Hypotaurine promotes longevity and stress tolerance via the stress response factors DAF-16/FOXO and SKN-1/NRF2 in Caenorhabditis elegans†
Qin-Li
Wan
,
Xiaodie
Fu
,
Xiao
Meng
,
Zhenhuan
Luo
,
Wenyu
Dai
,
Jing
Yang
,
Chongyang
Wang
,
Hao
Wang
* and
Qinghua
Zhou
 *
*
The First Affiliated Hospital, Biomedical Translational Research Institute, Jinan University, Guangzhou, Guangdong 510632, China. E-mail: haowang@jnu.edu.cn; gene@email.jnu.edu.cn; Fax: +86 20 85222787; Tel: +86 20 85222787
First published on 11th November 2019
Abstract
Hypotaurine, an important sulfur-containing and nonpeptidic amino acid, is a precursor of taurine and an antioxidant. Our previous study indicated that hypotaurine levels are associated with the ageing of Caenorhabditis elegans (C. elegans). However, whether hypotaurine plays a role in the lifespan regulation of C. elegans and the mechanism remains undetermined. Here, we found that hypotaurine enhances oxidative stress resistance and ameliorates ageing in C. elegans. Our results show that hypotaurine regulates a variety of pathways and leads to the upregulation of some age-related genes to extend lifespan. We also found that the stress response-related transcription factors DAF-16/FOXO and SKN-1/NRF2 contribute to the beneficial longevity conferred by hypotaurine. Moreover, our results demonstrate that hypotaurine induced lifespan extension by regulating the insulin/IGF-1 signaling (IIS) pathway, the reproductive signaling pathway and DR-like mechanisms. Additionally, our results also indicated that mitochondrial function also plays a crucial role in the lifespan extension induced by hypotaurine. Taken together, these data indicate that hypotaurine delays the ageing of C. elegans, due, at least in part, to its antioxidant activity, which in turn regulates IIS, and reproductive and DR-related pathways, thereby inducing the activity of the transcription factors DAF-16 and SKN-1.
Introduction
Ageing is universally characterized as the deterioration of tissue and cellular integrity that eventually leads to organ failure. In humans and most vertebrates, ageing is associated with a higher incidence of some diseases, including type 2 diabetes, cancer, hypertension, Alzheimer's disease and Parkinson's disease.1,2 The effects of ageing are large social and economic burdens. Therefore, understanding the basic molecular mechanisms of ageing and interventions to promote healthy ageing are urgently needed, and drugs that can influence lifespan and healthspan are of high putative value. To date, accompanied by the development of anti-ageing drugs, several FDA-approved drugs, including rapamycin,3 metformin,4 trametinib5 and lithium,6 are known to extend lifespan in a wide range of model organisms; others, including urolithin A,7 JZL1848 and TES-991,9 have not yet been approved by the FDA but also show great potential in affecting lifespan and healthspan.Food-derived compounds have great potential to regulate lifespan due to their fewer toxic and side effects. Recently, several similar compounds that ameliorate ageing have been identified, such as resveratrol,10 urolithin A,7 branched-chain amino acids11 and blueberry extracts.12 Here, taurine and hypotaurine are very important sulfur-containing and nonpeptidic amino acids. It has been reported that taurine and hypotaurine are food materials that mainly exist in poultry, fish, shellfish, shrimp, squid, algae, and other animals (including mammals).13 In many animals (mammals and humans), taurine, as an antioxidative agent, is involved in a variety of crucial biological functions, including cell proliferation regulation, detoxification, immunomodulation and oxidative stress inhibition.14,15 Hypotaurine, a precursor of taurine, showed more powerful antioxidant activity than did taurine due to its faster reaction with superoxide radicals and hydroxyl.16 Indeed, hypotaurine, as an antioxidant, has also been reported to play an important role in the hepatoprotective effect against oxidative stress-mediated liver injuries.17,18 Additionally, it has been reported that hypotaurine, due to its antioxidant activity, can quench oxidants released by human neutrophils, inhibit lipid peroxidation, and prevent the inactivation of superoxide dismutase by hydrogen peroxide.19,20
Several lines of evidence have demonstrated that there is an inseparable relationship between oxidative damage and lifespan regulation in several different species. Some antioxidants have also been suggested to alleviate ageing, such as hydralazine,21 cytoprotective polyphenol22 and polysaccharides,23 which promote oxidative stress resistance and affect lifespan and healthspan. Indeed, taurine, as an antioxidant, also effectively delayed ageing by promoting resistance to oxidative stress.24 However, whether and how another antioxidant, hypotaurine, a precursor of taurine, delays ageing has not been elucidated.
In our previous study, we found that the level of hypotaurine decreased with age.25 Continuing previous research, in this work, we use Caenorhabditis elegans (C. elegans) as a model organism to explore whether hypotaurine can prolong lifespan and to describe the molecular mechanism by which hypotaurine extends lifespan. C. elegans possesses several characteristics that make it an excellent model for ageing research, including short lifespan, conserved developmental programmes, genetic tractability and a fully sequenced genome. In addition, ageing studies in C. elegans have provided a wealth of information about the molecular mechanisms that regulate ageing, such as insulin/insulin-like growth factor (IGF), dietary restriction (DR), reproductive signaling pathways and mitochondrial function.26,27 In this work, our results showed that hypotaurine significantly extended the lifespan of C. elegans due to its antioxidant activity by activating the stress-related transcription factors DAF-16/FOXO and SKN-1/NRF2 and regulating several age-related signaling pathways, including IIS, reproductive signaling and DR-like signaling.
Materials and methods
Nematode strains and maintenance
The C. elegans strains used in this work were obtained from the Caenorhabditis Genetic Center (CGC) (University of Minnesota, USA), which is supported by the NIH NCRR. Maintenance, synchronization and RNAi treatment were conducted as previously described.28,29 Wild-type N2 (Bistol) was used as the reference stain. All of the following strains were used in this work: the wild-type C. elegans strain N2, CF1038 daf-16(mu86) I, EU1 skn-1(zu67) IV, VC199 sir-2.1(ok434) IV, TK22 mev-1(kn1) III, CB1370 daf-2(e1370) III, CF1903 glp-1(e21444) III, MQ887 isp-1(qm150) IV, CB4876 clk-1(e2519) III, CL2166 (dvIs19 [(pAF15) gst-4p::GFP::NLS]) III, CL2070 (dvIs70 [hsp-16.2p::GFP + rol-6(su1006)]), SJ4005 (zcIs4 [hsp-4::GFP] V), SJ4100 (zcIs13[hsp-6::GFP]), DA1116 eat-2(ad1116) II, RB1206 rsks-1(ok1255) III and RB754 aak-2(ok524) X.All compounds used in this work were purchased from Sigma-Aldrich (Munich, Germany). Hypotaurine was dissolved in PBS, and NGM plates with compound were equilibrated overnight before use.
Lifespan assays
All lifespan assays were performed at 20 °C, except for those with CF1903, according to the standard protocols as previously described.29 For CF1903, L1 worms were grown at 20 °C for 12 h, then grown at 25 °C until the L4 larval or young adult stage to eliminate the germline, and then the worms were switched back to 20 °C for the lifespan assay.29 After bleaching, aged synchronized eggs were incubated in M9 buffer overnight and placed on solid nematode growth medium (NGM) at the L1 stage. Then, 100 late L4 larvae or young adults were transferred to plates seeded with dead Escherichia coli (E. coli), 10 μM 5-fluro-2′-deoxyuridine (FUdR, Sigma) and the indicated compounds. The animals were then transferred to fresh plates every other day to ensure drug potency and scored every day. Animals that failed to exhibit mechanical stimulation-induced movement were scored as dead. Statistical analysis and mean lifespan were obtained by SPSS software. P values were calculated by a log-rank test. Experiments were repeated at least twice. The mean, SEM, p value and lifespan value are summarized in Table S1,† ESI.Paraquat stress resistance assay
The resistance to oxidative stress induced by paraquat was analysed using a previously described method.30 Briefly, age-synchronized young adults were transferred to fresh plates containing 5 mM paraquat and with or without 10 mM hypotaurine. The survival rate was determined as described for the lifespan assay until all the animals died.Measurement of reactive oxygen species (ROS)
To measure the intracellular ROS accumulation in C. elegans, L1 larvae of wild-type N2 were treated with the appropriate compound or control until they reached the young adult stage; each group contained 50–100 individuals. Then, animals treated with or without the compound were transferred to plates with 10 mM 2′7′-dichlorofluorescein diacetate (H2DCF-DA) and incubated in the dark at 20 °C for 1 h. After incubation, animals labelled with H2DCF-DA were picked onto agar pads containing 5 mM imidazole and then imaged using a Nikon Ti2-U fluorescence microscope. The relative fluorescence intensity was evaluated using ImageJ software, and the p value was calculated using a two-tailed Student's t test.Quantitative RT-PCR assay
Total RNA was isolated from approximately 3000 synchronized day 1 adult worms per condition with the phenol–chloroform extraction method. Afterwards, RNA concentrations were quantified using a NanoDrop spectrophotometer. Then, cDNA was prepared using a High Capacity cDNA Transcription kit (Applied Biosystems) following the manufacturer's protocol and using 500 ng of RNA. SYBR Green Select Master Mix (Applied Biosystems) was used to quantify mRNA levels in a CFX96 real-time system (Bio-Rad). For each gene, triplicate biological samples and three technical replicates were performed. The results were calculated by the 2−ΔΔCt method after normalizing to the reference gene cdc-42.31P values were calculated using two-tailed Student's t test. The primers used in this publication are summarized in Table S2,† ESI.Fluorescence microscopic imaging
GST-4::GFP, HSP-16.2::GFP, HSP-6::GFP and HSP-4::GFP activity were assayed using strains CL2166, CL2070, SJ4100 and SI4005, respectively. For CL2166, age-synchronized young adult transgenic worms were treated with 10 mM hypotaurine and solvent control (PBS) for 12 h. Then, the animals were picked onto agar pads and imaged at 20× magnification using a Nikon Ti2-U fluorescence microscope. For CL2070, SJ4100 and SJ4005, synchronized L1 larvae were incubated on plates with or without 10 mM hypotaurine until they reached the young adult stage, and then all animals were heated at 35 °C for 2 h to induce the expression of heat shock proteins. The animals were moved to agar pad and imaged at 20× magnification using a Nikon Ti2-U fluorescence microscope after the animals recovered for 12 h at 20 °C. The total GFP signal for every animal was quantified by ImageJ software. The data shown are the average number of pixels in every transgenic strain (n ≥ 30) at each indicated treatment. P values were calculated using two-tailed Student's t test.Results
Hypotaurine treatment increases C. elegans lifespan
To examine whether hypotaurine plays a role in lifespan regulation, we first analysed the effect of hypotaurine on the lifespan of C. elegans (Fig. 1A). We exposed wild-type N2 to increasing concentrations of hypotaurine ranging from 0.02 to 10 mM on E. coli OP50, which were heat inactivated to avoid the metabolism of the compound. Our results showed that all concentrations significantly increased the lifespan compared with vehicle, with the maximum lifespan observed at 10 mM (Fig. 1B–D). Therefore, in all subsequent experiments, hypotaurine was applied at a concentration of 10 mM.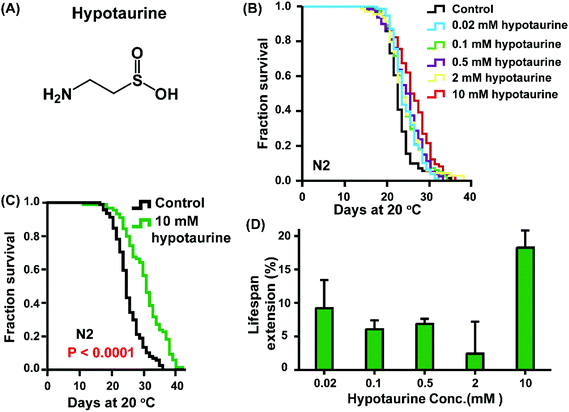 | ||
| Fig. 1 Hypotaurine extends the lifespan of C. elegans. (A) Chemical structure of hypotaurine. (B) Lifespan curves of wild-type N2 worms cultured at increasing concentrations (0.02–10 mM). (C) Survival analysis of animals exposed to either hypotaurine (10 mM) or vehicle (PBS). (D) Dose-dependent analysis of the effect of hypotaurine on the lifespan of C. elegans. Error bars represent the mean ± SD. Lifespan was analysed using the Kaplan–Meier test, and p values were calculated using the log-rank test. Experiments were repeated at least twice, and detailed lifespan values are summarized in ESI Table 1.† | ||
Hypotaurine treatment activates the DAF-16 and SKN-1 transcription factors
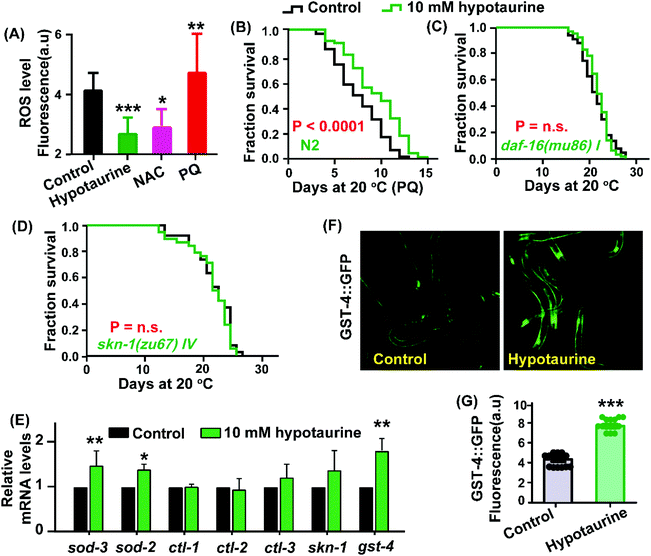
Considering that hypotaurine is an antioxidant, we first examined the effect of hypotaurine on internal levels of ROS in wild-type animals using the 2′7′-dichlorofluorescin diacetate (H2DCF-DA) assay. In this assay, H2DCF-DA is a free radical sensor that becomes deacetylated by intracellular esterases and then emits fluorescence correlating with intracellular ROS levels. Our results showed that under exposure to 10 mM hypotaurine, the worms showed significantly decreased levels of ROS compared with the ROS levels in nontreated controls (Fig. 2A). Furthermore, to assess whether hypotaurine-mediated lifespan extension is associated with antioxidant activity, we determined the effect of hypotaurine on lifespan under the oxidative stress conditions induced by 5 mM paraquat in wild-type worms. Our results showed that hypotaurine significantly protected wild-type animals from oxidative stress, suggesting that the pro-longevity effect of hypotaurine depends on its antioxidative capacity, at least in part (Fig. 2B).It is well known that antioxidant activity is closely related to pro-longevity effects on various organisms.32,33 To determine whether and to what extent known genetic mediators of lifespan extension and oxidative stress interventions may contribute to the beneficial lifespan of hypotaurine, we first examined the transcription factor DAF-16. DAF-16, the C. elegans FOXO orthologue, is a central regulator of longevity and oxidative stress tolerance, and many DAF-16 target genes encode proteins predicted to protect cells from oxidative stress.34 We found that the pro-longevity effect of hypotaurine was completely lost in the daf-16(mu86) mutant (Fig. 2C). In addition, we also found that hypotaurine treatment increased the endogenous mRNA levels of target genes of DAF-16 (sod-2 and sod-3), although the level of other target genes of DAF-16 (ctl-1, ctl-2 and ctl-3) remained unchanged (Fig. 2E). However, upregulation of sod-3 and sod-2 induced by hypotaurine were abolished in the daf-16 mutant (Fig. S1A,† ESI). Altogether, these results suggested that hypotaurine increased the lifespan of C. elegans by activating DAF-16/FOXO.
In addition to DAF-16, the C. elegans NRF2 orthologue SKN-1 is also a key oxidative stress response transcription factor that activates antioxidant and phase II detoxification genes, in turn detoxifying free radicals and conjugating electrophiles.35 We analysed the role of skn-1 in the lifespan extension induced by hypotaurine. We found that hypotaurine failed to extend the lifespan of the skn-1(zu67) mutant (Fig. 2D), and the mRNA level of gst-4, a target gene of skn-1 that responds to phase II detoxification, was significantly increased in animals treated with 10 mM hypotaurine compared with nontreated control animals (Fig. 2E). However, the elevation of gst-4 mRNA level induced by hypotaurine was abolished in the skn-1(zu67) mutant (Fig. S2A,† ESI). Additionally, the mRNA level of skn-1 was also moderately elevated but not to a significant level (Fig. 2E). Furthermore, we also characterized the effect of hypotaurine on the protein expression of GST-4 using the transgenic strain CL2166.36 Our results show that the fluorescence intensity of CL2166 was significantly increased in animals exposed to 10 mM hypotaurine compared with animals treated with the vehicle (Fig. 2F and G), but did not do the same in worms subjected to skn-1 RNAi (Fig. S2B–D, ESI†). These data indicated that the lifespan extension effect of hypotaurine was also conferred by activating SKN-1.
Hypotaurine extends the lifespan of C. elegans through the IIS pathway and the reproductive signaling pathway
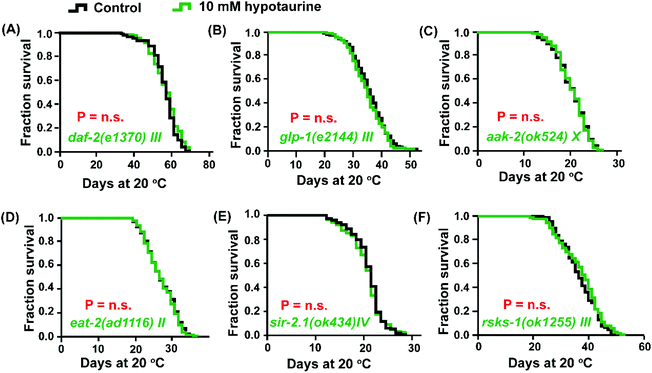
Both DAF-16 and SKN-1 are pivotal downstream effectors of the insulin/IGF-1 signaling (IIS) pathway that regulate lifespan.37 In addition, IIS pathway-mediated regulation of longevity is also associated with the modulation of ROS levels.38 Due to the activation of DAF-16 and SKN-1 by hypotaurine to achieve lifespan extension, we asked whether the IIS pathway plays a role in hypotaurine-mediated lifespan extension. Indeed, the effect of hypotaurine on lifespan could share similar phenotypic features with the IIS pathway because hypotaurine did not further lengthen the lifespan of the long-lived mutant of the insulin-like receptor daf-2 (Fig. 3A).In addition, the activation of DAF-16 and SKN-1 by hypotaurine was also reminiscent of the reproductive signaling pathway. In C. elegans, abolishing reproduction can significantly increase lifespan by regulating the transcription factors DAF-16 and SKN-1, at least in part.39,40 We assessed the effect of hypotaurine on the long-lived glp-1 (e2144) mutant, which is a temperature-sensitive mutant that is long-lived when cultured at the nonpermissive temperature due to the elimination of the germline.41 Our results showed that glp-1 harboured a similar mean lifespan when exposed to 10 mM hypotaurine and to the vehicle (Fig. 3B). In summary, the longevity phenotype induced by hypotaurine might depend on the inhibition of IIS and reproductive signaling, subsequently activating the downstream transcription factors DAF-16 and SKN-1.
Hypotaurine extends lifespan in a manner that mimics DR
It has been reported that in C. elegans, the constitutive activation of AMP-activated kinase (AMPK) elevates animal stress resistance and longevity in a DAF-16-dependent manner.42 To confirm whether hypotaurine activates DAF-16 due to the induction of AMPK activity, we analysed the effect of 10 mM hypotaurine on the short-lived aak-2(ok524) mutant, which is a loss of function mutant of one of the two α catalytic subunits of AMPK (AMPKα2). We found that, similar to the daf-16 mutant, hypotaurine could not further extend the lifespan of the aak-2 mutant (Fig. 3C).In C. elegans, AMPK is a conserved major sensor of cellular energy status, and both AMPK and DAF-16 respond to dietary restriction-induced longevity.43 The pro-longevity effect of hypotaurine via DAF-16 and AMPK raises the question of whether DR plays a role in the lifespan extension induced by hypotaurine. Therefore, we tested the effect of hypotaurine on the lifespan of the eat-2 mutant, which is a model that mimics DR due to impaired pharyngeal pumping and therefore reduced food intake.44 Indeed, the impairment of eat-2 expression completely inhibited the hypotaurine-induced effects on the lifespan of wild-type animals (Fig. 3D).
C. elegans SIR-2.1, a member of the sirtuin family, plays an important role in response to DR-mediated longevity by binding DAF-16 in a 14-3-3-dependent manner.45 To investigate whether the DR-like mechanism induced by hypotaurine requires sir-2.1, we examined the effect of hypotaurine on the sir-2.1 mutant. We found that the loss of function of sir-2.1 completely eliminated the pro-longevity effect of hypotaurine (Fig. 3E), suggesting that sir-2.1 is necessary for hypotaurine-induced longevity.
Another nutrient sensor, TOR, which is an important sensor of amino acid levels, is a mediator of the metabolic response to DR and of longevity.46,47 We found that 10 mM hypotaurine could not further increase the lifespan of the rsks-1 mutant, indicating that the positive impact of hypotaurine on lifespan was conferred by the mTOR signaling pathway (Fig. 3F). rsks-1 is an orthologue of ribosomal protein S6 kinase (S6K) and a key target of the TOR pathway. This result indicated that the positive impact of hypotaurine on lifespan required the mTOR signaling pathway. Taken together, these results illustrated that the effects of hypotaurine on lifespan extension may be conferred by DR-like mechanisms.
Hypotaurine-induced lifespan extension depends on mitochondrial function
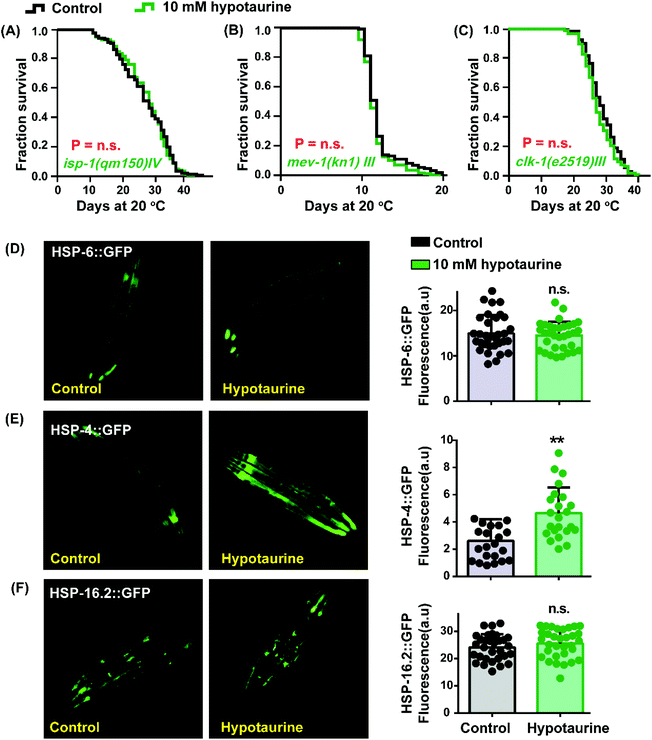
As mentioned before, the antioxidant activity of hypotaurine contributes to its lifespan-extending effect. Reactive oxygen species (ROS), as a byproduct of normal metabolism, are mainly generated in the mitochondria.48 Furthermore, the longevity of mitochondrial mutant strains has been correlated with resistance to oxidative stress and ROS-mediated signaling.49 These studies made us question whether mitochondrial function plays a role in hypotaurine-mediated lifespan extension. We detected the impact of hypotaurine on worms with mutations in isp-1, clk-1 and mev-1. The isp-1 gene encodes the Rieske iron–sulfur protein of mitochondrial respiratory chain complex III, and worms with mutated isp-1 were shown to be long-lived.50 Similar to isp-1, clk-1 encodes an orthologue of human COQ7 (coenzyme Q7, hydroxylase), and a clk-1 mutation was also shown to promote lifespan extension.51 However, the mutation of mev-1, which encodes the cytochrome b large subunit (Cyt-1/ceSDHC) in complex II of the mitochondrial electron transport chain, decreases lifespan.52 Our results showed that hypotaurine failed to extend the lifespan of the long-lived mitochondrial dysfunction isp-1 (Fig. 4A) and clk-1 mutants (Fig. 4C) and the short-lived mev-1 mutant (Fig. 4B). Altogether, our results demonstrated that hypotaurine extended the lifespan of C. elegans through mitochondrial signaling.Considering that the lifespan extension mediated by hypotaurine was associated with mitochondrial function, we determined the impact of hypotaurine on the mitochondrial unfolded protein response (UPRmit), which has been reported to be induced by the mitochondrial respiratory chain and to contribute to lifespan extension.53 Unexpectedly, the UPRmit (GFP fluorescence intensity of HSP-6::GFP) was unchanged in animals exposed to 10 mM hypotaurine compared with untreated control animals (Fig. 4D). Additionally, we also detected other unfolded protein responses (UPRER and UPRcytosolic) using the transgene strains SJ4005 and CL2070, respectively. Our results showed that hypotaurine notably increased the UPRER (Fig. 4E), but not the UPRcytosolic (Fig. 4F). In support of our result, it has been reported that the UPRER is associated with NRF2 to prolong lifespan.54 This finding also indicates that hypotaurine may induce the function of SKN-1/NRF2 to achieve lifespan extension by activating the UPRER.
Discussion
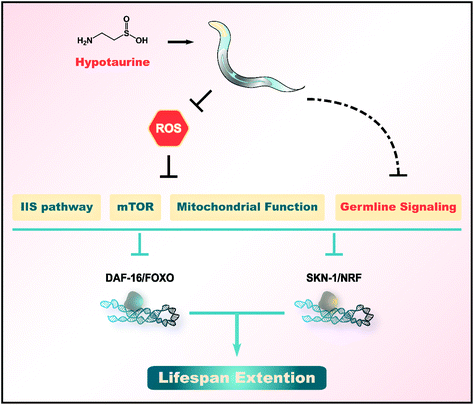
The oxidative stress and “free radical theory” represent widely accepted explanations of ageing.55,56 High levels of ROS are generated as byproducts by aerobic metabolism and contribute to the damage of many macromolecules, such as DNA, lipids and proteins.55,57 An imbalance between the generation of ROS and their detoxification allows various oxygen radicals to escape and damage many macromolecules. This damage is thought to result in an accelerated decline in physiological functions and an enhanced risk of death. Therefore, numerous studies have indicated the impact of supplementation with antioxidants on ageing.Here, we found that hypotaurine, a sulfur-containing amino acid and antioxidant, promotes the healthspan and lifespan of C. elegans (Fig. 1). Hypotaurine acts through IIS and the reproductive signaling pathway, as well as DR-like mechanisms, to induce the function of DAF-16/FOXO and SKN-1/NRF2 to extend lifespan. In addition, hypotaurine also affects lifespan extension by influencing mitochondrial function (Fig. 5).
In this work, we found that the pro-longevity effect of hypotaurine depends on the inhibition of the IIS pathway. To support our finding, a previous study reported that impaired insulin and IGF-1 signaling reduced ROS levels and notably increased the lifespan of animals.58 Additionally, our results illustrated that hypotaurine elevated the lifespan by mimicking the DR-like mechanism, including activating AMPK and sir-2.1, and inhibiting the mTOR signaling pathway (Fig. 3C–F). Indeed, a previous report indicated that the lifespan extension of yeast by inhibiting the mTOR signaling pathway is related to regulating the level of ROS.59 Likewise, researchers found that DR could activate SIRTs in mice, which induce SOD2 activation, and finally reduce ROS levels and oxidative stress.60 Consistent with this result, we also found that the mRNA level of sod-2 was significantly elevated when animals were exposed to 10 mM hypotaurine. Similarly, it has been reported that AMPK, an energy sensor, can induce the transcription factor DAF-16/FOXO, subsequently up-regulating various antioxidant genes to quench the ROS level.61 These results seem to indicate several possible mechanisms for hypotaurine-mediated lifespan extension: one possibility is that hypotaurine directly activates oxidative stress-related signaling, such as inhibiting the IIS pathway and mTOR signaling pathway, thereby activating the downstream transcription factor, including DAF-16 and SKN-1, finally resulting in alleviating oxidative stress and enhancing lifespan; another possibility is that hypotaurine, as an antioxidant, directly reduces the level of ROS to achieve lifespan extension, at the same time, it regulates several signaling pathways (i.e., IIS, mTOR, reproductive and DR-like pathways) that are beneficial for longevity, which in turn creates a virtuous cycle that delays ageing and enhances healthspan. In addition, in this work, we found that hypotaurine failed to extend the lifespan of the short-lived mev-1(kn1) mutant, which produces more intracellular ROS. This seems to be contradictory to the lifespan extension-mediated by hypotaurine depending on reduction of the ROS level. However, these effects were similar to some reported natural products which also can extend the lifespan of C. elegans, such as leaf extracts from Anacardium occidentale,62 chlorophyll63 and natural lignans from Arctium lappa.64 This result also suggests that the effect of hypotaurine to prolong life may not only depend on its antioxidant activity, but may also regulate other aging-related signaling pathways. Indeed, our results exhibited that other endogenous signaling pathways, such as the reproductive signaling pathway, other than a direct antioxidant mechanism are involved in the lifespan extension induced by hypotaurine.
It is well known that metabolism and ageing are intimately linked, and many approaches that interfere with metabolism can also affect longevity.65,66 Recently, several metabolites that can modulate ageing have been identified, such as eicosapentaenoyl ethanolamide,67 oxaloacetate,68 ketoglutarate,69 xanthine70 and allantoin.71 Endogenous metabolites, due to their low toxicity and limited side effects, show considerable advantages for regulating lifespan. Concomitantly, unbiased screening approaches to identify anti-ageing compounds have been performed by many studies and have made great progress.33 The most direct and effective method to discover anti-ageing metabolites is to include metabolome research. Recently, research has found that xanthine metabolism is associated with the ageing of organisms based on an unbiased multi-omics study and that supplementation with xanthine and its derivatives can extend the lifespan of C. elegans.70 In addition, similar to this study, in our previous study, we found that ageing is accompanied by changes in pyrimidine metabolism.25 In a subsequent study, we found that exogenously supplemented pyrimidine metabolites, including thymine, cytidine, orotate, beta-aminoisobutyrate and uridine, in food can significantly prolong the lifespan of C. elegans.29 In this work, based on our ageing-related metabolomics study in which the level of hypotaurine was related to the ageing of C. elegans, we found that hypotaurine supplementation in food prolonged the lifespan and healthspan of C. elegans. Therefore, our research provides a new way to develop new anti-ageing endogenous metabolites based on metabolomic studies.
Additionally, the role of the metabolic pathway of hypotaurine, an endogenous metabolite, in ageing is another problem that needs to be explored in future studies. In future research, we need to further clarify the detailed molecular mechanism by which hypotaurine delays ageing and to determine the optimal concentration that is beneficial for the health of the body.
Conflicts of interest
There is no conflict of interest in the submission of this manuscript, and the manuscript is approved by all authors for publication.Acknowledgements
We would like to thank the Caenorhabditis Genetic Center (CGC) for providing the worm strains, which is funded by the NIH Office of Research Infrastructure Programs (P40OD010440). This work was supported by the National Natural Science Foundation of China (81601299), the National Key R&D Program of China (2018YFC2002000), the Program of Introducing Talents of Discipline to Universities (111 Project, No. B16021), the Natural Science Foundation of Guangdong Province, China (2018A0303131003) and the China Postdoctoral Science Special Fund (2018M633288).References
- C. Lopez-Otin, M. A. Blasco, L. Partridge, M. Serrano and G. Kroemer, The hallmarks of aging, Cell, 2013, 153, 1194–1217 CrossRef CAS PubMed.
- J. Campisi, P. Kapahi, G. J. Lithgow, S. Melov, J. C. Newman and E. Verdin, From discoveries in ageing research to therapeutics for healthy ageing, Nature, 2019, 571, 183–192 CrossRef CAS.
- D. E. Harrison, R. Strong, Z. D. Sharp, J. F. Nelson, C. M. Astle, K. Flurkey, N. L. Nadon, J. E. Wilkinson, K. Frenkel, C. S. Carter, M. Pahor, M. A. Javors, E. Fernandez and R. A. Miller, Rapamycin fed late in life extends lifespan in genetically heterogeneous mice, Nature, 2009, 460, 392–395 CrossRef CAS.
- A. Martin-Montalvo, E. M. Mercken, S. J. Mitchell, H. H. Palacios, P. L. Mote, M. Scheibye-Knudsen, A. P. Gomes, T. M. Ward, R. K. Minor, M. J. Blouin, M. Schwab, M. Pollak, Y. Zhang, Y. Yu, K. G. Becker, V. A. Bohr, D. K. Ingram, D. A. Sinclair, N. S. Wolf, S. R. Spindler, M. Bernier and R. de Cabo, Metformin improves healthspan and lifespan in mice, Nat. Commun., 2013, 4, 2192 CrossRef.
- C. Slack, N. Alic, A. Foley, M. Cabecinha, M. P. Hoddinott and L. Partridge, The Ras-Erk-ETS-Signaling Pathway Is a Drug Target for Longevity, Cell, 2015, 162, 72–83 CrossRef CAS PubMed.
- J. I. Castillo-Quan, L. Li, K. J. Kinghorn, D. K. Ivanov, L. S. Tain, C. Slack, F. Kerr, T. Nespital, J. Thornton, J. Hardy, I. Bjedov and L. Partridge, Lithium Promotes Longevity through GSK3/NRF2-Dependent Hormesis, Cell Rep., 2016, 15, 638–650 CrossRef CAS.
- D. Ryu, L. Mouchiroud, P. A. Andreux, E. Katsyuba, N. Moullan, A. A. Nicolet-Dit-Felix, E. G. Williams, P. Jha, G. Lo Sasso, D. Huzard, P. Aebischer, C. Sandi, C. Rinsch and J. Auwerx, Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents, Nat. Med., 2016, 22, 879–888 CrossRef CAS.
- A. L. Chen, K. M. Lum, P. Lara-Gonzalez, D. Ogasawara, A. B. Cognetta 3rd, A. To, W. H. Parsons, G. M. Simon, A. Desai, M. Petrascheck, L. Bar-Peled and B. F. Cravatt, Pharmacological convergence reveals a lipid pathway that regulates C. elegans lifespan, Nat. Chem. Biol., 2019, 15, 453–462 CrossRef CAS PubMed.
- E. Katsyuba, A. Mottis, M. Zietak, F. De Franco, V. van der Velpen, K. Gariani, D. Ryu, L. Cialabrini, O. Matilainen, P. Liscio, N. Giacche, N. Stokar-Regenscheit, D. Legouis, S. de Seigneux, J. Ivanisevic, N. Raffaelli, K. Schoonjans, R. Pellicciari and J. Auwerx, De novo NAD(+) synthesis enhances mitochondrial function and improves health, Nature, 2018, 563, 354–359 CrossRef CAS PubMed.
- S. J. Park, F. Ahmad, A. Philp, K. Baar, T. Williams, H. Luo, H. Ke, H. Rehmann, R. Taussig, A. L. Brown, M. K. Kim, M. A. Beaven, A. B. Burgin, V. Manganiello and J. H. Chung, Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases, Cell, 2012, 148, 421–433 CrossRef CAS PubMed.
- J. Mansfeld, N. Urban, S. Priebe, M. Groth, C. Frahm, N. Hartmann, J. Gebauer, M. Ravichandran, A. Dommaschk, S. Schmeisser, D. Kuhlow, S. Monajembashi, S. Bremer-Streck, P. Hemmerich, M. Kiehntopf, N. Zamboni, C. Englert, R. Guthke, C. Kaleta, M. Platzer, J. Suhnel, O. W. Witte, K. Zarse and M. Ristow, Branched-chain amino acid catabolism is a conserved regulator of physiological ageing, Nat. Commun., 2015, 6, 10043 CrossRef CAS.
- H. Wang, J. Liu, T. Li and R. H. Liu, Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans, Food Funct., 2018, 9, 5273–5282 RSC.
- R. Tevatia, J. Allen, D. Rudrappa, D. White, T. E. Clemente, H. Cerutti, Y. Demirel and P. Blum, The taurine biosynthetic pathway of microalgae, Algal Res., 2015, 9, 21–26 CrossRef.
- T. Bouckenooghe, C. Remacle and B. Reusens, Is taurine a functional nutrient?, Curr. Opin. Clin. Nutr. Metab. Care, 2006, 9, 728–733 CrossRef CAS.
- G. Agnello, L. L. Chang, C. M. Lamb, G. Georgiou and E. M. Stone, Discovery of a substrate selectivity motif in amino acid decarboxylases unveils a taurine biosynthesis pathway in prokaryotes, ACS Chem. Biol., 2013, 8, 2264–2271 CrossRef CAS.
- O. Aruoma, B. Halliwell, B. M. Hoey and J. Butler, The antioxidant action of taurine, hypotaurine and their metabolic precursors, Biochem. J., 1988, 256, 251–255 CrossRef CAS.
- T. Sakuragawa, T. Hishiki, Y. Ueno, S. Ikeda, T. Soga, A. Yachie-Kinoshita, M. Kajimura and M. Suematsu, Hypotaurine is an Energy-Saving Hepatoprotective Compound against Ischemia-Reperfusion Injury of the Rat Liver, J. Clin. Biochem. Nutr., 2010, 46, 126–134 CrossRef PubMed.
- M. Acharya and C. A. Lau-Cam, Comparison of the protective actions of N-acetylcysteine, hypotaurine and taurine against acetaminophen-induced hepatotoxicity in the rat, J. Biomed. Sci., 2010, 17(Suppl. 1), S35 CrossRef.
- T. R. Green, J. H. Fellman, A. L. Eicher and K. L. Pratt, Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils, Biochim. Biophys. Acta, 1991, 1073, 91–97 CrossRef CAS.
- L. Pecci, G. Montefoschi, M. Fontana, S. Duprè, M. Costa and D. Cavallini, in Taurine 4, Springer, 2002, pp. 163–168 Search PubMed.
- E. Dehghan, Y. Zhang, B. Saremi, S. Yadavali, A. Hakimi, M. Dehghani, M. Goodarzi, X. Tu, S. Robertson, R. Lin, A. Chudhuri and H. Mirzaei, Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway, Nat. Commun., 2017, 8, 2223 CrossRef.
- S. Davinelli, J. C. Bertoglio, A. Polimeni and G. Scapagnini, Cytoprotective Polyphenols Against Chronological Skin Aging and Cutaneous Photodamage, Curr. Pharm. Des., 2018, 24, 99–105 CrossRef CAS.
- J. Wang, S. Hu, S. Nie, Q. Yu and M. Xie, Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides, Oxid. Med. Cell. Longevity, 2016, 2016, 5692852 Search PubMed.
- A. F. Aydın, J. Çoban, I. Doğan-Ekici, E. Betül-Kalaz, S. Doğru-Abbasoğlu and M. Uysal, Carnosine and taurine treatments diminished brain oxidative stress and apoptosis in D-galactose aging model, Metab. Brain Dis., 2016, 31, 337–345 CrossRef.
- Q. L. Wan, X. Shi, J. Liu, A. J. Ding, Y. Z. Pu, Z. Li, G. S. Wu and H. R. Luo, Metabolomic signature associated with reproduction-regulated aging in Caenorhabditis elegans, Aging, 2017, 9, 447–474 CAS.
- K. Seo, E. Choi, D. Lee, D. E. Jeong, S. K. Jang and S. J. Lee, Heat shock factor 1 mediates the longevity conferred by inhibition of TOR and insulin/IGF-1 signaling pathways in C. elegans, Aging Cell, 2013, 12, 1073–1081 CrossRef CAS PubMed.
- C. J. Kenyon, The genetics of ageing, Nature, 2010, 464, 504–512 CrossRef CAS.
- T. Stiernagle, Maintenance of C. elegans, C. elegans, 1999, 2, 51–67 Search PubMed.
- Q. L. Wan, X. Meng, X. Fu, B. Chen, J. Yang, H. Yang and Q. Zhou, Intermediate metabolites of the pyrimidine metabolism pathway extend the lifespan of C. elegans through regulating reproductive signals, Aging, 2019, 11, 3993–4010 Search PubMed.
- S. Q. Zheng, X. B. Huang, T. K. Xing, A. J. Ding, G. S. Wu and H. R. Luo, Chlorogenic Acid Extends the Lifespan of Caenorhabditis elegans via Insulin/IGF-1 Signaling Pathway, J. Gerontol., Ser. A, 2017, 72, 464–472 CAS.
- D. Hoogewijs, K. Houthoofd, F. Matthijssens, J. Vandesompele and J. R. Vanfleteren, Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans, BMC Mol. Biol., 2008, 9, 9 CrossRef.
- A. Pole, M. Dimri and G. P. Dimri, Oxidative stress, cellular senescence and ageing, AIMS Mol. Sci., 2016, 3, 300–324 CAS.
- D. Barardo, D. Thornton, H. Thoppil, M. Walsh, S. Sharifi, S. Ferreira, A. Anžič, M. Fernandes, P. Monteiro and T. Grum, The DrugAge database of aging-related drugs, Aging Cell, 2017, 16, 594–597 CrossRef CAS PubMed.
- C. T. Murphy, S. A. McCarroll, C. I. Bargmann, A. Fraser, R. S. Kamath, J. Ahringer, H. Li and C. Kenyon, Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans, Nature, 2003, 424, 277–283 CrossRef CAS.
- J. M. Tullet, J. W. Green, C. Au, A. Benedetto, M. A. Thompson, E. Clark, A. F. Gilliat, A. Young, K. Schmeisser and D. Gems, The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms, Aging Cell, 2017, 16, 1191–1194 CrossRef CAS.
- M. Ravichandran, S. Priebe, G. Grigolon, L. Rozanov, M. Groth, B. Laube, R. Guthke, M. Platzer, K. Zarse and M. Ristow, Impairing L-Threonine Catabolism Promotes Healthspan through Methylglyoxal-Mediated Proteohormesis, Cell Metab., 2018, 27, 914–925 e915 CrossRef CAS.
- S. Ogg, S. Paradis, S. Gottlieb, G. I. Patterson, L. Lee, H. A. Tissenbaum and G. Ruvkun, The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans, Nature, 1997, 389, 994–999 CrossRef CAS.
- K. Zarse, S. Schmeisser, M. Groth, S. Priebe, G. Beuster, D. Kuhlow, R. Guthke, M. Platzer, C. R. Kahn and M. Ristow, Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal, Cell Metab., 2012, 15, 451–465 CrossRef CAS.
- M. C. Wang, E. J. O'Rourke and G. Ruvkun, Fat metabolism links germline stem cells and longevity in C. elegans, Science, 2008, 322, 957–960 CrossRef CAS PubMed.
- M. J. Steinbaugh, S. D. Narasimhan, S. Robida-Stubbs, L. E. Moronetti Mazzeo, J. M. Dreyfuss, J. M. Hourihan, P. Raghavan, T. N. Operana, R. Esmaillie and T. K. Blackwell, Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence, eLife, 2015, 4, e07836 CrossRef.
- D. Garigan, A. L. Hsu, A. G. Fraser, R. S. Kamath, J. Ahringer and C. Kenyon, Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation, Genetics, 2002, 161, 1101–1112 CAS.
- E. L. Greer, D. Dowlatshahi, M. R. Banko, J. Villen, K. Hoang, D. Blanchard, S. P. Gygi and A. Brunet, An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans, Curr. Biol., 2007, 17, 1646–1656 CrossRef CAS PubMed.
- E. L. Greer and A. Brunet, Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans, Aging Cell, 2009, 8, 113–127 CrossRef CAS PubMed.
- B. Lakowski and S. Hekimi, The genetics of caloric restriction in Caenorhabditis elegans, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 13091–13096 CrossRef CAS PubMed.
- A. Berdichevsky, M. Viswanathan, H. R. Horvitz and L. Guarente, C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span, Cell, 2006, 125, 1165–1177 CrossRef CAS PubMed.
- T. Vellai, K. Takacs-Vellai, Y. Zhang, A. L. Kovacs, L. Orosz and F. Muller, Genetics: influence of TOR kinase on lifespan in C. elegans, Nature, 2003, 426, 620 CrossRef CAS PubMed.
- L. T. MacNeil, E. Watson, H. E. Arda, L. J. Zhu and A. J. Walhout, Diet-induced developmental acceleration independent of TOR and insulin in C. elegans, Cell, 2013, 153, 240–252 CrossRef CAS PubMed.
- F. Weinberg, R. Hamanaka, W. W. Wheaton, S. Weinberg, J. Joseph, M. Lopez, B. Kalyanaraman, G. M. Mutlu, G. R. Budinger and N. S. Chandel, Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 8788–8793 CrossRef CAS PubMed.
- W. Yang and S. Hekimi, Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans, Aging Cell, 2010, 9, 433–447 CrossRef CAS PubMed.
- S. L. Rea, N. Ventura and T. E. Johnson, Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans, PLos Biol., 2007, 5, e259 CrossRef PubMed.
- P. L. Larsen and C. F. Clarke, Extension of life-span in Caenorhabditis elegans by a diet lacking coenzyme Q, Science, 2002, 295, 120–123 CrossRef CAS PubMed.
- S. Yanase, K. Yasuda and N. Ishii, Adaptive responses to oxidative, damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span, Mech. Ageing Dev., 2002, 123, 1579–1587 CrossRef CAS PubMed.
- J. Durieux, S. Wolff and A. Dillin, The cell-non-autonomous nature of electron transport chain-mediated longevity, Cell, 2011, 144, 79–91 CrossRef CAS PubMed.
- J. M. Hourihan, L. E. Moronetti Mazzeo, L. P. Fernandez-Cardenas and T. K. Blackwell, Cysteine Sulfenylation Directs IRE-1 to Activate the SKN-1/Nrf2 Antioxidant Response, Mol. Cell, 2016, 63, 553–566 CrossRef CAS PubMed.
- K. B. Beckman and B. N. Ames, The free radical theory of aging matures, Physiol. Rev., 1998, 78, 547–581 CrossRef CAS PubMed.
- R. S. Sohal, R. J. Mockett and W. C. Orr, Mechanisms of aging: an appraisal of the oxidative stress hypothesis, Free Radical Biol. Med., 2002, 33, 575–586 CrossRef CAS PubMed.
- T. Finkel and N. J. Holbrook, Oxidants, oxidative stress and the biology of ageing, Nature, 2000, 408, 239–247 CrossRef CAS PubMed.
- M. Xie and R. Roy, Increased levels of hydrogen peroxide induce a HIF-1-dependent modification of lipid metabolism in AMPK compromised C. elegans dauer larvae, Cell Metab., 2012, 16, 322–335 CrossRef CAS PubMed.
- Y. Pan, E. A. Schroeder, A. Ocampo, A. Barrientos and G. S. Shadel, Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling, Cell Metab., 2011, 13, 668–678 CrossRef CAS PubMed.
- X. Qiu, K. Brown, M. D. Hirschey, E. Verdin and D. Chen, Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation, Cell Metab., 2010, 12, 662–667 CrossRef CAS PubMed.
- A. B. Hwang, E. A. Ryu, M. Artan, H. W. Chang, M. H. Kabir, H. J. Nam, D. Lee, J. S. Yang, S. Kim, W. B. Mair, C. Lee, S. S. Lee and S. J. Lee, Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E4458–E4467 CrossRef CAS PubMed.
- C. Duangjan, P. Rangsinth, X. Gu, M. Wink and T. Tencomnao, Lifespan Extending and Oxidative Stress Resistance Properties of a Leaf Extracts from Anacardium occidentale L. in Caenorhabditis elegans, Oxid. Med. Cell. Longevity, 2019, 2019, 9012396 Search PubMed.
- E. Wang and M. Wink, Chlorophyll enhances oxidative stress tolerance in Caenorhabditis elegans and extends its lifespan, PeerJ, 2016, 4, e1879 CrossRef PubMed.
- S. Su and M. Wink, Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans, Phytochemistry, 2015, 117, 340–350 CrossRef CAS PubMed.
- T. Finkel, The metabolic regulation of aging, Nat. Med., 2015, 21, 1416–1423 CrossRef CAS PubMed.
- C. Lopez-Otin, L. Galluzzi, J. M. P. Freije, F. Madeo and G. Kroemer, Metabolic Control of Longevity, Cell, 2016, 166, 802–821 CrossRef CAS PubMed.
- M. Lucanic, J. M. Held, M. C. Vantipalli, I. M. Klang, J. B. Graham, B. W. Gibson, G. J. Lithgow and M. S. Gill, N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans, Nature, 2011, 473, 226–229 CrossRef CAS PubMed.
- D. S. Williams, A. Cash, L. Hamadani and T. Diemer, Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO dependent pathway, Aging Cell, 2009, 8, 765–768 CrossRef CAS PubMed.
- R. M. Chin, X. Fu, M. Y. Pai, L. Vergnes, H. Hwang, G. Deng, S. Diep, B. Lomenick, V. S. Meli and G. C. Monsalve, The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR, Nature, 2014, 510, 397 CrossRef CAS PubMed.
- A. Gioran, A. Piazzesi, F. Bertan, J. Schroer, L. Wischhof, P. Nicotera and D. Bano, Multi-omics identify xanthine as a pro-survival metabolite for nematodes with mitochondrial dysfunction, EMBO J., 2019, 38, e99558 CrossRef PubMed.
- S. Calvert, R. Tacutu, S. Sharifi, R. Teixeira, P. Ghosh and J. P. de Magalhaes, A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans, Aging Cell, 2016, 15, 256–266 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo02000d |
| This journal is © The Royal Society of Chemistry 2020 |