A ‘Trojan horse strategy’ for the development of a renewable leather tanning agent produced via an AlCl3-catalyzed cellulose depolymerization†
Abstract
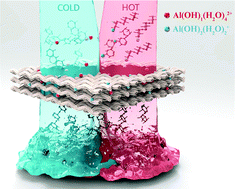
The development of renewable and non-toxic tanning agents substantially contributes toward the sustainable expansion of the leather industry. Herein, we report a green methodology to synthesize a novel tanning agent consisting of Al-oligosaccharides complexes produced via an efficient AlCl3-catalyzed cellulose depolymerization. Our experimental procedure allowed the production of a high purity tanning agent by the effective removal of the cellulose-decomposed small oxygenates and macromolecular oligosaccharides, via a liquid–liquid extraction using THF as the solvent. This also promoted the decolorization of the leather and enabled the efficient penetration of Al into the collagen matrix of skin. On this matter, experimental work combined with theoretical modeling were used to elucidate the tanning mechanism. In a first step, [Al(OH)1(H2O)4]2+ and [Al(OH)2(H2O)2]+ species weakly interacted or coordinated with the O-1 bond of the oligosaccharides produced. This interaction efficiently prevented the overload of Al species onto the leather surface, thus enhancing their penetration into leather matrix. Then, the active Al species were released from the Al-oligosaccharides complex and strongly coordinated with the –NH2 groups of the collagen fibers present in the leather, which helped to stabilize the fiber bundles, and therefore, contributed to achieve a satisfactory tanning performance.
- This article is part of the themed collection: 2019 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...