperature, beyond their critical values. SCF have a liquid-like density, which induces a solvating power mimicking liquid-like characteristics, and their gas-like viscosity results in higher mass transfer. Carbon dioxide (CO2) is the most widely used supercritical fluid because of its intrinsic properties (inert, non-toxic, non-inflammable), low cost, abundant availability, and facile recovery from the product and it also possesses moderate critical temperature and pressure (Tc = 31.1 °C, Pc = 7.38 MPa). As a function of pressure and temperature, changes in the density of the fluid in its supercritical state permit variable solvating power, allowing for selective extractions. The high volatility at atmospheric pressure implies that after depressurization extracts are solvent-free. Supercritical CO2 is a typical non-polar solvent; its solvent power based on polarity can be placed between pentane and toluene.19 To enhance the solubilization of polar substances, a polar co-solvent (ethanol or methanol) can be added to supercritical CO2. Carbon dioxide is generally recognized as a safe (GRAS) solvent, so products containing extracts obtained with “food grade” carbon dioxide are safe in terms of human health. Several studies have been performed on natural product extraction using supercritical CO2, but the high working pressure (Pc = 7.38 MPa) has limited its industrial applications and is the prime concern during scale-up. The extraction mechanism of supercritical CO2 can be concisely divided into four stages; primarily, the diffusion of the supercritical fluid into a porous sample matrix also known as static extraction occurs where the sample is allowed to equilibrate with the supercritical fluid for a pre-determined period, immediately followed by dynamic extraction within the matrix; the third stage is the diffusion of the solutes out of the matrix, and finally the recovery of the analytes from the sample during decompression. Supercritical fluid extraction (SFE) from solid materials is achieved with autoclaves and installations and it comprises four main parts: (i) a volumetric pump to ensure correct pumping of the fluid, and the pump can be preceded by a cooler which brings gaseous components to a liquid state (ii) a heat exchanger, (iii) an extractor, where pressure is established and maintained by a back pressure regulating valve, (iv) a separator (Fig. 3).The SFE from solids is divided into macroscopic steps: the extraction and the separation of the solute from the solvent. To perform SFE, the fluid has to be brought to its supercritical state. In order to achieve this, the fluid is sequentially pressurized and heated before it enters the extractor. After reaching the desired pressure and temperature, the SFE percolates in the extractor, with an ascending or descending flux enabling the extraction of the solute contained in the matrix. Separation of the solute through SFE takes place in the separator, where the SCF turn into a gaseous state, thereby yielding the extract which is separated by the assistance of gravity. Extracts are therefore collected at the bottom of the separator. Depending on the equipment, the gas can be recycled by re-injecting it into the system or can be released into the atmosphere.
Applications
Extraction by SFE with CO2 (SFE-CO2) is well-known at both academic and industrial levels and therefore is the subject of numerous publications. Large-scale SFE is especially known since the late 1970s for the decaffeination of coffee and tea. Applications of SFE-CO2 for the extraction of natural substances such as oils and fats, flavors and fragrances and pigments from various plants, microorganisms or by-products will continue to be the main theme of research activities. The SFE-CO2 extraction of natural substances is described in several reviews.20–25Essential oils (EOs) were traditionally extracted from seeds, roots, flowers and leaves using hydrodistillation. Thermal degradation, hydrolysis and solubility of some compounds in water may alter the flavour of the desired chemical compounds. The SFE-CO2 technique is an efficient alternative to avoid these problems.26–29 The optimum operating conditions for the extraction of EOs by the SFE-CO2 method are pressure in the range of 90–250 bar and temperature ranging from 40 to 50 °C.
For example, Conde-Hernandez et al.30 published their work on the utilization of the SFE-CO2 technique for the isolation of EO from rosemary. The parameters considered were temperature (40 and 50 °C) and pressure (10.34 and 17.24 MPa) at two levels and the maximum EO recovery was between 1.41 and 2.53 g EO per 100 g of dry rosemary (% w/w) for the conditions selected.
Vági et al.31 compared the extracts produced from marjoram (Origanum maorana L.) using supercritical CO2 (50 °C and 45 MPa) and ethanol Soxhlet extraction. Extraction yields were 3.8 and 9.1% respectively. Nevertheless, the supercritical extract comprised 21% essential oil, while the alcoholic extract contained only 9% volatile oil substances.
Oil extraction is generally effectuated using hexane, which imparts negative effects as the solvent is petroleum-based and toxic. Produced from fossil energies, n-hexane, which is one of the main constituents of industrial hexane, is suspected to be reprotoxic making its usage at the industrial scale questionable. Since the early 1980s, the use of SFE-CO2 in the field of extraction of fats and oils from various plant or animal sources has been studied extensively.32 For example, Salgin et al. employed the SFE process for the extraction of jojoba oil and determined the extraction efficiency as a function of process parameters such as pressure, temperature, the particle size of jojoba seeds, and the flow rate of CO2.33
Carotenoids are intricate compounds that are prone to oxidation and are sensitive to light and heat. SFE-CO2 is a promising method to recover them from plants instead of organic solvents and hot water.34 In accordance, Lima et al. described the extraction of carotenoids from carrot peels by SFE-CO2 utilizing ethanol as a co-solvent. With respect to the validated model, the optimal conditions for the maximum mass yield (5.31%) were found to be 58.5 °C, 306 bar and 14.3% of ethanol, and to be 59 °C, 349 bar and 15.5% ethanol for carotenoid recovery (86.1%).35
Success story
Industrial processing with SCF has been a reality for several years. Since the early 1970s patents on coffee decaffeination or hop extraction have been garnering modest attention, giving rise to a great number of industrial units. In 2009, Perrut36 estimated that 300 industrial units used SCF. For supercritical extraction performed on solid materials, the main consumer-oriented applications are related to the food and perfume industries: aroma and flavor extraction (hops, vanilla, ginger, roses, etc.) and coffee and tea decaffeination. For example, Maxwell House Coffee (a division from General Foods) has reported the processing of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of coffee per year. The plant is equipped with a 60 m3 extractor and has the ability to function on a semi-continuous scale.37 The removed caffeine is further sold to pharmaceutical or food companies for industry-specific applications.
000 tons of coffee per year. The plant is equipped with a 60 m3 extractor and has the ability to function on a semi-continuous scale.37 The removed caffeine is further sold to pharmaceutical or food companies for industry-specific applications.
Liquefied gas technology
Process and procedure
In the last few decades, the quest for new and alternative solvents with enhanced physical properties has revived the interest in the use of liquefied gases as extraction solvents. To name a few n-propane, n-butane and dimethyl ether are examples of liquefied gases that operate at a lower pressure (200–1000 kPa), for extraction purposes. The added advantage includes the feasibility of these gases to remain in the liquid state requiring gentle pressure (<1 MPa) and their ability to become easily evaporated at low temperatures. Therefore, Liquefied Gas Extraction (LGE) processes can be performed at room temperature, with low energy consumption and reduced traces of residual solvent in the biomass. In addition, LGE also preserves the quality of both raw materials and extracts, thereby reducing the number of post-treatment steps. Moreover, their chemical structures make them suitable for the extraction of lipophilic compounds essentially providing an alternative to replace existing solvents. In this sense, liquefied gas extractions serve as a potential alternative technology in accordance with the principles of green extraction of natural products. The operating mechanism of LGE can be broadly classified into two modes: batch mode or semi-continuous mode. At its infancy, LGE was performed in the conventional way similar to that of solid–liquid extraction where the solid was soaked or immersed in a pre-determined volume of solvent. But, quickly scientific community realized that semi-continuous modes were effective as it was easier to evaporate the solvent. Hence, in the semi-continuous extraction processes, the solvent is continuously evaporated and recycled. In theory, a limited amount of solvent can be used indefinitely until the plant material is exhausted. As semi-continuous processes are not equilibrium limited, even solvents with relatively poor partitioning coefficients but high selectivity can be used with higher efficiency (Fig. 4).Another key component of the semi-extraction processes is that it can be classified into two modes based on the flow direction of the solvent: isobaric and non-isobaric (pressure-driven).
Applications
Various experimental studies evaluated the potentiality of the LGE technique (Table 2). In particular, propane, n-butane or dimethyl ether (DME) as liquified solvents have been extensively investigated for the extraction of fats and oils, antioxidants and aromas.38–48 Zanqui et al.49 proposed n-propane as a solvent for the extraction of chia (Salvia hispanica L.) oil, resulting in a good extraction yield (28.16%) similar to the Soxhlet method and SC CO2, within a short time period (1 h). Moreover, the oil extracted using n-propane showed the best oxidation stability and the highest contents in polyunsaturated fatty acids (829 mg g−1 oil), particularly, omega 3 (628 mg g−1 oil) and omega 6 (201 mg g−1 oil). Therefore, liquefied gas extraction using n-propane was found to be the most efficient method for the extraction of chia oil. Similarly, Goto et al.50 proposed an extraction process using dimethyl ether (DME) directly for lipid extraction from wet microalgae. They showed that lipid extraction using DME was just as effective as the Bligh–Dyer method (yield = 40.1%), set as the reference. Moreover, owing to its unique physicochemical properties DME can extract lipids from microalgae without any preliminary drying and cell disruption steps. As a result, the number of steps and the energy consumption of the overall process could be considerably reduced. Bier et al.46 extracted terpenes from agro-industrial waste using liquid petroleum gas (LPG), which is a mixture of isomers of propane and butane. This technique was compared to extractions using the Soxhlet method with n-hexane. It was ascertained that LPG in batch mode at 35 °C resulted in higher yields (5.36% vs. 3.88% using Soxhlet) and high-quality essential oils, limonene (95.3%), α-pinene (0.4%) and β-pinene (0.2%) were the major constituents. Similarly, Nenov et al.47 described the extraction of an essential oil from Ceylon cinnamon tree (Cinnamomum verum) using 1,1,1,2-tetrafluoroethane. The analyzed extract profile indicated the presence of cinnamal (77.3%) and coumarin (4.3%), with physicochemical properties identical to essential oils described in the literature, using either classical extraction methods or SC CO2.| Matrix | Analytes | Experimental conditions | Analysis and detection | Ref. |
|---|---|---|---|---|
| Red grape | Anthocyanins | Expeller | UV-visible, HPLC | 63 |
| Fresh passion fruit | Polyphenols | Twin-screw extruder | UV-visible, | 65 |
| Cashew apple | Carotenoids | Helical-type press | HPLC-DAD-MS | 66 |
| Sunflower seed | Vegetable oil | Twin-screw extruder | Iodine and acid value | 62 |
| Green Banana | Polyphenols | Twin-screw extruder | UV-visible, DPPH, HPLC-DAD, HPLC-MS | 68 |
| Carrots | β-Carotene, polyphenol | 67 | ||
| Jatropha seed | Vegetable oil | Twin-screw extruder | UPLC-MS | 62 |
| Coriander | Vegetable oil | Twin-screw extruder | UV-visible, | 62 |
| Insect | Protein | Twin-screw extruder | Microanalyser | 64 |
Success story
The technology of liquefied gases as alternative solvents for solid–liquid vegetable extraction has been successfully validated by CELSIUS (Villette-de-Vienne, France) based on its patented process “NECTACEL”. Since 2014 several industrial projects in the theme of liquefied gases were developed for applications as pharmaceutics, cosmetics, aromas, perfumes, nutraceuticals and food additives. CELSIUS Sarl has been an integral participant in the research activities, and a pioneer in the design of machinery for promoting the smooth transition from laboratory studies to the industrial stage. The NECTACEL pilot equipment designed by CELSIUS is available in industrial capacities of 1, 200 and 500 liters.Extrusion technology
Process and procedure
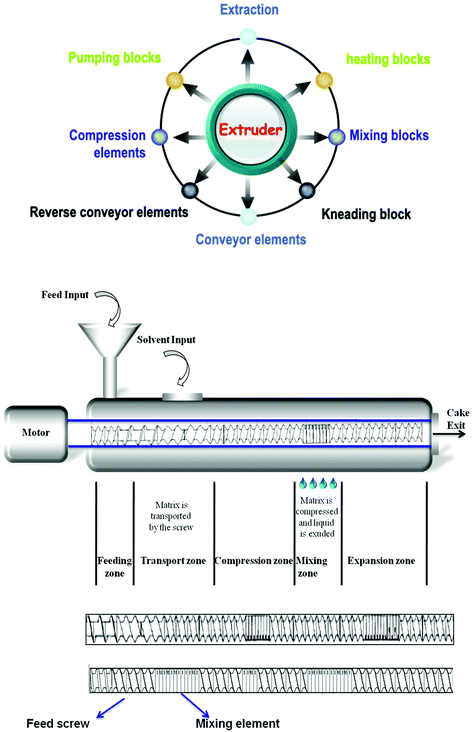
Food products manufactured by extrusion technology have become an increasingly familiar type in our daily diets. The basic principle is that ingredients are injected under pressure in a barrel, they are stirred using one or two rigid screws and then they emerge at the other end through a grid also known as a shaping dye. The primary unit operation that occurs in the barrel is the mixing of ingredients which are initially fed and also added to it at different stages of the extrusion process. The secondary unit operation involves heating of the mixture of ingredients. Towards the end of the extrusion process, the ingredients are mixed intently, where the mechanical action involved enables the processor to obtain very specific textures such as crispy, soft inside, crunchy, texturizing, super expansion, etc.Extrusion is a versatile industrial process with industry-friendly traits. Indeed, it cements its position by offering the following functionalities: continuous production line, flawless quality achieved with a high level of process automation, cleaning of production lines and their interoperability make it possible to produce various products with characteristic differences on the same line without loss of availability of the production tool. The extrusion process can be compared to a chemical reactor in which the products are shaped while undergoing continuous mechanical and thermal treatment. What once required multiple interventions is now a sequential continuous process combined in a single machine. The advantages of this technology result in enormous space and energy savings compared to traditional processes. Extruders are also highly adaptable, almost all process parameters can be modified according to the type of product desired; the speed of rotation and the configuration of the screws, temperature profile, pressure or material flow rates. These characteristics make it possible to modify the texture, shape, and the humidity level in the final product.
The pressing technique is the foundation and the primary unit operation for the production of wine, fruit juices and oils. Two types of presses are mainly used: hydraulic and screw, but screw press has evolved progressively to replace hydraulic presses. Savoir et al.51 conducted a large review of influential factors in hydraulic and screw pressing.
Over the last thirty years52 single-screw and twin-screw extrusion processes have emerged as a convenient tool for the formulation of several food products and in animal feed. Today, twin-screw extrusion is applied in many sectors such as the agri-food sector53 and plastic processing.
The interest of extrusion technology for the fractionation of agro resources is linked to the multiplicity of unitary actions that it allows achieving (Fig. 5).
