Open Access Article
Open Access ArticleProtective effects of alfalfa saponins on oxidative stress-induced apoptotic cells
Yalei
Cui†
ab,
Boshuai
Liu†
a,
Xiao
Sun
a,
Zidan
Li
a,
Yanyan
Chen
a,
Zhiguo
Guo
a,
Hua
Liu
a,
Defeng
Li
ab,
Chengzhang
Wang
ab,
Xiaoyan
Zhu
ab and
Yinghua
Shi
 *ab
*ab
aCollege of Animal Science and Veterinary Medicine, Henan Agricultural University, Zhengzhou, Henan 450002, China. E-mail: annysyh@henau.edu.cn; Fax: +8613803855266
bHenan Key Laboratory of Innovation and Utilization of Grassland Resources, Zhengzhou, Henan 450002, China
First published on 6th August 2020
Abstract
As is known, alfalfa saponins can be used as a feed additive in a pig's diet and the addition of alfalfa saponins to a pig's diet could improve its antioxidant capacity. However, the mechanism by which alfalfa saponins exert their antioxidant effects has not been studied. To address this issue, H2O2-induced rat intestinal epithelial cells were used to establish an oxidative stress model to explore the protective mechanism of alfalfa saponins in this study. The results demonstrated that alfalfa saponins could rescue the cell proliferation activity, elevate the amount of antioxidant enzymes and downregulate the release of MDA and LDH in H2O2-induced cells. The antioxidant activity of alfalfa saponins was achieved by restoring GSH homeostasis. Further results demonstrated that alfalfa saponins could inhibit cell apoptosis through activating the MAPK signaling pathway. These results elucidated the mechanism by which alfalfa saponins exert their antioxidant effects and provided a potential strategy for alleviating oxidative stress in monogastric animals.
Introduction
Reactive oxygen species (ROS) are normally generated during metabolic processes in all organisms.1,2 In general, small amounts of free radicals are constantly produced, which are also reduced or removed in time.3 But when this balance is disturbed by excessive ROS, oxidative stress would occur.4 Oxidative stress could result in cellular damage, which is often reflected by a decreased amount of antioxidant enzymes, an increased amount of malondialdehyde (MDA) or lactate dehydrogenase (LDH), and even cell apoptosis.5–7 At present, early weaning of piglets is one of the main technical means to improve the breeding environment of sows and elevate the utilization efficiency of pigsty. However, due to the physiological developmental characteristics of piglets and the combined effects of environmental, nutritional and psychological factors, oxidative stress is a common state of weaned piglets and one of the major stresses that causes economic losses in piglet production.8,9In recent decades, the growing evidence demonstrates that compounds with antioxidant properties in the plant extracts could reduce or prevent the extent of oxidative stress in animals. Melissa officinalis in Brazil shows effective antioxidant effects in the prevention of various neurological diseases associated with oxidative stress in rats.10 The flower extract of Etlingera elatior has powerful antioxidant effects against lead-induced oxidative stress.11 Furthermore, increasing attention has been paid to saponins that defend against oxidative stress. Notoginsenoside R1 could rescue cellular damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation.12 Ginsenoside Rg1 plays protective roles in hydrogen peroxide-induced injury in human neuroblastoma cells.13 Alfalfa saponins, extracts of Alfalfa (Medicago sativa), are natural bioactive compounds, which consist of a sugar moiety glycosidically linked to a hydrophobic aglycone (sapogenin), mainly pentacyclic triterpenoid.14 Our previous study has reported that alfalfa saponins were found to be a potential source of natural antioxidants due to their marked antioxidant activity.15 However, the mechanism of anti-oxidation of alfalfa saponins in monogastric animals, especially in intestinal epithelial cells in vitro, has not been investigated.
To explore the antioxidant effects of alfalfa saponins on monogastric animal epithelial cells, rat intestinal epithelial cells (IEC-6 cell line) were used as experimental materials, and H2O2 was exploited as a stressor to establish an oxidative stress model. In order to reveal the protective effects of alfalfa saponins on rat intestinal epithelial cells in oxidative stress, the influence of alfalfa saponins on the cell proliferation activity and anti-oxidation of IEC-6 was explored. Furthermore, the mechanism of alfalfa saponins on IEC-6 cell apoptosis under oxidative stress was investigated, providing a new method for improving the antioxidant stress resistance of intestinal epithelial cells.
