Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The potential of decarbonising rice and wheat by incorporating carbon capture, utilisation and storage into fertiliser production
A.
Gonzalez-Diaz†
 *,
L.
Jiang†
*,
L.
Jiang†
 *,
A. P.
Roskilly
and
A. J.
Smallbone
*,
A. P.
Roskilly
and
A. J.
Smallbone
Department of Engineering, Durham University, Durham, UK. E-mail: Abigail.gonzalez-diaz@durham.ac.uk; long.jiang@durham.ac.uk
First published on 6th January 2020
Abstract
This paper aims to evaluate the reduction in greenhouse gas emissions of rice and wheat and their supply chains by incorporating carbon capture, utilisation and storage into fertiliser production mainly from the ammonia process, which is a part of the fertiliser that produces most of the carbon dioxide. Greenhouse gas emissions of these grains without carbon capture, utilisation and storage are provided from the results of life cycle assessment in the literature. After that, carbon dioxide emission from fertiliser production is quantified. The alternative considered for utilisation is enhanced oil recovery and it is compared with the conventional way of oil production. The effects of carbon capture, utilisation, and storage on greenhouse gas reduction are presented in terms of the supply chains of rice and wheat to make people conscious about the use and optimisation of food. The reduction of greenhouse gas is around 6–7% in the rice supply chain e.g. rice milk, spoons of uncooked rice and 14–16% in the wheat supply chain e.g. pasta, one slice of bread. Although the alternative for carbon dioxide storage demonstrates marginally higher greenhouse gas reduction, enhanced oil recovery may offer an economic incentive from additional oil production that could reduce the cost of rice and wheat.
1. Introduction
Global carbon dioxide (CO2) emissions have continued to rise significantly increasing the potential for catastrophic climate change. In the recent Paris Agreement on climate change established with the Intergovernmental Panel on Climate Change (IPCC), a new goal has been set to limit temperature rise to 2 °C.1 The agricultural sector also contributes to worldwide greenhouse gas (GHG) emissions,2,3 with a share that is 10–12% of CO2 equivalent. Considering indirect emissions from other activities related to agriculture such as fertiliser production, land use change, food storage, packaging etc., this share can be up to one-third of the total GHG emissions.4 It is acknowledged that fertilisers are basically produced from ammonia. Of the total ammonia production in the world, 85% of the product is used to produce fertilisers for growing human and animal food.5 It then follows that the feedstock used in ammonia production will play a significant role in the amount of energy consumption and CO2 emissions produced during food production.It is estimated that ammonia production consumes almost 1.2% of total global primary energy which contributes to 0.93% of GHG emissions.6 About 70% of the ammonia production in the world is based on steam methane reforming (SMR) technology, and this is mainly because SMR is considered to be the best proven technology which is cost-effective and has low energy consumption.5 Further reduction of CO2 emissions to near zero from ammonia production could be only realised by using appropriate CO2 capture, utilisation, and storage (CCUS) technology.7 As such, this could prove to be a feasible approach to reduce GHG emissions of the fertiliser in food cultivation. Current studies have mainly focused on the gas separating technologies of SMR processes e.g. PSA, TSA or membrane which aim to obtain and recover a high purity product gas.8,9 Thus, during the process, high purity CO2 is generated as a product in the intermedium process. Additional CO2 is generated by burning additional fossils to increase the temperature in the SMR reactor as well as to generate steam and electricity for use in the process. The industrial sector (including ammonia and fertiliser synthesis) has not received the same attention as power plants for the deployment of carbon capture and storage (CCS) due to its associated costs and no economic incentive.10 However, there are opportunities for CO2 utilisation (CU) based on ammonia production because the CO2 concentration in the flue gas is higher than those in other processes e.g. power plants which are usually in the range from 4% to 20%.11 Thus CO2 from an ammonia plant at high purity is ready for CU e.g. for enhanced oil recovery (EOR), polymers, urea, CH4, methanol, etc.12 Although CU faces some challenges e.g. low energetic level and reactivity, CU could reduce the cost of capturing additional CO2 and its storage process when compared with that of CCS.13 Currently, CO2-EOR is considered as an available technology that has been used successfully in North America to increase the oil production from depleted fields. Large amounts of the injected CO2 could be retained in storage.14 Most importantly, EOR offers an opportunity where the CO2 can be sold in high-volumes to a customer. In addition, revenue for selling CO2 could be an incentive to accelerate the deployment of CCS in the industry. However, CU is an energy and material intensive process. Thus, to clarify whether it allows for a net reduction of environmental impacts, every alternative for CO2 utilisation must be evaluated in terms of a life cycle perspective.15 Another alternative to reduce GHG emissions in food cultivation is the use of organic fertilisers. A number of research studies have investigated this issue in terms of energy use, GHG emissions, and cost-effectiveness when compared with that using conventional fertilisers.16,17 From a technical perspective, although the environmental impact e.g. aquatic and human toxicity potential, eutrophication and acidification potential is reduced by using organic fertilisers, it makes little contribution to the reduction of global warming potential (GWP).18 Comparably, a few studies claim that organic food could be better than the conventional food with regard to life cycle assessment (LCA) and the results are much associated with raw material inputs and CO2 emissions.19,20 Thus, an alternative method is expected to be figured out which could be a good solution to this CO2 issue for the food when compared with organic food.
This paper aims to evaluate the CO2 emission reduction in rice and wheat by incorporating CCUS into the supply chain via the ammonia plant, which is the main source when CO2 is regarded to be produced from fertilisers. These grains are selected to be research objects because they provide most of the world's food supply.21 The general technical route is shown in Fig. 1. The GHG emissions of grains without CCUS are compared with those using CO2 storage and utilisation. EOR is selected and analysed as an alternative for CU. Several previous research studies have presented the LCA of rice and wheat. However, they have not considered CCUS for reducing CO2 emissions that are generated by grain production. Although the information is obtained from LCA studies, only GWP is evaluated. It is worth noting that this study is the first evaluation to quantify the amount of CO2 reduced by incorporating CCUS in fertiliser production which could give more insights and inspirations to the general public. The framework of this paper is illustrated as follows: GHG emissions for the selected grains from different references are presented in section 2. To estimate the overall capture rate of the ammonia plant, technical assessment is then carried out and described in section 3. After that, in the same section, GHG emissions for grains with CCUS are estimated followed by conclusions in section 4.
 | ||
| Fig. 1 Alternatives to decarbonised selected food: rice and wheat incorporating CCUS in fertiliser production. | ||
2. GWP by selected food
World ammonia (NH3) production in 2018 was 176.5 million tonnes per year22 and represented around 1% of the total world CO2 emissions. It is predicted to increase to 234 million tonnes per year in 2021.23 Ammonia production from natural gas using a steam methane process produces around 1.6 tonnes CO2 per tonne NH3 and consumes 28 GJ per tonne NH3.5 This will result in around 374.4 million tonnes of CO2 per year in 2021. If CCS is incorporated at 90% capture level in all ammonia plants in the world, 336.96 million tonnes of CO2 per year could be avoided. In this work, rice and wheat are selected to evaluate the reduction of CO2 if CCUS is incorporated. The reason for selecting these crops (rice, wheat) is because they supply most of the world's food21 and consume a large amount of fertiliser.Global warming or CO2 equivalent is presented, which is compounded for CO2: 1, CO: 2, CH4: 21, and N2O: 310 according to IPCC.24 The information that comes from different LCA studies is required before estimating the reduction in global warming by incorporating CCUS in selected crop cultivation.