Twin-screw extruder mechanism: a conveying action is actuated by a forward pitch screw; a monolobe screw facilitates radial compression and shearing actions. A bilobe screw exerts a significant mixing and shearing action, thus expediting the conveying and axial compression actions in combination with a forward pitch screw. A reversed pitch screw carries out intensive shearing and considerable mixing thereby exerting a strong axial compression in combination with a forward pitch screw. The plethora of operation steps can be carried out in any desired order depending on the transformations required. The characteristics of screw elements mainly their position or spacing (pitch, stagger angle, and length) determine the screw profile/configuration (Fig. 6). The material is introduced through the feeding zone which is then conveyed by the screw work to the other end, where the transition mixture is texturized by the shaping dye. The key components influencing the performance are realized by configuration arrangements (product transformation, residence time distribution, and mechanical energy input) during extrusion processing. All parameters such as screw and sleeve profiles, the thermal regime, and the screw drive system contribute to the achievement of the desired profile under optimal conditions.
 | ||
| Fig. 6 Development of agitated mills (a), ball mills; attritors (b, 1928);72 sand mills “Sand Mills” (c; 1950);73 agitated disc mills (d).71 | ||
Application in sustainable extraction
The recovery of waste from the processing of food and by-products is achieved using the extrusion process. A significant volume of agro and food industry by-products is generated, many of which have been processed by only extrusion or in synergy with other processing techniques including extrusion. This justifies the immense presence of extrusion in various agro-food manufacturing sectors such as fruit and vegetables, dairy products, meat, seafood, and cereal processing, residues from the production of starch, syrup, and alcohol, and residues from the processing of oilseeds. Offiah et al.54 presented the possibilities for developing novel products by extrusion and creating even newer markets for these products to choose from.About one-third of food for human consumption is wasted, from initial agricultural production through processing to consumption. Industries are increasingly interested in recovering this food waste in order to enhance their value and streamline their manufacturing operation for the complete valorization of the raw materials utilized in the production process. With technological advances in food processing techniques, these wastes could become useful by-products or residues that could be transformed into finished products for various applications.55–57 These residues could become an additional resource for manufacturers if cost-effective recycling or reprocessing methods are employed. A diversified range of extruded foods has been developed from by-products from the food industries.58 Several of these industrial by-products and residues have found useful applications in extruded products. Waste produced from fruit and vegetables accounts for about 30% of processed materials.59 These by-products are rich in antioxidants, fatty acids, fibres, minerals, vitamins, polyphenols, carotenoids, etc.59–61 Many health benefits have been attributed to these functional components.58 The use of extrusion to recover this waste adds value to these by-products increasing the overall sustainability for the food processing industries and reducing the negative environmental effects that would result from their disposal. Therefore, the extrusion process can be an integral part of a sustainable development approach.
Success story
Extrusion technology is used in many fields and particularly in the food industry such as (Table 2): oil extraction (olives, cocoa butter, oleaginous seeds); concentration in dry matter (sugar pulp, wastes from the food industry); juice extraction from grapes, fruits and tomatoes.In their review, Offiah et al.54 presents many examples of row food products and by-products processed using extrusion. Vegetable oils present a valuable class of bioresources. The quality of the oil obtained depends on its extraction process. The oilseed processing industries are increasingly interested in thermomechanical pressing for sustainable development. Uitterhaegen et al.62 presented in their review the use of twin-screw extrusion for the extraction of vegetable oil as well as the recent research and technological advances in the optimization of oil extraction processes.
Monrad et al.63 have studied extraction of anthocyanins and flavan-3-ols from red grape pomace using an expeller process. This work demonstrated that this process allowed extraction of 68% of monomeric anthocyanins for crude and 58% of total flavan-3-ols and enhancements of antioxidant activities by the ORAC assay were 58% compared to those obtained with conventional procedures. Nowadays, the use of alternative protein sources is widely investigated to ensure a sufficient and nutritious diet for society. Insects are considered a sustainable and efficient alternative. Alam et al.64 have studied the use of hot melt extrusion to produce pellets made of insect flour used alone or mixed in different ratios with cornflour. These investigations show that it is possible to include insect flour during the extrusion process as an alternative potential source of proteins.
Delvar et al.65 used a twin-screw extruder to extract chemical constituents of interest with possible bio-activity from purple passion fruit (whole fruit or co-products of juice production) and to prepare an oil-in-water emulsion directly by the extrusion process. Passion fruit is rich in phenolic compounds that can be used in the cosmetics or food industries. Moreover, passion fruit seeds contain 30% of oil rich in unsaturated fatty acids. This innovative approach could help reduce processing costs and time because the final product which is crude oil in a water emulsion could be used directly in the natural formulation which requires lipophilic compounds emulsified in a water system. Twin-screw extrusion makes it possible to extract a large quantity of polyphenol which represents 67.5% of the whole fruit extractable polyphenols.
Pinto de Abreu et al.66 studied the extraction of carotenoids from the press cake of cashew apple. This work demonstrated that pressing allowed an increase in the total carotenoid content by a factor of 10. Recently Viacara et al.67 worked on postharvest wounding stress in carrots. Wounding increased the total free and bound phenolic content, whereas the carotenoid content was unaltered. In the sequential application of wounding stress, extrusion induced higher accumulation of total free and bound phenolics, whereas in the case of carotenoids, the sequential application induced degradation of all-trans β-carotene and furthered its trans–cis isomerization.
New research work investigated the combined effect of drying (oven and freeze-drying) and extrusion on the starch bioaccessibility and phenolic profile of green banana flours.68 A combination of freeze-drying and extrusion resulted in flours with the best quality in terms of retention of phenolic compounds since freeze-drying helps to preserve epicatechin meanwhile extrusion increases the bioaccessibility of flavonols and phenolic acid due to the disruption of the tissue matrix.
Mechanochemical technology
Process and procedure
Size reduction of materials is a vital step, and humans have developed grinding techniques that make it possible, for example, to reduce the seeds to flour, making them easier to handle and cook, or to prepare the pigments used in rock paintings. To do this, they used tools such as grinding wheels or pestle mortars. These very simple tools, operated by a single person, generate a mechanical force to press the fruit into juice or transform mixtures of sand, clay and other materials into the mortar. This mechanical action promotes or generates chemical reactions in itself, which is why we speak of mechanochemistry. Over the years, grinding techniques have evolved rapidly to meet the growing demands of oil processing to overcome the intricate challenges associated with the pre-conditioning of oilseeds to enhance oil recovery and yield.Mechanochemistry is the study of physicochemical transformation generated by mechanical force, it is an interdisciplinary science based on chemistry and mechanical engineering, which investigates the chemical or physicochemical changes of substances subjected to high energy mechanical force.
The mechanochemical technique was first developed and accepted as such to prepare inorganic compounds such as cement or metal oxides for new batteries. It will take more time for the world of organic synthesis and chemists to seriously consider the use of mechanical techniques to synthesize drugs, materials, and dyes or to extract active compounds. Isolated work using tools as basic as a mortar and pestle showed the possibility of extraction by the means of mechanochemistry. For better incorporation of mechanochemistry in size reduction applications, chemists have recently turned to ball mill type equipment capable of generating greater grinding efficiency and better reproducibility with less user effort and larger quantities of product to be processed.
This approach contributes to the development of green and sustainable chemistry because it makes it possible to avoid or considerably reduce the use of organic solvents which are often harmful. In addition, it is known that the use of solvents causes dilution of the reacted molecules. However, the speed of a reaction is mediated by the dilution: generally, the greater the dilution, the slower the reaction and the lower the probability that the desired constituents will come in contact for the reaction to occur. Grinding avoids the dilution problem of solvent by facilitating constant contact between the reagent and mixture thereby promoting exhaustive and complete reactions.
The basic principle on which mechanochemistry for extraction is based on is the cell disruption and the extraction of compounds of interest. Studies have shown that mechanochemical treatment resulted in the destruction of cell walls and increased the total contact surface area due to the change of the smooth surface to an open porous structure.69
Cellular destruction can be defined as the loss of integrity of the cell wall or membrane. It leads to the release of intracellular contents. Different levels of cell destruction depending on the degree of micronization of the debris and the selectivity of the release of the molecules can be observed.
• Permeabilization: perforation of the membrane and cell walls allowing the release of intracellular molecules.
• Destruction: walls and membranes are broken to release intact organelles.
• Disintegration: the walls and membranes of organelles are ruptured resulting in smaller cellular debris.
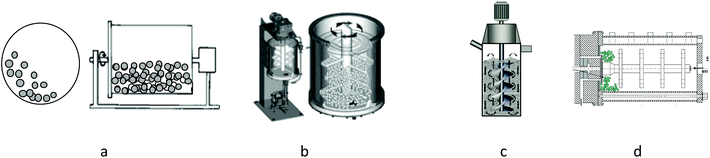
The magnitude of destruction influences the release of internal molecules, the higher the degree of destruction, the less the selectivity in the release of molecules is witnessed as they tend to release simultaneously. Cellular destruction is a crucial step: it determines the total costs of production as well as the quantity and quality of extractable intracellular metabolites.70 The mechanism of the mechanochemical assisted extraction technique is to obtain reduced particle size, destroy the call wall, decompose cellulose, and accelerate the dissolution kinetics that can increase the extraction efficiency and decrease the processing time. By mechanochemical treatment, the rigid cell walls are completely destroyed, which eliminates the diffusion resistance between the plant matrix and the solvent, thus increasing the release of active compounds. Currently, ball mills have closed grinding chambers (to avoid the formation of gas bubbles), vertical or horizontal, which can be equipped with a double jacketed for cooling. The balls are retained in the mill by a slit, screen or centrifugation system71 (Fig. 6).
Depending on the geometry of the chamber and the agitator, several types of agitated ball mills can be distinguished. Three examples of geometries are shown in Fig. 7: shredders with a disc agitator, shredders with a finger/counter-finger agitator and ring chamber shredders.
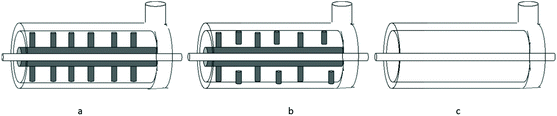 | ||
| Fig. 7 Different ball mill geometries (a) agitating disc mills; (b) finger to finger mills; annular gap mill (c).71 | ||
Bead milling has recently been introduced for plant crushing. The process involves the use of a multitude of parameters (Fig. 8, bead size and density, bead materials, filling rate, agitation rate, grinding time, suspension formulation, etc.) offering wide adjustment and optimization possibilities. This process has the advantage of offering a high destruction efficiency for a wide variety of plants and microorganisms ranging from yeasts to bacteria74–81 or microalgae.
Applications in sustainable extraction
The conventional extraction methods such as decoction, percolation, Soxhlet extraction, and heat reflux extraction are generally used for the extraction of macro- or micro-chemical components from plants. These methods are relatively safe and simple but the drawback of high time-consuming operation, requiring a large amount of organic solvent, and having poor extraction efficiency can't be overlooked. The possible degradation of thermolabile compounds is a major disadvantage of conventional methods. More efficient and environmentally friendly extraction techniques must be developed in order to prevent pollution and reduce costs in order to pursue further studies, particularly in the field of sustainable development.82,83Mechanochemical extraction was introduced as a pre-treatment for intensifying the extraction of bioactive compounds. Green mechanical extraction has many advantages such as increasing the yield of bio-active products, extraction and solubilization of substances in water and at room temperature instead of using organic solvents, reducing extraction time and simplified purification steps.84,85
Success story
Mechanochemical extraction has been successfully employed for the extraction of active compounds from plants. The use of mechanochemistry in the extraction process of active components from plants potentially improves the extraction efficiency, facilitates extraction processes and can also prevent the reliance and intensive usage of harmful organic solvents.Xu et al.86 studied the selective extraction of gardenia yellow and geniposide from Gardenia jasmine based on selective extraction by mechanochemistry, which allowed the development of an easy separation technique with reduced environmental impacts. In their study, they articulated that energy and solvent consumption could be reduced and proposed a scheme for complete utilization of the Gardenia fruit. Their extraction method has proven to be both environmentally friendly and effective, serving as an alternative for the extraction of active compounds in the pharmaceutical industry.
Another study presents the extraction of water-insoluble triterpene acid from pine using pure water after milling with Na2CO3. In this case, the role of mechanochemical treatment is not only to increase the effective total contact surface area of the mixture components and to eliminate or decrease hindrances to diffusion but also to transform chemically the target substances into forms that are more soluble in water or the solvent. Korolev et al.87 reported their work on improving the aqueous extraction yield of triterpene acids from fir needles by 35.9%.
Based on the same principle, Xie et al.88 used a mechanochemical extraction technique to extract magnolol from Magnolia officinalis. After grinding with Na2CO3, magnolol was isolated and selectively extracted with water resulting in a 7% higher yield than that obtained by heat reflux extraction.
Mechanochemical assisted extraction was also developed for the extraction of rutin from Hibiscus mutabilis L.89 The mechanochemical assisted extraction is a valuable method for rapid manufacturing of other natural products, especially to extract compounds that exhibit lower solubility in water. Mechanochemical pretreatment enables these components to be transformed into water-soluble forms such as salt, glycosides and other complexes. In the case of rutin, the study revealed that the yield could improve by 31.7%.
Many researchers have proven the effectiveness of mechanochemical assisted extraction as an intensification pre-treatment for the extraction of bioactive compounds from plant materials. Numerous studies have been carried out on the use of mechanochemistry for the extraction of active compounds from plants such as the extraction of flavonoids from bamboo leaves90 or Sephora flavescens,91 flavonoids and terpene trilactones from Ginko leaves,92 antioxidants from Laurus nobilis,93 alkaloids84 from plants etc. Wu et al.83 presented in 2017 an overview of mechanochemical assisted extraction as an eco-friendly technology.