Results
Establishment of an oxidative stress model
To establish an oxidative stress model, the appropriate time and concentration of H2O2 to act on cells were first determined. To investigate the appropriate time for H2O2 to treat cells, the cell proliferation rate was analyzed after IEC-6 cells were treated with 150 μmol L−1 H2O2 for different time courses (0, 3, 6, 12, 24 and 48 h). The result demonstrated that the cell proliferation rate showed no significant difference in groups of cells without H2O2 treatment (P > 0.05, Fig. 1A). But the survival rate of IEC-6 cells significantly reduced in H2O2-treated cells when compared with that in cells without treatment for the same time course (3, 6, 12, 24 and 48 h), reducing to 81%, 71%, 62%, 51% and 33%, respectively (P < 0.01, Fig. 1A). As the survival rate of IEC-6 cells was 51% when cells were treated for 24 h, 24 h was selected as the appropriate time for H2O2 to act on the cells of this experiment. To determine the appropriate concentration of H2O2 to stimulate oxidative stress, 6 concentration gradients (0, 75, 100, 150, 200 and 300 μmol L−1) were set to treat IEC-6 cells. The cell survival rate decreased significantly after the cells were treated with 75, 100, 150, 200 and 300 μmol L−1 H2O2 for 24 h, reducing to 76%, 61%, 53%, 41% and 39%, respectively (Fig. 1B). From the above results, the median lethal concentration of H2O2 on IEC-6 cells was found to be 150 μmol L−1. Therefore, 150 μmol L−1 H2O2 was selected for oxidative stress research in subsequent experiments. According to the results obtained, an oxidative stress model was established by exploiting 150 μmol L−1 H2O2 treatment for 24 h.The effects of alfalfa saponins on the proliferation rate of IEC-6 cells
To explore the influence of alfalfa saponins on the proliferation rate of IEC-6 cells, the survival rate of IEC-6 cells was examined. Different concentration gradients (0, 25, 50, 100, 200, 400, and 800 μg mL−1) were set to treat IEC-6 cells for 24 h. The results indicated that there was no significant difference in cell viability when the cells were treated with 0–400 μg mL−1 alfalfa saponins, although the cell viability decreased with increasing concentration (P > 0.05, Fig. 2A). However, when IEC-6 cells were treated with 800 μg mL−1 alfalfa saponins, the cell activity significantly reduced (P < 0.01, Fig. 2A). To ensure that alfalfa saponins were not toxic to IEC-6 cells, the concentration of alfalfa saponins used was less than 50 μg mL−1 in the subsequent experiments. Thus, the low concentration of alfalfa saponins was 25 μg mL−1 and the high concentration of alfalfa saponins was 40 μg mL−1. In order to investigate whether alfalfa saponins could resist the cellular stress caused by H2O2, the cells were pre-incubated with low and high concentration (25 and 40 μg mL−1) alfalfa saponins for 24 h. The alfalfa saponin treated groups showed higher cell viability when compared with the control group but without significant difference (P > 0.05, Fig. 2B). However, 25 and 40 μg mL−1 alfalfa saponins could obviously rescue the cell viability in the oxidative stress model. The survival rate of IEC-6 cells increased by 40% and 67%, respectively (P < 0.01, Fig. 2B). Thus, 25 and 40 μg mL−1 were selected as the concentrations of alfalfa saponins to be used in subsequent experiments.The anti-oxidant activity of alfalfa saponins in oxidative damage of cells
