Efficacy of proprietary Lactobacillus casei for anti-tuberculosis associated gastrointestinal adverse reactions in adult patients: a randomized, open-label, dose–response trial
Song
Lin
ab,
Shanliang
Zhao
c,
Jiahong
Liu
d,
Jianwen
Zhang
a,
Chao
Zhang
a,
Haibo
Hao
a,
Yuxia
Sun
a,
Jing
Cai
a,
Yang
Yang
a,
Yan
Ma
a,
Yuanyuan
Li
a,
Jinyu
Wang
a and
Aiguo
Ma
 *a
*a
aThe College of Public Health, Qingdao University, 38 Dengzhou Road, Qingdao, 266021, Shandong province, China. E-mail: magfood@qdu.edu.cn; Fax: +86 532 83812434; Tel: +86 532 82991518
bDepartment of Medical Epidemiology and Biostatistics, Karolinska Institutet, Nobels Väg 12A, 171 77 Stockholm, Sweden
cLinyi People's Hospital East Branch, 27 Jiefang East Road, Lanshan District of Linyi 276000, Shandong province, China
dThe Qingdao Central Hospital, 127 Siliu South Road, Qingdao 266000, Shandong province, China
First published on 19th November 2019
Abstract
Anti-tuberculosis (TB) drugs can induce a series of gastrointestinal adverse events, which can seriously affect patients’ quality of life and may lead to treatment failure. Studies have shown that probiotics treatments can improve antibiotic-induced gastrointestinal symptoms. In this randomized, open-label, dose–response clinical trial, we investigated the preventive effects of Lactobacillus casei on anti-TB-induced gastrointestinal adverse events. In total, 429 adult patients who underwent intensive-phase anti-TB therapy were included and randomly assigned to consume one bottle of L. casei of per day (low-dose group, n = 142), two bottles of L. casei per day (high-dose group, n = 143), or no intervention (control group, n = 144) for 2 months. Each bottle of L. casei contained 10 billion colony-forming units of live L. casei. Patients’ daily gastrointestinal symptoms were recorded during the intervention period. After 2 months of L. casei consumption, 397 patients had completed the intervention. Both the high and low dose L. casei groups (37.6% and 29.4%, respectively) had lower incidences of anti-TB-associated total gastrointestinal adverse events than the control group (50.0%). The high and low dose L. casei groups (3.5 d and 5.8 d, respectively) also had shorter duration anti-TB-associated adverse gastrointestinal symptoms than the control group (6.2 d). Regarding individual symptoms, the higher L. casei dose resulted in a lower incidence of vomiting and appetite loss. Similar dose-dependent protective effects of L. casei were observed regarding the duration of vomiting and appetite loss. These findings indicated that daily L. casei consumption prevented anti-TB-associated gastrointestinal adverse events. This trial was registered at the Chinese Clinical Trial Register (ChiCTR-IOR-17013210).