Pulsed electric field and HVED
Process and procedure
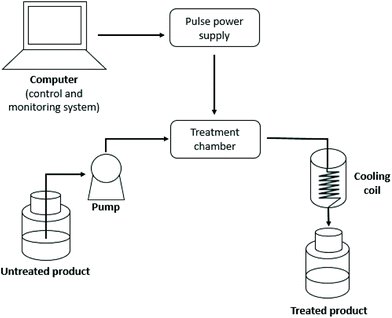
In recent years, industries have been immensely interested in the implementation of emerging technologies used for green extractions. Emerging non-thermal technologies that use high voltage to generate electric field and perform an extraction are pulsed electric field (PEF) technology and high voltage electrical discharge (HVED). These technologies are based on the phenomenon of electroporation in which cell membrane permeability is increased due to the electrical field applied forming pores on the cell membrane and extraction of intracellular components is enhanced through a diffusion process.94,95PEF is a technology where a material is placed between two electrodes, through which direct-current high-voltage pulses (kV) are applied in specific short time periods. The electric field is being generated by high voltage, and if the electric field is high enough, electroporation occurs.96 The process consists of pulse power supply that transforms the sinusoidal alternating current into pulses with adequate peak voltage and energy, and a treatment chamber where the high-voltage pulses are applied to the product (Fig. 9). In the treatment chamber, the product is being placed between two electrodes and the applied electric field, if presented between parallel electrodes, is homogeneous. The main PEF variable parameters include electric field strength, treatment time, frequency of the pulses and shape of the pulse waves.96,97 Electric field strength depends on the gap between the electrodes and on the applied voltage, while the treatment time depends on the number of pulses delivered and specific energy. The specific energy refers to the electrical energy needed for the generation of a high voltage pulse in the treatment chamber and it is expressed as kJ kg−1. Finally, the total specific energy for the treatment can be calculated from the specific energy of one pulse multiplied by the number of pulses applied during the treatment.96
Another promising innovative emerging technology for green extractions is HVED. HVED (cold plasma) has been recently researched for enhancing extractions of various compounds from different raw materials.98 In this technology, energy is presented directly in a liquid throughout a plasma channel formed by applying a current of high-voltage electrical discharge between two electrodes. Compared to PEF, HVED causes more damage to the product, influencing both the cell wall and membrane.94 The technology is based on the phenomenon of electrical breakdown in liquids that causes physical (i.e. shock waves, bubble cavitation) and chemical effects (i.e. formation of free radicals), which consequently promotes cell structure damage and the creation of membrane pores.94–99 The HVED extraction system is usually classified in three categories: batch, continuous and circulating extraction systems. The process mechanism is the same for all three categories, but the difference is between the device structures, which mostly refers to electrodes. In the batch system, the extraction is conducted in a batch treatment chamber and each of these treatment processes is independent. Usually, needle-plane electrodes are used with positive voltage applied on the needle. The high intensity electric field is concentrated at the needle electrode and the electrical discharge can consequently happen when the voltage is high enough. The continuous HVED extraction system has a continuous treatment chamber and it is more applicable in industries. In this system, a pair of stainless-steel electrodes is used, one of these electrodes is a high voltage electrode and the other one is grounded. These electrodes can be either parallel disc mesh electrodes (converged electric field type) or annular electrodes (annular gap type). The third circulating extraction system was designed to achieve a higher yield of extraction. The system consists of a high voltage pulsed generator which can generate high voltage pulses in several microseconds, a treatment chamber, an extracting tank and a transport unit. There are two stainless steel electrodes, needle (high voltage) and ring (grounded), and the needle electrode is placed in the centre of the ring electrode. This design allows larger process capacity with quite a small treatment chamber.100
Applications in sustainable extraction
The main advantage of using PEF and HVED in extraction processes compared to conventional extraction techniques that use heating is that PEF and HVED are non-thermal techniques. This leads to the preservation of thermolabile components presented in food and increases the yield and quality of the obtained extract.96 Furthermore, these processes have low energy consumption that is in accordance with the principles of green extraction. In general, energy consumption of extraction processes can be reduced following few general approaches: using innovative techniques, process optimization and energy recovery released during the process.101 Some authors have applied these approaches for sustainable extractions of food and natural products.A study for the evaluation of the effect of both PEF and HVED on the extraction of sesame cake compounds was conducted. Both PEF and HVED were used with different energy inputs as pre-treatments to diffusion. Results showed that the disintegration index and the polyphenol and protein contents increased with energy inputs, until 83 kJ kg−1. Also, both pre-treatments improved the extraction yields of polyphenols, lignans and proteins from sesame cake and improved the kinetics of diffusion. According to these results, it can be concluded that PEF or HVED can reduce the use of organic solvents and the need for high temperatures to improve diffusion that is of great interest for the industry.94
As wine production is one of the most important agricultural activities worldwide, there is a generation of huge amounts of waste and by-products. Various green alternative methods, including PEF and HVED, were analysed for the extraction of bioactive compounds from winery wastes and by-products. PEF was shown to be effective at extracting natural components from wine grapes, grape pomace and vine shoots. Results showed increased extraction yields of total polyphenols, anthocyanins and proteins. On the other side, HVED also resulted in increased extraction yields of polyphenols from grape pomace, grape seeds and vine shoots; moreover, it was presented as a more energy efficient extraction method compared to PEF.99
Newer studies were also directed towards PEF extractions of valuable compounds from the meat and fish industry.102 Researchers have investigated the ability of PEF to facilitate the extraction of functional proteins from waste chicken meat and obtained a fraction enriched with proteins with possible antioxidant properties.95 This technology was also shown to be effective at extracting high yields of proteins from mussel and by-products from the fish processing industries and for the extraction of calcium and chondroitin sulphate from fishbones.102 Furthermore, PEF treatments for extractions from microorganisms, like microalgae, yeasts or bacteria, represent a promising resource for obtaining high-value products, including proteins, lipids, carbohydrates and pigments (i.e. carotenoids, chlorophylls). Microalgae, as a renewable resource, present a promising and sustainable source of biocompounds.96 A study that used PEF as a pretreatment method for lipid extraction from microalgae Chlorella pyrenoidosa grown in wastewater was conducted. Following the pretreatment, the yield of fatty acid methyl esters was 12% higher compared to the traditional method using ultrasound. Results showed that PEF was an effective method for cell disruption enhancing lipid extraction which is a major step of biodiesel production from microalgae.103
A frequently researched application of HVED was in the extractions of various bioactive compounds from plant sources. Researchers have efficiently extracted phenolic compounds, oils, polysaccharides and proteins from different plant-based materials, including fruits, vegetables, coffee ground, herbs etc. Newer studies are directed towards extractions from waste and by-products in the food industry as it saves enormous amounts of valuable compounds naturally present in plants that is wasted during processing.100 A continuous HVED extraction system, the annular gap type, was designed and optimized for flavonoid extraction from peanut shells. The optimal treatment conditions included: 25% ethanol concentration, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL g−1 liquid to solid ratio, 13 kV and 60 mL min−1 flow rate of the material. Under these conditions, the maximum yield of flavonoids was 0.948 ± 0.014%. The treatment with the annular gap type resulted in a shorter duration and higher efficiency for the flavonoid extraction, compared to the converged electric field type treatment chamber and conventional warm maceration, and had no effects on the composition of the extracted flavonoids.104 In a recent study, the potential of HVED for the extraction of phenolic compounds and antioxidants from olive leaves has been investigated. Olive leaves act as a waste in olive oil production, but are also considered medicinal and aromatic herbs rich in phenolic compounds. Olive leaf extracts, obtained by green solvents (water and ethanol) and HVED as green technology, were in line with the principles of green chemistry and sustainability while having high nutritive value. The process was optimized and the highest values of phenolics were obtained under the following conditions: HVED treatment for 9 min with argon gas, 20 kV and 50% ethanol.5 Even though HVED is a potential technology for extractions with many advantages (reducing solvent consumption, low process temperature, increased extraction yield compared to other methods), there are still some disadvantages that should be solved before implementing this technology in industries. It was shown that many free radicals are produced during the treatment with HVED which have a negative impact on target compounds since they can oxidize such compounds and produce cell damage. The production of free radicals should be controlled by controlling the energy input and optimizing the process parameters. Hence, more experiments need to be conducted to investigate the disadvantages of HVED and scale-up factors before industry application.100
1 mL g−1 liquid to solid ratio, 13 kV and 60 mL min−1 flow rate of the material. Under these conditions, the maximum yield of flavonoids was 0.948 ± 0.014%. The treatment with the annular gap type resulted in a shorter duration and higher efficiency for the flavonoid extraction, compared to the converged electric field type treatment chamber and conventional warm maceration, and had no effects on the composition of the extracted flavonoids.104 In a recent study, the potential of HVED for the extraction of phenolic compounds and antioxidants from olive leaves has been investigated. Olive leaves act as a waste in olive oil production, but are also considered medicinal and aromatic herbs rich in phenolic compounds. Olive leaf extracts, obtained by green solvents (water and ethanol) and HVED as green technology, were in line with the principles of green chemistry and sustainability while having high nutritive value. The process was optimized and the highest values of phenolics were obtained under the following conditions: HVED treatment for 9 min with argon gas, 20 kV and 50% ethanol.5 Even though HVED is a potential technology for extractions with many advantages (reducing solvent consumption, low process temperature, increased extraction yield compared to other methods), there are still some disadvantages that should be solved before implementing this technology in industries. It was shown that many free radicals are produced during the treatment with HVED which have a negative impact on target compounds since they can oxidize such compounds and produce cell damage. The production of free radicals should be controlled by controlling the energy input and optimizing the process parameters. Hence, more experiments need to be conducted to investigate the disadvantages of HVED and scale-up factors before industry application.100
Success story
Although HVED is still in laboratory level investigations and no industry applications for extractions were noted, the use of PEF at the industrial scale is constantly increasing. ELEA Technology from Germany is a leading supplier of PEF, a successful company that was created to commercialise all PEF technologies initially developed by German Institute of Food Technologies (DIL). Currently, ELEA has developed and installed over 125 ELEA PEF systems that are in full-scale operation around the world in various food and beverage sectors. There are several more manufacturers that have developed PEF equipment and recognized the need for food industry application such as Pulsemaster from The Netherlands, Scandinova from Sweden, Wek-tec from Germany, and Heat and Control from the USA.Ohmic, infrared (IR) and ultraviolet (UV) heating
Process and procedure
The conventional thermal processing systems involve heat energy transferred through conduction and convection from a hot medium to a cooler product. This may cause a large temperature gradient and uneven temperature distribution in the product. Therefore, various innovative heating techniques have been developed which are faster, more efficient and energy saving methods.97Ohmic heating, or Joule heating, is the process where electric current is passed through the food (material) of resistance which causes heat to be released (the Joule effect). The equipment for ohmic heating consists of a heating cell, electrodes that are directly in contact with the heating material, an AC power source and a treatment chamber where the product is placed.97 According to Ohm's law, the amount of dissipated heat (energy) is directly related to the applied voltage and the electrical conductivity of the product. Various factors have an influence on the ohmic heating rate of food: electrical conductivity, field strength, particle size, concentration, ionic concentration, and electrodes. The key parameter in the design of an effective ohmic heater is electrical conductivity (S/m), a measure of how well a material accommodates the movement of electric charge. Electrical conductivity is different through a heterogeneous material and that leads to differences in the conversion of electrical current to thermal energy, which is the main limitation of ohmic heating.105
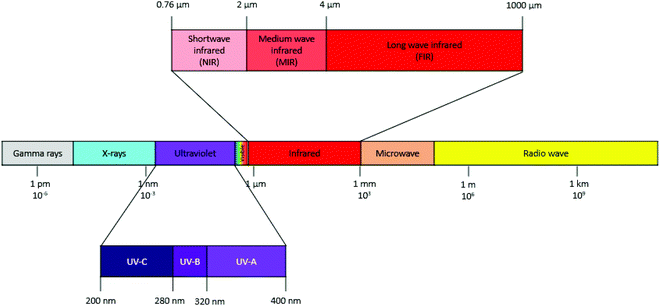
Infrared (IR) radiation is a type of electromagnetic radiation dispersed from an infrared emitter (lamp) resulting from the vibrational and rotational energy of molecules.97 Consequently, thermal energy is generated following the absorption of radiating energy and the heating effectiveness is associated with the line of sight between the radiating source and the product. This is the main reason for the high energy efficiency of infrared systems to initiate an extraction. The infrared region of the electromagnetic spectrum (Fig. 10) ranges from 0.76 μm to 1000 μm divided in three zones: shortwave infrared, also called “near” or “high intensity” (NIR: 0.76–2 μm), medium wave infrared, also known as “middle” or “medium intensity” infrared (MIR: 2–4 μm), and long wave infrared, also called “far” or “low intensity” infrared (FIR: 4–1000 μm).13 The main features of infrared irradiation include high heat transfer capacity, fast and simple process control, direct heat penetration into the product, no heating of surrounding air, low operating and maintenance costs, and a relatively safe and clean process.97
 | ||
| Fig. 10 The electromagnetic spectrum with ultraviolet and infrared irradiation zones (NIR – near infrared, MIR – middle infrared, FIR – far infrared, UV – ultraviolet). | ||
Ultraviolet (UV) light treatment uses light in the electromagnetic spectrum from 100 to 400 nm and is usually used in industry for extending the shelf-life of the product. Gas discharge leads to the emission of UV light at wavelengths depending on the composition, excitation, ionization and kinetic energy of these elements. Mercury lamps are used as a standard. Traditionally, low pressure mercury vapor lamps at 254 nm were used for food surface disinfection and treatment of food liquids. However, several alternative UV source types have been developed and are applied in the food industry, such as excimer lamps, pulsed lamps (i.e. xenon) and light-emitting diodes (LEDs). The UV spectrum is generally divided into UV-A (wavelength 320–400 nm), UV-B (wavelength 280–320 nm) and UV-C (wavelength 200–280 nm).97 UV-C light is also called non-ionising radiation since it does not produce chemical residues, by-products or radiation. It is a simple, dry and cold process with low maintenance and cost requirements. Therefore, there is an increasing interest in using UV-C light in food processing including extractions of natural food compounds.97,107
Applications in sustainable extraction
Ohmic heating, IR and UV light are fast and environmentally-friendly methods that have been applied in a wide range of food processing techniques. Ohmic heating can be applied to a broad variety of foods, including liquids and semi-solid and solid foods accompanied by a suitable carrier liquid. Beyond heating, the electric field under ohmic heating causes electroporation of cell membranes and therefore can be used for extractions with reduced energy requirements during processing. Electric field with strength under 100 V cm−1 allows an enhanced extraction process. An increased extraction yield was obtained in the extraction of natural components from various plant products.105 Moreover, there is growing interest in science to apply ohmic heating in food waste extractions. For this reason, a study for the optimization of pectin extraction from orange juice waste assisted by ohmic heating was conducted. Results showed that the optimum ohmic heating method for extraction takes place at 90 °C and with a voltage gradient of 15 V cm−1. The ohmic method could extract pectin more significantly than the conventional method due to more intensive rupturing of the cell wall, and it was observed that the extracted pectin had a high degree of esterification and high emulsifying activity.108 The effect of ohmic heating on oil extractions was also studied. In a study from Aamir and Jittanit (2017), the extraction of oil from Gac aril powder with ohmic heating was compared to conventional heating. Study results showed that the ohmic method provided significantly higher oil extraction efficiency and that the extracted oil also contained greater amounts of red-color pigments such as β-carotene and lycopene, compared to the conventional method.109 Another study analysed the extraction of Pulicaria undulata essential oil by ohmic hydrodistillation. Ohmic-assisted hydrodistillation is a promising green process where internal heating and the electroporation phenomenon occur owing to the electrical current passing through the plant material. Compared to conventional hydrodistillation, the extraction by ohmic-assisted hydrodistillation needed a much shorter time and afforded a significantly higher extraction yield. A chemical profile of the obtained essential oils showed that the oils obtained by ohmic-assisted hydrodistillation had more content of 1,8-cineole and chrysanthenone, and the oils were more effective in terms of antibacterial and antioxidant activities. Finally, the electrical energy consumption was almost half of that measured for conventional hydrodistillation. According to this and on the basis of equivalent CO2 emission, ohmic-assisted hydrodistillation may well be categorized as a green extraction technology.110 More studies should be conducted to analyse the usage of ohmic heating in food containing fats and oils for better process understanding. Because of less awareness and higher initial costs of the technology, its usage is still limited.105IR radiation has been in use for a long time, but only in the last two decades, the advantages of IR application in different chemical processes, including natural products extraction, have been investigated. Mainly FIR radiation has been employed for these reactions. Various natural compounds, such as polyphenols, flavonoids, mannitol, sucrose, glucose, fructose, capsaicin, quercetin and others, have been successfully extracted from different vegetable specimens.106 A new method of infrared-assisted extraction coupled to headspace solid-phase microextraction has been developed for the fast determination of the volatile components in tobacco. Compared with conventional water-bath heating and non-heating extraction methods, the developed method greatly improved the extraction efficiency and was showed to be a simple, time-saving, cost-effective, highly efficient, and environmentally friendly approach.111 Recently, Rajha et al. (2019) have patented a new infrared extraction technique and combined it with deep eutectic solvents, as a green alternative to conventional organic solvents, for the extraction of polyphenols from pomegranate peels. This extraction process was conducted at 50 °C for 90 min and the IR extraction technique was shown as the most efficient method compared to ultrasound and solid–liquid extraction methods. The use of deep eutectic solvents with IR allowed the extraction with a higher yield of polyphenols and improved their biological activities.112
A literature search presented IR radiation as a clean and effective method for natural component extraction. Taking into account the possible usage of green energy, solvent-free extraction and other benefits, IR radiation was shown to be a good green approach.107
UV light is still in its developmental phase and only a few studies have been conducted on UV-assisted extractions. The effect of UV-C treatment has been tested in the extractions of phenols, flavonoids and vitamin C from honey pineapple, “pisang mas” banana and guava. From results, it was clear that UV-C treatment improved the polyphenolic profile of all three fruits. The total phenolic and flavonoid content increased with the increase of treatment time, but the content of vitamin C decreased. The study presented UV-C as a useful treatment for fresh-cut tropical fruits for enhancing their nutritional value and it plays a positive role in preventing several physiological and pathological processes for consumers.111 More recently, scientists have analysed the combination of supercritical fluid extraction followed by UV irradiation for the extraction of vitamin D from shiitake mushrooms (Lentinula edodes). By supercritical fluid extraction, high extraction yields of ergosterol and ergosterol derivatives were obtained. Hence, provitamin D2 was further converted to vitamin D2 by UV-light irradiation. The method also induced vitamin D4 formation, but in lower amounts than vitamin D2 or lumisterol2.113 Moreover, a study on the UV assisted extraction of flavonoids and allantoin from aqueous and alcoholic extracts of the medicinal plant Symphytum officinale (comfrey) was carried out. This plant protects skin against UV irradiation. UV-A, UV-B and UV-C radiations were assessed for 10 min in comfrey aqueous and alcoholic extracts. The experiment showed that UV irradiation enhanced the yields of the active ingredient of comfrey extracted with methanol, whereas it improves flavonoids, reducing the power and allantoin levels of comfrey extracted by the aqueous infusion method. UV-radiation reduced the levels of flavonoids, reducing the power and allantoin when the comfrey was extracted using alcohols.114 These research studies have raised many questions resulting in the need for further investigation of UV irradiation for sustainable extractions.