To explore the role of alfalfa saponins in cellular oxidative damage, the expression levels of T-AOC, CAT and GSH-PX were examined. The results demonstrated that 25 and 40 μg mL−1 alfalfa saponins could gradually elevate the amount of T-AOC in cells without H2O2 treatment but there was no significance in these two groups of different concentrations (P > 0.05, Fig. 3A). In the oxidative stress model, the cells suffered a significant decrease of the expression level of T-AOC (P < 0.05, Fig. 3A); 25 and 40 μg mL−1 alfalfa saponins could rescue the expression level of T-AOC in the oxidative stress model but without significance compared with cells with H2O2 treatment (P > 0.05, Fig. 3A). The enzyme activity of CAT gradually increased (P > 0.05) while the enzyme activity of GSH-PX could be significantly increased by 25 and 40 μg mL−1 alfalfa saponins in cells without H2O2 treatment compared with the negative control group (cells without treatment). In the oxidative stress model, the enzyme activity of CAT and GSH-PX decreased significantly but could be rescued by pre-incubation of cells with 25 and 40 μg mL−1 alfalfa saponins (Fig. 3A).MDA and LDH are often used as indicators of the degree of cellular damage. Therefore, the amounts of MDA and LDH were analyzed. Compared with the negative control group, the amount of MDA significantly decreased when cells were treated with 25 and 40 μg mL−1 alfalfa saponins (P < 0.01). However, the amount of MDA elevated obviously in the oxidative stress model while the cells pre-incubated with 25 and 40 μg mL−1 alfalfa saponins showed a smaller amount of MDA, indicating that alfalfa saponins could protect cells from peroxide (Fig. 3B). In addition, 25 and 40 μg mL−1 alfalfa saponin had little effects on the release of LDH compared with that of cells without treatment (P > 0.05, Fig. 3C). However, H2O2 treated cells showed a greater release of LDH. Further investigation demonstrated that the pre-incubation of cells with 25 and 40 μg mL−1 alfalfa saponins can significantly reduce the LDH release of H2O2 treated cells by 45% and 56%, suggesting that alfalfa saponins can reduce the LDH release in a dose-dependent manner (P < 0.01, Fig. 3C). Taken together, alfalfa saponins could protect cells from peroxide, reducing cellular damage caused by H2O2.
The anti-oxidative effects of alfalfa saponins by restoring GSH homeostasis
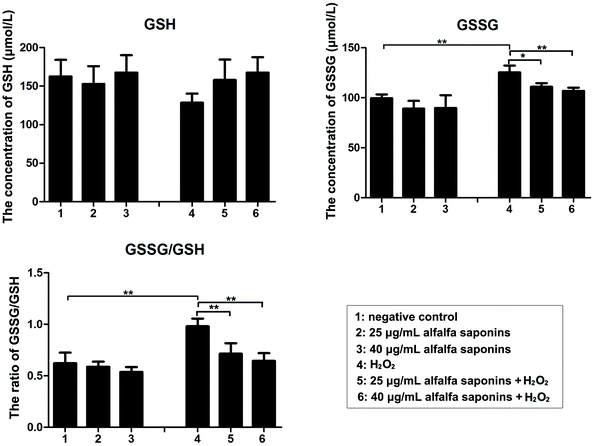
To explore the regulatory mechanism of alfalfa saponins on oxidative damage of cells, the amounts of GSH and GSSG were detected. Compared with the negative control group, the intracellular GSSG concentration and GSSG/GSH ratio in the H2O2 treated group were significantly increased (P < 0.01, Fig. 4). After 25 and 40 μg mL−1 alfalfa saponin pre-treatment, the GSSG content and GSSG/GSH ratio were gradually decreased compared with those in the H2O2 treated group, indicating that the antioxidative effects of alfalfa saponins on oxidative damage of cells are achieved by restoring GSH homeostasis.The downstream pathway of H2O2-induced cell apoptosis by alfalfa saponins
To further explore the mechanism of alfalfa saponins on H2O2-induced oxidative damage, the cell apoptosis rate was firstly investigated using a fluorescence microscope. The result demonstrated that cells treated with 25 and 40 μg mL−1 alfalfa saponins showed no significant difference compared with the negative control, while cells treated with H2O2 presented a higher apoptosis rate (Fig. 5A). However, the pre-incubation of cells with 25 and 40 μg mL−1 alfalfa saponins could significantly reduce the cell apoptosis rate (Fig. 5A). It was suggested that the antioxidant effect of alfalfa saponins on H2O2 induced oxidative stress in IEC-6 cells may be achieved by inhibiting cell apoptosis. In order to further determine the regulatory mechanism of alfalfa saponins on H2O2-induced IEC-6 cell apoptosis, the expression profiles of caspase-3, caspase-9, Bax and Bcl-2 were determined by western blot analysis. The results showed that there was no difference of pro-caspase-3 expression in the different groups. However, both 25 and 40 μg mL−1 alfalfa saponins gradually reduced the expression of cleaved caspase-3, caspase-9 and Bax in cells and increased the expression of the Bcl-2 gene. After treatment with H2O2, cleaved caspase-3, caspase-9 and Bax expression levels were significantly increased, and Bcl-2 gene expression was significantly decreased. 25 and 40 μg mL−1 alfalfa saponin pre-treatment in the oxidative stress model reduced intracellular cleaved caspase-3, caspase-9 and Bax, and increased bcl-2 gene expression, suggesting that the mechanism of alfalfa saponins against H2O2-induced IEC-6 cell apoptosis may be achieved by up-regulating Bcl-2 and down-regulating the expression of cleaved caspase-3, caspase-9 and Bax, thereby inhibiting mitochondrial apoptosis (Fig. 5B).In order to study the signaling pathway of alfalfa saponins on the anti-oxidation protection of IEC-6 cells, the expression of three signaling pathway proteins of MAPK was detected, including ERK1/2, JNK and p38. There was no significant difference in the total protein expression of ERK1/2, JNK and p38 (P > 0.05). Compared with the negative control group, the phosphorylation levels of ERK1/2, p38 and JNK in H2O2-treated cells significantly increased (P < 0.01), while the phosphorylation levels of the 3 proteins in 25 and 40 μg mL−1 alfalfa saponin pre-incubated groups were effectively reduced (P < 0.01, Fig. 5C). The results showed that alfalfa saponins could inhibit the activation of the MAPK signaling pathway in IEC-6 cells induced by H2O2, thus protecting IEC-6 cells.
In conclusion (Fig. 5D), alfalfa saponin pre-treatment could achieve antioxidative effects by restoring GSH homeostasis and inhibiting mitochondrial apoptosis through the MAPK signaling pathway, thereby further alleviating oxidative damage of cells.
Discussion
Alfalfa saponins could enhance the antioxidant system
Weaned piglets often suffer from oxidative stress.8,9 Our previous study has indicated that alfalfa saponins can be used as a feed additive in a pig's diet to improve its antioxidant capacity.15 However, the mechanism remains unknown. Studies have reported that H2O2 can be used to induce cells to establish an oxidative stress model, which is widely applied in the modeling of oxidative stress in intestinal epithelial cells.16,17 Thus, an oxidative stress model was established to simulate weaned piglets to explore the mechanism of alfalfa saponins in regulating oxidative stress. In general, the content of free radicals maintains a dynamic balance in cells. Once this balance is destroyed, it will cause oxidative damage of cells, leading to the content changes of the substrates related to redox. T-AOC can be used to indicate the total antioxidant capacity of organisms.18 GSH-PX is one kind of important antioxidant enzyme.19 CAT is widely distributed in living organisms, and it can promote the decomposition of H2O2 and protect cells from free radical damage.20 MDA is one kind of lipid peroxide metabolite which is produced through the non-enzymatic system when oxygen free radicals attack the membrane of polyunsaturated fatty acids (PUFA).21 LDH is a stable cytosolic enzyme that can be rapidly released into the extracellular plasm once the cell membrane is damaged.22 Therefore, the amounts of MDA and LDH indirectly reflect the degree of cellular damage. At present, researchers have proved that most saponins have certain antioxidant capacity.12,13 In this study, the survival rate of cells, and the activities of T-AOC, GSH-PX and CAT were improved by pre-incubation with alfalfa saponins. Moreover, the contents of MDA and LDH were significantly lower in an oxidative stress model pre-incubated with alfalfa saponins, indicating that alfalfa saponins could increase the antioxidant enzyme activity and enhance the ability of clearing free radicals.The antioxidative effects of alfalfa saponins are achieved by restoring GSH homeostasis
The oxidative cytotoxic agent, H2O2, typically considered as a cause of oxidative stress always induces cell apoptosis.23,24 GSH plays an important role in maintaining the redox homeostasis of organisms.25 Once the cells are damaged by oxidation, GSH participates in the antioxidant reaction to be oxidized to GSSG, resulting in an increase in the ratio of GSSG/GSH. A report has demonstrated that the saponins from Aralia taibaiensis could affect the GSH level in D-galactose-induced rats,26 and the administration of ginsenosid-Rd significantly increased GSH, but decreased GSSG, resulting in a decrease of the GSSG/GSH ratio in the oxidative damage related to aging in senescence-accelerated mice.27 Consistent with previous studies, the results of this study demonstrated that the antioxidative effects of alfalfa saponins on oxidative damage in IEC-6 cells may also be achieved by increasing the amount of GSH synthesis and reducing the ratio of GSSG/GSH to restore GSH homeostasis.Alfalfa saponins can inhibit cell apoptosis through the MAPK signaling pathway in the oxidative stress model
Apoptosis is one of the important outcomes of oxidative damage in cells. Caspase-3 is an executor of cell apoptosis and is directly involved in apoptotic events. Activated caspase-3 (cleaved caspase-3, 17 kDa) is activated when cells undergo apoptosis.28 In addition, anti-apoptotic genes (Bcl-2 and Bcl-xL) and pro-apoptotic genes (Bax, Bad, Bid, and Bnip3) in the Bcl-2 family are also involved in the regulation of apoptosis.29 The results of this study showed that alfalfa saponin pre-treatment reduced the expression of intracellular cleaved caspase-3, caspase-9 and Bax, and increased Bcl-2 expression in the oxidative stress model, suggesting that the mechanism of alfalfa saponins against H2O2-induced IEC-6 cell apoptosis may be through up-regulation of Bcl-2 and down-regulation of cleaved caspase-3, caspase-9 and Bax gene expression. The MAPK signal transduction pathway is involved in a series of physiological and biochemical reactions (such as cell proliferation, differentiation, metabolism, transformation and apoptosis). MAPKs are mainly composed of three subfamily proteins ERK1/2, p38 and JNK. Zhou Y et al. reported that ERK1/2, JNK and p38 MAPK could be significantly activated when oxidative damage occurs in intestinal epithelial cells.30 Moreover, the activation of these kinases will further promote the increase of the expression of proteoglycans, leading to the occurrence of apoptosis.31 The results of this study found that the degree of phosphorylation of the three proteins was significantly reduced by the addition of alfalfa saponins in the oxidative stress model. It is indicated that alfalfa saponins can prevent oxidative damage by inhibiting H2O2-induced activation of cell apoptosis, thereby protecting cells.Materials and methods
Cell culture
Rat intestinal epithelial cells (IEC-6 cell line) were purchased from BeNa Culture Collection, Beijing, China. IEC-6 cells were cultured in RPMI-1640 (Solarbio, Beijing, China) supplemented with 10% fetal bovine serum (FBS) at 37 °C with 5% CO2.The establishment of the oxidative stress model
To select the optimal concentration of H2O2, 30% H2O2 solution (Beijing Chemical Reagent Company, Beijing, China) was diluted with PBS to different concentrations, and fresh solutions of H2O2 with different concentrations were prepared daily. Cells (1.2 × 104 per well) were plated onto a 96-well plate. After being cultured for 24 h, the cells were treated with 0, 75, 100, 150, 200 and 300 μmol L−1 H2O2, respectively. After being treated with H2O2 for 24 h, the cell medium was discarded. The cell proliferation activity was monitored using a MTT solution cell proliferation assay kit (Solarbio, Beijing, China) according to the manufacturer's protocol. The cell proliferation rate (absorbance at 490 nm) was examined using an iMARKTM microplate reader (Bio-Rad, USA).To select the optimal time of H2O2, cells (1.2 × 104 per well) were plated onto a 96-well plate. After being cultured for 24 h, the cells were treated with 150 μmol L−1 H2O2 for 0, 3, 6, 12, 24 and 48 h, respectively. After being treated with H2O2, the cell medium was discarded. The cell proliferation activity was monitored as described above.
The determination of alfalfa saponin concentration in IEC-6 cells
To explore the appropriate concentration of alfalfa saponins in IEC-6 cells, cells (1.2 × 104 per well) were plated onto a 96-well plate. After being cultured for 24 h, the cells were treated with 0, 25, 50, 100, 200, 400 and 800 μg mL−1 alfalfa saponins, respectively. After being treated with alfalfa saponins for 24 h, the cell proliferation activity was monitored using the MTT solution cell proliferation assay kit (Solarbio, Beijing, China) according to the manufacturer's protocol.Cell proliferation assay
Cells (1.2 × 104 per well) were plated onto a 96-well plate. After being cultured for 24 h, the cells were subjected to different treatments. The control group (negative control) was without any treatment. The cells of 25 μg mL−1 and 40 μg mL−1 alfalfa saponin groups were treated with 25 and 40 μg mL−1 alfalfa saponins, respectively. The cells treated with 150 μmol L−1 H2O2 were named H2O2, while cells pre-incubated with 25 or 40 μg mL−1 alfalfa saponins and subsequently induced by H2O2 were separated into groups of 25 μg mL−1 alfalfa saponins + H2O2 or 40 μg mL−1 alfalfa saponins + H2O2. All groups were treated for 24 h, and then, the cell proliferation activity was monitored using the MTT solution cell proliferation assay kit (Solarbio, Beijing, China) according to the manufacturer's protocol.Detection of cellular antioxidant enzymes, MDA and GSSG/GSH
Cells (3 × 105 per well) were plated onto a 6-well plate. After being cultured for 24 h, the cells were subjected to different treatments as described above. After 24 h, the cells were collected and lysed with a sonicator to prepare a cell suspension. After centrifugation for 10 min at 1000 rpm, the cell supernatant was examined using T-AOC, GSH-PX, CAT, MDA and GSH/GSSG kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).LDH detection assay
Cells (1.2 × 104 per well) were plated onto a 96-well plate. After being cultured for 24 h, the cells were subjected to different treatments as described above. After the cells were treated for 24 h, 120 μL of cell supernatants from different groups was collected and plated onto a new 96-well plate to detect the amount of LDH using an LDH cytotoxicity assay kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer's protocol.Annexin V assay of apoptosis
Apoptosis assay of IEC-6 cells by using annexin V (Invitrogen) was performed according to the manufacturer's protocol. Briefly, cells (5 × 104 per well) were plated onto a 24-well plate. After being cultured for 24 h, the cells were subjected to different treatments as described above. After 24 h, the cells were washed twice with cold PBS. The cells were resuspended in 195 μL of Annexin V-FITC binding buffer, followed by the addition of 5 μL of Annexin V-FITC and 10 μL of propidium iodide (PI). The cells were incubated for 20 min in the dark, and then analyzed using a fluorescence microscope.Western blot analysis
Western blot analysis was performed using SDS-PAGE. Before transferring proteins to a nitrocellulose membrane (Bio-Rad, USA), the membrane was blocked with 5% milk at 4 °C overnight. The membrane was incubated with a primary antibody for 2 h and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Sigma-Aldrich, USA) for 2 h at room temperature. Proteins were detected using Western Lightning™ Plus-ECL Oxidizing Reagent Plus (Perkin Elmer, USA). The antibodies of Bcl-2 (26 kDa), caspase-3 (32/17 kDa), caspase-9 (46 kDa), and Bax (21 kDa) were purchased from PROTEINTECH GROUP, USA. The antibodies of p38 (43 kDa), p-p38 (thr180/tyr182, 43 kDa), ERK (44/42 kDa), p-ERK (thr202/tyr204, 44/42 kDa), JNK (45/53 kDa), and p-JNK (thr183/tyr185, 46/54 kDa) were purchased from Cell Signaling Technology, USA. The antibody of GAPDH (37 kDa) was purchased from TransGen Biotech, Beijing, China.Statistical analysis
The mean and standard deviations for triplicate assays were calculated by one-way analysis of variance (ANOVA). The statistical significance between different treatments was determined using Student's t test.Conclusion
Taken together, alfalfa saponins could elevate the amount of antioxidant enzymes and downregulate the release of MDA and LDH. The antioxidant activity of alfalfa saponins was achieved by restoring GSH homeostasis. Furthermore, alfalfa saponins could increase the survival rate of cells by activating the MAPK signaling pathway. Therefore, alfalfa saponins could function as a cellular oxidative damage inhibitor or a potential drug candidate, providing a new strategy for inhibiting cell apoptosis induced by oxidative stress in monogastric animals.Author contributions
YHS, YLC and BSL designed, conducted, analyzed and wrote the manuscript. XS, ZDL, YYC, GZG and HL helped in performing the experiments and analyzed the data. DFL, CZW and XYZ analyzed and wrote a part of the data.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
The authors are thankful for the financial support from the China Forage and Grass Research System (No. CARS-34).References
- C. G. Silva, R. S. Herdeiro, C. J. Mathias, A. D. Panek, C. S. Silveira, V. P. Rodrigues, M. N. Rennó, D. Q. Falcão, D. M. Cerqueira, A. B. M. Minto, F. L. P. Nogueira, C. H. Quaresma, J. F. M. Silva, F. S. Menezes and E. C. A. Eleutherio, Evaluation of antioxidant activity of Brazilian plants, Pharmacol. Res., 2005, 52, 229–233 CrossRef PubMed.
- T. Finkel and N. J. Holbrook, Oxidants, oxidative stress and the biology of ageing, Nature, 2000, 408, 239–247 CrossRef PubMed.
- V. I. Lushchak, Free radicals, reactive oxygen species, oxidative stress and its classification, Chem.-Biol. Interact., 2014, 224, 164–175 CrossRef CAS PubMed.
- B. Poljsak, D. Šuput and I. Milisav, Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants, Oxid. Med. Cell. Longevity, 2013, 2013, 956792 Search PubMed.
- H. Sies, Oxidative stress: a concept in redox biology and medicine, Redox Biol., 2015, 4, 180–183 CrossRef CAS PubMed.
- M. L. Urso and P. M. Clarkson, Oxidative stress, exercise, and antioxidant supplementation, Toxicology, 2003, 189, 41–54 CrossRef CAS PubMed.
- Z. Wu, H. Wang, S. Fang and C. Xu, Roles of endoplasmic reticulum stress and autophagy on H2O2–induced oxidative stress injury in HepG2 cells, Mol. Med. Rep., 2018, 18, 4163–4174 CAS.
- J. Yin, M. M. Wu, H. Xiao, W. K. Ren, J. L. Duan, G. Yang, T. J. Li and Y. L. Yin, Development of an antioxidant system after early weaning in piglets, J. Anim. Sci., 2014, 92, 612–619 CrossRef CAS PubMed.
- P. Zheng, B. Yu, J. He, G. Tian, Y. Luo, X. Mao, K. Zhang, L. Che and D. Chen, Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets, Br. J. Nutr., 2012, 109, 2253–2260 CrossRef PubMed.
- R. P. Pereira, R. Fachinetto, A. de Souza Prestes, R. L. Puntel, G. N. Santos da Silva, B. M. Heinzmann, T. K. Boschetti, M. L. Athayde, M. E. Bürger, A. F. Morel, V. M. Morsch and J. B. T. Rocha, Antioxidant Effects of Different Extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus, Neurochem. Res., 2009, 34, 973–983 CrossRef CAS PubMed.
- T. Jackie, N. Haleagrahara and S. Chakravarthi, Antioxidant effects of Etlingera elatior flower extract against lead acetate - induced perturbations in free radical scavenging enzymes and lipid peroxidation in rats, BMC Res. Notes, 2011, 4, 67–67 CrossRef PubMed.
- B. Ma, X. Meng, J. Wang, J. Sun, X. Ren, M. Qin, J. Sun, G. Sun and X. Sun, Notoginsenoside R1 attenuates amyloid-β-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation, Int. Immunopharmacol., 2014, 22, 151–159 CrossRef CAS PubMed.
- Z.-G. Sun, L.-P. Chen, F.-W. Wang, C.-Y. Xu and M. Geng, Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells, Neural Regener. Res., 2016, 11, 1159–1164 CrossRef CAS PubMed.
- S. Sen, H. P. S. Makkar and K. Becker, Alfalfa Saponins and Their Implication in Animal Nutrition, J. Agric. Food Chem., 1998, 46, 131–140 CrossRef CAS PubMed.
