Effect of transglutaminase cross-linking on the allergenicity of tofu based on a BALB/c mouse model
Jing
Bai
ab,
Junyu
Hui
ab,
Qiaoling
Lu
ab,
Anshu
Yang
 *ab,
Juanli
Yuan
c,
Jinyan
Gao
d,
Zhihua
Wu
*ab,
Juanli
Yuan
c,
Jinyan
Gao
d,
Zhihua
Wu
 ab,
Xin
Li
ad,
Ping
Tong
ab,
Xin
Li
ad,
Ping
Tong
 a and
Hongbing
Chen
a and
Hongbing
Chen
 *ab
*ab
aState Key Laboratory of Food Science and Technology, Nanchang University, Nanjing Dong Lu 235, Nanchang 330047, China. E-mail: yanganshu@ncu.edu.cn; chenhongbing@ncu.edu.cn; Fax: +86 791 88333708; Tel: +86 791 88333529
bSino-German Joint Research Institute, Nanchang University, Nanjing Dong Lu 235, Nanchang 330047, China
cSchool of Pharmacy, Nanchang University, Nanchang 330047, China
dSchool of Food Science and Technology, Nanchang University, Nanchang 330047, China
First published on 4th December 2019
Abstract
Soybean products are limited in terms of safe consumption because of the sensitization of raw materials. In this study, the allergenicity of cross-linked tofu with microbial transglutaminase (MTG) was evaluated on the basis of a BALB/c mouse model. The mice were randomly divided into five groups. Cholera toxin was used as an adjuvant to sensitize the mice through intragastric administration, and tofu was given orally to investigate its sensitization effect on the mice. The allergy symptoms, body temperature, and weight of the mice were detected. The immunoglobulin E (IgE), immunoglobulin G (IgG), and spleen cytokines of the mice were determined through an enzyme-linked immunosorbent assay. The regulation of the differentiation balance of the different subsets of splenic T lymphocyte (Th1, Th2) and regulatory T cells (Tregs) in the mice was measured through flow cytometry. Results showed that the mice administered with MTG-cross-linked tofu had fewer allergic symptoms compared with those of the control group. The concentrations of serum-specific IgE and IgG, plasma histamine, and mast cell protease 1 (mMCP-1) significantly decreased. The Th2-related cytokine levels reduced, and the IFN-γ levels increased. The proportion of Th2 cells decreased, and the proportion of CD4+CD25+Foxp+ Tregs increased as the percentage of Th1 cells increased. Therefore, the sensitization of enzymatic cross-linked tofu decreased.
1. Introduction
Tofu is a traditional food with soybean as a raw material, and it contains eight essential amino acids required by the human body; moreover, tofu is popular among consumers because of its nutritional characteristics of high protein and low cholesterol.1 However, Soybean has 11S and 7S globulins, which are the two main protein components and the main allergens in tofu. These allergens are also found in tofu gel, and their protein structures can be changed during the processing of tofu. Some studies have reported that soybean protein subunits are dissociated and rearranged to form granules with different sizes after soybean milk is heated.2 The formation of tofu gels can change the spatial structure of soy proteins through various processes, including natural soybean protein denaturation, subunit dissociation–association, and aggregation. After raw soybean milk is heated, the molecular conformation of soybean protein (glycinin and β-conglycinin) changes from a natural folding state to an unfolding state, and sulfhydryl and hydrophobic groups within the molecule are exposed.3 Aggregates are formed by disulfide bonds and hydrophobic interactions between non-exposed groups.4 Previous studies showed the α-helix, β-sheet, β-turn and random coil of soybean protein are transformed into one another during bean curd production, and the structure of a tofu gel network mainly exists in the form of β-sheet.5 Tofu is a derivative of soybean milk, and its allergenicity has been widely explored. However, only a few studies have examined the systemic allergenicity of soymilk and tofu. Adachi et al.6 found that β-conglycinin nearly completely disappears after soymilk is digested by the stomach for 20 min, but the β-conglycinin structure remains intact after tofu is digested for 120 min. Therefore, patients who ingest tofu suffer from anaphylactic shock induced by food-dependent exercise. Song et al.7 found that the immunoglobulin E (IgE) immunoreactivity of tofu is approximately 20 times higher than that of soymilk.In tofu processing, microbial transglutaminase (MTG) can enhance the strength of tofu gel and improve the formability and quality of tofu.8 Yasir et al.9 found that protein in soybean milk is more easily crosslinked by MTG after boiling than before boiling. Many studies have investigated the use of transglutaminase to modify proteins and alleviate allergenicity of allergen proteins.10–13 Related studies have confirmed that transglutaminase cross-linking modification can promote gluten protein to produce T cell binding epitopes associated with celiac disease, thereby enhancing its allergenicity.14 Porta et al.15 found that transglutaminase cross-linking improves the digestibility of egg mucin and reduces its antigenicity. A previous study showed significantly decreased antigenicity levels of β-lactoglobulin and its cross-linking products treated with cysteine reduction.16 Leszczynska et al.17 found that transglutaminase modification reduces the allergenicity of flour protein. Therefore, MTG cross-linking can alter the sensitization of various allergenic proteins.
Animal models are the most commonly used direct methods to evaluate the potential allergenicity of food in vivo. Such models have been widely used to study the cellular and molecular mechanisms of various IgE-mediated allergic diseases. BALB/c mice, which are widely used in studying highly sensitive diseases, are inbred strains with a high IgE response.18 For example, Gizzarelli et al.19 established an oral IgE-mediated soybean protein sensitization model in a BALB/c mice by using cholera toxin (CT) as an adjuvant. In addition to the levels of soybean protein-specific IgE and IgG1 antibodies, the levels of IL-4 and IL-5 increase. Yang et al.20 found that the potential allergenicity of SBM is reduced by solid-state fermentation induced by a mixture of Lactobacillus casei, yeast, and Bacillus subtilis in a BALB/c mouse model. Murakami et al.21 also found in percutaneous sensitizing soybean allergens in a mouse model (soybean globulins 7S and 11S). However, few reports have exploded the sensitization of tofu, especially those based on animal experiments. In this study, a BALB/c mouse model was used to explore the effect of enzymatic cross-linking on the potential allergenicity of tofu, providing a theoretical basis for developing high-quality hypoallergenic tofu products.
2. Materials and methods
2.1 Materials
The soybean variety ‘Dongnong 42’ was provided by the Northeast Agricultural University. MTG (food additive, enzyme activity: 102.3 U mL−1) was purchased from Taixing Yiming Biological Products Co., Ltd (Taixing, Jiangsu, China). Goat anti-mouse specific antibodies (IgG and IgE) were obtained from MBI Fermentas (SouthernBiotech, Birmingham, USA). Mouse IL-4, IL-5, IL-10, IL-13, IFN-γ enzyme-linked immunosorbent assay (ELISA) kits and a mouse mMCP-1 kit were acquired from eBioscience, Inc. (eBioscience, San Diego, USA). A mouse plasma histamine ELISA kit was obtained from Demeditec Company (Demeditec, Germany). FITC anti-mouse CD4, FE anti-mouse CD25, PE anti-mouse IL-4, Percp anti-mouse IFN-γ, and APC anti-mouse Foxp3 were also procured from eBioscience, Inc. (eBioscience, San Diego, USA). All reagents were of analytical grade.2.2 Preparation of samples
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/v) for 10 h. The soaked beans were washed, and raw soybean milk was prepared by beating twice at a soybean-to-water ratio of 1
3 (w/v) for 10 h. The soaked beans were washed, and raw soybean milk was prepared by beating twice at a soybean-to-water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5 (w/v). The raw soybean milk was boiled (75 °C for 5 min, 95 °C for 10 min), and the pH of soybean milk was adjusted to 6.0. When the temperature of the boiled soybean milk reduced to 85 °C–90 °C, 0.33% gluconic acid lactone was added and warmed for 30 min. Then, the samples were transferred to a plastic mold and pressed for 3 h. Single MTGase tofu was prepared using the same method with slight modifications. The temperature of soybean milk was reduced to 50 °C and maintained at this level for 10 min. MTG (5 U per g protein) was added and cross-linked at constant temperature for 3 h. The enzyme was then inactivated in a water bath at 90 °C for 5 min.
6.5 (w/v). The raw soybean milk was boiled (75 °C for 5 min, 95 °C for 10 min), and the pH of soybean milk was adjusted to 6.0. When the temperature of the boiled soybean milk reduced to 85 °C–90 °C, 0.33% gluconic acid lactone was added and warmed for 30 min. Then, the samples were transferred to a plastic mold and pressed for 3 h. Single MTGase tofu was prepared using the same method with slight modifications. The temperature of soybean milk was reduced to 50 °C and maintained at this level for 10 min. MTG (5 U per g protein) was added and cross-linked at constant temperature for 3 h. The enzyme was then inactivated in a water bath at 90 °C for 5 min.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (w/v) to prepare soybean milk. After cross-linking with MTG was performed at 50 °C for 3 h, the enzyme was inactivated as mentioned above. Then, 0.33% lactone was added, mixed, and placed in a water bath at 85 °C–90 °C for 30 min. Then, the sample was put into plastic mold and pressed for 3 h.
8 (w/v) to prepare soybean milk. After cross-linking with MTG was performed at 50 °C for 3 h, the enzyme was inactivated as mentioned above. Then, 0.33% lactone was added, mixed, and placed in a water bath at 85 °C–90 °C for 30 min. Then, the sample was put into plastic mold and pressed for 3 h.
2.3 Animals
Female BALB/c mice (SPF grade, weigh 20 ± 2 g, aged 4–6 weeks) were purchased from Hunan Slake Jingda Laboratory Animal Co., Ltd (Hunan, China) and all mice used in this study were cared for in accordance with the guidelines for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication 85-23, 1996), and all experimental procedures were approved by the Animal Care Review Committee, Nanchang University. The mice were fed with soybean meal-free diet and continuously propagated for two generations, and the second generation of the female mice (4 weeks old) was used as experimental animals. The mice drank tap water and ate freely. Their room temperature and humidity were set at 19 °C–25 °C and 55%–65%, respectively. Light was turned on for 10–12 h per day.2.4 Establishment of a mouse sensitization model
Mouse sensitization was performed in accordance with the method of Yang et al.22 with some modifications. The mice were randomly divided into five groups (n = 10), namely, PBS group (PBS), soybean protein control group (Control), gluconic acid lactone tofu group (GDL), enzymatic cross-linked tofu group (MTG), and composite coagulant tofu group (GDL-MTG). All the mice were sensitized orally challenged with a blunt needle on days 0, 7, 14, and 21, 28, which were given a certain dose of protein solution or tofu solution with the same amount of protein (5 mg protein/tofu protein + 10 μg CT). On day 35, the mice were orally challenged with 20 mg of the corresponding protein (Fig. 1). The negative control group received CT (10 μg) in PBS alone. After 30 min of the last sensitization, the sensitization symptom of the mice was scored (Table 1),23 and the body temperature and body weight of the mice were measured. The mice were euthanized by dislocation. Blood and spleens were collected, and different biomarkers were analyzed.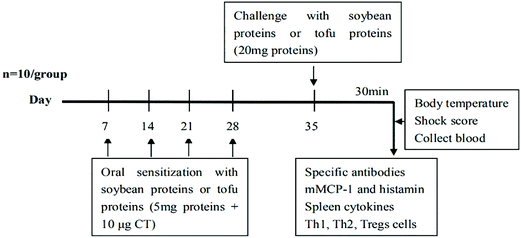 | ||
| Fig. 1 Experimental scheme of in BALB/c mice sensitized weekly by intragastric gavage using CT as adjuvant. | ||
| Score | Symptoms |
|---|---|
| 0 | No symptoms |
| 1 | Scratching nose and mouth |
| 2 | Swelling around the eyes and mouth; pillar erection; reduced activity; higher breathing rate |
| 3 | Shortness of breath; blue rash around the mouth and tail; higher breathing rate |
| 4 | No activity after stimulation, shivering and muscle contractions |
| 5 | Death by shock |
2.5 Detection of specific immunoglobulin and mMCP-1 in serum
As previously reported,24 specific IgG and IgE levels in serum were detected through indirect ELISA. A 96-well ELISA microplate was coated with 10 μg per well of soybean protein solution in 0.05 M carbonate buffer (pH 9.6) at 4 °C overnight. The plates were washed thrice with PBST. Then, 250 μL of defatted milk (3%, w/w) powder was added to each well for blocking, and the mixtures were incubated at 37 °C for 1 h. After the plates were washed with PBST, 100 μL of diluted serum was added to each well and incubated at 37 °C for 1 h. HRP-labeled goat anti-mouse IgG and IgE antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) were added and incubated at 37 °C for 1 h. After the plates were washed with PBST, 100 μL of HRP-streptomycin-labeled avidin (diluted to 1
5000) were added and incubated at 37 °C for 1 h. After the plates were washed with PBST, 100 μL of HRP-streptomycin-labeled avidin (diluted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) was added and incubated at 37 °C for 1 h. Finally, 100 μL of TMB solution was added and incubated at 37 °C for 30 min. The reaction was terminated by adding 50 μL of sulfuric acid (2 M), and absorbance was measured at 450 nm. The mMCP-1 levels in serum were quantified with an ELISA kit (eBioscience, San Diego, USA).
60) was added and incubated at 37 °C for 1 h. Finally, 100 μL of TMB solution was added and incubated at 37 °C for 30 min. The reaction was terminated by adding 50 μL of sulfuric acid (2 M), and absorbance was measured at 450 nm. The mMCP-1 levels in serum were quantified with an ELISA kit (eBioscience, San Diego, USA).
2.6 Detection of histamine levels
The blood of the mice was collected in anticoagulant tubes containing heparin sodium. The supernatant was centrifuged at 4000g for 10 min and stored at −80 °C. Histamine levels were detected by a mouse plasma histamine ELISA kit.2.7 Splenocyte culture and cytokine analysis
The spleens of the mice were separated aseptically and ground with an RPMI 1640 medium. The supernatant was discarded by centrifugation at 250g for 5 min, erythrocyte lysate was added for 5 min, and the mixture was suspended in a complete culture medium (RPMI-1640 containing 10% fetal bovine serum). The number of cells was adjusted to 4 × 106 mL−1 and placed in a 24-well cell culture plate. Then, the cells were stimulated with soybean protein or tofu protein and cultured for 72 h (37 °C, 5% CO2). The supernatant was collected and stored at −80 °C. The contents of IL-4, IL-5, IL-10, IL-13, and IFN-γ in the spleen cells of the mice were detected with a commercial ELISA kit.2.8 Detection of Th1/Th2 in splenocytes
The level of Th1/Th2 cells was indirectly detected in terms of the expression of levels intracellular IFN-γ and IL-4. A 200 μL suspension of splenic single cells (4 × 106 mL−1) was obtained, and FITC anti-mouse CD4 antibody was added, and the mixture was incubated in darkness for 30 min. The supernatant was centrifuged and discarded after fixative and rupture agents were added to the cells. Percp anti-mouse IFN-γ antibody and PE anti-mouse IL-4 antibody were added, and the mixture was incubated in darkness for 30 min. The single cells were resuspended in 300 μL of flow buffer, and Th1/Th2 cells were analyzed through flow cytometry.2.9 Detection of regulatory T cells (Tregs)
As a specific marker, CD4+CD25+Foxp3+ is often used to identify Treg cells. Splenic single cell suspensions were acquired. FITC anti-mouse CD4 and PE anti-mouse CD25 were added, and the mixtures were incubated in darkness for 30 min. The fixation/permeabilization was added to cells and cultured in darkness for 30 min. The permeabilization buffer was subsequently added, and the supernatant was discarded. Afterword, APC anti-mouse Foxp3+ antibody was added, incubated in darkness for 30 min, and washed once. The cells were resuspended in 300 μL of flow buffer, and Treg cells were analyzed through flow cytometry (BD Accuri C6 Plus, BD, USA).2.10 Statistical analysis
Flow cytometry related data was analyzed using BD Accuri™ C6 Plus software and FlowJo V10 software. Charts were drawn using Origin 9.1 software and statistical analysis using SPSS 20.0 software. One-way analysis of variance was conducted, and differences between the sample means were performed. A probability value of less than 0.05 was considered statistically significant.3. Results
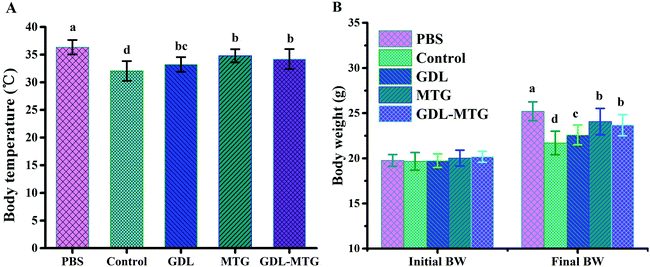
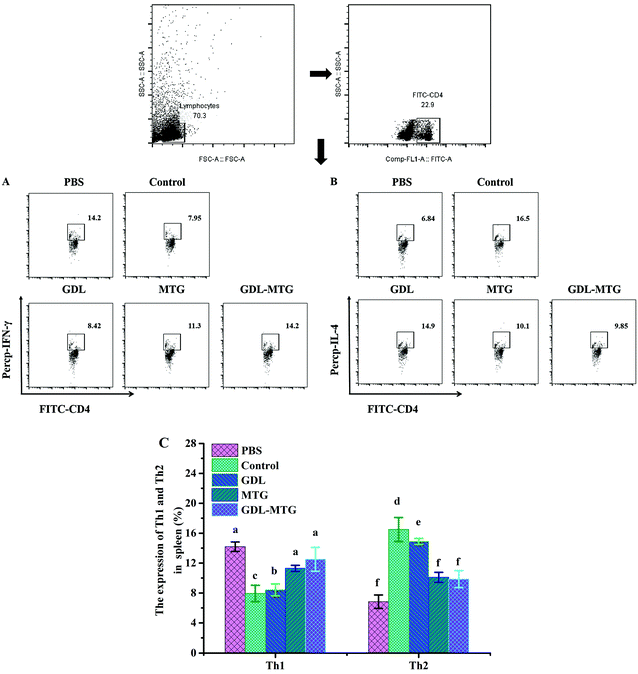
3.1 Allergy symptom, body temperature, and weight of mice
The allergenicity of enzymatic cross-linked tofu in vivo was studied in a mouse model of food allergy (Fig. 1). The allergy scores of the mice after stimulation are shown in Table 2. Some mice in the control and experimental groups showed different sensitized statuses. No allergy symptom was observed in the PBS group, and mild allergy symptoms were identified in the enzymatic cross-linked tofu group. The changes in the body weight and rectal temperature of the mice after the challenge are shown in Fig. 2. The average body temperature of the mice in the PBS group was 36.34 °C, and the body temperature of the mice in the soybean protein group (control) significantly decreased (p < 0.05). The body temperature of the GDL, MTG, and GDL-MTG groups was higher than that of the control group, and the MTG and GDL-MTG groups significantly differed from the control group (p < 0.05). Fig. 2B shows the change in body weight between the beginning and the end of the experiment. The weights of the mice in the PBS group were the largest, and those of the mice in the soybean group were the smallest. The weights of the mice in the three other groups were larger than those in the control group but were smaller than those in the PBS group. The weights of all the groups significantly differed.| Group | Score | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| PBS (n = 10) | 10 | 0 | 0 | 0 | 0 | 0 |
| Control (n = 10) | 4 | 1 | 3 | 2 | 0 | 0 |
| GDL (n = 10) | 5 | 1 | 2 | 2 | 0 | 0 |
| MTG (n = 10) | 7 | 1 | 2 | 0 | 0 | 0 |
| GDL-MTG (n = 10) | 8 | 1 | 1 | 0 | 0 | 0 |
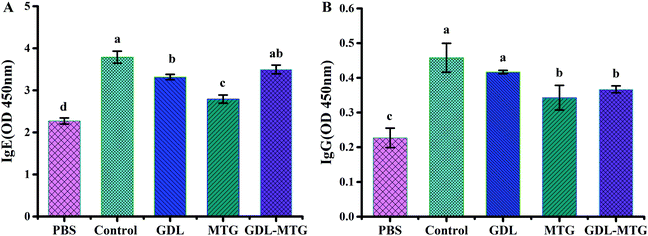
3.2 Specific IgE and IgG levels
Immunoglobulins are important parameters of allergies, and they should be considered in the assessment of the potential effects of any product on allergens. In food allergens, most allergic reactions are IgE mediated. Indirect ELISA was conducted to detect the levels of IgE and IgG in the serum of the mice (Fig. 3). The levels of IgE and IgG in the mouse serum of the control group were higher than those in the PBS group, indicating that the mice were in an allergic state. The levels of IgE and IgG antibodies in the serum of the mice in the MTG cross-linked tofu group were significantly higher than those in the PBS group but were significantly lower than those in the control group (p < 0.05).3.3 Serum mMCP-1 and plasma histamine levels
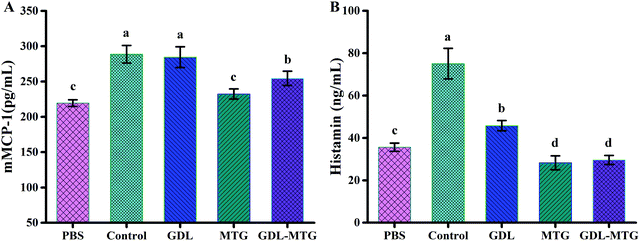
Mouse mast cell protease-I (mMCP-1) is stored and secreted by intestinal mucosal mast cells. mMCP-1 is not only a marker of an increase in mucosal mast cell proliferation and intestinal permeability, but also a new systemic serum marker of intestinal mast cell activation in mouse food allergy models. In this study, the contents of mMCP-1 were measured (Fig. 4). MTG enzymatic cross-linking significantly reduced the mMCP-1 and histamine levels. As shown in Fig. 4A, the contents of mMCP-1 in the PBS, control, and GDL groups were 219.50, 288.67 and 284.61 pg mL−1, respectively. The contents of mast cell protease in the MTG group (232.44 pg mL−1) and the GDL-MTG group (254.56 pg mL−1) decreased compared with those in the control group (288.67 pg mL−1), and these contents were higher than those in the PBS group. However, they were not significantly different from the PBS group.Activated mast cells or basophils undergo degranulation to release various allergenic mediators, such as histamine, cysteine, leukotriene, and prostaglandin D2.25 The trend of plasma histamine level was the same as the mMCP-1 level (Fig. 4B); that is, the histamine content in the control group was the highest (75.04 ng mL−1). The histamine levels of the MTG and GDL-MTG groups significantly decreased, and these levels did not significantly differ from those of the PBS group.
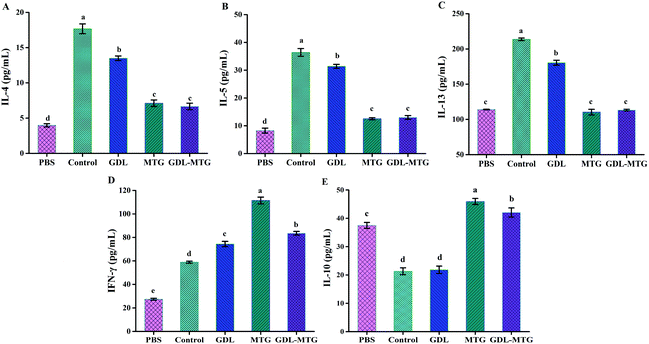
3.4 Spleen cytokine levels
Cytokines play a critical role in allergic reactions. In this study, the levels of the Th1-related cytokine IFN-γ; the Th2-related cytokines IL-4, IL-5, and IL-13; and the Treg-related cytokine IL-10 were detected by stimulating spleen cells in vitro. As shown in Fig. 5, the levels of IL-4, IL-5, and IL-13 (Th2 dependent) in the control group significantly increased, whereas their levels in the MTG and GDL-MTG groups were significantly lower than those in the PBS group. The two groups significantly differed from the PBS group. The levels of IFN-γ (Th1 dependent) in the MTG and GDL-MTG groups were also significantly higher than that in the control group (p < 0.05). The change in IL-10 was similar to that of IFN-γ; that is, the levels of IL-10 in the MTG and GDL-MTG groups were significantly higher than those in the control group (Fig. 5E).3.5 Differentiation and balance regulation of Th1/Th2
Th1 and Th2 can produce different cytokines to determine the function of cell subsets, participate in the proliferation and differentiation of related cell subpopulations, and make Th1 and Th2 cells exhibit mutual inhibition in function. When Th2 cells overreact, the body experience allergic reactions. In this study, the expression of Th1/Th2 in the spleen cells of the mice was determined (Fig. 6). The expression of Th1 in all the experimental groups decreased significantly compared with that in the PBS group (14.2%). The expression levels of control group and GDL group were the lowest (7.95%, 8.42%, respectively). Their levels significantly differ from those of the PBS group. There was no significant difference between the enzyme cross-linked tofu groups and the PBS group. Moreover, the expression of Th2 was consistent with that of Th1. In a word, in the enzymatic cross-linked tofu group, as the Th1 cell population increased, the expression of the Th2 cell population decreased significantly. This result coincided with changes in spleen cytokine levels (Fig. 5).3.6 Tregs
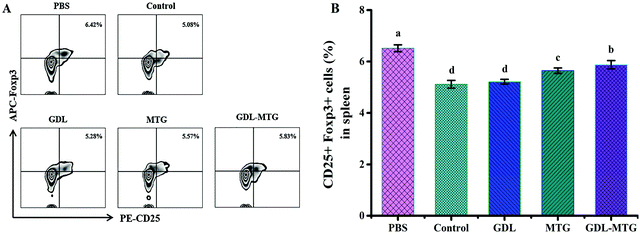
In allergic reactions, Tregs play an important role in maintaining immune tolerance and preventing the occurrence of autoimmune diseases. They can also maintain the differentiation balance of Th1/Th2. Tregs are T lymphocytes characterized by CD25+Foxp3+ in CD4+ cells. The level of CD25+Foxp3+ in the CD4+ cells in the spleen of the mice was identified through flow cytometry (Fig. 7). The results showed that the expression of CD25+Foxp3+ in the PBS group was the highest (6.42%), whereas the expression of CD25+Foxp3+ in the control group was approximately 21.0% lower than that in the PBS group. CD25+Foxp3+ in the three other groups increased, and the groups that had MTG were significantly different from the control group (p < 0.05).4. Discussion
Animal models can simulate food allergy mediated by human-specific immunoglobulin IgE, and they are suitable for evaluating food allergens.26,27 BALB/c mice are small inbred strains with a good production performance; they are easy to raise and have an immune system that is similar to that of humans in terms of overall structure. Thus, they are widely used in studies on highly sensitive diseases.28,29 As an adjuvant, CT can increase the uptake of allergens because its non-toxic subunits can bind to the receptors of intestinal epithelial cells.30,31 Yang et al.22 induced food allergy in BALB/c mice through CT-assisted gastric perfusion. BALB/c mouse models have been successfully applied in the detection of allergens, such as eggs and peanuts. In this study, the allergenic effects of enzymatic cross-linked tofu were systematically evaluated by the anaphylactic shock symptoms, body temperature and weight, serum mMCP-1, plasma histamine, cytokines, Th1/Th2, and Tregs of the animal models.Soybean allergy is a characteristic type I allergic reaction mediated by an antigen-specific IgE antibody. Christensen et al.32 showed that soybean protein can induce BALB/c mice to produce high levels of serum antibodies against glycinin and β-conglycinin. Our results showed that the levels of IgE and IgG in serum in the mice sensitized by MTG cross-linked tofu were significantly lower than those of the soybean protein group (Fig. 3). Therefore, the mice in the enzymatic cross-linked tofu group had less hypersensitivity than those in the soybean protein group, and the production of IgE and IgG antibodies was significantly reduced. Although it would not cause allergic reactions, the relative increase in IgE and IgG antibodies could also be induced when the enzymatic cross-linked tofu was ingested by the mice. Similarly, Gizzarelli et al.33 found that soybean protein can induce high levels of antigen-specific IgE and IgG1 in a IgE-mediated soybean sensitization model induced by intragastric immunity in mice. Mast cells play an important role in allergic diseases. After allergens are activated, the degranulation of mast cells releases many granular mediators, including histamine, leukotriene, and prostaglandin D2, etc.,25 thereby triggering a series of allergic reactions. Increased mMCP-1 levels can enhance the permeability of intestinal epithelial cells, promote Th2 inflammation, and lead to food allergy.34,35 Our results showed that the release of mMCP-1 and histamine in the mice fed with the soybean protein mice increased, whereas their release in the mice administered with enzymatic cross-linked tofu decreased. These results were consistent with those regarding the production of the above mentioned specific antibodies. These results fully indicated that enzymatic cross-linked tofu could reduce the allergy symptoms in mice.
Many studies have reported that cytokines play a crucial role in the regulation of allergic diseases.36,37 In our study, the soybean group induced low levels of IFN-γ and high levels of IL-4, IL-5, and IL-13 in the spleen lymphocytes of the mice, whereas the cytokine expression level of enzymatic cross-linked tofu was contrary to that of the soybean group (Fig. 5). Gizzarelli et al.33 established an oral intragastric IgE-mediated soybean protein sensitization model in BALB/c mice by using CT as an adjuvant. In addition to soybean protein specific IgE and IgG antibodies, the levels of IL-4 and IL-5 increased, and this finding was confirmed in our study. Enzymatic cross-linking tofu could significantly upregulate the level of IL-10. Hanakawa et al.38 obtained similar results in a mouse model intradermally injected with diphtheria toxin.
CD4+ T cells are the most complex immune cell populations known to function in cellular immune responses. In such responses, initial CD4+ T cells differentiate into T helper cells (Th), and the precursors of Th cells stimulated by antigens can differentiate into intermediate Th0. Under the action of different factors, Th0 selectively migrates and differentiates into Th1 or Th2 cells. IL-12 and IFN-γ can induce Th0 to differentiate into Th1 cells, IL-4 and IL-13 can promote Th0 to differentiate into Th2 cells, and IL-10 can promote the differentiation of Tregs. When the body is in a normal state, Th1 and Th2 are in a dynamic equilibrium. Once the body is stimulated by foreign antigens, the expression levels of Th1 and Th2 change, leading to specific types of immune response showing advantages and resulting in a Th1/Th2 imbalance.39,40 Studies have shown that this imbalance is a pathogenesis of allergic diseases.41 Perez-Mazliah et al.42 showed that, the imbalance of Th1/Th2 biased to Th2 can induce the enhancement of humoral immunity, leading to allergic reactions. In our study, MTG enzymatic cross-linked tofu increased the Th1 cell population in the mouse spleen and decreased the Th2 cell population, thereby causing the imbalance of Th1/Th2 differentiation (Fig. 7). These results were confirmed by cytokine analysis findings (Fig. 5). Tregs keep the body in a normal state by regulating the balance of Th1/Th2 expression. When an allergic reaction occurs in the body, the balance of Th1/Th2 tends to develop toward Th2 immune response.43 In our study, the levels of Tregs in the MTG and GDL-MTG groups were higher than those in the control group, and these changes were consistent with those in the Th1 and Th2 cell groups (Fig. 6). The allergic reaction of the MTG and GDL-MTG groups were remarkably weakened and were inclined to a tolerant state; thus, enzymatic cross-linked tofu could stimulate tregs secretion, shift the Th1/Th2 balance to the Th1 direction, and reduce sensitization. Healthy individuals are more tolerant of allergens than patients with allergy mainly because of the increased Tregs in vivo, which can induce the immune tolerance of exogenous antigens and thus inhibit allergic reactions.39,44 Therefore, the sensitization of enzymatic cross-linked tofu is reduced, which may be due to the fact that MTG can embed the sensitization epitopes originally exposed to the protein surface, or change the spatial structure of conformation epitopes in the allergic proteins during the preparation of cross-linked tofu.
In conclusion, a BALB/c mouse model was used to study the sensitization of enzymatic cross-linked tofu. The results showed that enzymatic cross-linked tofu exhibited hypoallergenic symptoms; the levels of IgE, IgG, mMCP-1, and histamine were lower than those of soybean protein. The enzymatic cross-linked tofu also downregulated the population of Th2 and Treg cytokines and upregulated the population of Th1 cytokines, which induced the balance of Th1/Th2 to shift toward Th1. Thus, sensitization decreased. Therefore, enzymatic cross-linking could reduce the sensitization of tofu, providing an effective means for hypoallergenic soybean products.
Conflicts of interest
All authors declare no conflict of interest.Acknowledgements
The authors are thankful for the support from National Natural Science Foundation of China (No. 31460439, 31760453, 31972186, 31601404), Young Scientist Training Program of Jiangxi Province (No. 20122BCB23006).References
- F. Rossi, G. E. Felis, A. Martinelli, B. Calcavecchia and S. Torriani, Microbiological characteristics of fresh tofu produced in small industrial scale and identification of specific spoiling microorganisms (SSO), LWT–Food Sci. Technol., 2016, 70, 280–285 CrossRef CAS.
- Y. Chen and T. Ono, Protein particle and soluble protein structure in prepared soymilk, Food Hydrocolloids, 2014, 39, 120–126 CrossRef CAS.
- Q. Zhang, W. Li, M. Feng and M. Dong, Effects of different coagulants on coagulation behavior of acid-induced soymilk, Food Hydrocolloids, 2013, 33, 106–110 CrossRef CAS.
- C. H. Wang and S. Damodaran, Thermal gelation of globular-proteins-influence of protein conformation on gel strength, J. Agric. Food Chem., 1991, 39, 433–438 CrossRef CAS.
- J. R. Zhu, H. Deng, A. S. Yang, Z. H. Wu, X. Li, P. Tong and H. B. Chen, Effect of microbial transglutaminase cross-linking on the quality characteristics and potential allergenicity of tofu, Food Funct., 2019, 10, 5485–5497 RSC.
- A. Adachi, T. Horikawa, H. Shimizu, Y. Sarayama, T. Ogawa, S. Sjolander, A. Tanaka and T. Moriyama, Soybean β-conglycinin as the main allergen in a patient with food-dependent exercise-induced anaphylaxis by tofu: Food processing alters pepsin resistance, Clin. Exp. Allergy, 2009, 39, 167–173 CrossRef CAS PubMed.
- Y. S. Song, C. Martinez-Villaluenga and E. G. De Mejia, Quantification of human IgE immunoreactive soybean proteins in commercial soy ingredients and products, J. Food Sci., 2008, 73, T90–T99 CrossRef CAS.
- Y. Chang, S. Shiau, F. Chen and F. Lin, Effect of microbial transglutaminase on the rheological and textural characteristics of black soybean packed tofu coagulating with Agar, LWT–Food Sci. Technol., 2011, 44, 1107–1112 CrossRef CAS.
- S. B. M. Yasir, K. H. Sutton, M. P. Newberry, N. R. Andrews and J. A. Gerrard, The impact of transglutaminase on soy proteins and tofu texture, Food Chem., 2007, 104, 1491–1501 CrossRef.
- Y. Li and S. Damodaran, In vitro digestibility and IgE reactivity of enzymatically cross-linked heterologous protein polymers, Food Chem., 2017, 221, 1151–1157 CrossRef CAS PubMed.
- R. Porta, C. V. L. Giosafatto, P. di Pierro, A. Sorrentino and L. Mariniello, Transglutaminase-mediated modification of ovomucoid: Effects on its trypsin inhibitory activity and antigenic properties, Amino Acids, 2013, 44, 285–292 CrossRef CAS PubMed.
- M. B. Villas-Boas, M. A. Fernandes, R. D. L. Zollner and F. M. Netto, Effect of polymerization with transglutaminase on in vitro digestion and antigenicity of beta-lactoglobulin, Int. Dairy J., 2012, 25, 123–131 CrossRef CAS.
- G. Xing, C. V. L. Giosafatto, X. Rui, M. Dong and L. Mariniello, Microbial transglutaminase-mediated polymerization in the presence of lactic acid bacteria affects antigenicity of soy protein component present in bio-tofu, J. Funct. Foods, 2019, 53, 292–298 CrossRef CAS.
- E. H. A. Dekking, P. A. Van Veelen, A. de Ru, E. M. C. Kooy-Winkelaar, T. Groneveld, W. F. Nieuwenhuizen and F. Koning, Microbial transglutaminases generate T cell stimulatory epitopes involved in celiac disease, J. Cereal Sci., 2008, 47, 339–346 CrossRef CAS.
- R. Porta, C. V. L. Giosafatto, P. di Pierro, A. Sorrentino and L. Mariniello, Transglutaminase-mediated modification of ovomucoid: Effects on its trypsin inhibitory activity and antigenic properties, Amino Acids, 2013, 44, 285–292 CrossRef CAS PubMed.
- M. B. Villas-Boas, K. P. Vieira, G. Trevizan, R. de Lima Zollner and F. M. Netto, The effect of transglutaminase-induced polymerization in the presence of cysteine on β-lactoglobulin antigenicity, Int. Dairy J., 2010, 20, 386–392 CrossRef CAS.
- J. Leszczynska, A. Lacka and M. Bryszewska, The use of transglutaminase in the reduction of immunoreactivity of wheat flour, Food Agric. Immunol., 2006, 17, 105–113 CrossRef CAS.
- C. Zhou, T. Ludmila, N. Sun, C. Wang, Q. Pu, K. Huang and H. Che, BALB/c mice can be used to evaluate allergenicity of different food protein extracts, Food Agric. Immunol., 2016, 27, 589–603 CrossRef CAS.
- F. Gizzarelli, S. Corinti, B. Barletta, P. Iacovacci, B. Brunetto, C. Butteroni, C. Afferni, R. Onori, M. Miraglia, G. Panzini, G. Di Felice and R. Tinghino, Evaluation of allergenicity of genetically modified soybean protein extract in a murine model of oral allergen-specific sensitization, Clin. Exp. Allergy, 2006, 36, 238–248 CrossRef CAS PubMed.
- A. S. Yang, L. L. Zuo, Y. F. Cheng, Z. H. Wu, X. Li, P. Tong and H. B. Chen, Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms, Food Funct., 2018, 9, 1899–1909 RSC.
- H. Murakami, T. Ogawa, A. Takafuta, E. Yano, N. Zaima and T. Moriyama, Identification of the 7S and 11S globulins as percutaneously sensitizing soybean allergens as demonstrated through epidermal application of crude soybean extract, Biosci. Biotechnol. Biochem., 2018, 82, 1408–1416 CrossRef CAS PubMed.
- H. Yang, J. Y. Gao, A. S. Yang, J. Lu and H. B. Chen, Allergenicity characteristics of germinated soybean proteins in a BALB/c mouse model, Regul. Toxicol. Pharmacol., 2015, 72, 249–255 CrossRef CAS PubMed.
- P. Rupa and Y. Mine, Oral immunotherapy with immunodominant T-cell epitope peptides alleviates allergic reactions in a BALB/c mouse model of egg allergy, Allergy, 2012, 67, 74–82 CrossRef CAS PubMed.
- A. S. Yang, C. Y. Long, J. H. Xia, P. Tong, Y. F. Cheng, Y. Wang and H. B. Chen, Enzymatic characterisation of the immobilised Alcalase to hydrolyse egg white protein for potential allergenicity reduction, J. Sci. Food Agric., 2017, 97, 199–206 CrossRef CAS PubMed.
- J. Li, F. Zhang and J. Li, The Immunoregulatory Effects of Traditional Chinese Medicine on Treatment of Asthma or Asthmatic Inflammation, Am. J. Chin. Med., 2015, 43, 1059–1081 CrossRef PubMed.
- C. Kanagaratham, B. F. Sallis and E. Fiebiger, Experimental models for studying food allergy, Cellul. Mol. Gastroenterol. Hepatolol., 2018, 6, 356–369 CrossRef PubMed.
- J. Huang, C. Liu, Y. Wang, C. Wang, M. Xie, Y. Qian and L. Fu, Application of in vitro and in vivo models in the study of food allergy, Food Sci. Human Wellness, 2018, 7, 235–243 CrossRef.
- T. Liu, S. Navarro and A. L. Lopata, Current advances of murine models for food allergy, Mol. Immunol., 2016, 70, 104–117 CrossRef CAS PubMed.
- M. K. Oyoshi, H. C. Oettgen, T. A. Chatila, R. S. Geha and P. J. Bryce, Food allergy: Insights into etiology, prevention, and treatment provided by murine models, J. Allergy Clin. Immunol., 2014, 133, 309–317 CrossRef CAS PubMed.
- E. V. Navolotskaya, V. B. Sadovnikov, V. M. Lipkin and V. P. Zav'Yalov, Binding of cholera toxin B subunit to intestinal epithelial cells, Toxicol. In Vitro, 2018, 47, 269–273 CrossRef CAS PubMed.
- S. Kirkeby and A. M. Lynge Pedersen, Modifications of cholera toxin subunit B binding to human large intestinal epithelium. An immunohistochemical study, Microb. Pathog., 2018, 124, 332–336 CrossRef CAS PubMed.
- H. R. Christensen, S. W. Bruun and H. Frokiaer, Antigenic specificity of serum antibodies in mice fed soy protein, Int. Arch. Allergy Immunol., 2003, 132, 58–67 CrossRef CAS PubMed.
- F. Gizzarelli, S. Corinti, B. Barletta, P. Iacovacci, B. Brunetto, C. Butteroni, C. Afferni, R. Onori, M. Miraglia, G. Panzini, G. Di Felice and R. Tinghino, Evaluation of allergenicity of genetically modified soybean protein extract in a murine model of oral allergen-specific sensitization, Clin. Exp. Allergy, 2006, 36, 238–248 CrossRef CAS.
- P. Tong, S. Chen, J. Y. Gao, X. Li, Z. H. Wu, A. S. Yang, J. L. Yuan and H. B. Chen, Caffeic acid-assisted cross-linking catalyzed by polyphenol oxidase decreases the allergenicity of ovalbumin in a BALB/c mouse model, Food Chem. Toxicol., 2018, 111, 275–283 CrossRef CAS PubMed.
- L. Xu, H. Yi, Z. Wu, W. Han, K. Chen, M. Zang, D. Wang, X. Zhao, H. Wang and C. Qu, Activation of mucosal mast cells promotes inflammation-related colon cancer development through recruiting and modulating inflammatory CD11b+Gr1+cells, Cancer Lett., 2015, 364, 173–180 CrossRef CAS PubMed.
- N. K. Kordulewska, A. Cieślińska, E. Fiedorowicz, B. Jarmołowska, K. Piskorz-Ogórek and E. Kostyra, Cytokines concentrations in serum samples from allergic children-Multiple analysis to define biomarkers for better diagnosis of allergic inflammatory process, Immunobiology, 2018, 223, 648–657 CrossRef CAS PubMed.
- Z. Fan, H. Che, S. Yang and C. Chen, Estrogen and estrogen receptor signaling promotes allergic immune responses: Effects on immune cells, cytokines, and inflammatory factors involved in allergy, Allergol. Immunopathol., 2019, 47, 506–512 CrossRef CAS PubMed.
- S. Hanakawa, A. Kitoh, R. Shibuya, T. Dainichi, T. Nomura, T. Honda, G. Egawa, A. Otsuka, S. Nakajima, M. Fujita and K. Kabashima, Percutaneous sensitization is limited by in situ inhibition of cutaneous dendritic cell migration through skin-resident regulatory T cells, J. Allergy Clin. Immunol., 2019, 144, 1343–1362 CrossRef CAS PubMed.
- R. M. Talaat, S. F. Mohamed, I. H. Bassyouni and A. A. Raouf, Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity, Cytokine, 2015, 72, 146–153 CrossRef CAS.
- M. I. Vargas-Rojas, H. Solleiro-Villavicencio and E. Soto-Vega, Th1, Th2, Th17 and Treg levels in umbilical cord blood in preeclampsia, J. Matern.-Fetal Neonat. Med., 2016, 29, 1642–1645 CrossRef CAS PubMed.
- H. S. Shin, H. See, S. Y. Jung, D. W. Choi, D. Kwon, M. Bae, K. Sung and D. Shon, Turmeric (Curcuma longa) attenuates food allergy symptoms by regulating type 1/type 2 helper T cells (Th1/Th2) balance in a mouse model of food allergy, J. Ethnopharmacol., 2015, 175, 21–29 CrossRef CAS PubMed.
- D. Perez-Mazliah and J. Langhorne, CD4T-cell subsets in malaria: TH1/TH2 revisited, Front Immunol., 2015, 5, 1–8 Search PubMed.
- E. M. M. Velez, C. M. Galdeano, E. Carmuega, R. Weill, M. E. B. Bonet and G. Perdigon, Probiotic fermented milk consumption modulates the allergic process induced by ovoalbumin in mice, Br. J. Nutr., 2015, 114, 566–576 CrossRef CAS PubMed.
- M. N. Rivas and T. A. Chatila, Regulatory T cells in allergic diseases, J. Allergy Clin. Immunol., 2016, 138, 639–652 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2020 |