Success story
The application of the above-mentioned technologies in extractions is still limited. However, there are several companies that have efficiently developed and applied these novel methods. For example, AseptoRay from Israel, was created to meet the need in the food and beverage industry for non-thermal effective pasteurization. They have designed machines that use UV technology for the treatment of a wide range of liquids. Alfa Laval from Sweden and C-Tech Innovation from UK are companies with long experience in heat transfer. They have developed ohmic heating technologies particularly for the rapid pasteurization or sterilization of products which retain most of their nutritional and sensorial properties. Infrared heating has a wide application in industries. However, it is worth mentioning that the Infrabaker Company from Belgium is an innovative company specialized in the development and production of infrared applications for the food processing industry. These early successes may help to resolve the disadvantages of using ohmic heating, UV and IR irradiation in food processing and possibly have a recent industrial application in sustainable extractions.High pressure processing
Process and procedure
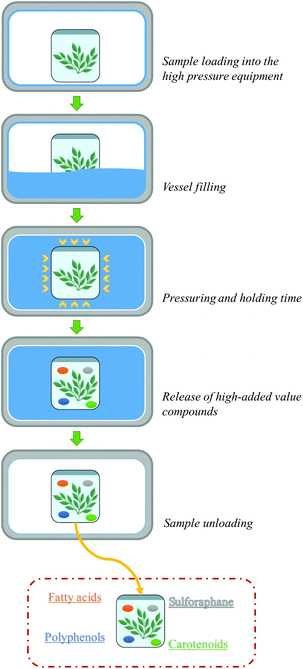
High pressure (HP) food processing technology consists of applying pressure (usually up to 600 MPa) at a defined temperature (at or around room temperature) and period (few minutes). The combination of pressure, temperature and processing time can be selected to achieve specific processing conditions.115–117 HP can be applied to inactivate both enzymes and microorganisms, associated with quality deterioration, preserve sensory attributes, extend the shelf life of food products, modify the bioaccessibility and bioavailability of nutrients and bioactive compounds and prevent the formation of toxic compounds, among other applications.115,118–121The basic design of HP equipment is mainly composed of the following components: a treatment vessel, closures or plugs, a yoke/wire-wound steel frame, a pressure pump, and a control system.122 The HP processing occurs by filling the vessel with a liquid (usually water) that transmits the pressure to the product (Fig. 11).
The procedure is carried out in a few minutes and follows the sequential steps: (i) loading the packaged product into the treatment vessel, (ii) filling with water, (iii) applying pressure at defined temperature, (iv) holding the pressure for a specific time, (v) releasing/discharging water, and (vi) finally unloading the treated product. Another remarkable characteristic of this technology is that a product of any shape can be processed.116 The main concepts that explain the application of HP and its effects on food involves the relationship between pressure and temperature, isostatic principle, Le Chatelier's principle, microscopic ordering, and the Arrhenius relationship.123 The first concept involving the relationship of pressure and temperature is caused by the intrinsic relationship between these two variables. Once the volume of food is reduced by an external pressure, it will change and a corresponding increase in temperature will be noticed. Consequently, the structure, phase and molecular organization within the food will be changed. This sequence of events is supported by the isostatic principle which states that the applied pressure is instantaneously and homogeneously distributed within the food item, independent of the food structure and geometry. The Le Chatelier's principle states that the equilibrium will change the volume of food to the corresponding equilibrium (reduced volume). A microscopic ordering also occurs during the HP treatment: the molecules will shift their organization towards a more organized and compact configuration. Finally, the Arrhenius relationship supports the modifications observed at the interatomic level. In this case, electrostatic interactions are largely influenced by shifts in pressure, which leads to alterations in bond strength. Consequently, physical properties dependent on electrostatic interactions are modified such as viscosity, density, acid–base equilibria, and process rates.124
HP processing aims to increase the shelf life of the treated food by reducing the load of viable microorganisms associated with spoilage. In addition, HP has been also associated with minimal impact on the sensory and nutritional characteristics of the treated food in comparison with conventional processing techniques. Low molecular weight molecules (particularly essential amino acids, vitamins, and those associated with flavor) are minimally affected by HP processing, which improves the preservation of original sensory properties and fresh-like characteristics of HP-treated foods. The effect is currently at the same level as a that obtained from thermal pasteurization.125,126
Application of HP in sustainable extraction
The use of HP has been explored as an extraction technique to recover high-added value compounds (i.e. polyphenols, carotenoids, isothiocyanates, fatty acids, essential oils, etc.) from a wide variety of sources (i.e. animal wastes, flowers, herbs, agri-food by-products) (Table 3).127| Source | Compounds | HP treatment and extracting conditions | Effect | Ref. |
|---|---|---|---|---|
| Radix (Angelica sinensis) | Ferulic acid | 100–500 MPa at 25 °C for 10 min (70% ethanol) | Extraction yield was increased using 300–500 MPa | 128 |
| Flos Sophorae flower | Flavonoids | 400–534 MPa at 25 °C for 5 min, 53–80% ethanol and a liquid to solid ratio of 13–47 mL g−1 | Increased extraction yield at 460 MPa, 75% ethanol solution and a liquid to solid ratio of 32 mL g−1 | 129 |
| Chilean papaya (Vasconcellea pubescens) seeds | Polyphenols, sulforaphane and fatty acids | 500 MPa at room temperature for 5, 10 and 15 min (80% ethanol) | The highest extraction yields were obtained after 15 min of extraction | 130 |
| Pomegranate peel (Punica granatum) | Phenolic compounds | 300 and 600 MPa at 20 °C for 5, 17.5 and 30 min (0, 40 and 80% ethanol) | Optimal extracting conditions were 470 MPa for 30 min using 55% ethanol for phenolic compounds; antioxidant activity was assay-dependent | 131 |
| Blueberry pomace (Vaccinium ashei Reade, Gardenblue) | Polyphenols and anthocyanins | 500 MPa at room temperature for 3 min (60% ethanol) | Increased total phenolic and anthocyanin recovery | 132 |
| Shrimp shells (Penaeus Vannamei Boone) | Astaxanthin | 100–600 MPa at room temperature for 5–20 min (acetone, dichloromethane or ethanol) | Extraction was enhanced using 200 MPa, 5 min, 20 mL g−1 and ethanol as a solvent | 133 |
Moreover, the main advantages associated with this technology are the improved extraction yield, reduced time to obtain the compounds and also the use of green solvents. For instance, the extraction of ferulic acid (an antioxidant, antimicrobial and inducer of brain blow flow) from radix (Angelica sinensis) was improved by the use of HP.128 The authors observed that the ferulic acid yield was improved from 1.06 (reflux extraction method) to 1.19 mg g−1 (HP method with pressures above 300 MPa). The authors also observed that the surface of the radix cell treated with HP was largely affected, which supported the higher extraction yield obtained in this kind of sample. A similar outcome was reported by using HP to obtain flavonoids from white buds of Flos Sophorae.129 The extraction was optimized using a response surface methodology where pressure, percentage of ethanol in the solvent and the liquid/solid ratio were selected as the main variables. According to the authors, the most efficient extracting conditions were 460 MPa, 75% ethanol solution and a liquid to solid ratio of 32 mL g−1, which yielded 203 mg flavonoids per g.
The extraction of bioactive compounds from (phenolic compounds, sulforaphane and fatty acids) from Chilean papaya (Vasconcellea pubescens) seeds was enhanced by combining extraction with HP technology.130 This result was obtained after treating the seeds at 500 MPa for 15 min that yielded the highest concentrations of phenolic compounds, sulforaphane and fatty acids in comparison with Soxhlet extraction and extractions carried out for 10 and 5 min. An interesting comparison between green technologies (ultrasound, microwave and HP) and a conventional extraction method for the extraction of polyphenols and anthocyanins was carried out using blueberry (Vaccinium ashei) pomace.132 A significant increase in the recovery of both polyphenols and anthocyanins was obtained only for HP (recovery of 70 and 40%, respectively) in comparison with the conventional heating method (recovery of 53 and 32%, respectively).
The use of HP to obtain phenolic compounds from pomegranate (Punica granatum) peel was also demonstrated to be a useful tool, as HP (470 MPa, 30 min, 55% ethanol solution as a solvent) allowed an increase in the yields.131 Interestingly, this study also indicated that the extracting conditions were of great importance for the compounds isolated. While 395 MPa maximized the extraction of anthocyanins, using 492 and 600 MPa maximized the recovery of flavonoids and tannins, respectively. Therefore, it is in full correspondence with the expected versatility and selectivity of this technique to obtain extracts rich in phenolic compounds. Another relevant outcome of using HP as an environmentally-friendly extraction technology was reported for the extraction of astaxanthin (a natural carotenoid used as a colorant in animal feed, food and cosmetics) from shrimp shells, a by-product of shrimp processing.133 The authors indicated that the maximum recovery of this carotenoid (71.1 μg g−1) was obtained by applying 200 MPa for 5 min with 20 mL ethanol per g shrimp shell. In addition, the authors also observed that the astaxanthin obtained from HP treatment displayed higher antioxidant activity than that isolated from the extraction at atmospheric pressure (43.6 μg g−1).
Therefore, carrying out the extraction with a solvent128,129 and using HP as pre-treatment134 are feasible strategies to improve the recovery of high-added value compounds. Moreover, the use of HP as an extraction technology is supported by the studies that reported significant improvements in obtaining high-added value compounds in relation to conventional extraction methods.130,132
Success story
The initial goal of HP processing was the improvement of the shelf life of food products using a non-thermal technology at the laboratory scale.135 This scenario progressed towards worldwide implementation of HP systems in modern times to process several packaged food products. Moreover, HP technology is an interesting solution available for professionals of the agri-food sector to change their equipment towards a more sustainable industrial activity. The current market of HP equipment is shared among companies such as Hiperbaric, Avure, and Multivac that are the main contributors to the development of this technology.135,136 As a leading company in the sector of HP equipment, Hiperbaric has been operating in this market since 1999. Its first application in 2002 was dedicated to treat dry cured meat products and later ready-to-eat products elaborated by Campofrío (Spain). The Hiperbaric equipment was then installed in Italy, Canada, USA, Japan, Australia, Peru, China and South Korea. Currently, its applications in food processing include meat, dairy and seafood products, juices, ready-to-eat meals, dips and salad dressings, infant food, vegetables, sandwich fillings, and wet salads, among others.137Solar energy for hydro and steam distillation
Process and procedure
Exploring solar-based technologies is a major advance in the development of more sustainable activities in the industrial sector, particularly for the generation of heat and electricity that supply energy for a wide variety of processes.138 Between these two interesting options, the solar thermal approach has a central role by converting solar energy into heat. This strategy is centered at collecting, concentrating and using it to start and maintain an industrial process. The main components are: a solar collector, a medium for heat transfer, a storage system, and a boiler (Fig. 12). Solar energy is collected and used to heat a selected medium (usually water) that is used to meet the demand for hot water and steam. Consequently, processes such as pasteurization, concentration, drying, washing, cleaning, and supporting thermochemical reactions can be performed.138,139The conversion of the Solar System into heating takes into account: (1) the physical properties of radiation, (2) the geometric relationship between the sun and the Earth, (3) the incidence of radiation, and (4) the characteristics of the irradiated surface. The physical fundamentals of radiation are the first step to understand the process, which considers that radiation travels at the speed of light and it is emitted by atoms and molecules returning from excited states (vibrational, rotational and electronic rearrangements). The geometric relationship between the sun and Earth is also considered since Earth rotation, revolution and inclination shift the incidence of radiation on its surface. In addition, it is also considered that the direct beams are parallel when reaching the surface of the Earth and the sun is a “disk” of uniform brightness. Regarding the characteristics of the irradiated surface, the incidence of radiation causes a simultaneous increase of surface's temperature as well as reflection and transmittance (in transparent materials). Consequently, the occurrence of these phenomena influences the surface absorption efficiency to convert solar energy into heating. Moreover, the losses associated with the thermal, re-radiation components and the geometry of the surface also affect the efficiency of the system.140 It is worth mentioning that the configuration of solar converting systems can be defined according to the coupled industrial operation. On the one hand, in processes consuming hot water, a stationary solar collector (mainly flat-plate collectors) can be used due to its range of indicative temperatures (from 60 to 240 °C). The flat collectors are characterized by the low concentration index (indicates the efficiency of a system to concentrate solar energy). On the other hand, processes demanding steam use a single- or two-axis tracking colleting system as the main options due to their higher concentration ratios (from 5 to 50 and from 100 to 1500, respectively) and indicative temperatures (in the range of 60–300 and 100–2000 °C, respectively).138,141
Application in sustainable extraction
Exploring this low-cost technology to obtain essential oils from vegetable sources is an innovative and green approach for the food sector. Although each equipment reported in the scientific literature was assembled in a particular way, all equipment follow the same basic configuration (Table 4): a solar collector coupled to an apparatus to produce steam, a distillation column, a condenser and a separator (usually a Florentine flask).142| Matrix | Components of solar hydrodistillation apparatus | Extraction yield | Ref. |
|---|---|---|---|
| Eucalyptus (Eucalyptus camaldulensis) and peppermint (Mentha peperita L.) leaves | Scheffler reflector, concentrator, steam receiver, distillation still, condenser and oil and a separator | 0.40 (peppermint) and 0.59 (eucalyptus) g/g | 143 |
| Orange (Citrus sinensis L.) peel | Solar reflector, secondary reflector, hydrodistillation unit, condenser and a Florentine flask | 1 g per 100 g | 144 |
| Peppermint (Mentha piperita L. and Mentha spicata L.) | Parabolic solar collector, hydrodistillation unit, condenser and a Florentine flask | 4.2–8.2 mL kg−1 | 145 |
| Peppermint (Mentha piperita L., Mentha spec., and Mentha spicata) | Scheffler reflector, secondary reflector, hydrodistillation unit, condenser and a Florentine flask | 2.00 (Mentha piperita L.), 2.50 (Mentha spec.) and 2.22 (Mentha spicata) mL kg−1 | 146 |
| Clove buds, cumin and fennel | Scheffler reflector, hydrodistillation unit, condenser and a Florentine flask | 0.76, 1.0 and 5.5 mL per 100 g for fennel, cumin and cloves, respectively | 147 |
| Melissa, peppermint and rosemary leaves, cumin seeds, and clove buds | Scheffler reflector, secondary reflector, hydrodistillation unit, condenser and a Florentine flask | 0.558 (Melissa leaves), 5.48 (rosemary leaves), 11.4 (cumin seeds), 11.9 (peppermint leaves), and 61.8 (clove buds) mL kg−1 | 148 |
| Cymbopogon citratus | Solar energy tube, a sunlight shade, a solar cell, a battery, a temperature controller, and an essential oil collection unit | 1.3 g per 100 g | 149 |
An interesting experiment assembled a solar energy harvesting system with a Scheffler reflector and a steam receiver to produce steam for distillation in the distillation column.143 The authors used leaves of eucalyptus (Eucalyptus camaldulensis) as well as peppermint (Mentha peperita L.) and obtained 0.59 and 0.40 g essential oil per g leaves for eucalyptus and peppermint, respectively. Additionally, the authors also estimated that the break-even point (when the accumulated profit is equal to the operational costs and investments) was about 181 days by taking into account the investment of $4.500 on materials and construction, the daily production of 72 mL of essential oil and 10 mL of pure essential oil for $4.50.
A similar experiment was conducted using a solar energy harvesting system equipped with a secondary reflector above the distillation column to produce steam for hydrodistillation of orange essential oil.144 The essential oil extraction obtained from this system was around 1 g per 100 g of orange peels. According to the authors, this process was faster than the conventional hydrodistillation systems (120 vs. 190 min to complete the extraction, respectively). An analogous solar energy harvesting system (parabolic solar collector) was used to isolate the essential oils of different peppermint species.145 The authors observed that the essential oil yields ranged from 4.2 to 8.2 mL kg−1 of leaves among species (Mentha spicata L. and Mentha piperita L., respectively). Another study also supports the use of solar energy harvesting systems coupled to hydrodistillation equipment to obtain essential oils from peppermint.146 In this case, the extraction yielded 2.00 (Mentha piperita L.), 2.50 (Mentha spec.) and 2.22 (Mentha spicata) mL essential oil per kg leaves.
The combination of hydrodistillation and solar energy was also used to obtain essential oils from clove buds, cumin and fennel.147 This study reported that differences among the samples (structure, composition, essential content, for instance) still play an important role in this green extraction approach. The authors indicate that clove buds, cumin and fennel required less energy to obtain 1 mL of essential oil (up to 0.5 kW h mL−1 of essential oil) than other herbs (pachouli and orange barks, energy consumption in the range of 2.7–2.8 kW h mL−1 of essential oil). In the same line, a study carried out with a Scheffler reflector and a secondary reflector to harvest solar energy afforded essential oils from melissa, peppermint and rosemary leaves, cumin seeds, and clove buds.148 The extraction of essential oils was influenced by the herb species wherein the highest yield was obtained from cumin seeds and peppermint leaves (11.4 and 11.9 mL essential oil per kg sample). Interestingly, the extraction carried out with melissa, peppermint and rosemary required less thermal energy than those performed with cloves and cumin to complete the extraction. Conversely, the extraction of essential oil from Cymbopogon citratus by either solar energy or the conventional hydrodistillation system was similar (around 1.3 g essential oil per 100 g leaves).149 According to an estimation performed by the authors, the conventional hydrodistillation method required 858 kW h to obtain the essential oil whereas the solar energy system was independent of electricity.
It is also worth mentioning that the solar energy harvesting systems proposed can also be simultaneously used with other thermal sources of energy and be integrated into a biorefinery to maximize the recovery of high added value compounds and minimize the residues from these activities. For instance, a study also explored the use of an auxiliary green energy source (in the case of days with low solar irradiation) for the hydrodistillation of eucalyptus and peppermint essential oils.143 The authors proposed and estimated the energy requirements for a biomass vertical boiler and indicated a 4.5-fold increase in the thermal energy demand in comparison with the solar energy harvesting system. Regarding the integration into a biorefinery, the solar energy harvesting systems coupled to hydrodistillation equipment can be used as a first step in biorefinery and reduce the holding time for further operation. This approach was explored for orange peels that were used to obtain phenolic compounds and pectin, which are natural ingredients used in the food industry to produce a wide variety of products.144 In this sense, the use of solar energy harvesting systems to obtain essential oils can be considered as the main green alternative to the isolation of this high-added value compounds. Moreover, these systems are reliable, have fast return of investment, and are easy to operate. Finally, the simultaneous use of these solar energy harvesting systems with auxiliary green thermal sources and the easy integration into a biorefinery approach are feasible solutions to advance toward a more sustainable extraction of essential oils.
Success story
The solar collector component is a useful solution to reduce the demands for electricity and fossil fuel to carry out hydrodistillation. The Scheffler concentrator was invented by Wolfgang Scheffler in order to harvest solar energy by focusing solar radiation into a single point. The possibility of tracking the sun movement is the main advantage of this apparatus that improves the time collecting solar energy.138,150 Akson's Solar is a company based in India that produces the Scheffler concentrator and was founded in 1997 to produce equipment to harvest solar energy. The company produces and sells, in addition to the Scheffler concentrator, a variety of water heating apparatus for house and commercial applications, photovoltaic plates to convert solar energy into electricity for both home and street lighting, feed pumps, and other equipment (biogas production and waste management, among others).151Dielectric heating and acoustic cavitation
History, experience and tradition in natural product extraction date back to ancient civilizations. Thanks to new enabling technologies for solid–liquid extraction the evolution of maceration, percolation and hydrodistillation is now a reality. These achievements may dramatically improve the sustainability of the process in agreement with the principles of green extraction and the concepts of circular economy.152 In brief the goals of a lower consumption of solvents, time and energy and a full preservation of phytocomplex integrity especially in the presence of thermo-sensitive components are achieved. Enabling technologies and cascade processes can offer an effective scalability and intensification of extraction procedures towards industrial applications. A positive economic impact with a cost-effective production of high-quality products will drive the innovation toward the industrial application. A higher efficiency and sustainability can be reached thanks to the design of new environmentally-friendly protocols that often use integrated techniques.153 Ultrasound- and microwave-assisted extraction procedures (UAE and MAE respectively) can play pivotal roles in achieving these objectives, both when they are used individually or in combination, leading to high active principle and oil yields, selectivity improvement, and high stability of the obtained product, often preserving the volatile fraction of the extract when natural flavor and taste are desired. These techniques can be dedicated to both pilot and industrial scales protocols, and several studies have compared their performance with those of the classic procedures.154An overview of the last ten years highlights the constant interest about this kind of eco-sustainable approach, especially with regard to the two techniques individually applied each with more than 7000 publications, rather than their use in tandem mode for which a rate of 10% of papers can be found. From 2009 to date, more than 200 patents are dealing with MAE and about 70 with ultrasound with 30 examples of combined use (Fig. 13).
Ultrasound and hydrodynamic cavitation
The strong mechanical effects generated by ultrasound dramatically enhance mass transfer during extraction protocols. The transmission of ultrasound through the medium gives rise to the phenomenon of acoustic cavitation, and microcavities were created when the pressure related to the acoustic waves breaks the cohesion of a liquid. These cavities start as microbubbles, which grow under the sound wave effect until they become unstable and violently collapse creating extreme localized conditions (high temperatures and pressures) inside the collapsing cavity. This cavity collapses generating shockwaves that produce mechanical effects which enhance the extraction efficiency thanks to effects such as: cell wall disruption, particle size reduction, intensive mixing and hot spots. Moreover, a structural modification of plant tissue occurs facilitating swelling and solvent permeation into the cells.155 Herbal and food components, such as oils, proteins, polysaccharides, and bioactive natural products can be effectively recovered by applying UAE.156 In a flow reactor, the effects of high-intensity ultrasound (strong acoustic cavitation) can dissolve or disperse/emulsify primary and secondary metabolites in a liquid that is generally water, preserving the full, natural composition of the original plant, but leading to extracts having much higher concentrations of primary and secondary metabolites. In a perspective of process intensification, the use of flow UAE is well placed, hinging the development of large-scale multiple transducer reactors that operate at high power density.Effective extraction processes can be carried out in rotor–stator hydrodynamic cavitation reactors. In such apparatus, rotating cylinders are periodically aligned with stator channels during high-speed rotation and the processed liquid is accelerated in the radial direction in the cavitation chamber and subjected to a pressure wave resulting in cavitation. The contact area of the solid/liquid surface increases when the cavitation bubbles collapse producing shockwaves.157
The choice of the solvent and the ratio with the plant material are strongly influenced by the nature of the phytocomplex and the type of application. It is noteworthy that the cavitation phenomenon is affected by the physical properties of the solvent; its intensity decreases as vapour pressure and surface tension increase. Moreover, the solid/liquid ratio can significantly influence the yield and efficacy of the extraction process. From the environmental point of view, UAE represents one of the best non-thermal technologies.158 UAE parameters as amplitude and pressure can be easily tuned as a function of the target specific objectives. It is noteworthy that frequencies higher than >300 kHz are not recommended as the acoustic cavitation intensity is reduced, and a stronger water sonolysis increases the radical generation and the correlated oxidative degradation. In spite of a rich literature reporting several studies comparing the extraction kinetics of UAE with classical methods, there is a lack of information on physical and chemical effects on target compounds.159 A study on the possible degradation effects of UAE on phenolic compounds was carried out by Zhang et al., verifying the stability of gallic acid under different extraction parameters (input power, frequency, temperature, and time) and solvents.160
UAE is generally performed in continuous mode, but the pulsed mode can sometimes provide the best performance over long extraction times.161 UAE can also be efficiently combined with other alternative techniques, such as supercritical CO2, resulting in improved rates and yields due to a faster solvation and smaller particle size.162 Moreover, ultrasound can assist SFE processes producing agitation where the use of mechanical stirrers is not possible.
In novel UAE dynamic systems, the raw materials are positioned in an extraction cylinder and subjected to solvent circulation; fresh solvents continuously flow through samples in an open system, while in closed systems, a pre-set volume of solvent always passes through the matrices. Flow cell design or the number of transducers are unique in every application, which greatly increases their potential for eventual patent protection. The cell wall disruption necessary to produce plant phytocomplexes has been obtained by intense acoustic cavitation (high power density) in a multiprobe flow reactor, fully preserving the chemical, biological, and functional properties of target molecules.163
Food component extraction in particular primary metabolites, (lipids, polysaccharides, proteins) phytochemicals, and flavours, together with the recovery of biomolecules from the agri-food waste, has been extensively studied.164
Thanks to its high nutritional value, spirulina is one of the most studied microalgae for the application in food supplements.165
A manothermosonication (MTS) as a green and innovative method to efficiently extract proteins from Arthrospira platensis cyanobacteria has been reported. This new hybrid extraction technique exploits the additive effects of heating, pressure and sonication, leading to an efficient cell disruption and mass transfer. This technique enabled the recovery of proteins (increase of 229%) and essential amino acids from the microalga in high yields. Microscopic observations (SEM and OM fluorescence) showed that acoustic cavitation caused fragmentation, sonoporation and detexturation of spirulina filaments, and 6 effective minutes of continuous MTS flow at a rate at 15 mL per hour (20 kHz probe), achieved 50% recovery of proteins. The best performance was achieved with a pressure of 2 bars, temperature of 24 °C and a US intensity of 55 W cm−2. The cost analysis however cannot justify the use of a pressurized process because UAE alone gives high-quality spirulina extracts rich in proteins, having also the possibility of industrial scale-up.
By using the response surface methodology, a UAE of mucilaginous materials (common mullein (Verbascum thapsus L.) flowers) carried out with water at a temperature of 67.5 °C, for 60 min, with a power of 371 W, and a solvent/plant material ratio of 40 v/w, achieved a maximum extraction yield of 5.75% of bioactive polysaccharides with a remarkable DPPH radical scavenging activity (67.7%)166
UAE is a technology that improves existing processes with equipment energy efficiency, ease of installation and/or of retrofitting, competitive energy costs, and low maintenance costs. Therefore, the scale-up of UAE processes became more attractive from an industrial point of view especially in the extraction of bioactives from plant and animal sources.167
An optimized reactor design is a crucial issue for efficient UAE because the most powerful US cavitation is found in the proximity of the horn tip, and the intensity decreases rapidly in a few centimetres. This technology uses no moving mechanical parts, and the erosion phenomenon on the transducer surface is generally acceptable. A great flexibility in product applications can be achieved through the design of features that comprise: automated frequency scanning with a maximum power delivery during the fluctuation of processing conditions, non-vibrational flanges on sonotrodes for the construction of high-intensity inline flow cells, and the construction of radial and hybrid sonotrodes. Existing facilities such as percolators and extractors can be easily modified by installing transducers and cooling systems. Integrated refrigeration systems can be adapted to sonoreactors of up to 1000 L in capacity, in order to keep the temperature constant during the extraction process.168 The absence of restrictions on solvent choice, matrix type, and moisture content expands its use to the extraction of any type of natural compound. Other UAE significant benefits for the industry are related to the enhancement of aqueous extraction processes, where solvents cannot be used, and to the improvement in generally recognized as safe solvent (GRAS) application. A β-cyclodextrin water solution can be considered as a green alternative to organic solvents in UAE, thanks to the possibility of encapsulation of apolar compounds or molecules with a lipophilic moiety, enhancing their stability, solubility, and bioavailability.169
Microwaves
In the last two decades, MAE has been widely applied in the extraction of volatile and non-volatile compounds from vegetal matrices.170 Belonging to non-ionizing radiation in the range of frequencies from 300 MHz up to 300 GHz, dielectric heating is used to maximize the extraction of naturally occurring compounds, phytonutrients and functional food ingredients saving solvents and time. MW volumetric heating interacts with polar molecules in cells’ cytoplasm and membranes leading to ionic conduction, dipole rotation and rapid diffusion. Molecules and structures with high dielectric constants can absorb more energy with a fast heating.171The huge demand of phytochemicals as ingredients for the production of formulated health products, nutraceuticals and cosmetics found in MAE a good ally to recover anthocyanins, flavonoids and saponins.172
Despite the contrasting opinions on the use of MW in transparent solvents, literature data reported the applications of MAE with a wide range of non-polar media. In fact, the direct heating of the MW-absorbing matrix allows the extraction of thermosensitive compounds rapidly released into the cold non-polar solvent, avoiding degradation. MAE of tomato peels with ethyl acetate significantly improved all-trans and total lycopene extraction yields, instead of conventional procedures where the cis-isomer is predominant. A higher yield of all-trans lycopene (13 mg per 100 g) was achieved in 1 min irradiation (400 W) with evident structural disruption shown by electron micrographs (TEM).173
Current biorefinery strategies are exploiting the abundant lignocellulosic biomass to recover cellulose, hemicellulose and lignin. After steam explosion pre-treatment of Phragmites australis to deconstruct the matrix and solubilise the hemicelluloses,174 MAE at 200 °C was used to extract lignin with the green solvent γ-valerolactone leading to a cellulose-rich pulp with a delignification of 75%.
The extraction selectivity can be improved by varying the solvent mixtures and by the rehydration of dried matrices to enhance their dielectric susceptibility.
The growing attention paid to environmental protection and clean technologies has led to the development of studies aimed at using solid-state and solvent-free extraction processes under MW irradiation. One example is the solvent-free microwave extraction (SFME) method, employed in essential oil extraction prior to vegetal matrix soaking in water.175,176
Rosmarinus officinalis essential oil has been obtained in a semi industrial pilot-scale study, thanks to a combination of MW heating and dry distillation in 30 min at atmospheric pressure, in a 75 L MW reactor. Despite the absence of solvent, the product obtained showed similar characteristics to that resulting from 2 hours of conventional hydrodistillation.176
Solvent-free microwave assisted hydrodiffusion combined with gravity (MHG) was studied for the recovery of bioactive compounds from both Undaria pinnatifida and Laminaria ochroleuca edible brown seaweeds, and the collected liquid phase was evaluated for functional hydrogel formulations with antioxidant properties.177 MHG is an efficient extraction technique for vegetal matrices with high moisture content. The experiments are carried out in an open vessel multimode MW extractor by collecting liquid extracts drained by gravity on a condenser outside the MW cavity. Working parameters (power, time) can be optimized as a function of the seaweed type, in order to achieve the maximum values of the total phenolic content. Similar to MHG, microwave dry-diffusion and gravity allow the achievement of caraway essential oil green extraction without using any organic solvent or water in less than 1 hour.178
Other innovative extraction methods under dielectric heating and acoustic cavitation
Solid-state microwave disruption (SSMD) has been described as an innovative technique for bioactive extraction from vegetal matrices. Differently from the more common MAE methods, this procedure avoids the use of solvents for biomass sample pre-treatment, allowing the disruption of the sample microstructure. In the absence of dielectric solvents around the biomass, the intracellular moisture can absorb a higher portion of MW energy, the system is then heated and the intracellular pressure increases as a consequence of moisture vaporization.179 A SSMD for the extraction of antioxidants and antidiabetic compounds (e.g. eupatorin, 3′-hydroxy-5,6,7,4′-tetramethoxyflavone and sinensetin) from Java (Orthosiphon stamineus) tea leaves has been reported by See et al.180 The efficiency of the proposed protocol has been evaluated by comparing its results with those of MAE (50 mL g−1 of 80% EtOH, 150 W, 12 min), and UAE (30 mL g−1 of 70% EtOH, 45% amplitude, 34 min). This aim requires a simple solvent elution step under room conditions (elution: 30 mL g−1 of ethanol for 60 min of soaking time) after SSMD treatment for 10 min at 300 W. The resulting extraction yields are the following: 4.65 mg g−1 for the proposed solid-state extraction with less energy consumption and lower volume equipment, 4.45 mg g−1 for MAE, and 4.18 mg g−1 for UAE.Polysaccharides such as laminarin and fucoidan obtained from brown macroalgae have a potential broad range of industrial applications thanks to their biological activities (i.e. antioxidant, antitumoral, anticoagulant and anti-inflammatory activities) that can be employed for the design of functional foods and nutraceuticals. Innovative extraction techniques such as MAE and especially UAE can greatly contribute to the future of a marine based bio-economy.181
The recycle of food waste and by-products has huge potential for obtaining functional ingredients from a kind of matrix that, only in EU, is generated in amounts that reaches 100 Mt.182 Nowadays, there is a tendency to replace conventional food waste processing, such as incineration or composting, with those able to valorise it through the recovery of substance with added value. Among these treatments that fall in the “2nd generation food waste management”,183 US and MW assisted treatments are of primary interest.
A minimal loss of US energy during extraction procedures that usually operate at 16–30 kHz can be achieved by the use high power ultrasonic probes. Moreover, the intermittent switch between the active and inactive times of the US processor during the extraction process, namely pulsed mode (PUAE), can lead to the preservation of heat sensitive biomolecules thanks to a lower heat generation. An innovative and eco-compatible strategy to valorise Castanea sativa bud by-products was developed by Turrini et al.184 using water/ethanol/glycerol (50/20/30 v/v/v) as the extraction solvent. A titanium (7 mm i.d.) sonotrode, and an operating frequency of 26 kHz, effective output of 200 W, were used to recover about 12% of the corresponding fresh weight buds in secondary metabolites as catechins, tannins, flavonols and cinnamic acids.
The wide range of pectin applications that involves not only the food industry but also pharmaceutical and cosmetic products has made innovative approaches necessary to overcome the limitations of conventional processes for its extraction from plant food wastes and by-products. Although an increase in pectin yields, together with a decreased extraction time and temperatures, has been shown in the literature by applying UAE, the combination of US with heating (UAHE) at lower temperatures, which is not required for conventional techniques, can maximize yields preserving the colour and microstructure of pectin. Moreover, hot-solvent MAE resulted to be more efficient than the conventional acidic solution extraction of these polysaccharides. Pomelo fruit, lime, sour orange, cocoa, sugar beet and papaya are among the matrices whose industrial processing can produce enormous amounts of waste and by-products. It is possible to recover substances with high added value from them, such as pectin, thanks to the optimized MAE conditions, also when MW was used for sample pre-treatments in order to increase the efficacy of other extractive procedures such as hot acid extraction of cocoa husk.185
One of the applications that have been most studied in recent years is that related to the intensification of eco-sustainable processes for the industrial production of olive oil.186 The release of oil from vacuoles can be amplified with the MW thermal effect, limiting at the same time the amount of produced waste water thanks to the reduction in water usage.187
The improvement of the olive oil extraction under high-power US on olive paste before malaxation has been reported by Bejaoui et al.188 The quick heating of the olive paste from 20 to 28 °C increases the industrial virgin oil yield by 1% and the oil extractability by 5.74% without modifying the quality of the obtained product in control parameters such as fatty acid composition and volatile aromatic compounds, rather than increasing the tocopherol, chlorophyll, and carotenoid content.
The intensive mass transfer generated by the acoustic cavitation process and shockwaves with a high-power US device introduced at an industrial plant that can process at 2 tons per h increased virgin olive oil extraction yields and the phenol content to 22.7% and 10.1%, respectively, when compared to traditional processes, preserving legal and commercial quality indices.189 It is noteworthy that the positive effects of US on both oil extractability and the enhancement of quality parameters reduced progressively when olives at higher maturity indices were used, compared to those with olives at the medium-early ripening stage. The simultaneous use of a low-frequency US device, a MW apparatus and a horizontal spring heat exchanger has been employed to design and assemble an industrial combined pilot plant (ICP), with the aim to investigate the real possibility of introducing these innovative technologies to obtain an olive oil with better nutritional properties.190 This versatile plant may operate with all three technologies simultaneously or individually with great advantages compared to conventional processes in terms of oil quality and total phenolics content. A continuous process from the milling to the solid–liquid separation phase has been described. Thanks to a rapid temperature adjustment of the olive paste following crushing, this method allows a malaxation time reduction to 20 min. A continuous conditioning process of the olive paste with only several minutes of treatment has been obtained by using a MW apparatus in addition to a heat exchanger. The low-frequency US apparatus allows an average increase in extractability ranging from 2.30 to 3.85%.
The eco-sustainable ultrasound–microwave-assisted extraction (UMAE) of prebiotic oligosaccharides from purple sweet potatoes (Ipomoea batatas L.) lasts for only 100 s under a US and MW power of 300 and 200 W respectively.191 Thanks to the generation of micro-fractures and disruption of potato tissue cell walls, this innovative coupled extraction technique performed at low temperature allows the improvement in extraction yields, as compared to MAE, UAE and conventional hot-water extraction. The same extraction system was previously reported as a green and efficient procedure able to increase the yield of total oligosaccharides, trisaccharides, and tetrasaccharides from lotus (Nelumbo nucifera Gaertn.) seeds to 76.6%, 17.5%, and 27.2%, respectively.192
An US-MW assisted extraction, preceded by combined enzyme treatment, was used on Lavandula angustifolia to enhance the yield and quality of the essential oil, when compared with the Clevenger-type extraction with enzymatic pre-treatment.193 The homogenized dried flowers in deionized distilled water with cellulase and hemicellulase were shaken at 40 °C for 60 min followed by 1 h sonication and MW irradiation leading to plant tissue dismantling and efficient distillation.
Future trends
Green extraction technologies are “mature” but “underused”. Industries which invest on these techniques have ROS (return of investments) less than 3 years, with high added value products, and secure markets. There is great access to Intellectual Property not on technology but on techniques, procedures and products for a specific application. But there are still some key challenges and barriers:• There is no advertising when companies use these green extraction techniques. They don't want to tell about the innovation made inside the company, sometimes because consumers are not confident with the technology, or because they have no IP, or they want to keep it secret.
• Availability of the appropriate pilot and test facilities, most of the academic laboratories have facilities from 1 to 10 liters maximum, but companies want to make tests in platforms with 100 to 1000 liters minimum and also to have suitable place for producing batches.
• Weak competitive position of European equipment suppliers. The equipment suppliers have limited resources for innovation and the view was expressed that creating alliances with industry and institutes/universities for providing innovation to them would be desirable for improving their competitive position.
• Inadequate number of technical specialists. There is concern about the low number of academia potentially resulting in future skill gaps. Additionally, the development of multidisciplinary skills is considered important for the future where integration/understanding of several technologies will become important.
• A (too) low number of start-up companies in this field in Europe.
The use of innovative extraction techniques such as ultrasound, microwave, instant controlled pressure drop, sub or super-critical fluid, pulsed electric fields, extrusion, ohmic, UV, IR, and solar assisted extraction (Table 5) allows the total recovery of bioactive compounds from plant matrices and also reduced extraction time, energy consumed, and less solvent. Conventional extraction techniques (maceration, decoction, leaching, percolation, digestion, hydro and steam distillation, etc.) are limited by the diffusion of solvents into biomass, due to the rigid structures of cell walls of plants. The solution could be to enhance the diffusion of solvents and to disrupt cell walls. For example, ultrasound and electric pulse fields allow a high disruption of cell which permits the acceleration of the mass transfer, thus recovering lipids in a short time. On the other hand, heating by microwave induces combined mass and heating transfer which permits the destruction of cells and liberation of primary and secondary metabolites. We cannot really compare the techniques; each technique is a performant for a class of compounds and for specific plants.
| Name | Investment | Sample size | Extraction time | Main disadvantages | Main advantages |
|---|---|---|---|---|---|
| Investment: low (less than 100 kEuros) and high (more than 200 kEuros). Extraction time: short (less than 1 hour) and long (more than 12 hours). | |||||
| Maceration | Low | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L 000 L |
Long | Limited by solubility | Large scale |
| Ultrasound | Low | 600 L | Short | Problem for separation | High cell disruption |
| Microwave | Medium | 150 L | Short | Hot spots | Cell disruption |
| DIC | High | 100 L | Short | High energy consumption | High cell disruption |
| SFE | High | 300 L | Medium | Need of know-how | Enhanced mass transfer |
| PEF | High | Continuous | Medium | Difficult operation | Electroporation of wall cells |
| Extrusion | Low | Continuous | Short | Impact of plant classes | Easy to use |
| Solar | Low | >1000 L | Long | Depends on climate | Easy to use |
| Ohmic, UV | Medium | >1000 L | Medium | Precise conditions | Enhanced transfer |
“What you see is what you extract”, with this sentence Choi and Verpoorte195 pointed that solvent extraction is one of the most important steps in sample preparation for phytochemical analysis but we can also generalize it to industrial production via the extraction of aromas, colors, antioxidants, fats and oils and fine chemicals for the food, cosmetic, perfumery, and pharmaceutical industries.
The ideal alternative solvents suitable for green extraction should have high solvency, high flash points with low toxicity and low environmental impacts, be easily biodegradable, obtained from renewable (non-petrochemical) resources at a reasonable price and should be easy to recycle without any deleterious effect to the environment. Finding the perfect solvent that meets all the aforementioned requirements is a challenging task, thus the decision for the optimum solvent will always be a compromise depending on the process, the plant and the target molecules. The objective of this comprehensive review is to furnish a vivid picture of current knowledge on green techniques used with or without green solvents. For example, sub- and super-critical fluid solvents are treated as techniques because they need specific apparatus to be used properly and safely.196–199
Education is the key for a sustainable future of humanity, we need to renew undergraduate education in the chemical sciences via sustainability to foster creativity using research, visualization and connectivity resources. Green extraction to obtain sustainable reagents and ingredients could be a success story to learn green chemistry and engineering. There is a need to conceive “kits” for practical work for universities or secondary schools, for north and south countries, for example, to make juice out of oranges, to make solvent free extraction of essential oil using simple laboratory glassware and a household microwave oven, and therefore to use essential oil mainly d-limonene as a synthon to produce s-carvone by oxidation. There is also an urgent need for solving the difficulty in communicating chemical innovations. There is a need to share our knowledge as scientists and specialists not only by scientific articles and books but also by providing education in primary schools and universities all around the world, by producing digital contents, and by making videos with several connectivity resources.194 These videos will include both courses on green extraction and also visual experiments which could be replicated easily in other universities north and south.
Conflicts of interest
There is no conflict of interest to declare.References
- H.-J. Bart, Extraction of Natural Products from Plants – An Introduction, in Industrial Scale Natural Products Extraction, ed. H.-J. Bart and S. Pilz, Wiley-VCH, Weinheim, 2011 Search PubMed.
- M. Macquer, Eléments de chimie théorique, Edition Hérissant, Paris, 1756 Search PubMed.
- J. French, The art of distillation, Editions Cotes, London, 1651 Search PubMed.
- F. Chemat, M. A. Vian and G. Cravotto, Int. J. Mol. Sci., 2012, 13, 8615–8627 CrossRef CAS PubMed.
- K. Allaf and P. Vidal, Feasibility Study of a Process of Drying/Swelling by Instantaneous Decompression Toward Vacuum of in Pieces Vegetables in View of Rapid Re-Hydration Gradient Activity Plotting, University of Technology of Compiègne UTC N°CR/89/103 industrial SILVALAON Partner, 1989 Search PubMed.
- K. Allaf, C. Besombes, B. Berka, M. Kristiawan, V. Sobolik and T. Allaf, in Enhancing Extraction Processes in the Food Industry, CRC Press, USA, 2011, pp. 255–302 Search PubMed.
- F. Chemat, N. Rombaut, A. Meullemiestre, M. Turk, S. Perino, A. S. Fabiano-Tixier and M. Abert Vian, Innovative Food Sci. Emerging Technol., 2017, 41, 357–377 CrossRef CAS.
- S. Mkaouar, N. Bahloul, A. Gelicus, K. Allaf and N. Kechaou, Sep. Purif. Technol., 2015, 145, 139–146 CrossRef CAS.
- V. Sanchez-Valdepenas, E. Barrajon, S. Vegara, L. Funes, N. Marti, M. Valero and D. Saura, Ind. Crops Prod., 2015, 76, 545–549 CrossRef CAS.
- C. Nguyen Van, PhD Thesis, Université de La Rochelle, 2010.
- T. Allaf, F. Fine, V. Tomao, C. Nguyen, C. Ginies and F. Chemat, OCL, 2014, 21(3), A301 CrossRef.
- M. H. Eikani, N. Khandan and E. Feyzi, Food Bioprod. Process., 2019, 117, 241–249 CrossRef CAS.
- M. Kristiawan, V. Sobolik and K. Allaf, J. Chromatogr. A, 2008, 1192, 306–318 CrossRef CAS PubMed.
- T. Allaf, V. Tomao, C. Besombes and F. Chemat, Chem. Eng. Process., 2013, 72, 24–30 CrossRef CAS.
- N. Rombaut, A. S. Tixier, A. Billy and F. Chemat, Biofuels, Bioprod. Biorefin., 2014, 8, 530–544 CrossRef CAS.
- K. Allaf, E. Debs-Louka, N. Louka, N. Cochet and G. Abraham, FR94/14832, 1994.
- T. Allaf and K. Allaf, Instant Controlled Pressure Drop (D.I.C.) in Food Processing, Food Engineering Series, Springer New York, New York, NY, 2014 Search PubMed.
- E. Badens, Les Techniques de l'Ingénieur, Editions T.I., 2012, Réf: CHV4010 Search PubMed.
- G. Lumia, in Eco-extraction du végétal, ed. F. Chemat, Dunod, Paris, 2011, pp. 231–258 Search PubMed.
- D. M. Samppatrao and K. Dhindra, Mater. Sci. Technol., 2019, 2, 463–484 Search PubMed.
- P. F. F. R. da Silva, A. P. T. Rocha-Santos and A. C. Duarte, Trends Anal. Chem., 2016, 76, 40–51 CrossRef.
- M. Herrero, A. P. Sánchez-Camargo, A. Cifuentes and E. Ibáñez, Trends Anal. Chem., 2015, 71, 26–38 CrossRef CAS.
- M. Herrero, J. A. Mendiola, A. Cifuentes and E. Ibánez, J. Chromatogr. A, 2010, 1217, 2495–2511 CrossRef CAS.
- E. Reverchon and I. De Marco, J. Supercrit. Fluids, 2006, 38(2), 146–166 CrossRef CAS.
- B. Díaz-Reinoso, A. Moure, H. Domínguez and J. C. Parajó, J. Agric. Food Chem., 2006, 54(7), 2441–2469 CrossRef PubMed.
- M. Yousefi, M. Rahimi-Nasrabadi, S. M. Pourmortazavi, M. Wysokowski, T. Jesionowski, H. Ehrlich and S. Mirsadeghi, Trends Anal. Chem., 2019, 118, 183–193 CrossRef.
- M. Akgun, N. A. Akgun and S. Dincer, J. Supercrit. Fluids, 1999, 15, 117–125 CrossRef CAS.
- T. Fornari, G. Vicente, E. Vázquez, M. R. García-Risco and G. Reglero, J. Chromatogr. A, 2012, 1250, 34–48 CrossRef CAS PubMed.
- S. M. Pourmortazavi and S. S. Hajimirsadeghi, J. Chromatogr. A, 2007, 1163, 2–24 CrossRef CAS.
- L. A. Conde-Hernandez, J. R. Espinosa-Victoria, A. Trejo and J. A. Guerrero-Beltran, J. Food Eng., 2017, 200, 81–86 CrossRef CAS.
- E. Vági, B. Simándi, Á. Suhajda and É. Héthelyi, Food Res. Int., 2005, 38, 51–57 CrossRef.
- M. J. H. Akanda, M. Z. I. Sarker, S. Ferdosh, M. Y. A. Manap, N. N. N. Ab Rahman and M. O. A. Kadir, Molecules, 2012, 17, 1764–1794 CrossRef CAS PubMed.
- U. Salgina, A. Calimli and B. Z. Uysal, J. Am. Oil Chem. Soc., 2004, 81, 293–296 CrossRef.
- R. Kumar Saini and Y. S. Keum, Food Chem., 2018, 240, 90–103 CrossRef PubMed.
- M. de Andrade Lima, D. Charalampopoulo and A. Chatzifragkou, J. Supercrit. Fluids, 2018, 133, 94–102 CrossRef CAS.
- M. Perrut, J. Supercrit. Fluids, 2012, 66, 359–371 CrossRef CAS.
- K. Benaissi, Les Techniques de l'Ingénieur, Editions T.I., 2013, Réf: CHV4015 Search PubMed.
- Z. Novello, J. Scapinello, J. D. Magro, G. Zin, M. D. Luccio, M. V. Tres and J. V. Oliveira, Ind. Crops Prod., 2015, 76, 697–701 CrossRef CAS.
- V. Rapinel, N. Rombaut, N. Rakotomanomana, A. Vallageas, G. Cravotto and F. Chemat, LWT - Food Sci. Technol., 2017, 85, 524–533 CrossRef CAS.
- D. Sparks, R. Hernandez, M. Zappi, D. Blackwell and T. Fleming, J. Am. Oil Chem. Soc., 2006, 83, 885–891 CrossRef CAS.
- C. M. da Silva, A. B. Zanqui, A. K. Gohara, A. H. de Souza, L. Cardozo-Filho, J. V. Visentainer, L. Rovigatti Chiavelli, P. R. Bittencourt, E. A. da Silva and M. Matsushita, J. Supercrit. Fluids, 2015, 102, 1–8 CrossRef CAS.
- M. P. Corso, M. R. Fagundes-Klen, E. A. Silva, L. Cardozo, J. N. Santos, L. S. Freitas and C. Dariva, J. Supercrit. Fluids, 2010, 52, 56–61 CrossRef CAS.
- H. Kanda, P. Li, M. Goto and H. Makino, Energies, 2015, 8, 610–620 CrossRef CAS.
- H. Kanda, P. Li, T. Yoshimura and S. Okada, Fuel, 2013, 105, 535–539 CrossRef CAS.
- R. Hoshino, M. Ogawa, K. Murakami, Wahyudiono, H. Kanda and M. Goto, Solvent Extr. Res. Dev., 2017, 24, 47–60 CrossRef CAS.
- M. C. J. Bier, A. B. Medeiros, J. S. de Oliveira, L. C. Côcco, J. da Luz Costa, J. C. de Carvalho and C. R. Soccol, J. Supercrit. Fluids, 2016, 110, 97–102 CrossRef CAS.
- N. Nenov, V. Gochev, T. Girova, I. Stoilova, T. Atanasova, V. Stanchev and A. Stoyanova, J. Essent. Oil Bear. Plants, 2011, 14, 67–75 CrossRef CAS.
- R. Hoshino and Wahyudiono, J. Nutr. Food Sci., 2014, 4, 1–5 Search PubMed.
- A. B. Zanqui, D. R. de Morais, C. M. da Silva, J. M. Santos, U. R. L. Chiavelli, P. R. S. Bittencourt, M. N. Eberlin, J. V. Visentainer, L. Cardozo-Filho and M. Matsushita, J. Braz. Chem. Soc., 2015, 26(2), 282–289 CAS.
- M. Goto, H. Kanda, Wahyudionoand and S. Machmudah, J. Supercrit. Fluids, 2015, 96, 245–251 CrossRef CAS.
- R. Savoire, J.-L. Lenoisellé and E. Vorobiev, Food Bioprocess Technol., 2012, 6, 1–16 Search PubMed.
- J. M. Bouvier and O. Campanella, Extrusion processing technology: Food and non food biomaterials, Wiley, 2014, Print ISBN: 9781444338119, Online ISBN: 9781118541685, DOI:10.1002/9781118541685.
- M. S. Alam, J. Kaur, H. Khaita and K. Gupta, Crit. Rev. Food Sci. Nutr., 2016, 56, 445–473 CrossRef CAS PubMed.
- V. Offiah, V. Kontogiorgos and K. O. Falade, Crit. Rev. Food Sci. Nutr., 2018, 59, 2979–2998 CrossRef PubMed.
- S. Shilev, M. Naydenov, V. Vancheva and A. Aladjadjiyan, Composting of food and agricultural wastes, in Utilization of Byproducts and Treatment of Wastes in the Food Industry, ed. V. Oreopoutou and W. Russ, Springer Science and Business Media, New York, 2006, pp. 283–302 Search PubMed.
- B. P. Helkar, A. K. Sahoo and N. J. Patil, Int. J. Waste Resour., 2016, 6, 248 Search PubMed.
- K. Jayathilakan, K. Sultana, K. Radhakrishna and A. S. Bawa, J. Food Sci. Technol., 2012, 49, 278–293 CrossRef CAS PubMed.
- A. Altan and M. Maskan, Development of extruded foods by utilizing food industry by-products, in Advances in Food ExtrusionTechnology, ed. M. Maskan and A. Altan, CRC Press, Boca Raton, 2016, pp. 121–160 Search PubMed.
- E. Kasapidou, E. Sossidou and P. Mitlianga, Agriculture, 2015, 5, 1020–1034 CrossRef CAS.
- T. Varzakas, G. Zakynthinos and F. Verpoort, Foods, 2016, 5, 88–120 CrossRef PubMed.
- T. I. N. Ezejiofor, U. E. Enebaku and C. Ogueke, Biotechnol. J., 2014, 4, 418–481 Search PubMed.
- E. Uitterhaegen and P. Evon, J. Food Eng., 2017, 212, 190–200 CrossRef CAS.
- J. K. Monrad, M. Suarez, M. J. Motilva, J. W. King, K. Srinivas and L. R. Howard, Food Res. Int., 2014, 65, 77–87 CrossRef CAS.
- M. R. Alam, M. Scampicchio, S. Angeli and G. Ferrentino, J. Food Eng., 2019, 259, 44–51 CrossRef CAS.
- A. Delvar, P. de Caro, L. Candy, Y. Caro, A. S. C. Sing and C. Raynaud, J. Food Eng., 2019, 236, 388–397 CrossRef.
- F. Pinto de Abreu, M. Dornier, A. P. Dionisio, M. Carail, C. Caris-Veyrat and C. Dhuique-Mayer, Food Chem., 2013, 138, 25–31 CrossRef CAS PubMed.
- F. Viacara, J. Santana-Galvez, E. Heredia-Olea, E. Pérez-Carrillo, V. Nair, L. Cisneros-Zevallos and D. A. Jacobo-Velazquez, Food Chem., 2019, 125551, DOI:10.1016/j.foodchem.2019.125551.
- J. Pico, K. Xu, Z. Mohamedshah, M. G. Ferruzzi and M. M. Martinez, Food Chem., 2019, 297, 124990, DOI:10.1016/j.foodchem.2019.124990.
- Z. Pan, Y. Huang, Y. Wang and Z. Wu, Bioresour. Technol., 2017, 245,, 641–648 CrossRef.
- W. N. Phong, P. L. Show, C. F. Le, Y. Tao, J.-S. Chang and T. C. Ling, Biochem. Eng. J., 2018, 135, 83–90 CrossRef CAS.
- A. Kwade and J. Schwedes, Chapter 6 Wet Grinding in Stirred Media Mills, in Handbook of Powder Technology, ed. A. D. Salman, M. Hounslow and J. P. K. Seville, Elsevier Science B.V., 2007, pp. 251–382 Search PubMed.
- http://www.attritor.in/attritor_working.html .
- A. Gupta and D. Yan, Stirred Mills – Ultrafine Grinding, in Mineral Processing Design and Operations, 2nd edn, 2016, ch. 10, pp. 287–316. DOI:10.1016/B978-0-444-63589-1.00010-1.
- J. A. Currie, P. Dunnill and M. D. Lilly, Biotechnol. Bioeng., 1972, 14,, 725–736 CrossRef.
- H. Schütte, K. H. Kroner, H. Hustedt and M.-R. Kula, Enzyme Microb. Technol., 1983, 5, 143–148 CrossRef.
- J. R. Woodrow and A. V. Quirk, Enzyme Microb. Technol., 1982, 4, 385–389 CrossRef.
- Y. Chisti and M. Moo-Young, Enzyme Microb. Technol., 1986, 8, 194–204 CrossRef CAS.
- A. Heim, U. Kamionowska and M. Solecki, Future Food Eng., 2007, 83, 121–128 CrossRef.
- Z. D. V. L. Mayerhoff, T. T. Franco and I. C. Roberto, Sep. Purif. Technol., 2008, 63, 706–709 CrossRef CAS.
- S. Haque, S. Khan, M. Wahid, R. K. Mandal, D. Tiwari, S. A. Dar, D. Paul, M. Y. Areeshi and A. Jawed, RSC Adv., 2016, 6, 16348–16357 RSC.
- A. Meullemiestre, C. Breil, M. Abert-Vian and F. Chemat, Bioresour. Technol., 2016, 211, 190–199 CrossRef CAS PubMed.
- M. Wang, W. Bi, X. Huang and D. Chen, J. Chromatogr. A, 2016, 1449, 8–16 CrossRef CAS PubMed.
- J. Wu, T. Ju, Y. Deng and J. Xi, Trends Food Sci. Technol., 2017, 66, 166–175 CrossRef.
- S. Wang, R. Zhang, X. Song, M. Wei, T. Xie and J. Cao, ACS Sustainable Chem. Eng., 2019, 7, 197–207, DOI:10.1021/acssuschemeng.8b02902.
- M. Aslam, M. Syarhabil Ahmad, M. Atanassova and M. Ayaz Ahmad, Adv. Environ. Biol., 2017, 11, 84–90 CAS.
- W. Xu, J. Yu, W. Feng and W. Su, Molecules, 2016, 21, 540–553 CrossRef PubMed.
- K. Korolev, O. Lomovskii, O. Rozhanskaya and V. Vasil'ev, Chem. Nat. Compd., 2003, 39, 366–372 CrossRef CAS.
- J. Xie, H. Li, X. Zhu, P. Wang and W. Su, Chem. Eng. Process., 2011, 50, 325–330 CrossRef CAS.
- J. Xie, L. Shi, X. Zhu, P. Wang, Y. Zhao and W. Su, Innovative Food Sci. Emerging Technol., 2011, 12, 146–152 CrossRef CAS.
- J. Xie, Y. Lin, X. Shi, X. Zhy, W. Su and P. Wang, Ind. Crops Prod., 2013, 43, 276–282 CrossRef CAS.
- Q. Zhang, J. Yu, Y. Wang and W. Su, Molecules, 2016, 21, 989–1003 CrossRef PubMed.
- X. Zhu, Y. L. Mang, J. Xie, P. Wang and W. K. Su, Ind. Crops Prod., 2011, 34, 1041–1052 CrossRef CAS.
- E. Rincon, A. Balu, R. Luque and L. Serrano, Ind. Crops Prod., 2019, 141, 111805–111111 CrossRef CAS.
- J. R. Sarkis, N. Boussetta, C. Blouet, I. C. Tessaro, L. D. F. Marczak and E. Vorobiev, Innovative Food Sci. Emerging Technol., 2015, 29, 170–177 CrossRef CAS.
- S. Ghosh, A. Gillis, J. Sheviryov, K. Levkov and A. Golberg, J. Cleaner Prod., 2019, 208, 220–231 CrossRef CAS.
- J. M. Martínez, C. Delso, M. Maza, I. Álvarez and J. Raso, Ref. Modul. Food Sci., 2018 DOI:10.1016/B978-0-08-100596-5.22435-9.
- H. Neetoo and H. Chen, Alternative Food Processing Technologies, in Food Processing, John Wiley & Sons, Ltd, Chichester, UK, 2014, pp. 137–169. DOI:10.1002/9781118846315.ch7.
- I. Žuntar, P. Putnik and D. Bursać Kovačević, et al. , Foods, 2019, 8(7), 248 CrossRef PubMed.
- F. J. Barba, Z. Zhu, M. Koubaa, A. S. Sant'Ana and V. Orlien, Trends Food Sci. Technol., 2016, 49, 96–109 CrossRef CAS.
- Z. Li, Y. Fan and J. Xi, Food Chem., 2019, 277, 246–260 CrossRef CAS PubMed.
- Green Extraction of Natural Products, ed. F. Chemat and J. Strube, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2015. DOI:10.1002/9783527676828.
- B. Gómez, P.E. S. Munekata and M. Gavahian, et al. , Food Res. Int., 2019, 123, 95–105 CrossRef PubMed.
- S.-F. Han, W. Jin and Q. Yang, et al. , Renewable Energy, 2019, 133, 233–239 CrossRef CAS.
- L.-G. Yan, Y. Deng, T. Ju, K. Wu and J. Xi, Food Chem., 2018, 256, 350–357 CrossRef CAS PubMed.
- N. Kaur and AK Singh, Crit. Rev. Food Sci. Nutr., 2016, 56(14), 2338–2351 CrossRef CAS PubMed.
- J. Martínez and R. Miranda, Infrared Irradiation, an Excellent, Alternative Green Energy Source, in Green Chemistry, IntechOpen, 2019. DOI:10.5772/intechopen.83805.
- M. Alothman, R. Bhat and A. A. Karim, Innovative Food Sci. Emerging Technol., 2009, 10(4), 512–516 CrossRef CAS.
- H. Saberian, Z. Hamidi-Esfahani, H. Ahmadi Gavlighi and M. Barzegar, Chem. Eng. Process., 2017, 117, 154–161 CrossRef CAS.
- M. Aamir and W. Jittanit, Innovative Food Sci. Emerging Technol., 2017, 41, 224–234 CrossRef CAS.
- M. Seidi Damyeh and M. Niakousari, Ind. Crops Prod., 2017, 98, 100–107 CrossRef CAS.
- Y. Yang, Y. Pan and G. Zhou, et al., J. Sep. Sci., 2016, 39(21), 4192–4201 CrossRef CAS PubMed.
- H. N. Rajha, T. Mhanna, S. El Kantar, A. El Khoury, N. Louka and R. G. Maroun, LWT, 2019, 111, 138–146 CrossRef CAS.
- D. Morales, A. Gil-Ramirez, F. R. Smiderle, A. J. Piris, A. Ruiz-Rodriguez and C. Soler-Rivas, Innovative Food Sci. Emerging Technol., 2017, 41, 330–336 CrossRef CAS.
- M. S. M. Al-Nimer and Z. Wahbee, J. Intercult. Ethnopharmacol., 2017, 6(3), 280–283 CrossRef CAS PubMed.
- F. J. Barba, N. S. Terefe, R. Buckow, D. Knorr and V. Orlien, Food Res. Int., 2015, 77, 725–742 CrossRef.
- B. Mertens, in New Methods of Food Preservation, Springer US, Boston, MA, 1995, pp. 135–158 Search PubMed.
- F. J. Barba, L. R. B. Mariutti, N. Bragagnolo, A. Z. Mercadante, G. V. Barbosa-Cánovas and V. Orlien, Trends Food Sci. Technol., 2017, 67, 195–206 CrossRef CAS.
- F. J. Barba, M. J. Esteve and A. Frígola, Compr. Rev. Food Sci. Food Saf., 2012, 11, 307–322 CrossRef CAS.
- F. J. Barba, M. Koubaa, L. do Prado-Silva, V. Orlien and A. de S. Sant'Ana, Trends Food Sci. Technol., 2017, 66, 20–35 CrossRef CAS.
- J. Szczepańska, F. J. Barba, S. Skąpska and K. Marszałek, Food Chem., 2020, 307, 125549 CrossRef PubMed.
- İ. Önür, N. N. Misra, F. J. Barba, P. Putnik, J. M. Lorenzo, V. Gökmen and H. Alpas, J. Food Eng., 2018, 219, 129–136 CrossRef.
- V. M. Balasubramaniam, G. V. Barbosa-Cánovas and H. L. M. Lelieveld, in Food Engineering Series, Springer, 2016, pp. 39–65 Search PubMed.
- F. J. Barba, L. Ahrné, E. Xanthakis, M. G. Landerslev and V. Orlien, Innovative Technologies for Food Preservation Innovative Technologies for Food Preservation, Academic Press, 2018, ch. 2, pp. 25–51 Search PubMed.
- V. M. (Bala) Balasubramaniam, S. I. Martínez-Monteagudo and R. Gupta, Annu. Rev. Food Sci. Technol., 2015, 6, 435–462 CrossRef.
- I. Oey, M. Lille, A. Van Loey and M. Hendrickx, Trends Food Sci. Technol., 2008, 19, 320–328 CrossRef CAS.
- E. Rendueles, M. K. Omer, O. Alvseike, C. Alonso-Calleja, R. Capita and M. Prieto, LWT - Food Sci. Technol., 2011, 44, 1251–1260 CrossRef CAS.
- P. Putnik, J. M. Lorenzo, F. J. Barba, S. Roohinejad, A. Režek Jambrak, D. Granato, D. Montesano and D. Bursać Kovačević, Foods, 2018, 7, 106 CrossRef PubMed.
- J. Xi and S. Luo, Sep. Purif. Technol., 2016, 165, 208–213 CrossRef CAS.
- J. Xi and L. Yan, Sep. Purif. Technol., 2017, 175, 170–176 CrossRef CAS.
- V. Briones-Labarca, M. Plaza-Morales, C. Giovagnoli-Vicuña and F. Jamett, LWT - Food Sci. Technol., 2015, 60, 525–534 CrossRef CAS.
- E. M. C. Alexandre, P. Araújo, M. F. Duarte, V. de Freitas, M. Pintado and J. A. Saraiva, Food Bioprocess Technol., 2017, 10, 886–900 CrossRef CAS.
- H. Zhang, W. Tchabo and Y. Ma, Emirates J. Food Agric., 2017, 29, 815–819 CrossRef.
- J. Li, W. Sun, H. S. Ramaswamy, Y. Yu, S. Zhu, J. Wang and H. Li, J. Food Process Eng., 2017, 40 DOI:10.1111/jfpe.12353.
- J. M. George, H. B. Sowbhagya and N. K. Rastogi, Drying Technol., 2018, 36, 1107–1116 CrossRef CAS.
- H. W. Huang, S. J. Wu, J. K. Lu, Y. T. Shyu and C. Y. Wang, Food Control, 2017, 72, 1–8 CrossRef.
- W. M. Elamin, J. B. Endan, Y. A. Yosuf, R. Shamsudin and A. Ahmedov, J. Eng. Sci. Technol. Rev., 2015, 8, 75–83 CrossRef.
- Hiperbaric, high pressure processing for food & beverage products. https://www.hiperbaric.com/en/, 2019.
- S. Mekhilef, R. Saidur and A. Safari, Renewable Sustainable Energy Rev., 2011, 15, 1777–1790 CrossRef.
- H. Panchal, J. Patel and S. Chaudhary, J. Sol. Energy Eng. Trans. ASME, 2018, 140, 010801 CrossRef.
- H. Akbari, Principals of Solar Engineering, CRC Press, 3rd edn, 2019 Search PubMed.
- S. H. Farjana, N. Huda, M. A. P. Mahmud and R. Saidur, Renewable Sustainable Energy Rev., 2018, 82, 2270–2286 CrossRef.
- F. J. Barba, M. Gavahian, I. Es, Z. Zhu, F. Chemat, J. M. Lorenzo and A. Mousavi Khaneghah, J. Cleaner Prod., 2019, 220, 1121–1130 CrossRef CAS.
- A. Afzal, A. Munir, A. Ghafoor and J. L. Alvarado, Renewable Energy, 2017, 113, 22–29 CrossRef CAS.
- S. Hilali, A. S. Fabiano-Tixier, K. Ruiz, A. Hejjaj, F. Ait Nouh, A. Idlimam, A. Bily, L. Mandi and F. Chemat, ACS Sustainable Chem. Eng., 2019, 7, 11815–11822 CrossRef CAS.
- Y. Kulturel and S. Tarhan, J. Sci. Ind. Res., 2016, 75, 691–696 CAS.
- A. Munir, O. Hensel, W. Scheffler, H. Hoedt, W. Amjad and A. Ghafoor, Sol. Energy, 2014, 108, 548–559 CrossRef.
- A. Munir and O. Hensel, Agric. Eng. Int. CIGR J., 2010, 12, 107–114 Search PubMed.
- A. Munir and O. Hensel, Biosyst. Eng., 2010, 106, 268–277 CrossRef.
- H. Y. Yen and Y. C. Lin, Ind. Crops Prod., 2017, 108, 716–721 CrossRef CAS.
- A. Kumar, O. Prakash and A. K. Kaviti, Renewable Sustainable Energy Rev., 2017, 77, 890–898 CrossRef.
- Anonymous, 2019. AKSOŃs solar. https://www.aksonsolar.com/.
- F. Chemat, M. Abert-Vian and G. Cravotto, Int. J. Mol. Sci., 2012, 13(7), 8615–8627 CrossRef CAS PubMed.
- A. I. Talmaciu, I. Volf and V. I. Popa, Chem. Biodiversity, 2015, 12, 1635–1650 CrossRef CAS PubMed.
- L. Orio, L. Alexandru, G. Cravotto, S. Mantegna and A. Barge, Ultrason. Sonochem., 2012, 19, 591–595 CrossRef CAS PubMed.
- B. K. Tiwari, TrAC, Trends Anal. Chem., 2015, 71, 100–109 CrossRef CAS.
- K. Vilkhu, R. Mawson, L. Simons and D. Bates, Innovative Food Sci. Emerging Technol., 2008, 9(2), 161–169 CrossRef CAS.
- G. Cravotto, F. Mariatti, V. Gunjevic, M. Secondo, M. Villa, J. Parolin and G. Cavaglià, Pilot Scale Cavitational Reactors and Other Enabling Technologies to Design the Industrial Recovery of Polyphenols from Agro-Food By-Products, a Technical and Economical Overview, Foods, 2018, 7, 130 CrossRef PubMed.
- F. Chemat, N. Rombaut, A. Meullemiestre, M. Turk, S. Perino and A. S. Fabiano-Tixier, et al. , Innovative Food Sci. Emerging Technol., 2017, 41, 357–377 CrossRef CAS.
- D. Pingret, A. S. Fabiano-Tixier and F. Chemat, Food Control, 2013, 31, 593–606 CrossRef.
- Q. Zhang, H. Shen, X. Fan, Y. Shen, X. Wang and Y. Song, Ultrason. Sonochem., 2015, 22, 149–154 CrossRef CAS PubMed.
- Z. Pan, W. Qu, H. Ma, G. G. Atungulu and T. H. McHugh, Ultrason. Sonochem., 2011, 18, 1249–1257 CrossRef CAS PubMed.
- P. Santos, A. C. Aguiar, G. F. Barbero, C. A. Rezende and J. Martínez, Ultrason. Sonochem., 2015, 22, 78–88 CrossRef CAS PubMed.
- P. Daghero and G. Cravotto, Plant Composition Comprising the Phytocomplex of a Plant Species and Process for Preparing Same, EP Patent2520182A1, 2012, Priority Appl. IT 2011-TO390 Search PubMed.
- G. Cravotto and A. Binello, Low-Frequency, High-Power Ultrasound-Assisted Food Component Extraction, in Innovative Food Processing Technologies Extraction, Separation, Component Modification, and Process Intensification. ed. K. Knoerzer, P. Juliano and G. Smithers, Woodhead Publishing Series in Food Science, Technology, and Nutrition, Elsevier, ch. 1, 2016, vol. 302, pp. 3–29 Search PubMed.
- L. Vernès, M. Abert-Vian, M. El Maâtaoui, Y. Tao, I. Bornard and F. Chemat, Ultrason. Sonochem., 2019, 54, 48–60 CrossRef PubMed.
- N. Babamoradi, S. Yousefi and P. Ziarati, J. Food Process. Eng., 2018, 41, e12851 CrossRef.
- S. Zhao, O. Baik, Y. J. Choi and S. Kim, Crit. Rev. Food Sci. Nutr., 2014, 54, 1283–1297 CrossRef CAS PubMed.
- S. Nikitenko and F. Chemat, Ultrasound in process engineering: new look at old problems, in Green Process Engineering: From Concepts to Industrial Applications, ed. M. Poux, P. Cognet and C. Gourdon, CRC Press, Taylor & Francis Group, 2015, pp. 145–165 Search PubMed.
- S. Mantegna, A. Binello, L. Boffa, M. Giorgis, C. Cena and G. Cravotto, Food Chem., 2012, 130, 746–750 CrossRef CAS.
- F. Chemat and G. Cravotto, Microwave-assisted extraction for bioactive compounds: theory and practice. XII, in Series: Food Engineering Series, Springer Science, New York, NY 10013, USA, 2013, vol. 4, pp. 238 Search PubMed.
- H. Wang, J. Ding and N. Ren, TrAC, Trends Anal. Chem., 2016, 75(Supplement C), 197–208 CrossRef CAS.
- T. Belwal, S. M. Ezzat, L. Rastrelli, I. D. Bhatt, M. Daglia, A. Baldi, H. Prasad Devkota, I. Erdogan Orhan, J. Kumar Patra, G. Das, C. Anandharamakrishnan, L. Gomez-Gomez, S. Fazel Nabavi, S. Mohammad Nabavi and A. G. Atanasov, Trends Anal. Chem., 2018, 100, 82–102 CrossRef CAS.
- K. K. H. Y. Ho, M. G. Ferruzzi, A. M. Liceaga, M. F. San and Martín-González, LWT - Food Sci. Technol., 2015, 62, 160–168 CrossRef CAS.
- G. Cavalaglio, F. Cotana, M. Gelosia, E. Pompili, S. D'Antonio and D. Ingles, Environ. Prog. Sustainable Energy, 2017, 36(3), 736–741 CrossRef CAS.
- M. E. Lucchesi, F. Chemat and J. Smadja, J. Chromatogr. A, 2004, 1043, 323–327 CrossRef CAS PubMed.
- A. Filly, X. Fernandez, M. Minuti, F. Visinoni, G. Cravotto and F. Chemat, Food Chem., 2014, 150, 193–198 CrossRef CAS PubMed.
- L. López-Hortas, H. Domínguez and M. D. Torres, Process Biochem., 2019, 78, 100–107 CrossRef.
- A. Farhat, A. S. Fabiano-Tixier, F. Visinoni, M. Romdhane and F. Chemat, J. Chromatogr. A, 2010, 1217, 7345–7350 CrossRef CAS PubMed.
- C. H. Chan, H. K. Yeoh, R. Yusoff and G. C. Ngoh, J. Food Eng., 2016, 188, 98–107 CrossRef CAS.
- T. Y. See, R. Yusoff, C. H. Chan and G. C. Ngoh, Food Bioprod. Process., 2018, 109, 98–106 CrossRef CAS.
- M. Garcia-Vaquero, G. Rajauria, J. V. O'Doherty and T. Sweeney, Food Res. Int., 2017, 99, 1011–1020 CrossRef CAS PubMed.
- M. Arshadi, T. M. Attard, R. M. Lukasik, M. Brnčić, A. M. Da Costa Lopes and M. Finell, et al. , Green Chem., 2016, 18(23), 6160–6204 RSC.
- C. S. K. Lin, L. A. Pfaltzgraff, L. Herrero-Davila, E. B. Mubofu, S. Abderrahim, J. Clark and R. Luque, Energy Environ. Sci., 2013, 6, 426–464 RSC.
- F. Turrini, D. Donno, R. Boggia, G. L. Beccaro, P. Zunin, R. Leardi and A. M. Pittaluga, Food Res. Int., 2019, 115, 276–282 CrossRef CAS PubMed.
- M. Marić, A. N. Grassino, Z. Zhu, F. J. Barba, M. Brnčić and S. R. Brnčić, Trends Food Sci. Technol., 2018, 76, 28–37 CrossRef.
- M. L. Clodoveo, Foods, 2019, 8, 121 CrossRef CAS PubMed.
- A. Leone, A. Tamborrino, R. Romaniello, R. Zagaria and E. Sabella, Biosyst. Eng., 2014, 125, 24–30 CrossRef.
- M. A. Bejaoui, G. Beltrán, A. Sánchez-Ortiz, S. Sánchez and A. Jiménez, Eur. J. Lipid Sci. Technol., 2016, 118, 332–336 CrossRef CAS.
- A. Taticchi, R. Selvaggini, S. Esposto, B. Sordini, G. Veneziani and M. Servili, Food Chem., 2019, 289, 7–15 CrossRef CAS PubMed.
- A. Tamborrino, R. Romaniello, F. Caponio, G. Squeo and A. Leone, J. Food Eng., 2019, 245, 124–130 CrossRef CAS.
- Z. Guo, B. Zhao, H. Li, S. Miao and B. Zheng, Innovative Food Sci. Emerging Technol., 2019, 54, 51–63 CrossRef CAS.
- X. Lu, Z. Zheng, H. Li, R. Cao, Y. Zheng, H. Yu and B. Zheng, Ind. Crops Prod., 2017, 107, 546–557 CrossRef CAS.
- M. M. A. Rasheda, Q. Tonga, A. Nagi, J. P. Li, N. U. Khan, L. Chen, A. Rotail and A. M. Bakry, Ind. Crops Prod., 2017, 100, 236–245 CrossRef.
- F. Chemat, Lesson about the history of extraction, Avignon University, France. https://www.youtube.com/watch?v=tDc_2J3I-1A Search PubMed.
- Y. H. Choi and R. Verpoorte, Metabolomics: What you see is what you extract, Phytochem. Anal., 2014, 25, 289–290 CrossRef CAS PubMed.
- R. K. Henderson, C. Jimenez-Gonzalez, D. J. C. Constable, S. A. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. S. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- D. Prat, O. Pardigon, H. W. Flemming, S. Letetsu, V. Ducandas, P. Isnard, E. Guntrum, T. Senac, P. Cruciani and P. Hosek, Sanofi ‘s Solvent Selection Guide: A Step Toward More Sustainable Processes, Org. Process Res. Dev., 2013, 17, 1517–1525 CrossRef CAS.
- F. Chemat, M. Abert Vian, H. K. Ravi, B. Khadhraoui, S. Hilali, S. Perino and A.-S. Fabiano Tixier, Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects, Molecules, 2019, 24, 3007 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, p. 30 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |