Open Access Article
Open Access ArticleBolus rheology and ease of swallowing of particulated semi-solid foods as evaluated by an elderly panel†
Aarti
Ben Tobin
 a,
Mihaela
Mihnea
b,
Marie
Hildenbrand
b,
Ana
Miljkovic
b,
Gonzalo
Garrido-Bañuelos
b,
Epameinondas
Xanthakis
b and
Patricia
Lopez-Sanchez
a,
Mihaela
Mihnea
b,
Marie
Hildenbrand
b,
Ana
Miljkovic
b,
Gonzalo
Garrido-Bañuelos
b,
Epameinondas
Xanthakis
b and
Patricia
Lopez-Sanchez
 *bc
*bc
aAgriculture and Food, CSIRO Commonwealth Scientific and Industrial Research Organisation, Brisbane, Australia
bAgriculture and Food, Bioeconomy and Health, RISE Research Institutes of Sweden, Gothenburg, Sweden. E-mail: patricia.lopez-sanchez@ri.se
cFood Nutrition and Science, Chalmers University of Technology, Gothenburg, Sweden
First published on 1st September 2020
Abstract
Preparation of a bolus is a complex process with both food comminution and degree of lubrication with saliva playing an important role in a safe swallow. Swallowing disorders i.e. dysphagia, are especially present among the elderly population and often lead to choking and further health complications. The aim of this research was to investigate the relationship between the perception of ease of swallowing in the elderly and the rheological parameters of particulated foods, using broccoli purees as a model system. Particulated foods can be described as a concentrated dispersion of plant particles in a fluid phase. The effect of the fluid phase (Newtonian vs. shear thinning) and dispersed phase (plant particles with different size distribution and morphology) on the rheological properties of simulated boli was studied by characterising shear viscosity, viscoelasticity, yield stress, extensional viscosity and cohesiveness. Ease of swallowing and mouthfeel were evaluated by a semi trained healthy elderly panel (n = 19, aged 61 to 81). Ease of swallowing was correlated with the presence of yield stress and extensional viscosity in the bolus, characteristic of boli with xanthan gum as the fluid phase. Although the properties of the fluid phase played a dominant role in the ease of swallowing, compared to the dispersed phase, both components played a role in the rheological properties of the bolus and the perception of ease of swallowing by the elderly panel. These results provide insights into the design of personalised foods for populations with specific needs such as those suffering from swallowing disorders.
Introduction
Food structure and texture change from the moment food is chewed and mixed with saliva to form a cohesive mass or bolus. Due to the dynamic nature of the oral process, bolus properties determine swallowability to a larger extent than food properties. Populations with swallowing disorders i.e. dysphagia, struggle to consume regular foods, which often leads to choking and aspirational pneumonia due to food entering the airway instead of the oesophagus.1 Texture modification of foods is the most common clinical strategy to aid those suffering from dysphagia, where solid food is processed to reduce the particle size in order to eliminate or minimise the chewing phase during oral processing. Often these foods are reconstituted with the aid of thickeners to increase the visual appeal, which also increases the viscosity of the food. Increasing bolus viscosity reduces the flow of the food during swallowing, allowing sufficient time for individuals with dysphagia to close the airway before the arrival of the bolus, which they are unable to do with low viscosity boli.2 However, high viscosity boli may require additional effort to swallow and could leave residues in the pharynx, which can lead to further health complications. In addition to high shear viscosity, other rheological parameters such as extensional viscosity and viscoelasticity also play an important role in determining ease of swallowing.3,4 However, there is no single convention with respect to which rheological properties determine food swallowability, especially for complex systems such as plant-based particulated foods.Plant-based particulated foods such as soups, purees, and smoothies are suspensions of plant particles (cell clusters, single cells and cell wall fragments) in a fluid phase containing soluble polymers, organic acid and salts5 The food structure of plant-based particulated foods can be designed by using different processing conditions. For instance, by simply exchanging the order of thermal and mechanical treatments, broccoli and carrot purees had different particle shapes, size distributions and rheological properties.4,5 Among the most common texturisers added to foods for dysphagia is xanthan gum, a bacterial polysaccharide produced by fermentation which has been shown to maintain a coherent bolus during flow, leading to the perception of easy to swallow.6 Due to its structural rigidity and rod-like conformation of its linear, cellulosic backbone, xanthan behaves rheologically as a non-Newtonian shear-thinning fluid;7 this means that its viscosity decreases with increasing shear rate. Furthermore, xanthan gum presents an apparent yield stress i.e. a minimum stress is required to flow. The effect of xanthan on the rheological properties of plant cell wall systems has been previously investigated in carrot suspensions, showing that it depends on the particle concentration.8 At low concentrations, xanthan increased the viscoelastic properties of the suspension, whilst at high concentrations, such as those in reconstituted foods for dysphagia, xanthan did not show a significant effect, as the viscoelastic behaviour was dominated by the plant particle network, with xanthan molecules entrapped in the interstitial regions.
Understanding bolus rheology is a key parameter in designing foods for dysphagia. Shear viscosity, yield stress, and viscoelasticity (G′ and G′′) are useful parameters to describe bolus properties; however, they are not sufficient to describe bolus behaviour during oral processing. Bolus behaviour under extensional flow, such as the one in the pharyngeal cavity during swallowing, is of critical importance to determine bolus flow and ease of swallowing.9
In this study we used broccoli purees as a model system to investigate how the different components of plant-based particulated foods, i.e. dispersed and fluid phases, impacted bolus rheology and ease of swallowing. Suspensions were produced with two different dispersed phases i.e. different particle size distributions and morphologies and two different fluid phases i.e. water as Newtonian fluid and a xanthan solution as shear thinning fluid. Food boli were produced by mixing the purees with artificial saliva and analysed for shear and extensional viscosities, yield stress, viscoelasticity and cohesiveness, and correlated with the swallowing perception by elderly panellists. Our results contribute to the knowledge on how food composition affects bolus rheology and ease of swallowing and would aid in the design of personalised foods for people suffering from dysphagia.
Materials and methods
Materials
Broccoli (Brassica oleracea) was purchased from a local supermarket in Sweden and used for sample preparation after a maximum of 3 days storage at 4 °C. The chemicals and enzymes used to produce the artificial saliva were: ammonium carbonate (Sigma-Aldrich, USA), potassium chloride (Merck, Germany), sodium bicarbonate (Across organics, Spain), potassium dihydrogen phosphate (Merck, Germany), magnesium chloride hexahydrate (Emsure, Germany), calcium chloride dihydrate (Merck, Germany), alfa amylase (Sigma-Aldrich, USA) and mucin from porcine stomach type II (Sigma-Aldrich, USA). Xanthan gum was purchased from Sigma-Aldrich.Sample preparation
Each broccoli head was cut into 5–8 cm pieces and the large stalks of the broccoli were discarded. Broccoli pieces were cooked using two different methods: heating followed by blending (HB) or blending followed by heating (BH). For the HB method, the broccoli was covered with water and cooked at 90 °C (core temperature measured with a thermocouple) for 20 minutes. The broccoli was mixed with water at a mass ratio of 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water
1 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) broccoli and blended for 3 minutes at maximum speed in a kitchen blender (TURBO blender, Moulinex; France). For the BH method, the broccoli was mixed with water at the same water
broccoli and blended for 3 minutes at maximum speed in a kitchen blender (TURBO blender, Moulinex; France). For the BH method, the broccoli was mixed with water at the same water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) broccoli ratio as for the HB method, blended for 3 minutes and then heated using the same parameters as the HB method.
broccoli ratio as for the HB method, blended for 3 minutes and then heated using the same parameters as the HB method.
The samples were centrifuged for 30 minutes at 4000g (Centrifuge Universal 320|320 R, Hettich, Germany) and the pellet was collected from several batches, in order to produce sufficient broccoli particles for all the experiments.
The pellet obtained from each of the cooking methods (HB and BH) was mixed with two different fluid phases, resulting in two sets of samples. The first set was prepared by dispersing different concentrations of the pellet (1, 2, 4, 6 and 8 wt%) in water, and the second set by dispersing in 0.5 wt% xanthan solution at the same concentration as in water. The suspensions were obtained by gently adding the pellet to the fluid phase and mixing with a magnetic stirrer. The pellet will be referred to as the dispersed phase throughout the manuscript.
Simulated bolus
Artificial saliva was produced based on the formulation described by Minekus et al.10 with mucin added (2 g L−1).11 A bolus was prepared by mixing broccoli suspensions and artificial saliva at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ref. 10) resulting in final particle concentrations of 0.5, 1, 2, 3 and 4 wt%.
1 (ref. 10) resulting in final particle concentrations of 0.5, 1, 2, 3 and 4 wt%.
Dry matter
The measurement of dry matter was carried out with the dehydration of pre-weighed samples in a 105 °C oven for 24 h (UNP 100-800, Memmert, Schawabach, Germany) for 24 hours. Two replicates were measured.Light microscopy
Light microscopy was performed using a microscope (Microphot-FXA, Nikon) equipped with a video camera (DFK33UX264, the imaging Source Europe GmbH, Bremen Germany). The samples were diluted 10 times with deionised water. Two droplets were placed on a glass slide and covered. The samples were observed with a 10× dry objective lens and at least eight images were obtained per sample. NIS-ElementsD software was used to collect the images.Particle size distribution
The particle size distribution (PSD) was measured after centrifugation, by laser light scattering (Mastersizer; Malvern Instruments Ltd, Malvern, UK). A refractive index of 1.53 for particles and 1.33 for water was used. The particle absorption index was set to 0.01. The particle size distribution was calculated from the intensity profile of the scattered light using the instrument software (Mastersizer 3000). The volume based (d4,3) and area based (d3,2) diameters were obtained for every sample.Rheological measurements
Rheological measurements of shear viscosity, viscoelasticity and yield stress were performed on a strain-controlled rheometer (ARG2, TA Instrument, USA) using 40 mm diameter parallel plates. Paraffin oil was added at the edge of the sample and a solvent trap was used to prevent the sample from drying during the measurements. Measurements were performed at a gap of 1 mm and a temperature of 35 °C to mimic biological conditions in the oral cavity. Samples were held in the rheometer for 2 minutes, to ensure that the samples had equilibrated to 35 °C, prior to the measurement. The shear viscosity was measured using a steady state flow sweep by increasing the shear rate from 1 s−1 to 100 s−1. Viscoelastic properties were measured by using frequency sweeps from 10 to 0.1 Hz and a 0.1% strain selected from the linear viscoelastic region. From these measurements, the elastic modulus G′ and viscous modulus G′′ were obtained. Apparent yield stress was measured by the dynamic stress/strain sweep method, in which the elastic G′ and viscous moduli G′′ were measured as a function of the oscillation stress at a frequency of 1 Hz and a strain range between 0.01 and 300%. The apparent yield stress was obtained using TRIOS v.5.0 software (TA instruments, USA) to analyse plots of the storage modulus vs. oscillation stress. The used function creates two tangents to the curve and finds their intersection, the onset point, and the corresponding oscillation stress is selected as the apparent yield stress. Three replicates were measured.Extensional viscosity
Extensional viscosity was measured at room temperature using a hyperbolic contraction flow geometry12 mounted on an Instron testing instrument (5542 Instron Corporation, Canton, MA, USA), where the samples were forced through a hyperbolic nozzle and the pressure drop over the nozzle was measured. Measurements were performed using a die with an inlet radius of 10 mm and outlet radius of 0.83 mm, imposing a total Hencky strain of 8–10 to the samples. The extensional strain rates were in the range of 10 to 120 s−1, and the extensional viscosity was obtained as described previously.12,13 Extensional viscosity of a simulated bolus containing only 0.5 wt% xanthan gum mixed with artificial saliva to a final concentration of 0.25 wt% was used as a reference. Three replicates were measured.Cohesiveness
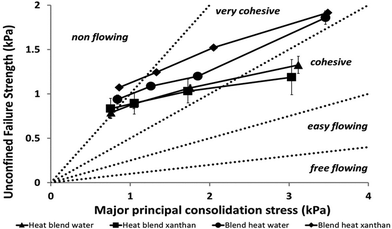
Cohesiveness of the different broccoli samples was determined with a Powder Flow Tester (PFT Powder Flow Tester, Brookfield Engineering Laboratories – Ametek Inc., USA) using the experimental methods described by Tobin et al.14 and Slettengren et al.15 with some modifications. A vane lid was used in the PFT measurements of the flow functions of the samples. The flow function tests were undertaken using the 263 cm3 volume shear cell. The standard flow function test program was used to measure the flow properties over the range of 4 major principal consolidation stresses in a geometric progression that generated values up to 3.5 kPa. The PFT was connected to a computer and Powder Flow Pro V1.2 software was used to collect the data. The flow functions of the broccoli samples represent the average values of at least 2 independent measurements.The ratio of the consolidation stress σ1 and unconfined yield strength σc defines the flowability ffc (eqn (1))15
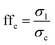 | (1) |
High flowability (ffc > 10) means that the sample is free-flowing, and low flowability (ffc < 1) means that the sample is non-flowing. When the flowability is determined at several consolidation stresses, it can be presented in a flow function plot.
Sensory evaluation
Descriptive sensory analysis was used to characterise the mouthfeel and ease of swallowing of broccoli suspensions. Four samples (6 wt% broccoli suspension, with HB or BH as the dispersed phase and water or xanthan as the fluid phase) were evaluated by 19 healthy panellists (14 females, 5 males) aged between 61 and 81 years (with an average age of 71). The panellists were selected from RISE's (Research Institutes of Sweden) consumer database, and most of the panellists had previously participated in sensory trials of different types of foods at RISE.One product familiarisation session was held for 1 hour prior to sensory evaluation of the samples. The list of sensory attributes was prepared based on previous studies.16–19 During the familiarisation session, the protocol and the different sensory attributes were explained and discussed with the panellists to ensure that they were familiar with the samples and the vocabulary used to define the sensory attributes. Reference samples were also evaluated to understand the scales for rating the attributes.
Formal sessions were conducted in the sensory laboratory of RISE Research Institutes of Sweden, in isolated booths equipped with computers, temperature control (22 °C) and daylight equivalent. Samples were served in opaque plastic cups, with each panellist receiving a 20 mL sample of each sample. Samples were randomised and presented with a three-digit code. Panellists were instructed to use a full 5 mL spoon to evaluate the various attributes of the samples.
The sensory attributes were rated on a 5 point categorical scale with 1 = low and 5 = high. The sensory vocabulary, definitions and sensory reference standards were described as follows:
•Difficulty in swallowing: The attribute of ease of swallowing was rated as difficulty in swallowing, where 1 would indicate that there was no difficulty in swallowing i.e. easy to swallow and 5 would indicate that the sample was not easy to swallow.
•Effort required to swallow: Effort required to prepare the bolus for swallowing from low (no effort) to high (a lot of effort).
•Mouth-coating: Sensation of a layer forming on the surfaces of the mouth, from low (water) to high (cream).
•Particle size perception: The proportion of large particles felt on the oral surfaces of the mouth compared to small particles, from low (not many large particles) to high (a lot of large particles).
•Smoothness: The velvety feeling of the sample in the mouth, from low (water) to high (potato puree).
•Residues in mouth: The ease at which the sample clears from the mouth after swallowing. From easy to clear to very difficult to clear.
All the sensory attributes were evaluated using three spoonfuls of samples and the following protocol. The first spoonful was to rate the ease of swallowing. The panellists were instructed to consume and swallow the sample naturally and to start the timer as they put the puree in their mouth and stop the timer once the puree was swallowed. With the second spoonful the panellists were instructed to compress and rub the sample with the tongue against the palate and rate the attributes of smoothness and particle size sensation. The third spoonful was used to rate mouth coating, and effort required to prepare the bolus for swallowing. After swallowing the third spoonful, they were asked to rate residues in mouth. The sensory data were collected using EyeQuestion v.3.8.6, Logic 8 BV software.
Statistical analysis
Panel performance was assessed using XLSTAT panel performance software to assess discrimination power. Sensory scores of each attribute were analysed by two-way analysis of variance ANOVA, with the sample and panellist (and their interactions) as the variation sources. A statistically significant difference was defined as p < 0.05. If a significant difference in means was indicated by the ANOVA, comparisons were performed using Tukey's honest significant difference (HSD) test. Sensory data were also analysed by Principal Component Analysis (PCA). All these analyses were performed with XLSTAT statistical software (Addinsoft, New York, NY, USA).The relationship between the sensory attributes and the different rheological parameters was explored throughout the use of Partial Least Squares Regression (PLS) and Hierarchical Cluster Analysis (HCA) using SIMCA 16.0.1.7928 software (Sartorium Stedim Data Analytics AB – Malmö, Sweden).
Results
Dispersed phase properties
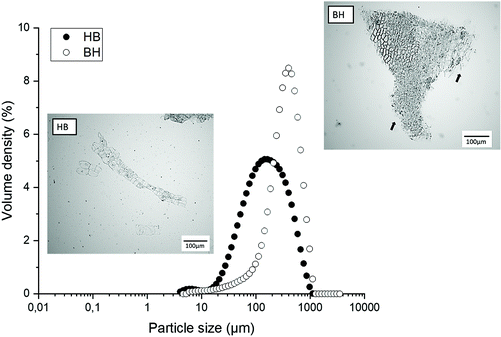
Fig. 1 shows that processing conditions impacted both the particle size distribution and particle morphology of the broccoli suspensions, resulting in samples with high polydispersity. The particle size determined from dynamic light scattering measurements showed that particles obtained from the heat and blend process (HB) had a smaller average particle size and broader particle size distribution, compared to the blend and heat (BH) process which was characterised by a larger average particle size and narrower particle size distribution. The average d4,3 (volume weighted mean) was 221.3 μm and 409.7 μm for HB and BH, respectively. The average d3,2 (surface weighted mean) was 90.5 μm and 199.5 μm for HB and BH, respectively.Particles in the HB samples were characterised by clusters of cells separated by the middle lamella leading to smooth surfaces; in contrast particles from the BH process were larger clusters of cells with regions of cells broken across the cell wall leading to rough surfaces. These results are in agreement with previous work,5 which also showed that smaller particles were obtained from the HB process; during the heating step the pectin from the middle lamella is depolymerized resulting in reduced cell adhesion, causing the tissues to be disrupted through the middle lamella during blending, whereas in the BH process, disruption could occur across the middle lamella or through the cell walls, resulting in clusters with broken cell wall fragments on the surface.
The total solid content of the HB and BH samples was 5.4 ± 1.1 wt% and 5.2 ± 0.7 wt% respectively. After centrifugation, as expected, the total solid content of the pellet from both samples increased to 8.4 ± 0.5 wt% and 8.3 ± 0.1 wt% for HB and BH, respectively. The average pH of the samples was 6.2 ± 0.1.
Rheological properties of simulated bolus
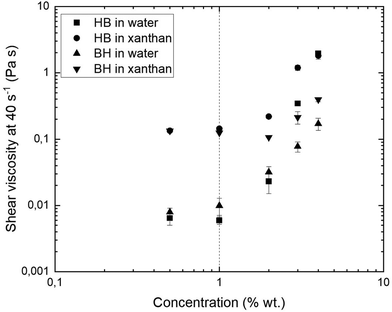
The viscosity of a suspension depends on the viscosity of the fluid phase and the particle phase volume. Therefore, it is expected that the bolus containing xanthan as the fluid phase, and the bolus in which particles pack in a less efficient manner, would have higher viscosities. In this case, this behaviour corresponds to HB samples. In the dilute region, no particle network can be created, and the viscosity is dominated by the fluid phase. In the intermediate region, the dependency on the concentration is more pronounced in the HB particles than BH particles, probably due to the differences in morphology of the particles and how they pack together, but also due to the chemical composition of the particles and their interactions with artificial saliva. The viscosity was higher in xanthan than the water samples, but this difference seemed to be reduced as the total particle concentration increases (especially for HB samples) to 4 wt%. At this concentration the viscosity was dominated by the particles and not the fluid phase as both HB samples in water and xanthan showed similar shear viscosities. Hemar et al.8 reported similar effects in rehydrated cell wall particle mixtures suspended in xanthan solution. They found that at low particle concentration, the suspension behaved as a dispersion of cell particles in a fluid phase of xanthan and that the resulting rheological properties were dominated by xanthan. At high particle concentrations, the xanthan molecules were confined to the interstitial space and thus contributed only marginally to the rheological properties. In a food bolus, saliva can act as a lubricant or a binder for the food particles. The results from this study suggest that the addition of artificial saliva to the samples with water could have a particle binding effect whilst in samples with xanthan, that effect was less apparent.
Broccoli suspensions with a particle concentration of 6 wt% and added artificial saliva (corresponding to a bolus with 3 wt% particle concentration after dilution with artificial saliva) presented a macroscopic aspect of vegetable purees. Therefore, 6 wt% broccoli suspensions were selected for sensory evaluation and further rheological characterisation was carried out with a 3 wt% simulated bolus to represent a potential bolus in the mouth.
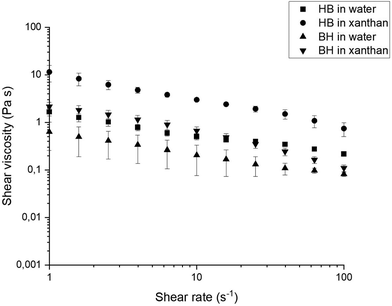
Fig. 3 shows shear viscosity vs. shear rate curves for boli at 3 wt% concentration of HB and BH particles suspended in water and xanthan solution. At this concentration the boli showed shear thinning behaviour, especially for samples containing xanthan solution as the fluid phase.22 Shear thinning behaviour has been proposed to be more appropriate for safe swallowing, because the flow of the bolus is reduced during swallowing, which allows time for the airway to be closed.
| Elastic modulus G′/Pa | Viscous modulus G′′/Pa | Apparent yield stress/Pa | |
|---|---|---|---|
| Heat and blend in water | 15.44 ± 3.40a | 1.78 ± 0.40a | 0.05 ± 0.01a |
| Heat and blend in xanthan | 53.60 ± 10.83b | 13.90 ± 3.40b | 0.40 ± 0.11b |
| Blend and heat in water | 12.08 ± 2.01a | 1.86 ± 0.37a | 0.03 ± 0.02a |
| Blend and heat in xanthan | 354.52 ± 74.01c | 76.96 ± 11.34c | 0.74 ± 0.25b |
Sensory evaluation
Table 2 summarises the results from the sensory evaluation of the broccoli purees. The fluid phase (water or xanthan) had a greater influence on the perception of ease of swallowing than the dispersed phase (HB vs. BH). Samples with xanthan as the fluid phase were perceived as less difficult to swallow i.e. easier to swallow and required less effort to prepare to swallow than those with water. However, the dispersed phase did impact the swallowing perception, as HB samples were easier to swallow and required less effort to prepare the bolus for swallowing than BH suspensions regardless of whether a thickener was added or not. This could be related to the fact that HB samples were perceived to have a smaller particle size, which is in agreement with the particle size measurements (Fig. 1). The type of dispersed phase had an important impact on the perceived particle size. HB samples were perceived as having a small particle size compared to BH samples. Previous studies have shown that concentrated plant-based suspensions with the softest and smallest particles lead to smooth textures;29 however, in our study the panellists were not able to perceive significant differences in smoothness. This could be because the particle size ranged from 30 to 400 μm in the previous study, whereas in this study the size range was narrower (200 to 400 μm). Furthermore, in the previous study the average age of the panel was 48 years and in our study the panellists were older with an average age of 71 years. In general all the samples were rated in the middle-upper part of the smoothness scale, suggesting that dispersion of plant particles with sizes in the range of 200 to 400 μm is not perceived as grainy, which is in agreement with the conclusions from the study by Appelqvist et al.29| Difficulty in swallowing | Effort required to prepare to swallow | Residues in-mouth | Mouth-coating | Particle size perception | Smoothness | |
|---|---|---|---|---|---|---|
| Heat and blend in water | 2.95ab | 2.11ab | 2.53b | 3.21ab | 1.79b | 3.21a |
| Heat and blend in xanthan | 1.90c | 1.74b | 2.26b | 2.42b | 1.47b | 3.32a |
| Blend and heat in water | 3.68a | 3.05a | 3.79a | 3.68a | 3.21a | 2.47a |
| Blend and heat in xanthan | 2.37bc | 2.05b | 2.47b | 2.68b | 2.11b | 2.74a |
Samples with xanthan as fluid phase left fewer residues in the mouth and were less mouth coating compared to samples with water; this was especially perceived in the case of BH samples. In general, samples prepared with xanthan as the fluid phase were perceived very similarly to each other, independently of the type of dispersed phase. It is worth noting that the panellist could discriminate among the samples with different fluid phase but not among the dispersed phase within the xanthan samples, except for the ease of swallowing.
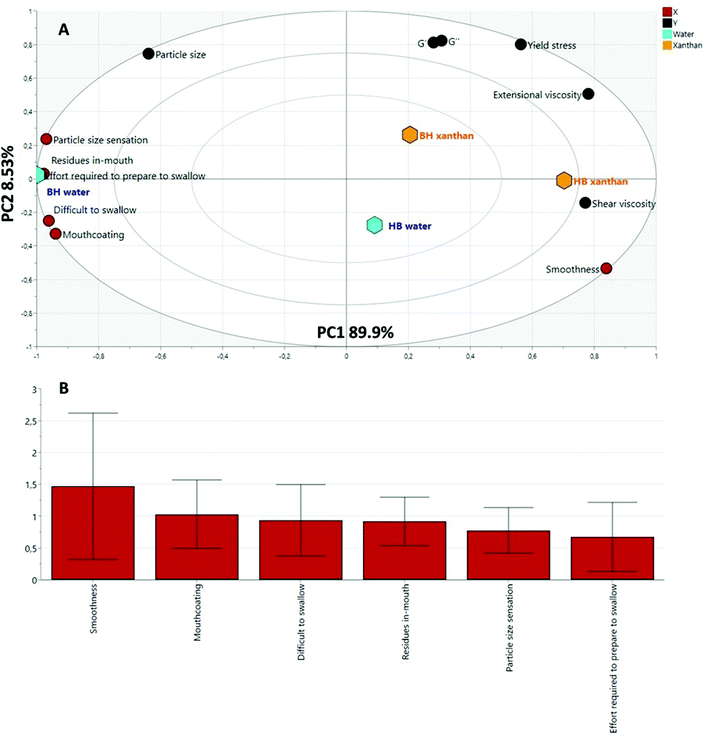
The PLS model was built introducing the sensory attributes as X variables and the rheological parameters as Y variables. As illustrated in Fig. 6A, the distribution of the samples along F1 (94.1%) is driven by the type of fluid used whereas the type of processing drives F2 (5.27%). HCA analysis unveiled two different groups in which BH in water was found to be different from the other three samples (HB water, BH xanthan and HB xanthan). These results are also strongly supported by the statistical differences in individual sensory attributes shown in Table 2. Thus, although the final characteristics of the samples are driven by the fluid phase, their perception was the result of the combination of the disperse and the fluid phase.
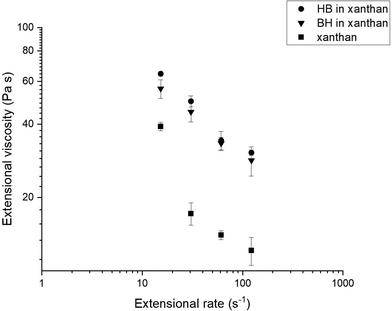
To assess which variables better describe the entire PLS model, a Variable Importance in Projection (VIP) plot (Fig. 6B) was generated. The VIP plot highlights the most important X variables (sensory attributes) describing the difference between samples in relation with Y variables (rheological parameters). Residues in mouth and particle size perception were found to be the sensory parameters to better represent the model. The perception of residues in mouth showed correlation with the particle size (0.61). Therefore, the larger the particle size, the greater the perception of residues in mouth. Mouth-coating showed a negative correlation with the extensional viscosity. The lower the extensional viscosity of the sample, the higher the mouth-coating sensation as it occurs for samples with water as a fluid phase. The more cohesive nature of the samples with xanthan would reduce the coating of oral surfaces.
Difficulty in swallowing had a high negative correlation with yield stress (−0.71) and especially with extensional viscosity (−0.92). This indicates that the higher the yield stress and extensional viscosity, the easier it is to swallow a bolus, as it was observed for xanthan samples. Furthermore, the higher the extensional viscosity and shear viscosity of the sample, the lower the effort required to swallow (−0.73 and −0.72 respectively). More detailed information regarding the relationship between the different variables is described on the correlation matrix (ESI Table S1†).
Conclusions
In this research we have investigated the contribution of the dispersed and fluid phase to the ease of swallowing of particulated foods during oral processing. We used broccoli suspensions as model systems and a food bolus was mimicked by mixing suspensions with artificial saliva. Our results indicate that the fluid phase has the highest impact on the perception of ease of swallowing, compared to the type of dispersed phase. Although the mouthfeel and ease of swallowing perceived during oral processing were driven by the fluid phase, they were the result of the combination of both the disperse and the fluid phase. This is likely due not only to the properties of the fluid phase itself, but also to how it interacts with the particles and affects the way they pack together in the bolus, leading to specific rheological properties. These findings support the hypothesis that the presence of yield stress and extensibility in the bolus, achieved by addition of xanthan as fluid phase, leads to particulated foods perceived as easy to swallow. In addition, suspensions with a broader particle size distribution and smaller particle size were easier to swallow, with good agreement between particle size measurements and particle size perception by the panel. The results highlight the importance of understanding the role of fluids and dispersed phase of complex food systems to design personalised foods with optimal sensory properties for specific populations such as those suffering from swallowing disorders.Funding
This work was supported by the Swedish Research Council Formas (2018-01346).Conflicts of interest
There are no conflicts to declare.Acknowledgements
Lisa Maria Oberrauter is kindly acknowledged for assistance with sensory evaluations. Jan-Willems Benjamin and Lubica Macakova are acknowledged for particle size measurements. Mats Stading is gratefully acknowledged for useful discussions.References
- C. M. Steele, W. A. Alsanei, S. Ayanikalath, C. E. Barbon, J. Chen, J. A. Cichero, K. Coutts, R. O. Dantas, J. Duivestein, L. Giosa, B. Hanson, P. Lam, C. Lecko, C. Leigh, A. Nagy, A. M. Namasivayam, W. V. Nascimento, I. Odendaal, C. H. Smith and H. Wang, The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review, Dysphagia, 2015, 30, 2–26 CrossRef.
- J. Chen and L. Lolivret, The determining role of bolus rheology in triggering a swallowing, Food Hydrocolloids, 2011, 25, 325–332 CrossRef CAS.
- M. Nystrom, W. M. Qazi, M. Bulow, O. Ekberg and M. Stading, Effects of rheological factors on perceived ease of swallowing, Appl. Rheol., 2015, 25, 40–48 Search PubMed.
- K. R. N. Moelants, R. Cardinaels, S. Van Buggenhout, A. M. Van Loey, P. Moldenaers and M. E. Hendrickx, A Review on the Relationships between Processing, Food Structure, and Rheological Properties of Plant-Tissue-Based Food Suspensions, Compr. Rev. Food Sci. Food Saf., 2014, 13, 241–260 CrossRef CAS.
- P. Lopez-Sanchez, J. Nijsse, H. C. Blonk, L. Bialek, S. Schumm and M. Langton, Effect of mechanical and thermal treatments on the microstructure and rheological properties of carrot, broccoli and tomato dispersions, J. Sci. Food Agric., 2011, 91, 207–217 CrossRef CAS.
- M. Nakauma, S. Ishihara, T. Funami and K. Nishinari, Swallowing profiles of food polysaccharide solutions with different flow behaviors, Food Hydrocolloids, 2011, 25, 1165–1173 CrossRef CAS.
- I. T. Norton, D. M. Goodall, S. A. Frangou, E. R. Morris and D. A. Rees, Mechanism and dynamics of conformational ordering in xanthan polysaccharide, J. Mol. Biol., 1984, 175, 371–394 CrossRef CAS.
- Y. Hemar, S. Lebreton, M. Xu and L. Day, Small-deformation rheology investigation of rehydrated cell wall particles–xanthan mixtures, Food Hydrocolloids, 2011, 25, 668–676 CrossRef CAS.
- E. K. Hadde and J. Chen, Shear and extensional rheological characterization of thickened fluid for dysphagia management, J. Food Eng., 2019, 245, 18–23 CrossRef.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. Wickham, W. Weitschies and A. Brodkorb, A standardised static in vitro digestion method suitable for food - an international consensus, Food Funct., 2014, 5, 1113–1124 RSC.
- M. R. C. Marques, R. Loebenberg and M. Almukainzi, Simulated Biological Fluids with Possible Application in Dissolution Testing, Dissolution Technol., 2011, 18, 15–28 CrossRef CAS.
- M. Nystrom, H. R. T. Jahromi, M. Stading and M. F. Webster, Hyperbolic contraction measuring systems for extensional flow, Mech. Time-Depend. Mater., 2017, 21, 455–479 CrossRef.
- K. Wikstrom and L. Bohlin, Extensional flow studies of wheat flour dough. I. Experimental method for measurements in contraction flow geometry and application to flours varying in breadmaking performance, J. Cereal Sci., 1999, 29, 217–226 CrossRef.
- A. B. Tobin, P. Heunemann, J. Wemmer, J. R. Stokes, T. Nicholson, E. J. Windhab and P. Fischer, Cohesiveness and flowability of particulated solid and semi-solid food systems, Food Funct., 2017, 8, 3647–3653 RSC.
- K. Slettengren, E. Xanthakis, L. Ahrne and E. J. Windhab, Flow Properties of Spices Measured with Powder Flow Tester and Ring Shear Tester-XS, Int. J. Food Prop., 2016, 19, 1475–1482 CrossRef.
- M. Sharma, E. Kristo, M. Corredig and L. Duizer, Effect of hydrocolloid type on texture of pureed carrots: Rheological and sensory measures, Food Hydrocolloids, 2017, 63, 478–487 CrossRef CAS.
- J. J. X. Ong, C. M. Steele and L. M. Duizer, Challenges to assumptions regarding oral shear rate during oral processing and swallowing based on sensory testing with thickened liquids, Food Hydrocolloids, 2018, 84, 173–180 CrossRef CAS.
- Z. Vickers, H. Damodhar, C. Grummer, H. Mendenhall, K. Banaszynski, R. Hartel, J. Hind, A. Joyce, A. Kaufman and J. Robbins, Relationships Among Rheological, Sensory Texture, and Swallowing Pressure Measurements of Hydrocolloid-Thickened Fluids, Dysphagia, 2015, 30, 702–713 CrossRef CAS.
- A. Tobin, Insights into Rational Design of Plant Based Foods to Improve Management of Dysphagia, PhD Thesis, The University of Queensland, 2014, DOI:10.14264/uql.2015.160.
- C. Leverrier, G. Almeida, L. Espinosa-Munoz and G. Cuvelier, Influence of Particle Size and Concentration on Rheological Behaviour of Reconstituted Apple Purees, Food Biophys., 2016, 11, 235–247 CrossRef.
- C. Leverrier, G. Almeida, P. Menut and G. Cuvelier, Design of Model Apple Cells Suspensions: Rheological Properties and Impact of the Continuous Phase, Food Biophys., 2017, 12, 383–396 CrossRef.
- N. Vilardell, L. Rofes, V. Arreola, R. Speyer and P. Clave, A Comparative Study Between Modified Starch and Xanthan Gum Thickeners in Post-Stroke Oropharyngeal Dysphagia, Dysphagia, 2016, 31, 169–179 CrossRef CAS.
- D. B. Genovese, J. E. Lozano and M. A. Rao, The Rheology of Colloidal and Noncolloidal Food Dispersions, J. Food Sci., 2007, 72, R11–R20 CrossRef CAS.
- L. Day, M. Xu, S. K. Øiseth, L. Lundin and Y. Hemar, Dynamic rheological properties of plant cell-wall particle dispersions, Colloids Surf., B, 2010, 81, 461–467 CrossRef CAS.
- P. Lopez-Sanchez, V. Chapara, S. Schumm and R. Farr, Shear Elastic Deformation and Particle Packing in Plant Cell Dispersions, Food Biophys., 2012, 7, 1–14 CrossRef.
- C. Macosco, Rheology:principles,measurements and applications, VCH publishers, New York, 1993 Search PubMed.
- K. Nishinari, M. Turcanu, M. Nakauma and Y. Fang, Role of fluid cohesiveness in safe swallowing, Sci. Food, 2019, 3, 5 Search PubMed.
- A. I. V. Ross, P. Tyler, M. G. Borgognone and B. M. Eriksen, Relationships between shear rheology and sensory attributes of hydrocolloid-thickened fluids designed to compensate for impairments in oral manipulation and swallowing, J. Food Eng., 2019, 263, 123–131 CrossRef CAS.
- I. A. M. Appelqvist, M. Cochet-Broch, A. A. M. Poelman and L. Day, Morphologies, volume fraction and viscosity of cell wall particle dispersions particle related to sensory perception, Food Hydrocolloids, 2015, 44, 198–207 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0fo01728k |
| This journal is © The Royal Society of Chemistry 2020 |