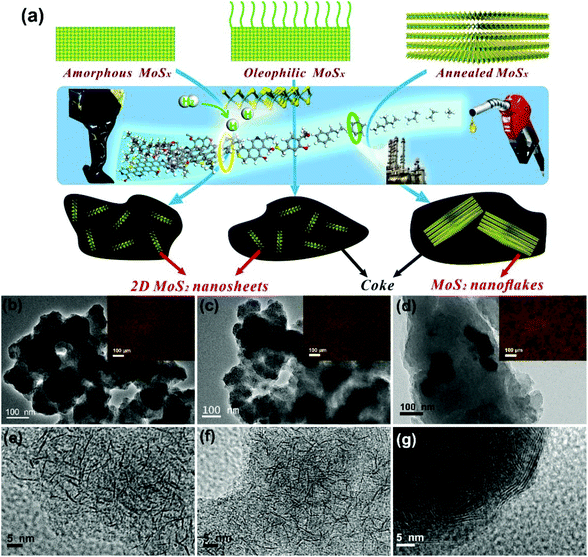
Amorphous molybdenum sulfide nanocatalysts simultaneously realizing efficient upgrading of residue and synergistic synthesis of 2D MoS2 nanosheets/carbon hierarchical structures†
Yajing
Duan
ab,
Yanglin
Liu
c,
Zhaojun
Chen
a,
Dong
Liu
 *d,
Enqiang
Yu
e,
Xiaodong
Zhang
a,
Hui
Fu
a,
Jinzhe
Fu
a,
Jiatao
Zhang
*d,
Enqiang
Yu
e,
Xiaodong
Zhang
a,
Hui
Fu
a,
Jinzhe
Fu
a,
Jiatao
Zhang
 *f and
Hui
Du
*f and
Hui
Du
 *a
*a
aCollege of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong 266071, China. E-mail: duhui@qdu.edu.cn; Tel: +86-532-85955529
bCollege of Physics, Qingdao University, Qingdao, Shandong 266071, China
cSchool of Computer Science and Technology, BIT, Beijing Institute of Technology, Beijing, 100081, China
dState Key Laboratory of Heavy Oil Processing, China University of Petroleum, Qingdao, Shandong 266580, China. E-mail: liudong@upc.edu.cn; Tel: +86-532-86980381
eChina Offshore Bitumen Co., Ltd, China National Offshore Oil Corp, Binzhou, Shandong 256600, China
fBeijing Key Laboratory of Construction-Tailorable Advanced Functional Materials and Green Applications, School of Materials Science & Engineering, Beijing Institute of Technology, Beijing 100081, China. E-mail: zhangjt@bit.edu.cn; Tel: +86-10-68918065
First published on 1st October 2019
Abstract
Slurry-phase hydrocracking employing a dispersed catalyst is an efficient technology for the upgrading of residue to clean fuels. The green synthesis of 2D nanosheet hierarchical composites when carrying out some intrinsic functionalities is interesting for both scientific and technological applications. Herein, we present our effort in simultaneously enhancing the residue upgradation and synergistic fabrication of 2D MoS2 nanosheets/carbon hierarchical structures based on amorphous molybdenum sulfide nanocatalysts. The amorphous molybdenum sulfide nanocatalysts (MoSx-AM, x ≈ 2.79) are constructed in an inverse microemulsion system and are then either modified by oleic acid to synthesize oleophilic MoSx nanocatalysts (MoSx-OL) or annealed to obtain MoS2 nanocatalysts with the typical layered structure (denoted as MoSx-AN). Benefiting from the decomposition into 2D MoS2 nanosheets with a length less than 20 nm during the residue slurry-phase hydrocracking, MoSx-AM and MoSx-OL exhibit superior hydrogenation and anti-coke activity compared with MoSx-AN. X-ray photoelectron spectroscopy and density functional theory (DFT) calculation reveal that the residue macromolecules with a polycyclic aromatic structure and heteroatoms show strong interaction with 2D MoS2 nanosheets, which disturbs the formation of a layered-structure of MoS2 and results in isolated 2D MoS2 nanosheets. The generated 2D MoS2 nanosheets with abundant active sites lead to the superior performance in residue slurry-phase hydrocracking. These results demonstrate a dispersed nanocatalyst for slurry-phase hydrocracking and also provide a perspective for the anti-coke of petroleum refining. In particular, this process also enabled the dispersed 2D MoS2 nanosheet/carbon hierarchical structure formation synergistically.
Introduction
In spite of the rapid development of renewable energy in recent years, oil energy remains the world's leading fuel, accounting for about a third of the global energy consumption.1 Whereas, with the reduction of quality crude oil reserves, low-quality petroleum resources such as vacuum residue (VR), extra-heavy oil, oil sands and natural bitumen play a momentous role in the growing supply of petroleum products.2,3 VR is the heaviest fraction of crude oil, as well as a vast and valuable feedstock for upgrading into light distillates. However, VR contains a high portion of asphaltenes, which would lead to severe precipitation and coke formation upon upgrading processes.4Slurry-phase hydrocracking, employing a dispersed catalyst which could avoid the deactivation by pore plugging suffered by the supported catalyst, is an efficient technology for the upgrading of VR.3,5 Since the dispersed catalyst contains no support, the free-radical mechanism has been proved to be the cracking mechanism of the slurry-phase hydrocracking process.6 During the slurry-phase hydrocracking of residue, hydrocarbon molecules are cracked into hydrocarbon free radicals at high temperature, and then the generated hydrocarbon free radicals would either crack continuously to obtain lighter products or condense to form coke. Meanwhile, active hydrogen formed on the dispersed catalyst could quench the hydrocarbon free radicals and then inhibit the condensing pathway.7,8 Therefore, for a residue slurry-phase hydrocracking process, it is important to employ a dispersed catalyst which is capable of converting the heavier oil fractions into light products, as well as inhibiting coke formation to ensure stable operation of the system. Traditionally, dispersed catalysts consist of the two catalyst types: water-soluble dispersed catalysts and oil-soluble dispersed catalysts, which could be transformed into common submicrometric particles of transition metal sulfides with catalytic activity by sulfurization.9–11 In recent years, interest in nanometer catalysts of sulfided transition metals (Mo, Ni, W, etc.) for residue slurry-phase hydrocracking has been constantly increasing.12–15
Mo-Based dispersed catalysts, including water-soluble Mo,16–18 oil-soluble Mo,4,19–24 and molybdenum disulfide (MoS2) nanocatalysts with different morphologies,12,25 have attracted more interest due to the high catalytic activity compared with the other transition metal-based dispersed catalysts. During the slurry-phase hydrocracking process, the water-soluble and oil-soluble Mo dispersed catalysts are converted to MoS2 through presulfurization or the in situ sulfurization reaction. According to the rim-edge theory, the catalytic activity of the MoS2 dispersed catalyst mainly arises from its disordered edge planes, while the basal planes are inert.26 Although the MoS2 catalysts with different nanostructures (nanoparticles, nanorods, nanoplatelets, nanoflowers and porous) have been used in residue slurry-phase hydrocracking, the hydrogenation and anti-coke activity are still unsatisfying because their dispersion was suppressed by the interconnected nanostructures.25 Therefore, it would be beneficial to the catalytic performance of MoS2 nanocatalysts if their suppressed agglomeration and stable dispersion were realized under high reaction temperature and pressure.