Introduction
Tuberculosis (TB) is an infectious disease caused by the bacillus Mycobacterium tuberculosis and the leading cause of mortality worldwide due to a single infectious agent.1,2 TB typically affects the lung (pulmonary TB) but can also affect other sites (extrapulmonary TB). In 2017, there were an estimated 10.0 million people who had developed TB, which caused an estimated 1.3 million deaths among HIV-negative people, with an additional 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths from TB among HIV-positive people. TB affects all countries and all age groups, but overall, 90% of cases occur among adults (aged ≥15 years), 64% in men, and a half are in five countries, including India (27%), China (9%), Indonesia (8%), and the Philippines (6%). Additionally, approximately 1.7 billion people are estimated to have a latent TB infection, among whom 5%–10% will develop an active TB disease during their lifetime.2
000 deaths from TB among HIV-positive people. TB affects all countries and all age groups, but overall, 90% of cases occur among adults (aged ≥15 years), 64% in men, and a half are in five countries, including India (27%), China (9%), Indonesia (8%), and the Philippines (6%). Additionally, approximately 1.7 billion people are estimated to have a latent TB infection, among whom 5%–10% will develop an active TB disease during their lifetime.2
Currently, the effective treatment for cases of drug-susceptible TB is a 6–8 month regimen of first-line drugs (e.g. isoniazid, rifampicin, ethambutol, and pyrazinamide) and second-line drugs (e.g. ofloxacin and moxifloxacin); an anti-TB treatment plan requires four drugs in combination during the intensive phase, while the continuation phase includes at least two anti-TB drugs.3 The long-term standardized treatment with anti-TB drugs has played a positive role in the control of the TB epidemic, but there are often many drug-related adverse reactions, such as adverse digestive system reactions, nervous system damage, allergic reactions, and blood system abnormalities.4 The main adverse reactions of the digestive system include adverse gastrointestinal reactions and liver disorders, which often occur during the 2 months of the intensive phase after the start of treatment.5,6 Anti-TB-associated adverse gastrointestinal reactions often manifest as nausea, vomiting, loss of appetite, diarrhea, and abdominal pain.7 The incidence of anti-TB-induced adverse gastrointestinal reactions has been reported to be as high as 11.7%, 23.7%, and 39.8% in young (15–30-years-old), middle-aged (30–60-years-old), and elderly (>60-years-old) Chinese patients, respectively.8 Symptomatic treatments (e.g. antiemetics and antidiarrheals) can alleviate these adverse reactions to a certain extent; however, they still cannot efficiently reduce the incidence and severity of adverse reactions, causing some patients to have to reduce the dose or interrupt anti-TB treatments.9 Therefore, it would be helpful to explore food or dietary supplements that could prevent anti-TB-induced adverse digestive system reactions.
Probiotics are microorganisms that confer a health benefit to the host when properly consumed. Meta-analyses of randomized controlled trials have shown that consumption of probiotics can influence mineral metabolism,10,11 glycolipid metabolism,12,13 and blood pressure.14,15 Furthermore, increased dietary intake of probiotics can improve gastrointestinal dysfunction symptoms caused by the extensive use of antibiotics. Two meta-analyses of randomized controlled trials reported that probiotics administration reduced the incidence of antibiotic-associated diarrhea.16,17 A meta-analysis has also shown that a single-agent Lactobacillus regimen can reduce the risk of developing antibiotic-associated diarrhea in adults, but not pediatric patients, compared with placebo.18 Hickson et al.,19 reported that consumption of a probiotic drink containing Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus can reduce the incidence of antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea.
An extensive literature search showed that no study has focused on the effects of probiotics for preventing the adverse gastrointestinal reactions of anti-TB treatment. Considering probiotics have been shown to have positive effects on gut physiology and intestinal barrier integrity, and to improve immune function,18,20 we hypothesized that L. casei could reduce the risk of anti-TB-associated adverse gastrointestinal reactions. Therefore, this study assessed the effect of L. casei on anti-TB-associated adverse gastrointestinal reactions, including nausea, vomiting, diarrhea, flatulence, loss of appetite, and constipation, during the intensive phase of treatment.
Methods
Trial design and participants
This was a randomized, open-label, dose–response trial registered at the Chinese Clinical Trial Register (ChiCTR-IOR-17013210). Adults aged 18–65-years-old with pulmonary TB were recruited from a chest hospital in Shandong province, China, between December 2017 and January 2019.Pulmonary TB was diagnosed by positive sputum culture-based tests according to the Chinese Guidelines.21 Women were excluded if they were pregnant or lactating. Additionally, patients were excluded for the following reasons: extra-pulmonary TB, diabetes mellitus, cardiovascular diseases, hematopoietic system diseases, gastrointestinal diseases, hepatitis B virus infection, hepatitis C virus infection, fatty liver disease, elevated liver enzymes, receiving hepatotoxic drugs other than anti-TB therapy, diagnosed with malignancy, severe mental illness, and probiotic supplementation within the previous 2 months. This study was approved by the Ethical Committee of the Qingdao City Center for Disease Control (201703) and Prevention and was performed in accordance with the Declaration of Helsinki. Before enrollment in the study, patients who agreed to participate signed an informed consent form.