- Y. H. Shi, J. Wang, R. Guo, C. Z. Wang, X. B. Yan, B. Xu and D. Q. Zhang, Effects of alfalfa saponin extract on growth performance and some antioxidant indices of weaned piglets, Livest. Sci., 2014, 167, 257–262 CrossRef.
- X. Cai, X. Chen, X. Wang, C. Xu, Q. Guo, L. Zhu, S. Zhu and J. Xu, Pre-protective effect of lipoic acid on injury induced by H2O2 in IPEC-J2 cells, Mol. Cell. Biochem., 2013, 378, 73–81 CrossRef CAS.
- E. Paszti-Gere, E. Csibrik-Nemeth, K. Szeker, R. Csizinszky, C. Jakab and P. Galfi, Acute oxidative stress affects IL-8 and TNF-α expression in IPEC-J2 porcine epithelial cells, Inflammation, 2012, 35, 994–1004 CrossRef CAS PubMed.
- F. Yu, J. Gao, Y. Zeng and C.-X. Liu, Effects of adlay seed oil on blood lipids and antioxidant capacity in hyperlipidemic rats, J. Sci. Food Agric., 2011, 91, 1843–1848 CrossRef CAS PubMed.
- R. Kohen and A. Nyska, Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification, Toxicol. Pathol., 2002, 30, 620–650 CrossRef CAS PubMed.
- O. M. Ighodaro and O. A. Akinloye, First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid, Alexandria J. Med., 2018, 54, 287–293 CrossRef.
- H. Jiang, R. Chen, S. Xue, H. Zhu, X. Sun and X. Sun, Protective effects of three remote ischemic conditioning procedures against renal ischemic/reperfusion injury in rat kidneys: a comparative study, Ir. J. Med. Sci., 2015, 184, 647–653 CrossRef CAS PubMed.
- H. Hong and G.-Q. Liu, Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin, Life Sci., 2004, 74, 2959–2973 CrossRef CAS PubMed.
- T. Sato, Y. K. Kaneko, T. Sawatani, A. Noguchi and T. Ishikawa, Obligatory Role of Early Ca2+ Responses in H2O2-Induced β-Cell Apoptosis, Biol. Pharm. Bull., 2015, 38, 1599–1605 CrossRef CAS PubMed.
- F. Di Meo, R. Cuciniello, S. Margarucci, P. Bergamo, O. Petillo, G. Peluso, S. Filosa and S. Crispi, Ginkgo biloba Prevents Oxidative Stress-Induced Apoptosis Blocking p53 Activation in Neuroblastoma Cells, Antioxidants, 2020, 9(4), 279 CrossRef CAS PubMed.
- L. Flohe, W. A. Günzler and H. H. Schock, Glutathione peroxidase: a selenoenzyme, FEBS Lett., 1973, 32, 132–134 CrossRef CAS PubMed.
- Y.-N. Li, Y. Guo, M.-M. Xi, P. Yang, X.-Y. Zhou, S. Yin, C.-X. Hai, J.-G. Li and X.-J. Qin, Saponins from Aralia taibaiensis Attenuate D-Galactose-Induced Aging in Rats by Activating FOXO3a and Nrf2 Pathways, Oxid. Med. Cell. Longevity, 2014, 2014, 320513 Search PubMed.
- T. Yokozawa, A. Satoh and E. J. Cho, Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice, J. Pharm. Pharmacol., 2004, 56, 107–113 CrossRef CAS PubMed.
- B. B. Wolf, M. Schuler, F. Echeverri and D. R. Green, Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation, J. Biol. Chem., 1999, 274, 30651–30656 CrossRef CAS PubMed.
- J. M. Adams and S. Cory, The Bcl-2 protein family: arbiters of cell survival, Science, 1998, 281, 1322–1326 CrossRef CAS PubMed.
- Y. Zhou, Q. Wang, B. M. Evers and D. H. Chung, Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis, Pediatr. Res., 2005, 58, 1192–1197 CrossRef CAS PubMed.
- G. Pearson, F. Robinson, T. Beers Gibson, B.-e. Xu, M. Karandikar, K. Berman and M. H. Cobb, Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions*, Endocr. Rev., 2001, 22, 153–183 CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |