Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The effect of culinary doses of spices in a high-saturated fat, high-carbohydrate meal on postprandial lipemia and endothelial function: a randomized, controlled, crossover pilot trial†
Kristina S.
Petersen
 a,
Connie J.
Rogers
a,
Connie J.
Rogers
 a,
Sheila G.
West
a,
Sheila G.
West
 ab,
David N.
Proctor
ab,
David N.
Proctor
 c and
Penny M.
Kris-Etherton
c and
Penny M.
Kris-Etherton
 *a
*a
aDepartment of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA. E-mail: pmk3@psu.edu; Tel: +1 (814) 863-2923
bDepartment of Biobehavioral Health, The Pennsylvania State University, University Park, PA, USA
cDepartment of Kinesiology, The Pennsylvania State University, University Park, PA, USA
First published on 20th March 2020
Abstract
Previously it has been shown that incorporation of >11 g of spices into a mixed meal blunts postprandial lipemia, which may reduce acute endothelial impairment. The effect of lower doses of spices remains unclear. The aim was to examine the postprandial effect of a meal high in saturated fat and carbohydrate inclusive of spices (2 g or 6 g) or exclusive of spices (0 g) on flow mediated dilation (FMD), lipids and lipoproteins, glucose, and insulin in men at-risk for cardiovascular disease. A 3-period randomized, controlled, crossover, pilot study was conducted. In random order, subjects consumed a high-saturated fat, high-carbohydrate meal (1076 kcal, 39 g saturated fat, 98 g carbohydrate) with 0 g, 2 g and 6 g of mixed spices. After meal consumption, blood was drawn hourly for 4 hours and FMD was measured at 2 and 4 hours. Serum lipids and lipoproteins, and insulin were measured in the fasting state and at each post-meal time point; plasma glucose was also assessed at each time point. Subjects were 13 men aged 52 ± 9 years that were overweight or obese (29.9 ± 3.1 kg m−2), and had an enlarged waist circumference (102.2 ± 8.9 cm). Time (p < 0.05) and treatment (p < 0.05) effects existed for FMD and triglycerides; no time by treatment interactions were detected. Post hoc testing showed that the meal with 6 g of spices lessened the postprandial reduction in FMD compared to the meal with no spices (−0.87 ± 0.32%; p = 0.031); no other pairwise differences were observed. Triglyceride levels were lower following the meal with 2 g of spices vs. the no spice meal (−18 ± 6 mg dL−1; p = 0.015); no difference was observed between the meal with 6 g of spice and the no spice meal (−13 ± 6 mg dL−1; p = 0.12). Glucose and insulin were unaffected by the presence of spices in the meal. In conclusion, this study provides preliminary evidence suggesting that lower doses of spices (2 and 6 g) than previously tested may attenuate postprandial lipemia and impairments in endothelial function caused by a high-saturated fat, high-carbohydrate meal.
Introduction
Humans spend approximately 18-hours per day in the postprandial (fed) state. Consumption of meals rich in fat, including saturated fat, induce postprandial lipemia,1 and a direct association exists between postprandial triglyceride levels and cardiovascular disease (CVD);2–5 the leading cause of death and disability globally and in the U.S.6,7 Non-fasting triglyceride levels better predict CVD risk than fasting levels.8 Hypertriglyceridemia promotes formation of remnant lipoprotein cholesterol2 that infiltrates the arterial wall, and causes endothelial impairment and atherosclerosis.9 Endothelial dysfunction, measured non-invasively by flow-mediated dilation (FMD), is strongly associated with the development of CVD.10 Since high-saturated fat meals are commonly consumed in the U.S. and other high-income countries11 strategies to blunt postprandial lipemia and attenuate impairments in endothelial function are required.Inclusion of relatively large doses of spices in a high-saturated fat meal attenuates postprandial lipemia.12,13 Two previous studies have shown that incorporation of approximately 14 g of spices (black pepper, cinnamon, cloves, ginger, garlic, oregano, paprika, rosemary and turmeric) in a meal containing ∼1000 kcal, ∼45 g of fat (predominantly saturated fat), and ∼98 g of carbohydrate lessened the postprandial increase in triglycerides compared to an isocaloric macronutrient matched meal containing no spices.12,13 In these studies, endothelial function was not assessed, thus the functional consequences of the spice-mediated reduction in lipemia on the vasculature is not clear.
Nakayama et al.14 observed a greater improvement in FMD at 1-hour following consumption of a relatively low-fat (10 g fat) curry containing 14.5 g of spices (clove, coriander, cumin, garlic, ginger, red pepper, turmeric) compared with a meal containing no spices in healthy men. This suggests that spices may affect vascular function independently of mechanisms related to post-meal lipemia. However, the post-prandial phase was not fully characterized since measurements were only taken at 1-hour.
Previous studies have tested doses of spices that are likely higher than what is customarily consumed as part of a meal in the U.S. Data for average U.S. population consumption of spices are not available, although based on the USDA Economic Research Services 2015 Supply and Disappearance data, ∼5 g day−1 of spices are available per person in the U.S (this does not take into account food spoilage and plate waste).15 Therefore, meal time consumption of spices is likely substantially lower than the doses previously tested. It remains unclear whether lower doses of spices, which may be more feasible for individuals in the U.S. to habitually consume as part of a mixed meal, exert similar effects on postprandial lipemia and FMD.
The aim of this pilot study was to investigate the postprandial effects of including spices, at culinary doses, in a meal rich in saturated fat and carbohydrates on FMD in men with enlarged waist circumference at-risk for CVD. Secondary outcomes were lipids and lipoproteins, glucose, and insulin. It was hypothesized that, in a dose–response manner, the presence of spices in the meal would attenuate the postprandial increase in triglycerides resulting in less acute impairment of FMD.
Methods
Study design
A three-period, randomized, controlled, crossover, pilot study was conducted. In random order, subjects consumed a high-saturated fat, high-carbohydrate meal containing 0 g, 2 g, and 6 g of spices. After meal consumption, blood was drawn hourly for 4 hours and FMD was measured at 2 and 4 hours. The primary outcome was FMD; secondary outcomes were lipids and lipoproteins, glucose, and insulin. Treatment order was randomized using a computer-generated scheme (http://www.randomization.com) and personnel conducting testing were blinded to the treatment order. Only the kitchen staff were aware of the treatment order during the data collection phase. The Institutional Review Board of the Pennsylvania State University approved the study (STUDY00005575) and the study was conducted in accordance with the Declaration of Helsinki of 1975. All subjects gave written informed consent before enrollment in the study. The trial is registered at http://clinicaltrials.gov (NCT03064958).Subjects
Males aged 40–65 years, with a BMI of 25 to 35 kg m−2, increased waist circumference (≥94 cm), and at least one other cardiovascular risk factor, i.e., triglycerides ≥ 150 mg dL−1; HDL-cholesterol < 40 mg dL−1; systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mm Hg; fasting glucose ≥ 100 mg dL−1 or C-reactive protein (CRP) > 1 mg dL−1 were eligible. Current use of nicotine products, type 2 diabetes, blood pressure > 160/100 mm Hg, prescription of anti-hypertensive, lipid lowering, or glucose lowering medication, CVD, liver or kidney disease were exclusion criteria. Originally, we aimed to enroll six subjects; however, a preliminary analysis for the inflammatory endpoints revealed a trend towards a modified response based on fasting blood glucose levels (normal range, <100 mg dL−1vs. range for impaired fasting glucose, ≥100 mg dL−1). Subsequently, an additional 7 subjects were recruited with the goal of having half of the sample with a fasting glucose value within the normal range (<100 mg dL−1) and the remainder in the range for impaired fasting glucose (≥100 mg dL−1); one subject was excluded from the inflammation analyses because of a low white cell count on the testing days indicative of an acute infection. These results will be reported in a subsequent manuscript.Recruitment
Subjects were recruited from the State College PA area using flyers placed on the University campus and in the community. Advertisements for the study were also placed on our website, and sent via University email lists; individuals who had previously participated in our studies were contacted. Individuals expressing interest in the study were provided with further information about the study. To determine eligibility a telephone screening comprising questions based on key inclusion and exclusion criteria was conducted. Individuals that were eligible based on the telephone screening, attended a screening appointment at the Clinical Research Center. After a 12 hours fast and alcohol avoidance for 48 hours, height, weight, blood pressure, and waist circumference were measured. Weight was measured in light clothing after removal of shoes, and blood pressure was measured by trained research nurses using a validated sphygmomanometer. Waist circumference was measured at the iliac crest by trained research nurses while participants were standing, feet shoulder-width apart with clothing removed from their waistline. Two nurses completed each measurement to ensure the tape was correctly positioned and parallel to the floor. Two measurements were taken to 0.1 cm and averaged. If measurements differed by more than 0.5 cm, a third measurement was taken. A blood draw was taken and sent to a commercial laboratory (Quest Diagnostics, Pittsburgh PA) for measurement of lipids, lipoproteins, glucose, CRP, blood chemistry, and complete blood count.Composition of the test meals
Table 1 shows the nutrient composition of the test meal that was used for all three-conditions. The test meal was comprised of a corn muffin, coconut chicken entrée, and a cookie; spices were incorporated into these meal items. This meal was used as the experimental challenge because it was previously shown to induce postprandial lipemia.12Table 2 shows the spices and the quantity that was incorporated into the three meal challenges. The test spices included basil (percentage of total dose 6.6%); bayleaf (3.2%); black pepper (3.9%); cinnamon (11.4%); coriander (6.6%); cumin (6.6%); ginger (12.9%); oregano (9.4%); parsley (6.9%); red pepper (6.6%); rosemary (5.1%); thyme (3.2%); and turmeric (17.5%). These spices were tested on the basis of literature showing cardiovascular benefits of the spices individually. In addition, they are among the most widely consumed spices in the U.S. All of the spices were in the dried form and provided by McCormick Science Institute (Hunt Valley, MD, USA). The meals were prepared by a Registered Dietitian in the metabolic kitchen at the Penn State Clinical Research Center. Spices were weighed with a balance accurate to 0.01 gram (Mettler Toledo, Columbus, OH, USA).| Nutrient profilea | Value |
|---|---|
| a Presented nutrient values were determined using the Nutrient Data System for Research (Minneapolis, MN). | |
| Energy (kcal) | 1076 |
| Protein (g) | 43 |
| Protein (% kcal) | 16 |
| Carbohydrates (g) | 98 |
| Carbohydrates (% kcal) | 36 |
| Total fat (g) | 60 |
| Total fat (% kcal) | 50 |
| Saturated fat (g) | 39 |
| Saturated fat (% kcal) | 33 |
| Dietary fiber (g) | 3 |
| Spicesa | Control meal (0 g) | 2 g mealb | 6 g mealb |
|---|---|---|---|
| a The spices were in the dried form and provided by McCormick Science Institute (Hunt Valley, MD, USA). b Spices were weighed with a balance accurate to 0.01 gram (Mettler Toledo, Columbus, OH, USA). | |||
| Basil | 0 | 0.13 | 0.4 |
| Bayleaf | 0 | 0.06 | 0.2 |
| Black pepper | 0 | 0.08 | 0.23 |
| Cinnamon | 0 | 0.23 | 0.68 |
| Coriander | 0 | 0.13 | 0.4 |
| Cumin | 0 | 0.13 | 0.4 |
| Ginger | 0 | 0.26 | 0.76 |
| Oregano | 0 | 0.19 | 0.56 |
| Parsley | 0 | 0.14 | 0.41 |
| Red pepper | 0 | 0.13 | 0.4 |
| Rosemary | 0 | 0.1 | 0.31 |
| Thyme | 0 | 0.06 | 0.2 |
| Turmeric | 0 | 0.35 | 1.05 |
Outcome assessments
After a 12 hours fast, no vigorous physical activity for 12 hours, and avoidance of alcohol and over-the-counter medications for 48 hours, participants visited the Clinical Research Center on three separate occasions, separated by at least 3 days. Polyphenols reach maximum plasma concentration within hours of ingestion (range 1.5–5.5 hours) and the excretion half-life is within one day (range 1.3–20 hours), therefore a 3-day washout period was used for the study.16 All testing was commenced between 6:30 am and 9 am, and testing time was kept consistent within-subjects. Upon arrival, in the fasting state, FMD was performed, and a catheter was positioned and a fasting blood sample was taken by trained research nurses. After baseline testing was completed, the meal was provided and the subjects consumed the meal within 15 minutes. At 60, 120, 180, and 240 minutes after completion of the meal, blood was collected. At 120 and 240 minutes FMD was performed. During the 4-hour post-meal testing period subjects rested quietly in a hospital-style clinic room and were allowed to watch television, use a computer or tablet, read, or sleep. Subjects consumed water ad libitum during the post-meal period.Flow mediated dilation
In the fasting state and at 120 and 240 minutes post meal consumption FMD was measured. These time points were examined because previous literature shows that phenolic compounds reach peak plasma level 2 hours following consumption;16,17 however, peak triglyceride response occurs 4 hours post meal.2 Thus, these time points correspond with the proposed mechanisms by which spices may modulate FMD. In the supine position, participants rested in a darkened room for 5 minutes prior to testing. All of the ultrasound examinations were completed by a single sonographer using a GE Logiq e (General Electric Company, Boston MA) ultrasound imaging system with a 10 MHz linear array transducer. Continuous, longitudinal, images of the brachial artery at 5 to 10 cm above the elbow on the right arm were recorded at five frames per second during baseline (1 minute), occlusion (5 minutes), and post-deflation (2 minutes). Occlusion was induced by inflation of a blood pressure cuff on the forearm (distal to the target artery) to 250 mm Hg using an automated device (D. E. Hokanson, Inc., Bellevue, WA, USA).Automated edge detection software (Brachial Analyzer; MIA, Iowa City, IA, USA) was used to measure artery diameter continuously throughout the recording by two trained scorers. Baseline diameter was defined as the average of all of the images collected over the 1 minute of the baseline recording. Peak artery diameter was determined as the largest diameter recorded in the first 2 minutes of the post-deflation period. Percent change in brachial diameter at peak dilation compared to baseline was calculated. The average of the two scorers’ values was used for analysis; if the two scorers' FMD value differed by more than 2 percentage points, a third person scored the scan and the average of the two values within 2 percentage points were used for analysis. Flow velocity was measured using duplex-pulsed Doppler with the ultrasound beam at two time points: at the beginning of baseline and immediately after cuff release. Flow (ml min−1) was calculated, based on the average of five cardiac cycles at each time point, using the following equation: velocity time integral × cross-sectional area of the vessel (π*(brachial artery diameter at baseline/2)2) × heart rate. Reactive hyperemia was calculated as the change in flow after cuff release and was calculated as (peak flow − baseline flow)/baseline flow × 100.
Lipids, lipoproteins, glucose, and insulin
Prior to meal consumption, and at 60, 120, 180, and 240 minutes after meal consumption whole blood was collected in a serum separator tube for analysis of lipids, lipoproteins, and insulin. The serum separator tube was kept at room temperature for 30 minutes to clot and centrifuged for 15 minutes. Aliquots were frozen at −80 °C and analyses run in batches at the end of the study. Samples were sent to a commercial laboratory (Quest Diagnostics, Pittsburgh, PA, USA) for analysis of triglycerides, total cholesterol, HDL cholesterol, and non-HDL cholesterol by spectrophotometry. Insulin was assessed by immunoassay. LDL-cholesterol was calculated using the Martin–Hopkins equation, which is appropriate for non-fasting samples.18Whole blood was also collected into blood collection tubes containing lithium heparin for measurement of glucose. This tube was centrifuged immediately upon collection for 15 minutes. Frozen aliquots were sent to a commercial laboratory (Quest Diagnostics, Pittsburgh, PA, USA) in batches at the end of the study for analysis of glucose by spectrophotometry.
Statistical analyses
Statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA). Normality of the residuals was assessed using Q–Q plots in combination with the Kolmogorov–Smirnov statistic and associated p-value (PROC UNIVARIATE). For variables with an approximately non-normal distribution, log-transformations were performed to normalize the distribution. The mixed models procedure (PROC MIXED) was used for all analyses with subjects modeled as a repeated factor. Covariance structure selection was based on optimizing the fit statistics based on the Bayesian Information Criterion; for all outcomes compound symmetry provided the best fit. In the primary analyses, between-treatment mean differences at each time point, for all outcomes, were assessed by the presence of main effects for time, treatment, and a time by treatment interaction. In addition, time, treatment, and time by treatment interactions were also assessed for the change in each variable from the pre-meal value. When a main interaction for time, treatment or time by treatment existed, post-hoc testing was conducted and the Tukey–Kramer method was used to adjust for multiple comparisons. Visit (1, 2 or 3) was included in the models to assess for the presence of carry-over effects; when a visit by treatment interaction was not detected visit was removed from the model. Spearman correlations were used to assess the relationship between the change in FMD and the change in triglycerides in the post-meal period using PROC CORR. Since subjects were a repeated factor, correlations were assessed by time-point and treatment separately. For log-transformed variables, data are presented as the least-squared geometric mean and the 95% confidence interval; for all other variables data are presented as least-squared means and standard errors, unless otherwise stated. Based on a previous study,13 6 subjects provides 80% power to detect a 27 ± 32 mg dL−1 difference in triglycerides (α = 0.05); comparable data to inform a power calculation for FMD were not available; however, based on the known variance of FMD,19 6 subjects provides 80% power to detect a 1.9 ± 2.3% difference in FMD between the treatments. Based on the actual sample size of 13, the study was powered to detect an FMD difference of 1.1 ± 2.3% and a triglyceride difference of 15 ± 32 mg dL−1 with 80% power (α = 0.05).Results
A total of 13 men completed the study. Sixty-three men were telephone screened, 28 attended a clinic screening appointment; 13 men did not meet the inclusion criteria, and 15 men qualified for the study. Two men were enrolled but did not complete the study; one subject withdrew prior to day 1 testing because of personal reasons, and one subject was intolerant of the FMD procedure at baseline on day 1 of testing and withdrew. Subjects were 52 ± 9 years of age, had a BMI in the overweight to obese range (29.9 ± 3.1 kg m−2), and had enlarged waist circumference (102.2 ± 8.9 cm) (Table 3). One subject had one qualifying CVD risk factor, five subjects had two risk factors, four subjects had three risk factors, one subject had four risk factors, and two subjects had five risk factors. In the fasting state, there were no differences between the test days or by randomization for FMD (p = 0.66, p = 0.17, respectively), triglycerides (p = 0.61, p = 0.47), glucose (p = 0.78, p = 0.19), insulin (p = 0.89, p = 0.84), total cholesterol (p = 0.74, p = 0.52), non-HDL cholesterol (p = 0.73, p = 0.33), LDL-cholesterol (p = 0.54, p = 0.43), and HDL-cholesterol (p = 0.95, p = 0.28). No evidence of carry-over effects was detected.| Characteristic | Mean ± standard deviation |
|---|---|
| Age (years) | 52 ± 9 |
| Weight (kg) | 92.5 ± 11.5 |
| BMI (kg m−2) | 29.9 ± 3.1 |
| Waist circumference (cm) | 102.2 ± 8.9 |
| Systolic blood pressure (mm Hg) | 121 ± 11 |
| Diastolic blood pressure (mm Hg) | 78 ± 5 |
| Glucose (mg dL−1) | 97.3 ± 11.5 |
| Total cholesterol (mg dL−1) | 201.8 ± 35.0 |
| LDL-cholesterol (mg dL−1) | 131.9 ± 28.3 |
| HDL-cholesterol (mg dL−1) | 44.2 ± 9.3 |
| Triglycerides (mg dL−1) | 128.9 ± 61.1 |
| CRP (mg L−1) | 1.8 ± 2.1 |
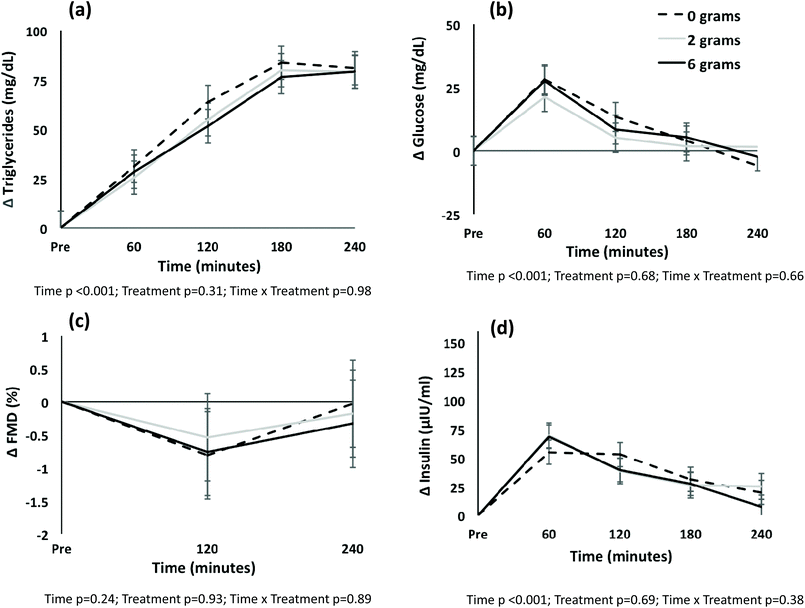
Both time and treatment effects existed for mean FMD and brachial artery dilation (both p < 0.05); no time by treatment interaction was present (both p > 0.05) (Table 4). Post-hoc testing showed that mean FMD differed between the meal with no spices and the meal with 6 g of spices (mean difference −0.87 ± 0.32%; p = 0.031); no difference was detected between the meal with 2 g of spices and the no spice meal (p = 0.40). Similarly, a difference in brachial artery dilation existed between the meal with no spices and the meal with 6 g of spices (mean difference −0.04 ± 0.02 mm; p = 0.038). No time, treatment or time by treatment interaction was observed for the change in FMD from pre-meal values (Fig. 1C). Baseline flow, peak flow, and reactive hyperemia did not differ by treatment, and no time by treatment interaction was observed (p > 0.05); a time effect was observed whereby baseline and peak flow tended to decline from pre-meal to 240 minutes, and reactive hyperemia increased over time (Table 4).
| Variable | 0 g spice meal | 2 g spice meal | 6 g spice meal | P value time | P value treatment | P value time × treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T120 | T240 | T0 | T120 | T240 | T0 | T120 | T240 | ||||
| Data are least squared means ± SEM.a Data non-normally distributed so presented as geometric mean (95% CI); PROC MIXED (SAS Institute, Cary, NC, USA) was used for analyses with subjects modeled as a repeated factor. | ||||||||||||
| FMD (%) | 4.91 ± 0.54 | 4.10 ± 0.54 | 4.94 ± 0.54 | 5.11 ± 0.54 | 4.57 ± 0.54 | 5.53 ± 0.54 | 5.73 ± 0.54 | 4.97 ± 0.54 | 5.8 5± 0.54 | 0.024 | 0.040 | 0.99 |
| Baseline brachial artery diameter (mm) | 5.19 ± 1.56 | 5.27 ± 1.56 | 5.26 ± 1.56 | 5.22 ± 1.56 | 5.26 ± 1.56 | 5.22 ± 1.56 | 5.14 ± 1.56 | 5.27 ± 1.56 | 5.1 5± 1.56 | 0.050 | 0.27 | 0.58 |
| Peak brachial artery diameter (mm) | 5.44 ± 0.16 | 5.48 ± 0.16 | 5.52 ± 0.16 | 5.48 ± 0.16 | 5.49 ± 0.16 | 5.51 ± 0.16 | 5.44 ± 0.16 | 5.53 ± 0.16 | 5.45 ± 0.16 | 0.25 | 0.80 | 0.57 |
| Peak dilation (mm) | 0.25 ± 0.03 | 0.21 ± 0.03 | 0.26 ± 0.03 | 0.26 ± 0.03 | 0.23 ± 0.03 | 0.28 ± 0.03 | 0.29 ± 0.03 | 0.26 ± 0.03 | 0.30 ± 0.03 | 0.024 | 0.048 | 0.98 |
| Baseline flow (ml min−1)a | 260 (194, 344) | 228 (171, 302) | 206 (156, 276) | 302 (228, 403) | 233 (176, 311) | 226 (169, 302) | 273 (206, 365 | 240 (181, 321) | 213 (159, 284) | 0.009 | 0.52 | 0.95 |
| Peak flow (ml min−1)a | 1353 (1141, 1604) | 1300 (1097, 1541) | 1367 (1153, 1636) | 1510 (1274, 1790) | 1339 (1130, 1588) | 1353 (1141, 1620) | 1525 (1274, 1808) | 1287 (1086, 1525) | 1422 (1188, 1686) | 0.03 | 0.41 | 0.53 |
| Reactive hyperemia (% change) | 447 ± 64 | 517 ± 64 | 602 ± 64 | 443 ± 64 | 533 ± 64 | 554 ± 65 | 509 ± 64 | 478 ± 64 | 593 ± 65 | 0.01 | 0.89 | 0.59 |
Time (p < 0.001) and treatment (p = 0.018) main effects were present for mean triglyceride levels (Table 5). Post hoc pairwise testing showed that triglycerides were statistically different at all times from pre-meal to 180 minutes (all p < 0.038); triglyceride levels at time 180 and 240 minutes were not different (p > 0.99). The treatment effect was attributable to a difference between the meal with no spices and the meal with 2 g of spices (mean difference 18 ± 6 mg dL−1; p = 0.015); the difference between the meal with no spices and the meal with 6 g of spices was attenuated to non-significance after adjustment for multiple comparisons (mean difference 13 ± 6 mg dL−1; p = 0.12). No treatment or time by treatment interaction was observed when the change in triglycerides from pre-meal levels was plotted (Fig. 1A).
| Variable | 0 g spice meal | 2 g spice meal | 6 g spice meal | P value time | P value treatment | P value time × treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T60 | T120 | T180 | T240 | T0 | T60 | T120 | T180 | T240 | T0 | T60 | T120 | T180 | T240 | ||||
| Data presented as least-squared means ± SEM unless otherwise stated.a Data non-normally distributed so presented as geometric mean (95% CI); PROC MIXED (SAS Institute, Cary, NC, USA) was used for analyses with subjects modeled as a repeated factor. | ||||||||||||||||||
| Triglycerides (mg dL−1) | 131 ± 20 | 163 ± 20 | 195 ± 20 | 215 ± 20 | 212 ± 20 | 117 ± 20 | 143 ± 20 | 172 ± 20 | 197 ± 20 | 196 ± 20 | 124 ± 20 | 152 ± 20 | 175 ± 20 | 200 ± 20 | 203 ± 20 | <0.001 | 0.018 | 0.99 |
| Total cholesterol (mg dL−1)a | 191 (171, 213) | 194 (174, 215) | 192 (174, 215) | 192 (174, 215) | 194 (176, 217) | 189 (171, 211) | 187 (169, 209) | 192 (174, 215) | 194 (176, 217) | 196 (178, 219) | 187 (167, 206) | 189 (169, 209) | 189 (169, 209) | 187 (169, 209) | 192 (172, 213) | 0.17 | 0.029 | 0.91 |
| Non-HDL cholesterol (mg dL−1)a | 153 (134, 174) | 156 (137, 178) | 158 (138, 178) | 158 (138, 179) | 159 (140, 181) | 150 (132, 171) | 150 (132, 169) | 154 (136, 176) | 158 (138, 179) | 159 (140, 181) | 147 (129, 167) | 148 (132, 169) | 150 (132, 171) | 150 (133, 171) | 154 (136, 176) | 0.007 | 0.005 | 0.96 |
| LDL-cholesterol | 128 (113, 145) | 129 (113, 145) | 125 (111, 141) | 124 (110, 140) | 126 (111, 143) | 129 (112, 144) | 124 (110, 140) | 125 (111, 141) | 125 (111, 143) | 128 (112, 144) | 124 (109, 140) | 123 (109, 138) | 122 (107, 137) | 119 (106, 136) | 123 (109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138) 138) |
0.47 | 0.018 | 0.96 |
| HDL-cholesterol (mg dL−1) | 37 (33, 41) | 37 (33, 41) | 36 (32, 40) | 34 (31, 38) | 35 (32, 38) | 38 (35, 43) | 37 (33, 41) | 37 (33, 41) | 36 (33, 40) | 36 (32, 40) | 38 (35, 43) | 38 (34, 42) | 37 (33, 41) | 36 (32, 39) | 36 (32, 40) | <0.001 | 0.006 | 0.92 |
| Glucose (mg dL−1) | 101 ± 5 | 129 ± 5 | 114 ± 5 | 105 ± 5 | 95 ± 5 | 98 ± 5 | 120 ± 5 | 103 ± 5 | 100 ± 5 | 100 ± 5 | 99 ± 5 | 126 ± 5 | 107 ± 5 | 104 ± 5 | 96 ± 5 | <0.001 | 0.25 | 0.77 |
| Insulin (μIU mL−1)a | 6 (3, 9) | 47 (29, 77) | 39 (24, 63) | 27 (16, 45) | 17 (10, 27) | 5 (3, 9) | 52 (32, 86) | 33 (20, 56) | 27 (16, 44) | 20 (12, 32) | 5 (3, 9) | 52 (32, 86) | 33 (20, 55) | 23 (14, 38) | 8 (5, 13 | <0.001 | 0.14 | 0.20 |
A time effect existed for glucose (p < 0.001) and insulin (p < 0.001). No treatment or time by treatment interaction was detected for mean glucose or insulin (Table 5), or change from pre-meal levels (Fig. 1B). A treatment effect was observed for total cholesterol (p = 0.029), non-HDL cholesterol (p = 0.005), LDL-cholesterol (p = 0.018), and HDL-cholesterol (p = 0.006) (Table 5). Post hoc pairwise testing showed that for total cholesterol, non-HDL cholesterol and LDL-cholesterol, the treatment effect was because of a difference between the meal with no spices and the meal with 6 g of spices (geometric mean difference total cholesterol 1.03, 95% CI 1.00, 1.05 mg dL−1, p = 0.003; non-HDL 1.04, 95% CI 1.01, 1.07 mg dL−1, p = 0.004; LDL-cholesterol 1.04, 95% CI 1.00, 1.07 mg dL−1, p = 0.026). LDL-cholesterol also differed between the meal with 2 g of spices and the meal with 6 g of spices (geometric mean difference 1.03, 95% CI 1.00, 1.06 mg dL−1; p = 0.048). HDL-cholesterol differed between the meal with no spices and the meal with 2 g of spices (geometric mean −1.03, 95% CI −1.06, −1.01 mg dL−1, p = 0.01) and the meal with 6 g of spices (−1.03, 95% CI, −1.06, −1.01 mg dL−1, p = 0.01).
At 120 minutes following the meal with 2 g of spices, a nominally significant inverse correlation existed between the change in triglycerides and the change in FMD (rho = −0.54; p = 0.057); after the meal with 6 g of spices (rho = −0.45; p = 0.13) and the meal with no spices (rho = 0.11; p = 0.71) the correlation was non-significant. Similarly, at 240 minutes following the meal with 6 g of spices a significant negative correlation was observed between the change in triglycerides and the change in FMD (rho = −0.62; p = 0.025); significant correlations between the change in triglycerides and the change in FMD were not observed after the 2 g meal (rho = −0.45; p = 0.13) or the meal with no spices (rho = −0.35; p = 0.25).
Discussion
Relatively high doses (>11 g) of commonly consumed spices – above levels currently consumed in the U.S. – may lessen the detrimental effects of high-saturated fat consumption in the 4–6 hours following a meal.12–14 The present study provides preliminary data that lower doses of spices (2 and 6 g), which may be consumed as part of a mixed meal in countries such as the U.S., may improve the postprandial triglyceride and FMD response to a high-saturated fat, high-carbohydrate meal in men with enlarged waist circumference. However, no time by treatment interactions were observed, and no differences were detected in the change from baseline analysis so these data should be interpreted cautiously. These pilot data suggest that incorporation of 6 g of spices into a high-saturated fat, high-carbohydrate meal attenuates postprandial impairment in FMD (∼0.9%) compared to an equivalent meal without spice. Likewise, the postprandial triglyceride excursion was lowered by the meal containing 2 g of spices compared with the no spice meal; the meal containing 6 g of spices also tended to blunt postprandial triglycerides but this did not reach statistical significance after adjustment for multiple comparisons (p = 0.12). Mean total cholesterol, non-HDL cholesterol, and LDL-cholesterol were higher after the 6 g meal vs. the no spice meal; HDL-cholesterol levels were lower after both the 2 g and 6 g meals. Glucose and insulin were unaffected by spice.Previously, postprandial studies examining the effect of spices have demonstrated reductions in postprandial lipemia12,13 and oxidative stress,20,21 and improvements in FMD.14 We have replicated the attenuation in postprandial lipemia previously observed;12,13 albeit the reductions were less than previously observed, but this is not unexpected because of the lower dose of spices given. Mean triglyceride levels over the 4-hour period were ∼10% lower following the 2 g meal vs. the meal with no spices, and ∼7% lower following the 6 g meal vs. the no spices meal. Skulas-Ray et al. observed a 31% reduction in triglycerides in the 4 hours following a high-fat, high-carbohydrate meal with 14 g of spices compared to a matched meal without spices.12 Skulas-Ray et al. also observed a reduction in postprandial insulin levels after the meal with spices, which was not replicated in the present study but may be explained by the difference in the study populations. We included men with enlarged waist circumference and at least one other risk factor for CVD whereas Skulas-Ray et al. included overweight but otherwise healthy men.
A recent 3-period, dose–response, 24 hour crossover trial examined the effect of isocaloric, macronutrient matched vegetable curries differing in the dose of fresh and dried spices (doses reported as total polyphenol content in gallic acid equivalents; 130 ± 18, 556 ± 19.7 and 1113 ± 211.6 mg per meal).22 The spice doses are not directly comparable to our study because of the inclusion of both fresh (onion, garlic, ginger) and dried spices. The meals contained 0 g, 6 g, and 12 g of dried spices (turmeric, cinnamon, coriander seeds, cumin seeds, Indian gooseberry ‘amla’, cayenne pepper, clove powder). Haldar et al. found a linear dose–response reduction in glucose in the 3 hours following the test meal and a non-significant linear dose response reduction in insulin.22 Interestingly, a dose–response increase in triglycerides was observed 3 hours following the meal; however, the authors state this could have been because the meals were not completely matched for vegetables and the highest spice meal included less eggplant, which is known to affect lipid absorption. The curry included 19 g of total fat (fat type not reported), which is a substantially lower dose than given in our study, which may also explain the different results reported. This study did not include any assessment of post-meal vascular function.
The attenuation in post-meal FMD impairment that we observed following the high-saturated fat, high-carbohydrate meal with 6 g of spices vs. the no spice meal is a novel finding. A meta-analysis of 35 clinical studies, including 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 subjects, showed per 1% increase in FMD the risk of a cardiovascular event was reduced by 12%.23 Therefore the difference in FMD following the 6 g meal vs. the no spice meal (0.87%) in the present study would be expected to confer an ∼10% cardiovascular event risk reduction. Nakayama et al. reported that FMD was increased 1 hour following a curry with 14.5 g of spices (clove, coriander, cumin, garlic, ginger, red pepper, turmeric), compared to a meal containing no spices, in healthy males (n = 14).14 The mechanism for the improvement in FMD observed by Nakayama et al. is not clear since no difference in oxidative stress markers, malondialdehyde-modified LDL-cholesterol or 8-iso-prostane, was observed. However, inclusion of 11.25 g of spices in a meal containing 250 g of high fat ground beef reduced oxidative stress (plasma and urinary malondialdhyde) in the 6 hours following ingestion, compared to an equivalent meal without spices, in healthy subjects (n = 11).21 This result was replicated in men with type 2 diabetes (n = 18), although no difference was detected in postprandial glucose, insulin or triglycerides between the spiced and non-spiced meal.20 An improvement in endothelial function (reactive hyperemia peripheral artery tonometry) was detected with the spiced meal after 2 hours. There was a concurrent borderline non-significant increase in urine nitric oxide concentration. These studies provide some evidence that spices may affect the vasculature by modulating oxidative stress and inducing nitric oxide formation; endothelial dysfunction is characterized by reduced nitric oxide bioavailability.
280 subjects, showed per 1% increase in FMD the risk of a cardiovascular event was reduced by 12%.23 Therefore the difference in FMD following the 6 g meal vs. the no spice meal (0.87%) in the present study would be expected to confer an ∼10% cardiovascular event risk reduction. Nakayama et al. reported that FMD was increased 1 hour following a curry with 14.5 g of spices (clove, coriander, cumin, garlic, ginger, red pepper, turmeric), compared to a meal containing no spices, in healthy males (n = 14).14 The mechanism for the improvement in FMD observed by Nakayama et al. is not clear since no difference in oxidative stress markers, malondialdehyde-modified LDL-cholesterol or 8-iso-prostane, was observed. However, inclusion of 11.25 g of spices in a meal containing 250 g of high fat ground beef reduced oxidative stress (plasma and urinary malondialdhyde) in the 6 hours following ingestion, compared to an equivalent meal without spices, in healthy subjects (n = 11).21 This result was replicated in men with type 2 diabetes (n = 18), although no difference was detected in postprandial glucose, insulin or triglycerides between the spiced and non-spiced meal.20 An improvement in endothelial function (reactive hyperemia peripheral artery tonometry) was detected with the spiced meal after 2 hours. There was a concurrent borderline non-significant increase in urine nitric oxide concentration. These studies provide some evidence that spices may affect the vasculature by modulating oxidative stress and inducing nitric oxide formation; endothelial dysfunction is characterized by reduced nitric oxide bioavailability.
The previous studies conducted by Nakayama et al.14 and Li et al.20,21 were not conducted under conditions of a high saturated fat meal challenge. The curry used by Nakayama et al. only contained 10 g of fat (fat type not reported), and was high in carbohydrates (82 g). In the studies by Li et al., the nutrient composition of the hamburger meal was not reported but it is expected that the meal contained ∼10 g of saturated fat. Thus, the present meal challenge is contextually different from past research conducted since lipemia was induced, but is physiologically relevant since the meal is reflective of typical meal selections in U.S., especially fast-foods.24
In the present study, an inverse association existed between the change in triglycerides and the change in FMD. Greater post-meal triglyceride elevation was correlated with greater reductions in FMD, and triglyceride increases of a lower magnitude were associated with greater FMD post-meal. Interestingly, this was only observed following the meals containing 2 g and 6 g of spices; no correlations existed between the change in FMD and the change in triglycerides following the meal with no spices, which is likely because of the more uniform triglyceride response driven entirely by the saturated fat content of the meal. Therefore, in the context of meal-induced lipemia, it is likely that spices modulate endothelial function by affecting post meal triglyceride levels. It is well established that postprandial lipemia induces endothelial dysfunction;24 however, the mechanisms are largely unclear. In vitro experiments show triglyceride rich lipoproteins increase expression of pro-inflammatory cytokines and adhesion molecules, and impair endothelial cell-dependent vasodilation, which may explain lipemia induced endothelial dysfunction.24,25
Spices are known to inhibit digestive enzymes, which may impair small intestinal fat absorption, reduce chylomicron formation and circulating triglyceride levels. McCrea et al., demonstrated that in vitro Pancreatic Lipase (PL) and Phospholipase A2 (PLA2) were inhibited in a dose-dependent manner by a spice blend containing black pepper, cinnamon, cloves, ginger, garlic, oregano, paprika, rosemary and turmeric.13 When tested individually, cinnamon, cloves, and turmeric were the most potent inhibitors of PL and PLA2. In the present study, on a proportional basis, cinnamon and turmeric comprised close to one-third of the total spice dose in the test meals, and therefore inhibition of PL and PLA2 is hypothesized to a least partially explain the attenuation in triglycerides in the postprandial period.
While this study was a randomized, cross-over study, conducted under well-defined and controlled conditions with blinding of all personnel involved in data collection, the results should be viewed in light of some limitations. This study was limited by the small sample size and the relatively short-duration (4 hours), which only represents the early postprandial phase. Future studies should be conducted for a minimum of 6 hours post meal. In addition, blood samples were taken hourly and FMD was only measured at 2 and 4 hours; a greater sampling frequency may have increased the capacity to characterize the postprandial response. Subjects were males not prescribed lipid lowering, antihypertensive or glucose lowering medications and therefore these results cannot be extrapolated to females and those taking medication for lipid, blood pressure or glucose lowering. In addition, the evening meal prior to the testing days was not standardized and subjects were only instructed to avoid vigorous physical activity for 12 hours prior to testing. Finally, the total polyphenol content of the spice blend given was not assessed in this study, and since a spice blend was given it is unclear which spice(s) mediated the observed effects.
Conclusions
In conclusion, this study provides preliminary evidence that incorporation of lower doses of culinary spices (2 and 6 g), than previously tested, in a high-saturated fat, high-carbohydrate meal may affect post-meal lipemia and FMD in men with enlarged waist circumference. Future research is needed to replicate these pilot findings and the longer-term impact of culinary spice consumption on metabolic outcomes and vascular function should be evaluated.Conflicts of interest
This study was funded by McCormick Science Institute; the funder had no role in data analyses or manuscript preparation. In addition, the study was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1UL1TR002014-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.KS Petersen – has received travel reimbursement and an honorarium from McCormick Science Institute to discuss the study findings with a regulatory agency.
CJ Rogers – has received funding to conduct the present study.
SG West – has received funding to conduct the present study.
DN Proctor – has received funding to conduct the present study.
PM Kris – Etherton – has received funding to conduct the present study, travel reimbursement and an honorarium from McCormick Science Institute to discuss the study findings with a regulatory agency.
Acknowledgements
The authors would like to thank Cyndi Flanagan, Christa Oelhaf, Filamena Martin, and all the staff at the Penn State Clinical Research Center for their assistance with data collection. In addition, we would like to thank Amy Ciccarella and the staff in the metabolic kitchen for preparing the test meals, and Paul Wagner for conducting the FMD procedure. We also wish to thank Kristin Davis, Danette Teeter, Jennifer Fleming, Emily Johnston and Valarie Sullivan for their help with the study.References
- R. A. Vogel, M. C. Corretti and G. D. Plotnick, Effect of a single high-fat meal on endothelial function in healthy subjects, Am. J. Cardiol., 1997, 79, 350–354 CrossRef CAS.
- B. G. Nordestgaard, M. Benn, P. Schnohr and A. Tybjaerg-Hansen, Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women, J. Am. Med. Assoc., 2007, 298, 299–308, DOI:10.1001/jama.298.3.299.
- S. Bansal, J. E. Buring, N. Rifai, S. Mora, F. M. Sacks and P. M. Ridker, Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women, J. Am. Med. Assoc., 2007, 298, 309–316, DOI:10.1001/jama.298.3.309.
- A. S. Lindman, M. B. Veierød, A. Tverdal, J. I. Pedersen and R. Selmer, Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian Counties Study, Eur. J. Epidemiol., 2010, 25, 789–798, DOI:10.1007/s10654-010-9501-1.
- B. G. Nordestgaard, Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease, Circ. Res., 2016, 118, 547–563, DOI:10.1161/CIRCRESAHA.115.306249.
- E. J. Benjamin, P. Muntner, A. Alonso, M. S. Bittencourt, C. W. Callaway, A. P. Carson, A. M. Chamberlain, A. R. Chang, S. Cheng and S. R. Das, et al., Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association, Circulation, 2019, 139, e56–e66, DOI:10.1161/cir.0000000000000659.
- GBD 2016 Causes of Death Collaborators, Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016, Lancet, 2017, 390, 1151–1210, DOI:10.1016/s0140-6736(17)32152-9.
- A. F. Stalenhoef and J. de Graaf, Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL, Curr. Opin. Lipidol., 2008, 19, 355–361, DOI:10.1097/MOL.0b013e328304b63c.
- T. Nakamura, H. Takano, K. Umetani, K.-I. Kawabata, J.-E. Obata, Y. Kitta, Y. Kodama, A. Mende, Y. Ichigi and D. Fujioka, et al., Remnant lipoproteinemia is a risk factor for endothelial vasomotor dysfunction and coronary artery disease in metabolic syndrome, Atherosclerosis, 2005, 181, 321–327, DOI:10.1016/j.atherosclerosis.2005.01.012.
- Y. Inaba, J. A. Chen and S. R. Bergmann, Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis, Int. J. Cardiovasc. Imaging, 2010, 26, 631–640, DOI:10.1007/s10554-010-9616-1.
- P. J. Huth, V. L. Fulgoni, D. R. Keast, K. Park and N. Auestad, Major food sources of calories, added sugars, and saturated fat and their contribution to essential nutrient intakes in the U.S. diet: data from the National Health and Nutrition Examination Survey (2003–2006), Nutr. J., 2013, 12, 116, DOI:10.1186/1475-2891-12-116.
- A. C. Skulas-Ray, P. M. Kris-Etherton, D. L. Teeter, C.-Y. O. Chen, J. P. Vanden Heuvel and S. G. West, A High Antioxidant Spice Blend Attenuates Postprandial Insulin and Triglyceride Responses and Increases Some Plasma Measures of Antioxidant Activity in Healthy, Overweight Men, J. Nutr., 2011, 141, 1451–1457, DOI:10.3945/jn.111.138966.
- C. E. McCrea, S. G. West, P. M. Kris-Etherton, J. D. Lambert, T. L. Gaugler, D. L. Teeter, K. A. Sauder, Y. Gu, S. L. Glisan and A. C. Skulas-Ray, Effects of culinary spices and psychological stress on postprandial lipemia and lipase activity: results of a randomized crossover study and in vitro experiments, J. Transl. Med., 2015, 13, 1–12, DOI:10.1186/s12967-014-0360-5.
- H. Nakayama, N. Tsuge, H. Sawada, N. Masamura, S. Yamada, S. Satomi and Y. Higashi, A single consumption of curry improved postprandial endothelial function in healthy male subjects: a randomized, controlled crossover trial, Nutr. J., 2014, 13, 1–7, DOI:10.1186/1475-2891-13-67.
- United States Department of Agriculture/Economic Research Service, Spices: Supply and disappearance. Available online: http://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/ (accessed on August 27 2019).
- C. Manach, G. Williamson, C. Morand, A. Scalbert and C. Rémésy, Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies, Am. J. Clin. Nutr., 2005, 81, 230S–242S CrossRef CAS PubMed.
- J. Balzer, T. Rassaf, C. Heiss, P. Kleinbongard, T. Lauer, M. Merx, N. Heussen, H. B. Gross, C. L. Keen and H. Schroeter, et al., Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial, J. Am. Coll. Cardiol., 2008, 51, 2141–2149, DOI:10.1016/j.jacc.2008.01.059.
- S. S. Martin, M. J. Blaha, M. B. Elshazly, P. P. Toth, P. O. Kwiterovich, R. S. Blumenthal and S. R. Jones, Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile, J. Am. Med. Assoc., 2013, 310, 2061–2068, DOI:10.1001/jama.2013.280532.
- S. G. West, P. Wagner, S. L. Schoemer, K. D. Hecker, K. L. Hurston, A. Likos Krick, L. Boseska, J. Ulbrecht and A. L. Hinderliter, Biological correlates of day-to-day variation in flow-mediated dilation in individuals with Type 2 diabetes: a study of test–retest reliability, Diabetologia, 2004, 47, 1625–1631, DOI:10.1007/s00125-004-1502-8.
- Z. Li, S. M. Henning, Y. Zhang, N. Rahnama, A. Zerlin, G. Thames, C. H. Tseng and D. Heber, Decrease of postprandial endothelial dysfunction by spice mix added to high-fat hamburger meat in men with Type 2 diabetes mellitus, Diabetic Med., 2013, 30, 590–595, DOI:10.1111/dme.12120.
- Z. Li, S. M. Henning, Y. Zhang, A. Zerlin, L. Li, K. Gao, R.-P. Lee, H. Karp, G. Thames and S. Bowerman, et al., Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations, Am. J. Clin. Nutr., 2010, 91, 1180–1184, DOI:10.3945/ajcn.2009.28526.
- S. Haldar, S. C. Chia, S. H. Lee, J. Lim, M. K. Leow, E. C. Y. Chan and C. J. Henry, Polyphenol-rich curry made with mixed spices and vegetables benefits glucose homeostasis in Chinese males (Polyspice Study): a dose-response randomized controlled crossover trial, Eur. J. Nutr., 2019, 58, 301–313, DOI:10.1007/s00394-017-1594-9.
- Y. Matsuzawa, T. G. Kwon, R. J. Lennon, L. O. Lerman and A. Lerman, Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis, J. Am. Heart Assoc., 2015, 4(11), e002270, DOI:10.1161/jaha.115.002270.
- M. Miller, N. J. Stone, C. Ballantyne, V. Bittner, M. H. Criqui, H. N. Ginsberg, A. C. Goldberg, W. J. Howard, M. S. Jacobson and P. M. Kris-Etherton, et al., Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association, Circulation, 2011, 123, 2292–2333, DOI:10.1161/CIR.0b013e3182160726.
- K. G. Jackson, S. S. Poppitt and A. M. Minihane, Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants, Atherosclerosis, 2012, 220, 22–33 CrossRef CAS PubMed.
Footnote |
| † Clinical trial registry: NCT03064958 (https://clinicaltrials.gov) |
| This journal is © The Royal Society of Chemistry 2020 |