MoS2, with a graphene-like two-dimensional (2D) structure, exhibited not only good chemical stability but also excellent electrical properties.27 Moreover, carbon materials have attracted wide attention due to their stable physical, chemical and mechanical properties, and are also regarded as promising matrixes for composites.28–30 Recently, MoS2/carbon hierarchical hybrids with improved properties resulting from the synergistic interactions, and their possible applications in catalysis, energy conversion and storage have attracted considerable attention.31–34 Despite many synthesis routes for MoS2/carbon hybrids, such as solvothermal, electrospinning, vapour deposition technique, etc., it is interesting to fabricate abundant 2D MoS2 nanosheets/carbon hierarchical structures when carrying out some intrinsic functionalities.
Herein, an amorphous molybdenum sulfide (MoSx-AM) nanocatalyst was synthesized in an inverse microemulsion, which was then either modified by oleic acid to synthesize oleophilic MoSx nanocatalysts (MoSx-OL) or annealed at 700 °C to obtain MoS2 nanocatalysts with the typical layered structure (denoted as MoSx-AN). The effects of surface properties and crystal structure on the catalytic activity of MoSx nanocatalysts were investigated in detail. The experimental results have shown that the MoSx-AM and MoSx-OL nanocatalysts exhibit superior hydrogenation and anti-coke activity compared with MoSx-AN due to the secondary decomposition characteristic. In particular, this process also enabled dispersed 2D MoS2 nanosheet/carbon hierarchical structure formation synergistically, which implied the full conversion of the residue to clean fuels and valuable composites without any waste. Combined with density functional theory (DFT) calculations, our results suggested that the strong interaction between MoS2 nanosheets and residue molecules disturbs the formation of the layered-structure of MoS2 and results in isolated 2D MoS2 nanosheets which contributed to the superior catalytic activity and the fabrication of 2D MoS2 nanosheets/carbon hierarchical structures.
Experimental section
Synthesis of catalysts
All the reagents were purchased from Aladdin Industrial Inc. and were of analytical reagent grade and used without further purification. MoSx-AM nanocatalysts were synthesized using ammonium tetrathiomolybdate (ATTM) and hydroxylamine hydrochloride in a Triton X-100/octanol/cyclohexane/water inverse microemulsion system.35 In a typical procedure, 6.0 g of ATTM solution (0.1 M) was mixed with 7.2 g of Triton X-100, 4.8 g of octanol and 8.0 g of cyclohexane with vigorous stirring at 40 °C until a transparent inverse microemulsion of ATTM obtained, while 6.0 g of reducing agent solution containing hydroxylamine hydrochloride (0.3 M) and hydrochloric acid (0.8 M) was mixed with them with vigorous stirring at 40 °C to form a transparent inverse microemulsion of the reducing agent. After that, the inverse microemulsion of the reducing agent was added dropwise into the inverse microemulsion of ATTM with continuous stirring at 40 °C and stirred for an additional 2 h. Then the mixture was placed and aged for 48 h at 40 °C. The black precipitates were collected by filtration, washed with distilled water and ethanol several times, dried at 50 °C in a vacuum oven, and MoSx-AM were finally obtained.For comparison, MoSx-OL were synthesized by surface modification of MoSx-AM with oleic acid based on our previous study,36 and MoSx-AN were prepared by annealing MoSx-AM in a tube furnace in a mixed stream of H2/N2 (1/9, v/v) at 700 °C for 2 h.
Characterization of catalysts
XRD analysis was performed on a PANalyitcal X Pert PRO MPD X-ray diffractometer using Cu Kα radiation (λ = 1.5418 Å) with 2θ = 5–70°. The morphology, size and elemental mapping of the nanocatalysts were observed by high-resolution transmission electron microscopy (HRTEM, JEOL JEM-2100F). The samples were dispersed in hexane by ultrasound, dropped on a copper grid and observed at 200 kV. Fourier transform infrared spectroscopy (FTIR) spectra were recorded by using a Nicolet Magna-750 FTIR spectrometer with a resolution of 2 cm−1 in the range of 4000–400 cm−1. X-ray photoelectron spectroscopy (XPS) measurements of the catalysts were performed on a Physical Electronics 5600ci XPS system with an Al Kα radiation source.Test of catalytic activity
An ultra-heavy oil (SZVR) purchased from the China National Offshore Oil Corporation was used as the feedstock oil, the composition and characteristics of which are displayed in Table S1.† Slurry-phase hydrocracking of SZVR over the different MoSx nanocatalysts was carried out using a 300 mL batch-type autoclave fitted with a stirrer. In each experiment, 120 g of SZVR and MoSx nanocatalysts (100 ppm on the basis of Mo weight) were charged into the autoclave. The reactor was pressurized to 80 bar with hydrogen after purging with hydrogen three times, heated to 430 °C under vigorous stirring and its internal pressure increased as high as around 176 bar. After reaction for 1 h, the reactor was rapidly quenched and vented. The gas, liquid (naphtha, diesel, vacuum gas oil (VGO), VR) and solid products were separated and their yields were calculated according to previous studies.6,37 For the VR product, the saturate, aromatic, resin, and asphaltene (SARA) composition and Conradson carbon residue were analyzed.38 In a similar manner, the hydrocracking tests of SZVR with bulk MoS2, water-soluble Mo catalyst (ammonium molybdates)39 and oil-soluble Mo catalyst (molybdenum naphthenate)40 were carried out.The morphology and size of solid products were observed by using a VIHENT SZX10 optical microscope, while the size distribution of solid products was determined by using a MALVERN 3000E Mastersizer. Additionally, the solid products were further analysed by XRD, XPS and HRTEM with elemental mapping.
Computational details
Density functional theory (DFT) calculations were performed to study the interaction between MoS2 nanosheets and MoS2 nanosheets, graphene layer (G), N doped graphene (N-G), and S–N doped graphene (SN-G), denoted as MoS2/MoS2, MoS2/G, MoS2/N-G and MoS2/SN-G, respectively. MoS2 not only shows hydrocracking catalytic activity but also shows hydrodeoxygenation catalytic activity and has been used as a catalyst for the hydrodeoxygenation of oleic acid.41 Therefore, DFT calculations were not carried out between oleophilic MoS2 nanosheets and graphene structures in this work.A 4 × 4 unit cell of graphene layer was used as a matrix to build the N doped graphene layer and S–N doped graphene layer. The different graphene layers were matched to a 3 × 3 unit cell of the MoS2 nanosheet with a lattice mismatch of about 2.8%. In all cases, a vacuum layer of 15 Å was set in the perpendicular direction to avoid the spurious interaction between the periodic structures for all calculations. The generalized gradient approximation parameterized by Perdew–Burke–Ernzerhof (GGA-PBE) exchange–correlation functional42 and double-numeric quality basis set with the polarization functions (DNP) were employed, as well as the empirical dispersion-corrected density functional theory (DFT-D) approach proposed by Grimme.43,44 In order to simulate the conditions of slurry-phase hydrocracking, the calculations were performed in an NVT ensemble at 700 K.
The binding energy calculation of the composite slab was illustrated by the example of the MoS2/G composite as follows:
| Ebind = Eslab − EMoS2 − EG |
Results and discussion
Characterization of catalysts
Fig. 1a presents a schematic representation of the simple preparation and treatment of the three MoSx nanocatalysts; the synthesized MoSx-AM were surface modified with oleic acid to enhance the dispersion stability of MoSx nanocatalysts in toluene (Fig. 2a), and also were annealed at 700 °C to generate the 2D layered structure of MoS2. Fig. 1b and e exhibit the feature of MoSx-AM prepared by the inverse microemulsion method, and the products are irregular shaped nanoparticles without the typical layered structure of MoS2. After the surface modification with oleic acid, the size, shape and crystal structure of MoSx nanocatalysts barely changed (Fig. 1c and f). As shown in Fig. S1,† the elemental mapping results suggest the well-defined spatial distribution of Mo and S in MoSx-AM and MoSx-OL. Conversely, significant agglomeration occurs during the annealing of MoSx-AM. From the images of MoSx-AN (Fig. 1d and g), it is clearly stated that the size of annealed products increases to hundreds of nanometers and the well-stacked structure has an interlayer distance between MoS2 nanosheets of 0.63 nm.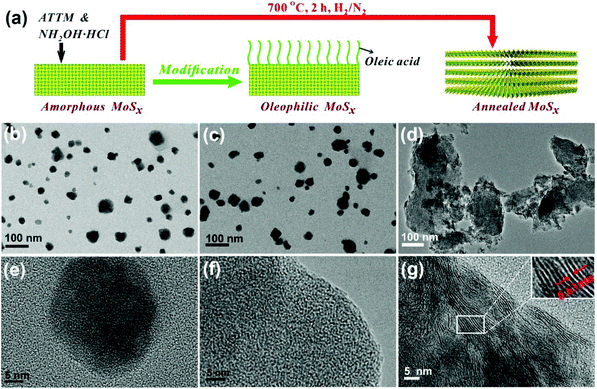 | ||
| Fig. 1 (a) Schematic illustration of the preparation of different MoSx nanocatalysts; TEM and HRTEM images of (b and e) MoSx-AM, (c and f) MoSx-OL and (d and g) MoSx-AN. | ||
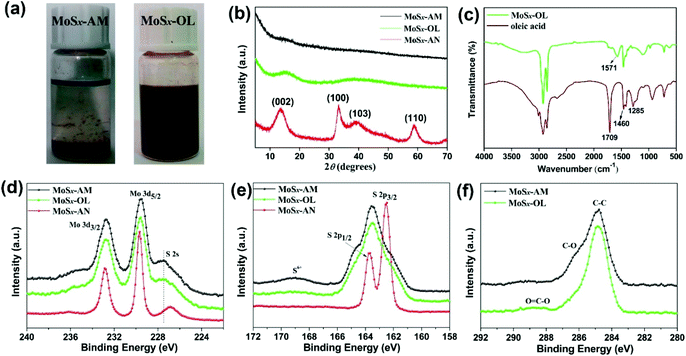 | ||
| Fig. 2 (a) Dispersion stability of MoSx-AM and MoSx-OL in toluene after 4 h; (b) XRD patterns and (d–f) XPS spectra of the MoSx nanocatalyst samples; (c) FTIR spectra of MoSx-OL and oleic acid. | ||
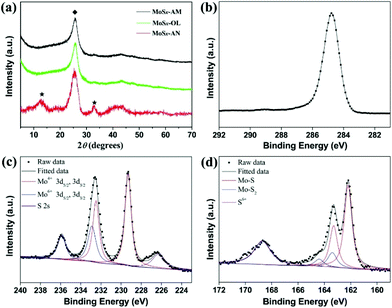
The three MoSx nanocatalysts were characterized by XRD as shown in Fig. 2b. XRD patterns clearly confirm no crystalline phase or agglomeration in MoSx-AM and MoSx-OL, also indicating that no agglomeration occurs during the surface modification process. In contrast, the diffraction peaks of MoSx-AN can be readily indexed to the hexagonal 2H-MoS2 (JCPDS 37-1492), in which the strong peaks at 14°, 33°, 39° and 59° can be assigned to the (002), (100), (103) and (110) planes. The distinct peak at 14° suggests the well stacked layered structure of annealed MoSx. The results are good in agreement with the results found in HRTEM analysis.
The incorporation of Triton X-100 or oleic acid into the samples has been confirmed by FTIR analysis as shown in Fig. S2† and Fig. 2c. Prior to surface modification, MoSx-AM basically display similar absorption bands with Triton X-100 (Fig. S2†), which indicates Triton X-100 adsorbing at the surface of MoSx-AM without a chemisorption pathway. Moreover, the characteristic absorption bands attributed to Triton X-100 disappear completely after the surface modification because of the replacement of Triton X-100 by oleic acid. As shown in Fig. 2c, different from the FTIR spectrum of oleic acid, the distinct bands of the carbonyl group at 1709 cm−1 and 1285 cm−1 disappear in the FTIR spectrum of MoSx-OL. Meanwhile, a new absorption band at 1571 cm−1 appears, which can be identified as the asymmetric stretching vibration of the COO− group.36 The absorption band of the COO− group shifted to a higher wavenumber of 1571 cm−1 compared to the isolated COO− group of 1550 cm−1, indicating the symmetrical coordination of the carboxylate group to the Mo atom.
The MoSx nanocatalyst samples were further characterized by XPS as shown in Fig. S3† and Fig. 2d–f. The XPS survey spectra confirm that the MoSx nanocatalyst samples contain Mo, S, C and O, meanwhile the peaks of C and O slash dramatically in the XPS spectrum of MoSx-AN. The binding energies of Mo 3d peaks are located at 229.6 eV and 232.8 eV as shown in the high-resolution XPS spectra of Mo 3d (Fig. 2d), which confirms the Mo4+ state of Mo in the MoSx nanocatalyst samples. Moreover, one more set of doublet peaks attributed to the 3d5/2 and 3d3/2 orbitals of Mo6+ are shown in the Mo 3d region of MoSx-AM and MoSx-AN (Fig. S4†). In the S 2p region of MoSx-AN, distinct doublet peaks at 162.5 eV and 163.7 eV are observed, which are attributed to S 2p3/2 and S 2p1/2 orbitals respectively (Fig. 2e). However, for MoSx-AM and MoSx-OL, two sets of doublet peaks at 162.3/163.5 eV and 163.6/164.8 eV are observed (Fig. S5†). The doublet peaks located at lower binding energies are assigned to the S 2p3/2 and S 2p1/2 orbitals of unsaturated S2− and terminal S22−, while the doublet peaks located at higher binding energies are assigned to apical S2− and bridging S22−.45,46 Additionally, the S6+ state of S is observed as the curve bread peak 2p spectra clearly indicate the amorphous morphology of MoSx-AM and MoSx-OL, which is consistent with the XRD results.
From the XPS results, the S/Mo atom ratios of MoSx-AM, MoSx-OL and MoSx-AN are 2.79, 2.75 and 2.01 respectively. Moreover, the incorporation of Triton X-100 or oleic acid into the samples is also confirmed by the C 1s region of the XPS spectra (Fig. 2f), and the results match those from the FTIR analysis.
Catalytic performance
We then investigated their residue slurry-phase hydrocracking performance to explore the contribution from the crystal structure and surface characteristics of MoSx nanocatalysts. It is well-known that hydrogen can be easily activated on the coordinatively unsaturated sites and sulfur ion vacancies of the molybdenum disulfide catalyst under the slurry-phase hydrocracking conditions, and then the activated hydrogen is subsequently transferred and quenched with nearby hydrocarbon free radicals, which ensure the coke precursor converting to liquid products rather than to coke.47–49 An ideal dispersed catalyst for slurry-phase hydrocracking should realize the effective and durable conversion of feedstock to light products. The MoSx nanocatalysts synthesized in this work display enhanced catalytic activities in residue slurry-phase hydrocracking than bulk MoS2, water-soluble Mo catalyst and oil-soluble Mo catalyst (Fig. 3 and Fig. S6†). The product distribution and conversion of SZVR slurry-phase hydrocracking with the three MoSx nanocatalysts are compared in Fig. 3a. Obviously, MoSx-AM and MoSx-OL exhibit a high feedstock oil conversion and a low coke yield compared to MoSx-AN, and low yields of light fractions (gas, naphtha) and heavy fractions (VR) are obtained with the application of MoSx-AM and MoSx-OL. Moreover, MoSx-AM and MoSx-OL display roughly similar catalytic activities for residue slurry-phase hydrocracking. In their case, the residue conversion increases from 73.1% obtained with MoSx-AN to higher than 76%, since the coke yield decreases from 1.53 wt% to lower than 0.4 wt%. We also found the same tendency in the SARA composition and Conradson carbon residue of VR products with different MoSx nanocatalysts (Fig. 3b). For MoSx-AM and MoSx-OL, they gain the similar low contents of heavy SARA fractions (resin and C7 asphaltene) and Conradson carbon residue of VR products compared to MoSx-AN. Given the above, MoSx-AM and MoSx-OL show a similar better catalytic activity in slurry-phase hydrocracking than MoSx-AN. This conclusion contradicts the common belief that crystallized MoS2 shows better catalytic activity for hydrogenation than amorphous molybdenum sulfide, and also contradicts our previous study that the catalytic activity of dispersed catalysts for slurry-phase hydrocracking is enhanced by oleophilic surface modification.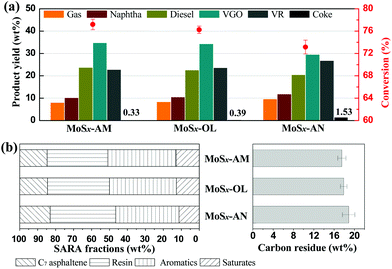 | ||
| Fig. 3 (a) Product distribution and conversion, and (b) SARA composition and Conradson carbon residue of VR products from SZVR slurry-phase hydrocracking with different MoSx nanocatalysts. | ||
Extraordinary conclusions require extraordinary evidence and since most of the added dispersed catalysts were captured by the solid products,20 extensive analysis of the solid products from SZVR slurry-phase hydrocracking with different MoSx nanocatalysts was performed. Analysis results of the solid products by microscope and TEM are shown in Fig. 4. As expected, the microscope images (insets in Fig. 4b–d) and size distribution (Fig. S7†) of solid products indicate that MoSx-AM and MoSx-OL outperformed MoSx-AN in terms of coke suppression in residue slurry-phase hydrocracking. TEM and HRTEM analyses of the solid products were carried out to explore the reason for catalytic activity differences of the MoSx nanocatalysts. As demonstrated in Fig. 4, MoSx-AM and MoSx-OL split into isolated 2D MoS2 nanosheets with a length less than 20 nm during the residue slurry-phase hydrocracking, which disperse uniformly in the solid products. The elemental mapping analysis of solid products also demonstrates the high dispersion of MoS2 nanosheets in coke (Fig. S8 and S9†). In contrast, little has changed for MoSx-AN, which disperse in the solid products remaining in its original structure and size (Fig. 4d, g and Fig. S10†). The hypothesis that explains the observed behavioral discrepancy is displayed in Fig. 4a. During the residue slurry-phase hydrocracking, the fission of MoSx nanocatalysts with the amorphous structure releases tremendous amounts of 2D MoS2 nanosheets into the feedstock. Due to the affinity of MoS2 for aromatic carbon that has been reported, the generated 2D MoS2 nanosheets can be well dispersed in the feedstock rather than stack into multi-layer MoS2 flakes. According to the “Rim-Edge” model,26 the ultra-dispersed 2D MoS2 nanosheets with abundant activity sites can facilitate the production of active hydrogen, which shows excellent catalytic activity for residue slurry-phase hydrocracking. Furthermore, the ultra-dispersed 2D MoS2 nanosheets also act as the coke capture agent. With the cracking of residue molecules, their condensation occurs as well. The generated coke deposited on 2D MoS2 nanosheets, meanwhile the 2D MoS2 nanosheets in feedstock adsorbed onto coke because of the affinity between them. Eventually, the solid products in the form of 2D MoS2 nanosheets/carbon hierarchical structures were synthesized synergistically.
Further evidence for the affinity of ultra-dispersed 2D MoS2 nanosheets for aromatic carbon was obtained from the XRD and XPS spectra. Fig. 5a demonstrates the XRD patterns of solid products from SZVR slurry-phase hydrocracking with different MoSx nanocatalysts. As expected, the diffraction peaks indexed to MoS2 are missing in the XRD patterns of solid products from SZVR slurry-phase hydrocracking with MoSx-AM and MoSx-OL. This indicates the ultra-dispersion of MoS2 in solid products, which is consistent with the HRTEM results. XPS results reveal that the solid products from SZVR slurry-phase hydrocracking with MoSx-AM mainly contain C, as well as Mo and S (Fig. S11†), in which the Triton X-100 dissociates from the catalyst surface after slurry-phase hydrocracking (Fig. 5b). Compared with MoSx-AM, the used catalyst in solid products shows a higher percentage of Mo6+ atoms (Fig. S4a and Fig. 5c), which could occur because of the bonding effect between Mo and heteroatoms (S, N, O) in the feedstock. Meanwhile, different from the sulphur-rich environment in MoSx-AM, the used catalyst in solid products has a higher percentage of S atoms with binding energy signals at 162.1/163.4 eV, which suggests the main chemical environment of the S atoms in the used catalyst is Mo–S. Elemental analysis by XPS reveals that the average S/Mo atom ratio of the solid products is 2.16. The stoichiometry result agrees with the TEM and XPS studies of the solid products.
 | ||
| Fig. 5 (a) XRD patterns of the solid products, (b–d) XPS spectra of the solid products from SZVR slurry-phase hydrocracking with MoSx-AM. | ||
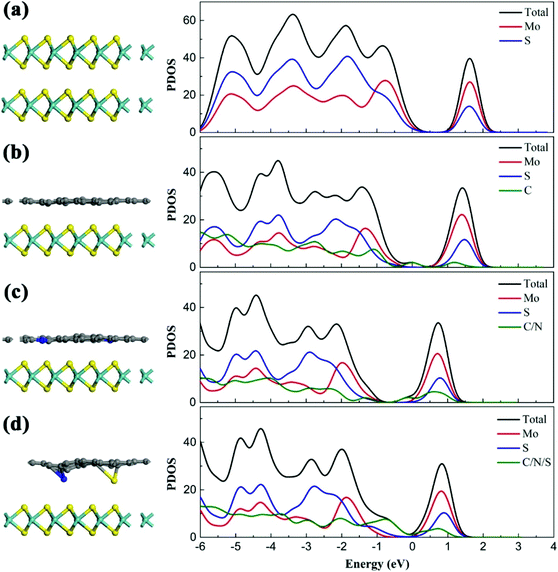
DFT calculation
In order to further investigate the interaction between the polycyclic aromatic structure of residue macromolecules and 2D MoS2 nanosheets, DFT studies of different composites (MoS2/MoS2, MoS2/G, MoS2/N-G and MoS2/SN-G) were performed in an NVT ensemble at 700 K (Fig. S12†). The most stable configurations and corresponding projected density of states (PDOS) of these composites are displayed in Fig. 6. The interlayer spacing of two MoS2 nanosheets is 6.43 Å after full relaxation (Fig. 6a), which is consistent with the d spacing of (002) planes of hexagonal MoS2.50 For MoS2/G, MoS2/N-G and MoS2/SN-G, the interlayer spacing is 3.36 Å, 3.33 Å and 3.34 Å, respectively. Normally, similar to hexagonal MoS2, the interaction between MoS2 and graphene is a relatively weak van der Waals interaction.51 However, a slight structural distortion is observed in MoS2/G at 700 K (Fig. 6b). For MoS2/N-G and MoS2/SN-G, after full relaxation, obvious structural distortions are observed in the doped graphene layers, especially around the doped nitrogen atoms. The calculated binding energies of these composites are MoS2/MoS2 (−0.65 eV), MoS2/G (−1.76 eV), MoS2/N-G (−2.09 eV) and MoS2/SN-G (−2.01 eV), respectively, which show an opposite tendency for their interlayer distances. We then calculated the projected density of states (PDOS) for the composites to understand electronic coupling, which may reveal the interaction mechanism between the polycyclic aromatic structure of residue macromolecules and the MoS2 nanosheet. As shown in Fig. 6 and Fig. S13,† the projected density of states (PDOS) of composites of the MoS2 nanosheet and graphene-based layers is shifted toward the lower energy state relative to MoS2/MoS2, which means more stable orbitals of MoS2 and graphene-based layers, and further proves the stronger interactions between them. Additionally, the adsorption of graphene-based layers results in a splitting of the S 2p orbitals along with a change in shape (Fig. S9†), suggesting that a more intense electronic activity occurs between graphene and the top layer of sulfur atoms in the MoS2 nanosheet.52,53 The DFT calculation results demonstrate the affinity of the MoS2 nanosheet for polycyclic aromatic carbon at high temperature and high pressure, which is consistent with the natural phenomenon of the C-MoS2 mixed-layer phase in molybdenite from Southern China.54 Thus, with the structural change from amorphous MoSx to MoS2 during the residue slurry-phase hydrocracking, the residue macromolecules with a polycyclic aromatic structure (especially, the condensed coke and semi-coke) show stronger affinity with MoS2 nanosheets, which intercalate into MoS2 nanosheets and disturb the formation of the layered-structure of MoS2. In that case, the secondary generated 2D MoS2 nanosheets with abundant active sites adsorb the coking precursors with a polycyclic aromatic structure, facilitate the hydrocracking, inhibit the condensation, and finally exhibit outstanding catalytic activity for residue slurry-phase hydrocracking.Conclusions
In summary, to study the effect of crystal and surface structure of molybdenum sulfide nanocatalysts on their performance in residue slurry-phase hydrocracking, we successfully constructed three molybdenum sulfide nanocatalysts. Benefiting from the decomposition into isolated 2D MoS2 nanosheets with a length less than 20 nm during the residue slurry-phase hydrocracking, MoSx-AM and MoSx-OL exhibit superior hydrogenation and anti-coke activity compared with MoSx-AN. During the slurry-phase hydrocracking, the residue macromolecules with a polycyclic aromatic structure and heteroatoms show strong interaction with MoS2 nanosheets, which disturbs the formation of the layered-structure of MoS2 and results in isolated 2D MoS2 nanosheets. Therefore, the generated 2D MoS2 nanosheets with abundant active sites lead to the superior performance in residue slurry-phase hydrocracking. In particular, the dispersed 2D MoS2 nanosheets/carbon hierarchical structures were synthesized synergistically, which have potential application as precursors of carbon composite for energy conversion and storage. These results not only demonstrate a dispersed nanocatalyst for slurry-phase hydrocracking to realize the full conversion of residue to clean fuels and valuable composites without any waste, but also provide a perspective for the fabrication of 2D MoS2 nanosheets/carbon hierarchical structures when carrying out some intrinsic functionalities.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (21808115, 21706140), China Postdoctoral Science Foundation (2019T120571, 2018M632623), as well as the Qingdao Municipal Science and Technology Bureau (No. 17-1-1-86-jch).References
- BP, BP Statistical Review of World Energy, 2017 Search PubMed.
- R. Sahu, B. J. Song, S. I. Ji, Y.-P. Jeon and C. W. Lee, A review of recent advances in catalytic hydrocracking of heavy residues, J. Ind. Eng. Chem., 2015, 27, 12–24 CrossRef CAS.
- L. C. Castañeda, J. A. D. Muñoz and J. Ancheyta, Current situation of emerging technologies for upgrading of heavy oils, Catal. Today, 2014, 220–222, 248–273 CrossRef.
- S. H. Kim, K. D. Kim and Y. K. Lee, Effects of dispersed MoS2 catalysts and reaction conditions on slurry phase hydrocracking of vacuum residue, J. Catal., 2017, 347, 127–137 CrossRef CAS.
- A. Hart, M. Greaves and J. Wood, A comparative study of fixed-bed and dispersed catalytic upgrading of heavy crude oil using-CAPRI, Chem. Eng. J., 2015, 282, 213–223 CrossRef CAS.
- H. Du, D. Liu, M. Li, P. Wu and Y. Yang, Effects of Temperature and Initial Hydrogen Pressure on the Isomerization Reaction in Heavy Oil Slurry-Phase Hydrocracking, Energy Fuels, 2015, 29, 626–633 CrossRef CAS.
- B. Browning, I. Pitault, F. Couenne, T. Jansen, M. Lacroix, P. Alvarez and M. Tayakout-Fayolle, Distributed lump kinetic modeling for slurry phase vacuum residue hydroconversion, Chem. Eng. J., 2019, 377, 119811 CrossRef.
- H. Du, D. Liu, H. Liu, P. Gao, R. Lv, M. Li, B. Lou and Y. Yang, Role of Hydrogen Pressure in Slurry-Phase Hydrocracking of Venezuela Heavy Oil, Energy Fuels, 2015, 29, 2104–2110 CrossRef CAS.
- M. J. Angeles, C. Leyva, J. Ancheyta and S. Ramírez, A review of experimental procedures for heavy oil hydrocracking with dispersed catalyst, Catal. Today, 2014, 220–222, 274–294 CrossRef CAS.
- M. T. Nguyen, N. T. Nguyen, J. Cho, C. Park, S. Park, J. Jung and C. W. Lee, A review on the oil-soluble dispersed catalyst for slurry-phase hydrocracking of heavy oil, J. Ind. Eng. Chem., 2016, 43, 1–12 CrossRef CAS.
- S. H. Kim, K. D. Kim, D. Lee and Y.-K. Lee, Structure and activity of dispersed Co, Ni, or Mo sulfides for slurry phase hydrocracking of vacuum residue, J. Catal., 2018, 364, 131–140 CrossRef CAS.
- R. Prajapati, K. Kohli and S. K. Maity, Slurry-Phase Hydrocracking of Residue with Ultradispersed MoS2 Catalysts Prepared by Microemulsion Methods, Energy Fuels, 2017, 31, 3905–3912 CrossRef CAS.
- D. Liu, H. Du, J. Zhang and G. Que, Reverse Microemulsion Synthesis and Characterization of Nano Nickel Sulfide Catalyst for Residue Slurry-Phase Hydrocracking, Energy Fuels, 2015, 29, 3353–3358 CrossRef CAS.
- Y. G. Hur, D. W. Lee and K. Y. Lee, Hydrocracking of vacuum residue using NiWS(x) dispersed catalysts, Fuel, 2016, 185, 794–803 CrossRef CAS.
- Y. G. Hur, M. S. Kim, D. W. Lee, S. Kim, H.-J. Eom, G. Jeong, M.-H. No, N. S. Nho and K.-Y. Lee, Hydrocracking of vacuum residue into lighter fuel oils using nanosheet-structured WS2 catalyst, Fuel, 2014, 137, 237–244 CrossRef CAS.
- K. P. Tian, A. R. Mohamed and S. Bhatia, Catalytic upgrading of petroleum residual oil by hydrotreating catalysts: a comparison between dispersed and supported catalysts, Fuel, 1998, 77, 1221–1227 CrossRef CAS.
- B. Fixari, S. Peureux, J. Elmouchnino, P. L. Perchec, M. Vrinat and F. Morel, New Developments in Deep Hydroconversion of Heavy Oil Residues with Dispersed Catalysts. 1. Effect of Metals and Experimental Conditions, Energy Fuels, 1994, 8, 588–592 CrossRef CAS.
- H. Ortiz-Moreno, J. Ramírez, R. Cuevas, G. Marroquín and J. Ancheyta, Heavy oil upgrading at moderate pressure using dispersed catalysts: Effects of temperature, pressure and catalytic precursor, Fuel, 2012, 100, 186–192 CrossRef CAS.
- S.-H. Kim, K.-D. Kim, H. Lee and Y.-K. Lee, Beneficial roles of H-donors as diluent and H-shuttle for asphaltenes in catalytic upgrading of vacuum residue, Chem. Eng. J., 2017, 314, 1–10 CrossRef CAS.
- H. Rezaei, X. Liu, S. J. Ardakani, K. J. Smith and M. Bricker, A study of Cold Lake Vacuum Residue hydroconversion in batch and semi-batch reactors using unsupported MoS2 catalysts, Catal. Today, 2010, 150, 244–254 CrossRef CAS.
- H. Rezaei, S. J. Ardakani and K. J. Smith, Comparison of MoS2 Catalysts Prepared from Mo-Micelle and Mo-Octoate Precursors for Hydroconversion of Cold Lake Vacuum Residue: Catalyst Activity, Coke Properties and Catalyst Recycle, Energy Fuels, 2012, 26, 2768–2778 CrossRef CAS.
- T. Jansen, D. Guerry, D. Gotteland, R. Bacaud, M. Lacroix, M. Ropars, C. Lorentz, C. Geantet and M. Tayakout-Fayolle, Characterization of a continuous micro-scale pilot unit for petroleum residue hydroconversion with dispersed catalysts: Hydrodynamics and performances in once-through and recycling mode, Chem. Eng. J., 2014, 253, 493–501 CrossRef CAS.
- R. Shen, C. Liu and G. Que, Hydrocracking of Liaohe vacuum residue on bimetallic oil soluble catalysts, Pet. Process. Petrochem., 1998, 29, 10–12 Search PubMed.
- B. Liu, K. D. Zhao, Y. M. Chai, Y. P. Li, D. Liu, Y. Q. Liu and C. G. Liu, Slurry phase hydrocracking of vacuum residue in the presence of presulfided oil-soluble MoS2 catalyst, Fuel, 2019, 246, 133–140 CrossRef CAS.
- S. Bano, S. W. Ahmad, S. I. Woo and F. Saleem, Heavy oil hydroprocessing: effect of nanostructured morphologies of MoS2 as catalyst, React. Kinet., Mech. Catal., 2015, 114, 473–487 CrossRef CAS.
- M. Daage and R. R. Chianelli, Structure-Function Relations in Molybdenum Sulfide Catalysts: The “Rim-Edge” Model, J. Catal., 1994, 149, 414–427 CrossRef CAS.
- W. Ye, M. Arif, X. Fang, M. A. Mushtaq, X. Chen and D. Yan, Efficient Photoelectrochemical Route for the Ambient Reduction of N2 to NH3 Based on Nanojunctions Assembled from MoS2 Nanosheets and TiO2, ACS Appl. Mater. Interfaces, 2019, 11, 28809–28817 CrossRef CAS PubMed.
- M. Arif, G. Yasin, M. Shakeel, M. A. Mushtaq, W. Ye, X. Fang, S. Ji and D. Yan, Hierarchical CoFe-layered double hydroxide and g-C3N4 heterostructures with enhanced bifunctional photo/electrocatalytic activity towards overall water splitting, Mater. Chem. Front., 2019, 3, 520–531 RSC.
- M. Arif, G. Yasin, M. Shakeel, X. Fang, R. Gao, S. Ji and D. Yan, Coupling of Bifunctional CoMn-Layered Double Hydroxide@Graphitic C3N4 Nanohybrids towards Efficient Photoelectrochemical Overall Water Splitting, Chem. – Asian J., 2018, 13, 1045–1052 CrossRef CAS PubMed.
- X. Fang, R. Gao, Y. Yang and D. Yan, A Cocrystal Precursor Strategy for Carbon-Rich Graphitic Carbon Nitride toward High-Efficiency Photocatalytic Overall Water Splitting, iScience, 2019, 16, 22–30 CrossRef CAS PubMed.
- P. Trogadas, V. Ramani, P. Strasser, T. F. Fuller and M.-O. Coppens, Hierarchically Structured Nanomaterials for Electrochemical Energy Conversion, Angew. Chem., Int. Ed., 2016, 55, 122–148 CrossRef CAS.
- D. Deng, K. S. Novoselov, Q. Fu, N. Zheng, Z. Tian and X. Bao, Catalysis with two-dimensional materials and their heterostructures, Nat. Nanotechnol., 2016, 11, 218–230 CrossRef CAS PubMed.
- X. Zhang, Z. Lai, C. Tan and H. Zhang, Solution-Processed Two-Dimensional MoS2 Nanosheets: Preparation, Hybridization, and Applications, Angew. Chem., Int. Ed., 2016, 55, 2–25 CrossRef.
- Y. Wang, R. Wei, B. Zhang, H. Lv, D. Xu, Q. Hao and B. Liu, Template-Assisted Self-Sulfuration Formation of MoS2 Nanosheets Embedded in Ordered Mesoporous Carbon for Lithium Storage, ACS Appl. Energy Mater., 2019, 2, 6158–6162 CrossRef CAS.
- M. Liu, X. Li, Z. Xu, B. Li, L. Chen and N. Shan, Synthesis of chain-like MoS2 nanoparticles in W/O reverse microemulsion and application in photocatalysis, Chin. Sci. Bull., 2012, 57, 3862–3866 CrossRef CAS.
- H. Du, D. Liu, H. Wu, W. Xia, X. Zhang, Z. Chen, Y. Liu and H. Liu, Surface Modification of Nickel Sulfide Nanoparticles: Towards Stable Ultra-Dispersed Nanocatalysts for Residue Hydrocracking, ChemCatChem, 2016, 8, 1543–1550 CrossRef CAS.
- H. Du, D. Liu, X. Zhang, Z. Chen, H. Xia, F. Lu, Y. Zhang, K. Xia and R. Jia, Effects of Surface Modification on the Catalytic Performances of Nickel Sulfide Nanocatalysts for Residue Hydrocracking: A Monte Carlo Simulation and Experimental Study, ChemCatChem, 2017, 9, 1329–1336 CrossRef CAS.
- M. V. Kök, Ö. Karacan and R. Pamir, Kinetic Analysis of Oxidation Behavior of Crude Oil SARA Constituents, Energy Fuels, 1998, 12, 580–588 CrossRef.
- D. Liu, X. Kong, M. Li and G. Que, Study on a Water-Soluble Catalyst for Slurry-Phase Hydrocracking of an Atmospheric Residue, Energy Fuels, 2009, 23, 958–961 CrossRef CAS.
- H. Du, M. Li, D. Liu, Y. Ren and Y. Duan, Slurry-phase hydrocracking of heavy oil and model reactant: Effect of dispersed Mo catalyst, Appl. Petrochem. Res., 2015, 5, 89–98 CrossRef CAS.
- A. N. Varakin, A. V. Fosler, S. P. Verevkin, A. A. Pimerzin and P. A. Nikul'shin, Hydrodeoxygenation of Oleic Acid on Supported and Unsupported MoS2 and NiMoS2 Catalysts for the Production of Green Diesel Fuel, Chem. Technol. Fuels Oils, 2019, 54, 686–697 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- S. Grimme, Accurate description of van der Waals complexes by density functional theory including empirical corrections, J. Comput. Chem., 2004, 25, 1463–1473 CrossRef CAS.
- S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS.
- P. Cao, J. Peng, J. Li and M. Zhai, Highly conductive carbon black supported amorphous molybdenum disulfide for efficient hydrogen evolution reaction, J. Power Sources, 2017, 347, 210–219 CrossRef CAS.
- L. R. L. Ting, Y. Deng, L. Ma, Y. Zhang, A. A. Peterson and B. S. Yeo, Catalytic Activities of Sulfur Atoms in Amorphous Molybdenum Sulfide for the Electrochemical Hydrogen Evolution Reaction, ACS Catal., 2016, 6, 861–867 CrossRef CAS.
- E. Furimsky, Catalysts for Upgrading Heavy Petroleum Feeds, Elsevier, Amsterdam, Netherlands, 2007 Search PubMed.
- S. G. Jeon, J. G. Na, C. H. Ko, K. B. Yi, N. S. Rho and S. B. Park, Preparation and application of an oil-soluble CoMo bimetallic catalyst for the hydrocracking of oil sands bitumen, Energy Fuels, 2011, 25, 4256–4260 CrossRef CAS.
- A. Almarshed, A. Hart, G. Leeke, M. Greaves and J. Wood, Effectiveness of Different Transition Metal Dispersed Catalysts for In situ Heavy Oil Upgrading, Ind. Eng. Chem. Res., 2015, 54, 10645–10655 CrossRef CAS.
- X. Zhang and Y. Xie, Recent advances in free-standing two-dimensional crystals with atomic thickness: design, assembly and transfer strategies, Chem. Soc. Rev., 2013, 42, 8187–8199 RSC.
- W. Zan, W. Geng, H. Liu and X. Yao, Influence of interface structures on the properties of molybdenum disulfide/graphene composites: A density functional theory study, J. Alloys Compd., 2015, 649, 961–967 CrossRef CAS.
- Z. Guo, N. Liao, M. Zhang and W. Xue, Theoretical approach to evaluate graphene/PANI composite as highly selective ammonia sensor, Appl. Surf. Sci., 2018, 453, 336–340 CrossRef CAS.
- J. C. Swart, E. van Steen, I. M. Ciobícă and R. A. van Santen, Interaction of graphene with FCC-Co(111), Phys. Chem. Chem. Phys., 2009, 11, 803–807 RSC.
- L. Kao, D. R. Peacor, J. R. M. Coveney, G. Zhao, K. E. Dungey, M. D. Curtis and J. E. Penner-Hahn, A C/MoS2 mixed-layer phase (MoSC) occurring in metalliferous black shales from southern China, and new data on jordisite, Am. Mineral., 2001, 86, 852–861 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9gc02855b |
| This journal is © The Royal Society of Chemistry 2020 |