2.1 Rice
It is extensively acknowledged that rice is regarded as one of the major cereal crops for more than half of the world's population.25 The cultivation of rice was expected to increase from 510.5 millions of tonnes in 2017 to 565.6 millions of tonnes in 2025.26 This could be mainly attributed to the fact that the demands will increase from 512 millions of tonnes in 2017 to 563.2 millions of tonnes in 2025.26 Its cultivation is one that contributes to the global climate change through emissions of CO2, CO, CH4, and N2O. But at the same time, rice cultivation and production are sensibly affected by climate change that could not cover the demands in the future.GHG emissions by rice cultivation in countries from China, the United States, etc., where most of the rice is produced, are presented in Table 1. The GHG depends on location, size of the farms, the variety of rice grains, and yield, among others.27 The amount of fertiliser used in rice cultivation varies by locations and local farming methods. For instance, in 2014, the amount of fertiliser was 570 kg per hectare in China, 290 kg in Bangladesh, 210 kg in Indonesia, and 130 kg in the United States. It is demonstrated that the variation is mainly because it depends on the fertility of the underlying soil.28 In Table 1, it can be observed that India and Japan present the highest and lowest emissions, respectively.
| Cultivation type | Country | System boundary | Unit | Sore | Source |
|---|---|---|---|---|---|
| a The higher global warming is related to the lower yield, which is 50% lower than in China. | |||||
| Conventional | China | Up to-farm gate | kgCO2 eq per tonne | 1700–1500 | 18 and 24 |
| Japan | Cradle-to-farm gate | kgCO2 eq per kg | 1.46 | 29 | |
| USA | Cradle-to-farm gate | kgCO2 eq per kg | 1.77 | 29 | |
| Thailand | Cradle-to-farm gate | kgCO2 eq per kg | 2.97 | 30 | |
| Bangladesh | Cradle-to-farm gate | kgCO2 eq per kg | 3.15 | 28 | |
| India | Production-to-farm gate | kgCO2 eq per kg | 5.65a | 31 | |
| Iran | No specified | kgCO2 eq per tonne | 277.21 | 32 | |
| Malaysia | Cradle-to-gate | tonne CO2 eq per tonne | 1.39 | 33 | |
2.2 Wheat
Wheat is the most important crop in the world, which is essential for many human diets.34 The main countries that export wheat are the United States, Canada, Australia, the European Union, and Argentina.35Table 2 presents a global warming impact of wheat cultivation. In 2018, wheat and rice production were 736.1 million and 511.4 million tonnes, respectively.36 GHG emissions by wheat cultivation and production are lower than those of rice. However, the amount of global wheat production is higher. Therefore, it is important to look for the alternatives to reduce GHG emission in its cultivation.| Country | System boundary | Unit | Sore | Source |
|---|---|---|---|---|
| a An average from nine states from the USA. | ||||
| Sweden | Up to-farm gate | kgCO2 eq per kg | 0.2–0.6 | 31 |
| Australia | Cradle-to-farm gate | kgCO2 eq per tonne | 304–487 | 37 |
| Europe | No specified | kgCO2 eq per tonne | 381 | 38 |
| USA | Cradle-to-farm gate | gCO2 eq per tonne | 356a | 39 |
| Iran | Cradle-to-gate | kgCO2 eq per tonne | 380 | 40 |
| Poland | Cradle-to-farm gate | kgCO2 eq per tonne | 364 | 41 |
2.3 CO2 emissions by fertiliser production in cultivation
Fertiliser production and utilisation is one of the most representative contributors of GWP in rice and wheat production which is successively dominated by CO2, N2O, and CH4.2,28 For example, the GWP of urea (fertiliser) production and supply in wheat represent around 34%, where 26%, 6.4% and 1.6% are related to CO2, N2O, and CH4, respectively.28Table 3 shows the contribution of GHG by fertiliser production in rice and wheat for Bangladesh, Thailand, China, Japan, Sweden, and Australia. Due to the lack of data, one of the assumptions considered in this work is that the percentage of CO2 generated by fertiliser production only comes from the ammonia process, even when the fertiliser system involves other equipment e.g. urea unit after the ammonia plant. The participation of CO2, N2O and CH4 in GHG generated in ammonia production are 91.92%, 7.97%, and 0.11% respectively, according to the cradle-to-gate LCA study presented in ref. 42. 91.92% of CO2 is generated by (a) fuel gas combustion in the primary and secondary reformers (93.4%), (b) compressors used to transport natural gas (4.18%), and (c) the steam generation required by the system (2.38%).| Food type | Country | Percentage of GHG by fertiliser production |
|---|---|---|
| a 11% of CO2 emission by fertiliser production includes manufacture/transport. b 11% of CO2 emission by fertiliser production includes the input of fertilisers and pesticides, rice seed production and transportation stages. c Due to the lack of information, this value is taken from the information provided for Bangladesh and Thailand28,30 considering that China is located close to these countries. | ||
| Paddy rice | Bangladesh | 11%a (ref. 28) |
| Thailand | 11%b(ref. 30) | |
| China | 11%c | |
| Japan | 7% (ref. 29) | |
| Wheat | Sweden | 24% (ref. 3) |
| Australia | 26% (ref. 37) | |
3. GHG emission assessment of rice and wheat by incorporating CCUS
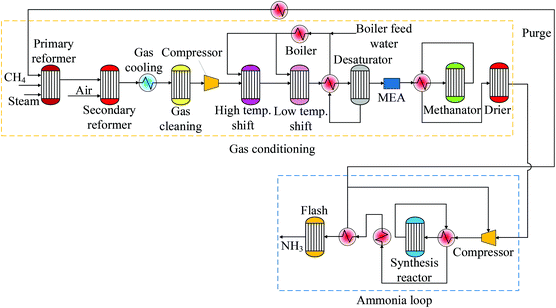
In ammonia production, the CO2 capture process is an important part of the system. However, CO2 should be stored or used in order to mitigate GHG emissions. Three scenarios to mitigate GHG are evaluated in this work for rice and wheat, i.e. (1) grain cultivation and production, (2) grain cultivation and production with CO2 storage, and (3) grain cultivation and production with CO2-EOR. The system boundaries for rice and wheat are cradle-to-farm gate which include fertiliser production, cultivation, harvesting, planting, irrigation etc. The boundary for CO2 storage starts from CO2 transport to the storage site, and for CO2-EOR starts from CO2 transport to fuel combustion. The functional unit for comparative analysis is 1 tonne of grain (rice or wheat). The boundaries for the system are shown in Fig. 2.Fertilisers e.g. ammonium nitrate, calcium ammonium nitrate, ammonium sulphate, and urea are produced using ammonia. CO2 in the process of fertiliser production is generated mainly from fossil fuels used during ammonia production, and a less percentage of CO2 is generated during the production of phosphorites and sulphuric acid (H2SO4) ammonia production.43 In order to estimate the overall capture rate by incorporating CCUS in fertiliser production using SMR, a detailed assessment of the integrated process is carried out in terms of H2 production and ammonia plant via the Haber–Bosch process. The production process is simulated in Aspen Plus to determine mass and energy balance which is based on an ammonia plant with a capacity of 1270 tonnes per day.
3.1 Ammonia process
Ammonia production adopts a well-established SMR process, which is generally composed of a SMR reactor, water shift reactor (WSR), CO2 separator, methanator, compressor, and ammonia reactor. Reactions (1) and (2) occur in SMR and WSR reactors, respectively.44Table 4 presents operating parameters and assumptions used in the simulation of SMR, WSR, and carbon capture (CC).45| CH4 + H2O = CO + 3H2 | (1) |
| CO + H2O = CO2 + H2 | (2) |
A schematic diagram of the whole SMR process is shown in Fig. 3 which is simulated in Aspen Plus using Peng Robinson's equation.44 The detailed processes are illustrated as follows: first methane (CH4) is mixed with steam at 510 °C and 30 bar. The mixed components enter the primary SMR reactor where reaction (1) occurs. After that, compressed air is mixed with the exhaustive flue gas from the primary SMR and flows into the second SMR reactor. O2 that comes from the air reacts with the remaining CH4 to increase the temperature to 950 °C, and N2 is used to produce ammonia. The syngas basically composed of CO, CO2, H2, CH4, and H2O is cooled down to 350 °C and exchanges heat with feed water used in the SMR. Reaction (2) occurs in WSR, and the syngas is cooled at 38 °C. After that, the syngas is cleaned from CO2. The CO2 is separated from the flue gas in an absorber column by using monoethanolamine (MEA) at an efficiency of 80% to achieve the purity of 95%. The syngas that contains H2 is delivered at 17 bar to the methanator.48 CO2 and CO are poisons for many types of catalysts. Thus, the residual CO and CO2 remaining after cleaning the syngas must be removed by converting to methane and water, as presented in reactions (3) and (4), through a nickel or ruthenium catalyst with H2 in the methanator.
| CO + 3H2 = CH4 + H2O | (3) |
| CO2 + 4H2 = CH4 + 2H2O | (4) |
First, the SMR reported in ref. 49 is reproduced to validate the model developed in Aspen Plus and to estimate the efficiencies of the SMR and WSR. After that, the model is updated to the capacity of 1270 tonnes per day of ammonia based on the industrial and commercial size reported in ref. 50. Additional assumptions considered in the SMR are elaborated as follows: composition of natural gas is 100% methane; the separation of water in the condenser is complete; heat losses through the equipment are neglected. The final step is the ammonia production which consists of the following steps: syngas compression and ammonia process. The syngas contains high concentrations of H2 and N2, which are compressed at 202.6 bar51 and delivered to the finally reactor where reaction (5) takes place.
| N2 + 3H2 = 2NH3 | (5) |
In this study, CO2 is removed using a MEA-based capture plant. It consists of an absorber where the CO2 is captured by the amine solvent at 30 wt% and a stripper where the CO2 is separated from the MEA solution.
Mass balance of the main raw material and ammonia production is shown in Table 5. It presents the main results of the ammonia process, and 28.5 tonnes per h of methane is used to generate 53.2 tonnes per h of ammonia. During the ammonia production process, 81.6 tonnes per h of CO2 is generated, and 53.2 tonnes per h is captured for utilisation and only 15 tonnes per h is emitted to the atmosphere. Although 90% of CO2 is captured in the capture plant, additional fuel is burned to generate heat and steam required by the ammonia plant. Then, the overall capture rate in the ammonia plant is 77.5%. This information is used to estimate the amount of CO2 mitigated in grain production, which is used for CO2 storage or EOR.
| Component | Amount |
|---|---|
| CH4 (tonne per h) | 28.5 |
| CH4 additional fuel in furnace (tonne per h) | 3.0 |
| Steam (tonne per h) | 96.2 |
| H2 to ammonia reactor (tonne per h) | 10.6 |
| N2 (tonne per h) | 47.8 |
| Ammonia (tonne per h) | 53.2 |
| CO2 captured (tonne per h) | 66.6 |
| CO2 emitted (tonne per h) | 15.0 |
| CO2 capture (%) | 77.5 |
Additional information for the capture plant is presented in Tables 6 and 7. The overall efficiency of the process from SMR to the ammonia reactor could reach 66%. The CC process is simulated to estimate energy consumption and CO2 emissions of the ammonia process. The composition and mass flow rate of the syngas are presented in Table 6, which serve as the input parameters for the CO2 capture plant. The syngas flow rate is 135.2 tonnes per h, and only one post-combustion capture train is necessary to capture 90% of CO2. The size of the train is defined in the literature when considering a maximum of approximately 292.5 tonnes per h of the absorber column. This is mainly due to the economic limits of the size of the absorber that are based on pressure drop constraints to ensure a stable operating condition with appropriate liquid and gas distributions.52,53Table 7 presents key results of the capture plant. The steam required to regenerate the solvent is 212 tonnes per h at 4 bar and the specific reboiler duty is 3.65 GJ per tonne CO2. The steam required is supplied by the same ammonia process.
| Items | Values |
|---|---|
| Syngas mass flow rate (tonne per h) | 135.2 |
| CH4 (mol %) | 0.25061 |
| H2O (mol %) | 0.62123 |
| CO (mol %) | 2.04756 |
| H2 (mol %) | 62.4948 |
| CO2 (mol %) | 16.5042 |
| N2 (mol %) | 18.0394 |
| Items | Values |
|---|---|
| Syngas temperature (°C) | 150 |
| Total steam required by the capture plant (tonne per h) | 212 |
| Reboiler temp (°C) | 120 |
| Reboiler steam pressure (bar) | 4 |
| Reboiler solvent pressure (bar) | 16.5 |
| Lean solvent mass flow rate (tonne per h) | 1494 |
| Lean loading (molCO2 molMEA−1) | 0.27 |
| Rich loading (molCO2 molMEA−1) | 0.457 |
| CO2 captured (tonne per h) | 66.6 |
| Reboiler duty (MW) | 63.94 |
| L/G ratio (mol mol−1) | 6.74 |
| Specific reboiler duty (GJ per tonCO2) | 3.65 |
| Total PCC auxiliary power consumption (MW) | 0.573 |
3.2 GHG emission reduction in grains by incorporating CO2 storage in fertiliser production
The first alternative to reduce the CO2 from fertiliser production in this work is to incorporate CO2 storage. Assuming a high capture rate of 77.5% estimated in the previous section and using information presented in Tables 1–3 (only for countries whose percentage of CO2 in GHG by fertiliser production is reported in the literature), the amount of CO2 for storage is estimated. Because the fertiliser used in rice and wheat farming depends on several factors, it cannot be assumed to be constant for all the countries. Additional emissions for transporting the CO2 from the fertiliser plant to the storage reservoir are taken into consideration based on ref. 54 and 55. Thus, it is estimated by assuming an average distance of 500 km, and an emission factor (EF) emitted per kg CO2 transported by pipeline is considered. The mass flow rate of CO2 captured and transported by pipeline (Mcap/grain) is the CO2 captured and stored from the fertiliser reported in Table 8. Then, the CO2 emitted through transport is estimated as presented in eqn (6) and (7) in terms of the exemplified paddy rice that is cultivated in Bangladesh: | (6) |
 | (7) |
| Country | GHG emission by grain without CCUS | Percentage of GHG by fertiliser | Total CO2 emitted by fertiliser production without capture | M cap/grain | ECemit/grain | ECtrans/grain | Total CO2 emitted by fertiliser production with CCS |
|---|---|---|---|---|---|---|---|
| Unit | kgCO2 eq per tonne grain | % | kgCO2 eq per tonne grain | kgCO2 per tonne grain | kgCO2 eq per tonne grain | kgCO2 eq per tonne grain | kgCO2 eq per tonne grain |
| a 11% of CO2 emission by fertiliser production includes: manufacture/transport. b 11% of CO2 emission by fertiliser production includes: input of fertilisers and pesticides, rice seed production and transportation stages. c This value is an average of 1700 and 1500 kgCO2 per tonne. d Due to the lack of information, this value is taken from information provided for Bangladesh and Thailand.28,30 | |||||||
| Paddy rice | |||||||
| Bangladesh | 3150 | 11a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
297.48 | 230.55 | 66.93 | 0.229 | 67.16 |
| Thailand | 2970 | 11b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
280.48 | 217.37 | 63.11 | 0.216 | 63.32 |
| China | 1600c | 11d | 151.10 | 117.10 | 34.00 | 0.116 | 34.11 |
| Japan | 1460 | 729 | 137.88 | 68.00 | 31.02 | 0.068 | 31.09 |
| Wheat | |||||||
| Sweden | 400 | 243 | 37.78 | 63.87 | 8.50 | 0.063 | 8.56 |
| Australia | 304 | 2637 | 28.71 | 52.59 | 6.46 | 0.052 | 6.51 |
It is worth noting that LCA reported in the literature for rice and wheat production includes fertiliser production. Then, the fertiliser process includes the ammonia plant. As a result, the CO2 capture plant is also included since CO2 separation is part of the ammonia process. In the ammonia plant, the CO2 is generated at high purity as part of the process, therefore only the CO2 generated by transporting is considered.
Table 9 shows the total GHG emissions with CCS. ECemit/grain is the amount of CO2 that is not captured, and which is emitted to the atmosphere. When CO2 is captured and stored in the ammonia plant to produce fertilisers and use in paddy rice cultivated in Bangladesh, Thailand, and China, the GHG emission is reduced by 7.31% and in Japan by 4.62%. In the case of wheat flour cultivated in Sweden and Australia, the incorporation of CCS has higher impact on GHG emission reduction by 15.92% and 17.28%, respectively. Although the annual wheat production and the percentage of GHG reduction in wheat flour production is higher than those for rice, the total amount of CO2 generated for rice is higher than that for wheat. This is mainly because the amount of GHG generated during rice cultivation and production is much higher than that for wheat. The CO2 could be reduced from 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 838 million tonnes per year to 25
838 million tonnes per year to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 018 million tonnes per year in rice, and from 24
018 million tonnes per year in rice, and from 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 472 million tonnes per year to 5547 million tonnes per year in wheat flour.
472 million tonnes per year to 5547 million tonnes per year in wheat flour.
| Country | GHG emission by grain without CCUS | GHG emission by grain with CO2 storage | Reduction |
|---|---|---|---|
| Unit | kgCO2 eq per tonne grain | kgCO2 eq per tonne grain | % |
| Paddy rice | |||
| Bangladesh | 3150 | 2919.68 | 7.31 |
| Thailand | 2970 | 2752.84 | 7.31 |
| China | 1600 | 1483.01 | 7.31 |
| Japan | 1460 | 1392.07 | 4.65 |
| Wheat flour | |||
| Sweden | 400 | 336.19 | 15.95 |
| Australia | 304 | 251.46 | 17.28 |
3.3 GHG emission reduction in grains by incorporating CO2-EOR in fertiliser production
As a common application for CU, EOR is selected in this work, and its general schematic diagram is shown in Fig. 4. It is one of the potential alternatives for CU and is a proven technique used to increase the crude oil production extracted from an oilfield. The EOR has been identified to be profitable at a commercial scale, which could be quite beneficial for the economy in the UK,56 the United States,57 Mexico,58etc. When the pressure of an oil reservoir is depleted through primary and secondary production, the use of the CO2 can be a tertiary recovery method. This technology includes injecting CO2 into the reservoir to dissolve in the oil. CO2 makes the oil reduce its viscosity59 because CO2 is miscible with oil.60 Simplified calculations can give an idea related to the reduction of CO2 emissions and the benefit for the co-production of grain and crude oil. The CO2 accounted for is the one generated by transporting CO2, burning the oil extracted by injecting CO2, and the remaining 22.5% of the CO2 that is not captured in the ammonia plant.For LCA of EOR, oil and electricity are the primary product and the coproduct.54 In this work, there are two products: (1) grain (rice or wheat) as the primary product and (2) oil as a coproduct. According to ref. 54 and 55, the credit (CO2 reduction for CCU) in LCA related to the GHG emissions associated with the electricity is assigned only to the oil as a single product. In this work, the credit or additional CO2 emission generated by oil production via EOR is assigned to grains. The credit or additional CO2 equivalent by the incremental oil is estimated by the difference between CO2 equivalent generated by EOR and by a conventional way to produce oil.
| Country | GHG emission by grain without CCUS | Percentage of GHG only by fertiliser | M cap/grain | Total CO2 emitted by rise and wheat production |
|---|---|---|---|---|
| Unit | kgCO2 eq per tonne grain | % | kgCO2 per tonne grain | kgCO2 per tonne grain |
| a 11% of CO2 emission by fertiliser production includes: manufacture/transport. b 11% of CO2 emission by fertiliser production includes: input of fertilisers and pesticides, rice seed production and transportation stages. c This value is an average of 1700 and 1500 kgCO2 per tonne. d Due to the lack of information, this value is taken from information provided for Bangladesh and Thailand.28,30 | ||||
| Paddy rice | ||||
| Bangladesh | 3150 | 11a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
230.55 | 66.93 |
| Thailand | 2970 | 11b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
217.37 | 63.11 |
| China | 1600c | 11d | 117.10 | 34.00 |
| Japan | 1460 | 729 | 68.00 | 31.02 |
| Iran | 277.21 | 11 | 20.29 | 5.89 |
| Malaysia | 1390 | 11 | 101.73 | 29.54 |
| Wheat | ||||
| Sweden | 400 | 243 | 63.87 | 8.50 |
| Australia | 304 | 2637 | 52.59 | 6.46 |
| Iran | 380 | 26 | 65.74 | 8.07 |
| Poland | 364 | 26 | 62.97 | 7.73 |
1. The CO2 equivalent per tonne of grain by transporting CO2 from the fertiliser plant to the oil field is estimated using eqn (6).
2. The CO2 equivalent emitted by the segment EOR operation is based on ref. 54 and 55 using eqn (8) and (9), and the following parameters: the incremental oil per tonne of CO2 injected (ϕuf) of 1.49 bbl per tonne CO2,61 and the CO2 emitted per incremental oil is 100 kgCO2 eq per bbl. For example, for paddy rice produced in Bangladesh, CO2 emitted by one barrel of incremental oil is explained by using the amount of CO2 for an EOR of 230.55 kgCO2 per tonne (0.230 tonnes CO2 per tonne) paddy rice which is presented in Table 11.
 | (8) |
 | (9) |
 | ||
| Fig. 5 System boundary of the life cycle of CO2 emission (a) of incremental oil via EOR; (b) of conventional oil production. | ||
| Country | E trans/grain | E EOR/grain | ECds/grain | Total CO2 emitted by oil production EOR |
|---|---|---|---|---|
| Unit | kgCO2 eq per tonne grain | kgCO2 eq per tonne grain | kgCO2 eq per tonne grain | kgCO2 eq per tonne grain |
| Paddy rice | ||||
| Bangladesh | 0.229 | 34.35 | 166.61 | 201.19 |
| Thailand | 0.216 | 32.39 | 157.09 | 189.69 |
| China | 0.116 | 17.45 | 84.63 | 102.19 |
| Japan | 0.068 | 10.13 | 49.14 | 59.34 |
| Iran | 0.020 | 3.02 | 14.66 | 17.71 |
| Malaysia | 0.101 | 15.16 | 73.52 | 88.78 |
| Wheat flour | ||||
| Sweden | 0.063 | 9.52 | 46.16 | 55.74 |
| Australia | 0.052 | 7.84 | 38.00 | 45.89 |
| Iran | 0.065 | 9.79 | 47.51 | 57.37 |
| Poland | 0.063 | 9.38 | 45.51 | 54.95 |
3. The CO2 emitted by the last segment (oil transport refining, fuel transport, and fuel combustion) termed the downstream segment is estimated and the parameter ECoil = 485 kgCO2 eq per bbl.54 For example, with respect to paddy rice produced in Bangladesh, CO2 emitted by downstream segments is explained according to following eqn (10):
 | (10) |
Based on the same amount of oil generated by EOR, CO2 equivalent by using conventional oil production is estimated to determine the increment or the reduction of CO2 equivalent. The boundary of the life cycle for conventional oil production is shown in Fig. 5b, which covers two segments: (1) oil extraction and production, and (2) oil transport, refining, fuel transport and combustion. The CO2 equivalent for the first segment is estimated based on ref. 62 and the second on ref. 54 by using eqn (9) in EOR. The GHG emission in the first is 9.2 gCO2 eq MJ−1 LHV. This amount excludes oil transport because it is considered in the second segment (downstream segment). 9.2 gCO2 eq MJ−1 LHV is converted to 54.3 kgCO2 eq per bbl by using the following information on oil:63 a LHV of 43.2 MJ kg−1 and density of 0.86 kg l−1. Then, it is converted from kgCO2 eq per bbl kgCO2 eq per tonne grain. An example for the paddy rice from Bangladesh is described by using eqn (11):
 | (11) |
Total CO2 emitted by incremental oil production from CO2-EOR per one tonne of rice and wheat is presented in Table 11. This result together with the total CO2 emitted by the conventional approach to produce oil are used to estimate the additional CO2 emission when EOR is implemented. Total CO2 equivalents by conventional oil in terms of rice and wheat are presented in Table 12, and the result is lower than that via EOR presented in Table 11. The difference between the total CO2 emitted by oil production via EOR and the total CO2 equivalent emitted by conventional oil production is presented in Table 13 column for “Additional CO2 emitted by CO2-EOR process”.
| Country | ECcop/grain | ECds/grain | Total CO2 equivalent emitted by conventional oil production |
|---|---|---|---|
| Unit | kgCO2 eq tonne grain | kgCO2 eq per tonne grain | kgCO2 eq per tonne grain |
| Paddy rice | |||
| Bangladesh | 18.67 | 166.61 | 185.27 |
| Thailand | 17.60 | 157.09 | 174.69 |
| China | 9.48 | 84.63 | 94.11 |
| Japan | 5.51 | 49.14 | 54.65 |
| Iran | 1.64 | 14.66 | 16.30 |
| Malaysia | 8.24 | 73.52 | 81.76 |
| Wheat flour | |||
| Sweden | 5.17 | 46.16 | 51.33 |
| Australia | 4.26 | 38.00 | 42.26 |
| Iran | 5.32 | 47.51 | 52.83 |
| Poland | 5.10 | 45.51 | 50.60 |
| Country | GHG emission by grain without CCUS | CO2 capture (CO2 reduced) (−) | Additional CO2 emitted by CO2-EOR processa (+) | Total GHG emission by grain with CO2-EOR | Reduction |
|---|---|---|---|---|---|
| Unit | kgCO2 per tonne grain | kgCO2 per tonne grain | kgCO2 eq per tonne grain | kgCO2 eq per tonne grain | % |
| a This amount is the difference between the total CO2 emitted by oil production EOR (Table 11) and the total CO2 emitted CO2 equivalent emitted by conventional oil production (Table 12). Because EOR emits more CO2 than conventional oil, this amount is added to the total GHG emissions. | |||||
| Paddy rice | |||||
| Bangladesh | 3150 | 230.55 | 15.91 | 2935 | 6.81 |
| Thailand | 2970 | 217.37 | 15.00 | 2768 | 6.81 |
| China | 1600 | 117.10 | 8.08 | 1491 | 6.81 |
| Japan | 1460 | 68.00 | 4.69 | 1397 | 4.34 |
| Iran | 277.21 | 20.29 | 1.40 | 258 | 6.81 |
| Malaysia | 1390 | 101.73 | 7.02 | 1295 | 6.81 |
| Wheat flour | |||||
| Sweden | 400 | 63.87 | 4.41 | 341 | 14.87 |
| Australia | 304 | 52.59 | 3.63 | 255 | 16.11 |
| Iran | 380 | 65.74 | 4.54 | 319 | 16.11 |
| Poland | 364 | 62.97 | 4.35 | 305 | 16.11 |
The difference in GHG emissions associated with oil production is assigned to grain production which leaves the LCA as a single primary product (grain). Therefore, total GHG emission by grains with CO2-EOR and the reduction of GHG emission are evaluated which are presented in Table 13. When CO2 is captured and used for EOR in the ammonia plant to produce fertilisers and use in paddy rice cultivated in Bangladesh, Thailand, and China, the GHG emission is reduced by 6.81% and by 4.34% in Japan. In the case of wheat flour cultivated in Sweden and Australia, the incorporation of CCS has a higher impact on GHG emission reduction by 14.87% and 16.11%, respectively. In this paper, if the oil production to cover the demands could be supplied by conventional oil production or EOR, the CO2 emissions to be quantified by conventional oil production or EOR will be based on the same amount of oil in both cases.
4. Impact on grain supply chains by incorporating CCUS in the fertiliser plant
Final GHG emissions (CO2 equivalent) from rice and wheat by incorporating CO2 storage and EOR are presented in Fig. 6. When comparing both candidates, CO2 storage presents a higher GHG emission reduction i.e. 6.8% than that of EOR. This is mainly because of CO2 transport from the ammonia plant to old wells, and the percentage of CO2 that is extracted together with the incremental oil.It is worth noting that unlike in power generation processes where the power or thermal energy can be replaced by renewable energy such as solar and wind, it is not possible to achieve that in the ammonia plant because most of the CO2 is generated from the process as explained in section 3.1. Therefore, CCUS could be the only solution to reduce GHG. For CC, it does not present any challenge in the ammonia plant since the CO2 is captured as a part of the process. For CO2-EOR, it faces a big challenge because CO2 selling price is greatly dependent on the oil price. It is indicated that EOR may produce even more CO2 from the incremental oil. It is beneficial that the demand of oil could be supplied by CO2-EOR instead of increasing the oil production from EOR and a conventional alternative. Then, EOR could provide an economic incentive, and develop experience and infrastructure that would reduce the cost of this technology, especially in developing countries where grain cultivation and its price play an important role in their economy.
Another option to reduce the adverse effect of fertilisers is the use of organic fertilisers. However, for a short term, it cannot be considered as a solution since a high demand of fertilisers could only be delivered via conventional pathways. As mentioned above, this alternative option significantly leads to reduction in terms of aquatic and human toxicity, eutrophication and acidification potential among others. However, it does not bring great benefit to GWP.18 Both alternatives of CO2 storage and CO2-EOR are important because in some countries there are no opportunities for EOR. In this circumstance, other alternatives for CU should be evaluated. The countries that supply most of the ammonia in the world are e.g. East Asia 30.6%, Africa 19.7%, East Europe and Central Asia 16% and North America 14.1%.22
Table 14 presents GHG emissions by a portion of diary food from rice and wheat using fertiliser production with CCUS.
In order to quantify the benefit of CCUS technology that could give the general insight, GHG emission reduction is presented in terms of dairy food portions made by rice and wheat flour. As shown in Fig. 7, GHG emission reduction for three spoons of rice is 24.5 grams with CCS and 22.5 grams with EOR; for 200 ml of rice milk is 17 grams with CCS and 16 grams with EOR. GHG emission reduction of 75 grams of uncooked wheat pasta with CCUS is 19 grams with CCS and 17.7 grams with CCUS. For one slice of bread, the reduction is 9.1 grams with CCS and 8.5 grams with CCUS. It is well known that CC is a technology that requires a large amount of investment. Therefore, it is very important to make people conscious about the use and optimisation of food in terms of quantifying the effect of CCUS on diary food and showing how difficult it is to reduce only around 6–7% of GHG in the rice supply chain and 14–16% in the wheat supply chain. It is concluded that CCUS could not reduce completely the GHG emissions on food. Using correctly the amount of food in places e.g. homes, restaurant, and schools could be another alternative that could be complemented with CCUS.
5. Conclusions
In this paper, the percentage of GHG emission reduction by incorporating CCUS in rice and wheat has been quantified. EOR has been selected as the method for CU. Conclusions are yielded as follows:(1) It is indicated that it is possible to reduce the GHG emissions per tonne of rice and wheat by 4.65–7.31% and 15.95–17.28% with CO2 storage as well as 4.34–6.81% and 14.87–16.11% with EOR, respectively.
(2) Although the alternative with CO2 storage presents a marginally higher GHG reduction, EOR could offer an economic incentive from additional oil production that could reduce the cost of rice and wheat when CCUS is incorporated and not necessary as an alternative to reduce GHG emissions.
(3) With CCUS, it essentially decarbonises the fertiliser production but still has a large GHG issue.
(4) Incorporation of CCUS is not only the alternative that could begin to solve the problem of GHG in food, but also could be complemented by using and optimising the amount of food in homes, hospitals, restaurants, etc.
Abbreviations
| CC | Carbon capture |
| CCS | Carbon capture and storage |
| CCUS | CO2 capture, utilisation and storage |
| CHP | Combined heat and power |
| CU | CO2 utilisation |
| EC | CO2 equivalent |
| EF | Emission factor |
| EOR | Enhanced oil recovery |
| Eq | Equivalent |
| GHG | Greenhouse gas |
| GWP | Global warming potential |
| HRSG | Heat recovery steam generator |
| IO | Incremental oil |
| IPCC | Intergovernmental Panel on Climate Change |
| IFA | International Fertiliser Industry Association |
| LCA | Life cycle assessment |
| LHV | Low heating value |
| M | Mass (kg·kg−1) |
| MEA | Monoethanolamine |
| NG | Natural gas |
| NRTL | Non-random two-liquid model |
| SMR | Steam methane reforming |
| T | Temperature (°C) |
| WSR | Water shift reactor |
| Y | Percentage |
| α | Emission factor |
| ϕ | Utilisation factor |
| cap | Capture |
| cop | Conventional oil production |
| cr | Capture rate |
| ds | Downstream |
| e | Emission |
| fer | Fertiliser |
| t | Transport |
| u | Utilisation |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by CCS from Industrial clusters and their Supply chains (CCSInSupply) funded by the Engineering and Physical Science Research Council of UK (EP/N024567/1).References
- Outlook WE, Energy Climate and Change, WEO, 2015 Search PubMed.
- C. Skinner, A. Gattinger, M. Krauss, H.-M. Krause, J. Mayer and M. G. A. van der Heijden, et al., The impact of long-term organic farming on soil-derived greenhouse gas emissions, Sci. Rep., 2019, 9(1), 1702 CrossRef PubMed.
- U. Sonesson, J. Davis and F. Ziegler, Food Production and Emissions of Greenhouse Gases. An overview of the climate impact of different product groups, 2009 Search PubMed.
- A. Gilbert, One-third of our greenhouse gas emissions come from agriculture, Farmers advised to abandon vulnerable crops in face of climate change, 2012, https://www.nature.com/news/one-third-of-our-greenhouse-gas-emissions-come-from-agriculture-1.11708, Accessed 27 June 2018.
- J. Brightling, Ammonia and the Fertiliser Industry: The Development of Ammonia at Billingham, Johnson Matthey Technol. Rev., 2018, 62(1), 32–47 CrossRef CAS.
- P. Gilbert and P. Thornley, Energy and Carbon Balance of Ammonia Production from Biomass Gasification, Poster at Bio-Ten Conference, Birmingham, 2010 Search PubMed.
- G. Collodi, G. Azzaro, N. Ferrari and S. Santos, Techno-economic Evaluation of Deploying CCS in SMR Based Merchant H2 Production with NG as Feedstock and Fuel, Energy Procedia, 2017, 114, 2690–2712 CrossRef CAS.
- M. Capocelli, M. Luberti, S. Inno, F. D'Antonio, F. Di Natale and A. Lancia, Post-combustion CO2 capture by RVPSA in a large-scale steam reforming plant, J. CO2 Util., 2019, 32, 53–65 CrossRef CAS.
- W. Shi, H. Yang, Y. Shen, Q. Fu, D. Zhang and B. Fu, Two-stage PSA/VSA to produce H2 with CO2 capture via steam methane reforming (SMR), Int. J. Hydrogen Energy, 2018, 43(41), 19057–19074 CrossRef CAS.
- P. C. Psarras, S. Comello, P. Bains, P. Charoensawadpong, S. Reichelstein and J. Wilcox, Carbon Capture and Utilization in the Industrial Sector, Environ. Sci. Technol., 2017, 51, 11440–11449 CrossRef CAS PubMed.
- I. Sharma, D. Friedrich, T. Golden and S. Brandani, Exploring the opportunities for carbon capture in modular, small-scale steam methane reforming: An energetic perspective, Int. J. Hydrogen Energy, 2019, 44(29), 14732–14743 CrossRef CAS.
- M. Voldsund, K. Jordal and R. Anantharaman, Hydrogen production with CO2 capture, Int. J. Hydrogen Energy, 2016, 41(9), 4969–4992 CrossRef CAS.
- Carbon capture utilisation and storage, SETIS. https://setis.ec.europa.eu/setis-reports/setis-magazine/carbon-capture-utilisation-and-storage/co2-feedstock-polymers2016.
- R. J. Stewart and S. Haszeldine, Carbon Accounting for Carbon Dioxide Enhanced Oil Recovery, Scottish Carbon Capture & Storage, 2014 Search PubMed.
- N. Thonemann and M. Pizzol, Consequential life cycle assessment of carbon capture and utilization technologies within the chemical industry, Energy Environ. Sci., 2019, 12(7), 2253–2263 RSC.
- E. Šarauskis, L. Masilionytė, D. Juknevičius, S. Buragienė and Z. Kriaučiūnienė, Energy use efficiency, GHG emissions, and cost-effectiveness of organic and sustainable fertilisation, Energy, 2019, 172, 1151–1160 CrossRef.
- J. Squalli and G. Adamkiewicz, Organic farming and greenhouse gas emissions: A longitudinal U.S. state-level study, J. Cleaner Prod., 2018, 192, 30–42 CrossRef.
- R. Zhao, L. Liu, L. Zhao, S. Deng and H. Li, Thermodynamic analysis on carbon dioxide capture by Electric Swing Adsorption (ESA) technology, J. CO2 Util., 2018, 26, 388–396 CrossRef CAS.
- S. Yodkhum, S. H. Gheewala and S. Sampattagul, Life cycle GHG evaluation of organic rice production in northern Thailand, J. Environ. Manage., 2017, 196, 217–223 CrossRef CAS PubMed.
- C. Adewale, J. P. Reganold, S. Higgins, R. D. Evans and L. Carpenter-Boggs, Agricultural carbon footprint is farm specific: Case study of two organic farms, J. Cleaner Prod., 2019, 229, 795–805 CrossRef.
- D. Pimentel, Energy Inputs in Food Crop Production in Developing and Developed Nations, Energies, 2009, 2(1), 1–24 CrossRef.
- nations Faaootu, World fertilizer trends and outlook to 2018, 2015.
- IFA, Fertilizer Outlook 2017–2021 Production & International Trade and Agriculture Services, 2017 Search PubMed.
- M. Wang, X. Xia, Q. Zhang and J. Liu, Life cycle assessment of a rice production system in Taihu region, China, Int. J. Sustainable Dev. World Ecol., 2010, 17(2), 157–161 CrossRef.
- Nations FFaAOotU, OECD-FAO Agricultural Outlook 2011–2030, 2009 Search PubMed.
- OECD/FAO, Agricultural outlook 2016–2025, OECD Publishing, Paris, 2016 Search PubMed.
- P. Roy, D. Nei, T. Orikasa, Q. Xu, H. Okadome and N. Nakamura, et al., A review of life cycle assessment (LCA) on some food products, J. Food Eng., 2009, 90(1), 1–10 CrossRef.
- A. N. Jimmy, N. A. Khan, M. N. Hossain and M. Sujauddin, Evaluation of the environmental impacts of rice paddy production using life cycle assessment: case study in Bangladesh, Model. Earth Syst. Environ., 2017, 3(4), 1691–1705 CrossRef.
- S. Hokazono and K. Hayashi, Variability in environmental impacts during conversion from conventional to organic farming: a comparison among three rice production systems in Japan, J. Cleaner Prod., 2012, 28, 101–112 CrossRef.
- S. Yodkhum, S. H. Gheewala and S. Sate, Life cycle GHG evaluation of organic rice production in northern Thailand, J. Environ. Manage., 2017, 196, 217–223 CrossRef CAS PubMed.
- S. H. Vetter, T. B. Sapkota, J. Hillier, C. M. Stirling, J. I. Macdiarmid and L. Aleksandrowicz, et al., Greenhouse gas emissions from agricultural food production to supply Indian diets: Implications for climate change mitigation, Agric., Ecosyst. Environ., 2017, 237, 234–241 CrossRef CAS PubMed.
- E. Habibi, Y. Niknejad, H. Fallah, S. Dastan and D. B. Tari, Life cycle assessment of rice production systems in different paddy field size levels in north of Iran, Environ. Monit. Assess., 2019, 191(4), 202 CrossRef PubMed.
- M. H. Abdul Rahman, S. S. Chen, P. R. Abdul Razak, N. A. Abu Bakar, M. S. Shahrun and N. Zin Zawawi, et al., Life cycle assessment in conventional rice farming system: Estimation of greenhouse gas emissions using cradle-to-gate approach, J. Cleaner Prod., 2019, 212, 1526–1535 CrossRef CAS.
- J. Balkovič, M. van der Velde, R. Skalský, W. Xiong, C. Folberth and N. Khabarov, et al., Global wheat production potentials and management flexibility under the representative concentration pathways, Glob. Planet. Change, 2014, 122, 107–121 CrossRef.
- (AWWPC) TfAWWPC, Winter Wheat Production Manual, 2013 Search PubMed.
- FAO, Food Outlook Biannual report on global food markets, 2018 Search PubMed.
- W. K. Biswas, L. Barton and D. Carter, Global warming potential of wheat production in Western Australia: a life cycle assessment, Water Environ. J., 2008, 22(3), 206–216 CrossRef CAS.
- R. Charles, O. Jolliet, G. Gaillard and D. Pellet, Environmental analysis of intensity level in wheat crop production using life cycle assessment, Agric., Ecosyst. Environ., 2006, 113, 216–225 CrossRef.
- B. G. O'Donnell, Life Cycle Assessment of American Wheat: Analysis of Regional Variations in Production and Transportation, Master of Science in Engineering, University of Washington, 2008 Search PubMed.
- M. Taki, F. Soheili-Fard, A. Rohani, G. Chen and H. Yildizhan, Life cycle assessment to compare the environmental impacts of different wheat production systems, J. Cleaner Prod., 2018, 197, 195–207 CrossRef.
- M. Holka, J. Jankowiak, J. Bieńkowski and R. Dąbrowicz, Life cycle assessment (LCA) of winter wheat in an intensive crop production system in Wielkopolska Region (Poland), Appl. Ecol. Environ. Res., 2016, 14, 535–545 CrossRef.
- A. Makhlouf, T. Serradj and H. Cheniti, Life cycle impact assessment of ammonia production in Algeria: A comparison with previous studies, Environ. Impact Assess. Rev., 2015, 50, 35–41 CrossRef.
- S. Monika and F. Tadeusz, Life cycle assessment of fertilizers: a review, Int. Agrophys., 2014, 28(1), 101 Search PubMed.
- A. Posada and V. Manousiouthakis, Heat and Power Integration of Methane Reforming Based Hydrogen Production, Ind. Eng. Chem. Res., 2005, 44(24), 9113–9119 CrossRef CAS.
- A. Zohrabian, M. M. Majoumerd, M. Soltanieh and S. Sattari, Techno-economic evaluation of an integrated hydrogen and power co-generation system with CO2 capture, Int. J. Greenhouse Gas Control, 2016, 44, 94–103 CrossRef CAS.
- X. D. Peng, Analysis of the thermal efficiency limit of the steam methane reforming process, Ind. Eng. Chem. Res., 2012, 51(50), 16385–16392 CrossRef CAS.
- M. Voldsund, K. Jordal and R. Anantharaman, Hydrogen production with CO2 capture, Int. J. Hydrogen Energy, 2016, 41, 4969–4992 CrossRef CAS.
- R. Soltani and M. A. Rosen, I. D. Assessment of CO2 capture options from various points in steam methane reforming for hydrogen production, Int. J. Hydrogen Energy, 2014, 39(35), 20266–20275 CrossRef CAS.
- D. R. Simbeck, Hydrogen costs with CO2 capture, Greenhouse Gas control technology 72005, 2005, p. 1059 Search PubMed.
- J. R. Bartels, A feasibility study of implementing an Ammonia Economy, PhD disertation, Iowa State University, 2008 Search PubMed.
- K. T. Alkusayer, Ammonia Synthesis for Fertilizer Production, WPI, 2015 Search PubMed.
- U. Desideri and A. Paolucci, Performance Modeling of a Carbon Dioxide Removal System for Power Plants, Energy Convers. Manage., 1999, 40, 1899–1915 CrossRef CAS.
- F. Rezazadeh, W. F. Gale, K. J. Hughes and M. Pourkashanian, Performance viability of a natural gas fired combined cycle power plant integrated with post-combustion CO2 capture at part-load and temporary non-capture operations, Int. J. Greenhouse Gas Control, 2015, 39, 397–406 CrossRef CAS.
- N. A. Azzolina, J. A. Hamling, W. D. Peck, C. D. Gorecki, D. V. Nakles and L. S. Melzer, A Life Cycle Analysis of Incremental Oil Produced via CO2 EOR, Energy Procedia, 2017, 114, 6588–6596 CrossRef CAS.
- N. A. Azzolina, W. D. Peck, J. A. Hamling, C. D. Gorecki, S. C. Ayash and T. E. Doll, et al., How green is my oil? A detailed look at greenhouse gas accounting for CO2-enhanced oil recovery (CO2-EOR) sites, Int. J. Greenhouse Gas Control, 2016, 51, 369–379 CrossRef CAS.
- P. Brownsort, K. Carruthers, D. Consulting, E. Energy, R. S. Haszeldine, G. Johnson, R. V. Kapila, A. Kemp, C. Littlecott, L. Mabon, E. Mackay, R. Macrory, B. Meyvis, P. Olden, R. Paisley, J. Paterson, G. E. Pickup, K. Piessens, R. J. Stewart, J. Turk, K. Turner, K. Welkenhuysen, M. Ball, I. Mann and G. Sim, CO2 storage and Enhanced Oil Recovery in the North Sea: Securing a low-carbon future for the UK, Edinburgh, 2015, https://pureportal.strath.ac.uk/en/publications/co-storage-and-enhanced-oil-recovery-in-the-north-sea-securing-a-.
- NETL/DOE, Carbon Dioxide Enhanced Oil Recovery, Untapped domestic energy supply and long term carbon storage solution, 2010, https://www.netl.doe.gov/sites/default/files/netl-file/CO2_EOR_Primer.pdf.
- R. Lacy, C. Serralde, M. Climent and M. Vaca, Initial assessment of the potential for future CCUS with EOR projects in Mexico using CO2 captured from fossil fuel industrial plants, Int. J. Greenhouse Gas Control, 2013, 19, 212–219 CrossRef CAS.
- Ltd SAOaG, An introduction to Enhanced Oil Recovery Techniques, 2013 Search PubMed.
- N. A. Azzolina, J. A. Hamling, W. D. Peck, C. D. Gorecki, D. V. Naklesa and L. S. Melzerc, A life cycle analysis of incremental oil produced via CO2 EOR, Energy Procedia, 2017, 6588–6596 CrossRef CAS.
- DOE/NETL, Carbon Dioxide Enhanced Oil Recovery. Untapped Domestic Energy Supply and Long Term Carbon Storage Solution, 2010, p. 8 Search PubMed.
- K. Vafi and A. R. Brandt, Reproducibility of LCA Models of Crude Oil Production, Environ. Sci. Technol., 2014, 48(21), 12978–12985 CrossRef CAS PubMed.
- C. Meili, N. Jungbluth and J. Annaheim, Life cycle inventories of crude oil extraction, ESU-services Ltd Fair consuting in sustainability, 2018 Search PubMed.
- BBC News, Climate change food calculator: What's your diet's carbon footprint?, https://www.bbc.co.uk/news/science-environment-46459714.
Footnote |
| † The first two authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2020 |