Randomization and intervention
Participants were simple-randomly allocated to three groups: (1) the low-dose L. casei group (1 × 1010 colony-forming units [CFU], one bottle [100 mL] of L. casei per day); (2) the high-dose L. casei group (2 × 1010 CFU, two bottles [100 mL] of L. casei per day); and (3) the control group (without L. casei intervention). The randomization sequence was generated by a blinded researcher using an online randomization generator (http://www.randomization.com). The study was open-label, and allocation was unmasked.The L. casei preparation was produced by Yakult Honsha Co., Ltd (Tokyo, Japan) in a sponsorship model. The L. casei preparations were in a liquid form and contained the L. casei Shirota strain, filtered water, skimmed milk, glucose, and fructose. Per 100 mL, these L. casei preparations provided approximately 10 billion CFU of L. casei Shirota, 66 kcal of energy, 1.2 g protein, and 15.7 g carbohydrate. All L. casei preparations were distributed to the participants twice per month. Participants were asked to avoid consuming any other products containing probiotic strains and to abstain from tobacco and alcohol products throughout the trial. Because L. casei is sensitive to the acidic environment of stomach, participants were asked to consume L. casei between 30–60 min after meals. Participants were asked to shake the bottles for resuspension before consumption.
Based on the Chinese Guidelines,22 all participants received intensive-phase anti-TB therapy comprising isoniazid, rifampicin, pyrazinamide, and ethambutol in addition to study interventions; after completing 2 months of treatment, participants were discharged from the study and continuation-phase anti-TB therapy was initiated.
Outcome measurements
Participants underwent baseline clinical evaluations, including physical examination, clinical symptoms, medical history, chest radiography, and collection of sputum samples for microscopy and culture, a urine sample to determine a kidney chemistry panel, and a blood sample to determine complete blood counts, a liver chemistry panel, and a virology panel. Compliance was assessed by scheduled personal interviews and counting the empty bottles returned every two weeks.The primary outcome of this study was intergroup differences in the incidence of total gastrointestinal adverse events based on the Rome III criteria.23 Secondary outcomes were intergroup differences in the incidence and duration of individual gastrointestinal adverse events (nausea, vomiting, diarrhea, flatulence, appetite, constipation). The duration of gastrointestinal adverse events was determined by the number of continuous days of these symptoms. Patient diaries recorded episodes of gastrointestinal adverse events.
Statistical analysis
The sample size was determined considering the expected adverse gastrointestinal reaction rate of 23.7%8 and 8.0% (one third of the control group) for L. casei intervention24 with 5% α (two-sided) and 90% power, resulting in 130 patients in each group. Given an anticipated 10% drop, we recruited 429 subjects in total.Statistical analyses were conducted with IBM SPSS Statistics software, version 23.0 (IBM Corporation, Armonk, NY, USA) and Stata 15.1 (StataCorp, College Station, TX, USA). All tests for significance were performed at α = 0.05 (two-sided). The baseline comparability of the treatment groups for subject characteristics was assessed with the ANOVA or χ2 tests, when applicable. Intergroup analysis to assess the percentages for each gastrointestinal symptom at the end of follow-up was performed using the χ2 test, Fisher's exact test, or Wilcoxon-type trend test, when applicable. Intergroup analysis to assess the duration of each gastrointestinal symptom at the end of follow-up was conducted using the Kruskal–Wallis H test or Wilcoxon-type test, when applicable.
Results
Characteristics of the participants
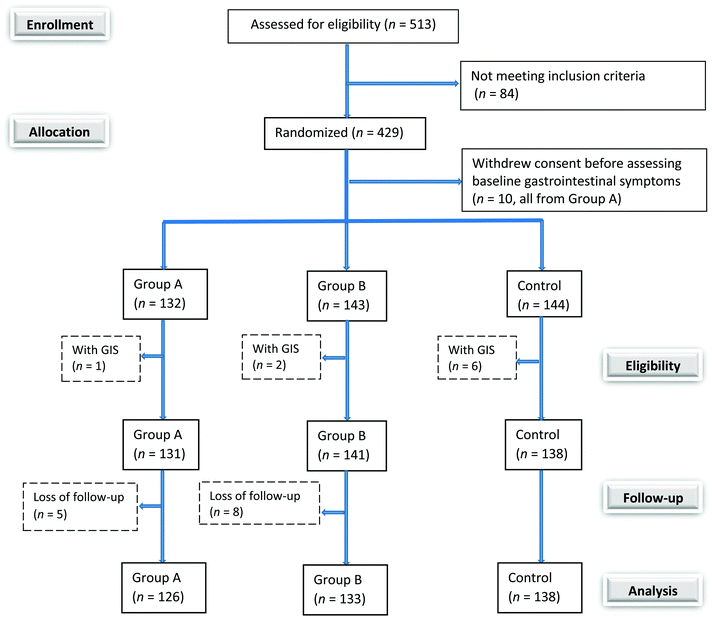
Among the 429 recruited patients, 144 were allocated to the control group, 142 to the low dose L. casei group, and 143 to the high dose L. casei group. Ten randomized patients who withdrew consent before assessing baseline gastrointestinal symptoms and nine randomized patients who presented gastrointestinal symptoms at the baseline were not included in this study. Thirteen patients did not complete the study, resulting in 3.2% loss to follow-up. During the intervention period, no adverse effects related to L. casei supplementation were observed, and all failures to complete study were due to personal reasons (Fig. 1). At the baseline, the demographic characteristics were similar among three groups, confirming the randomization efficiency (Table 1).| Characteristic | Group A (n = 132) | Group B (n = 143) | Control (n = 144) | p |
|---|---|---|---|---|
| Data are % (n) or mean (SD), unless otherwise stated. BMI: body mass index; WBC, white blood cell. Group A, anti-tuberculosis drugs plus 1 × 1010 colony-forming units of L. casei; group B: anti-tuberculosis drugs plus 2 × 1010 colony-forming units of L. casei; control, anti-tuberculosis drugs. | ||||
| Age (years) | 37.21 ± 14.48 | 36.20 ± 15.05 | 39.15 ± 16.49 | 0.256 |
| Men | 59.8% (79/132) | 56.6% (81/143) | 64.6% (93/143) | 0.384 |
| Height (m) | 1.70 ± 0.07 | 1.70 ± 0.07 | 1.69 ± 0.06 | 0.428 |
| Weight (kg) | 61.94 ± 9.68 | 59.77 ± 10.41 | 59.42 ± 10.23 | 0.086 |
| BMI (kg m−2) | 21.46 ± 2.83 | 20.71 ± 2.79 | 20.79 ± 2.93 | 0.058 |
| Marital status | 0.597 | |||
| Single | 32.6% (43/132) | 35.0% (50/143) | 31.2% (45/144) | |
| Married | 65.9% (87/132) | 64.3% (92/143) | 68.1% (98/144) | |
| Widowed/divorced | 1.5% (2/132) | 0.7% (1/143) | 0.7% (1/144) | |
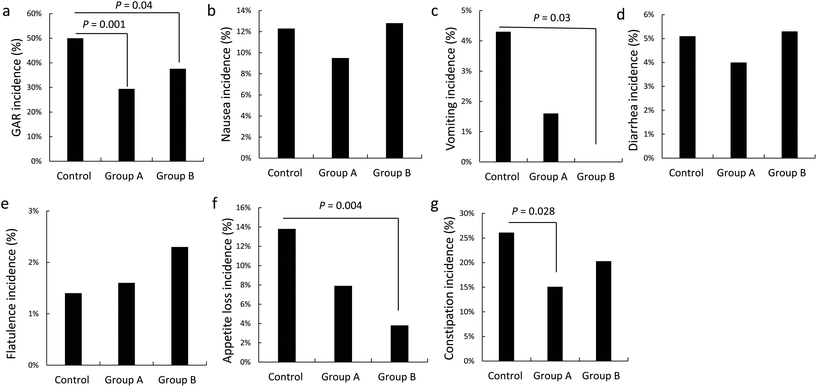
Incidence of anti-TB-associated gastrointestinal adverse events
Both L. casei doses were effective in reducing total gastrointestinal adverse events compared with the control group (Fig. 2a). A distinct dose–response relationship was observed, as the high L. casei dose resulted in a lower incidence of vomiting and appetite loss (Table 2, Fig. 2b and f). Compared with the control group, a greater reduction in constipation was observed in the low dose L. casei group (Fig. 2e). There were no statistical differences for other gastrointestinal adverse events (nausea, diarrhea, and flatulence) across the three trial groups (Fig. 2c, d, and g).| Group A (n = 126) | Group B (n = 133) | Control (n = 138) | p | |
|---|---|---|---|---|
| Data are % (n). GAE, gastrointestinal adverse events. Group A, anti-tuberculosis drugs plus 1 × 1010 colony-forming units of L. casei; group B: anti-tuberculosis drugs plus 2 × 1010 colony-forming units of L. casei; control, anti-tuberculosis drugs.a Non-parametric Wilcoxon-type trend test. | ||||
| GAE | 29.4% (37/126) | 37.6% (50/133) | 50.0% (69/138) | 0.034 |
| Nausea | 9.5% (12/126) | 12.8% (17/133) | 12.3% (17/138) | 0.913 |
| Vomiting | 1.6% (2/126) | 0 (0/133) | 4.3% (6/138) | 0.011 |
| Diarrhea | 4.0% (5/126) | 5.3% (7/133) | 5.1% (7/138) | 0.946 |
| Flatulence | 1.6% (2/126) | 2.3% (3/133) | 1.4% (2/138) | 0.616 |
| Appetite loss | 7.9% (10/126) | 3.8% (5/133) | 13.8% (19/138) | 0.003 |
| Constipation | 15.1% (19/126) | 20.3% (27/133) | 26.1% (36/138) | 0.232 |
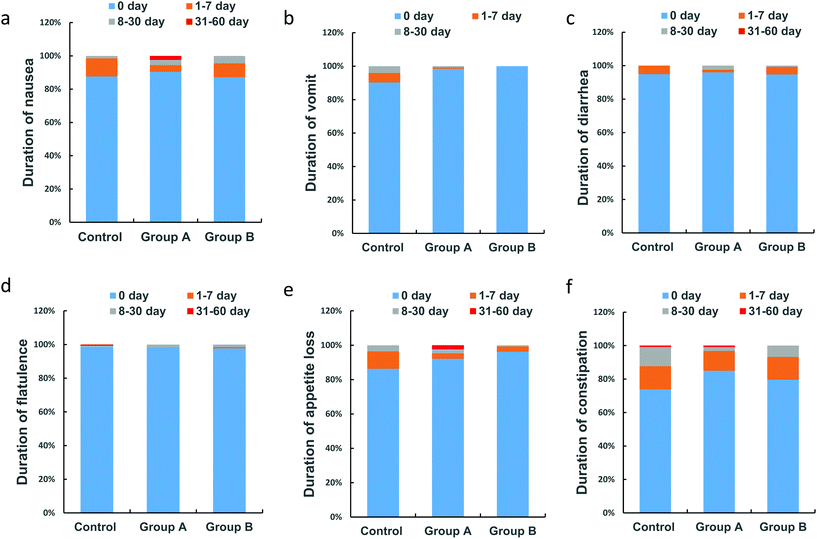
Duration of anti-TB-associated gastrointestinal symptoms
A dose–response relationship was observed with the high L. casei dose resulting in fewer days of continuous total gastrointestinal symptoms (Table 3). The average durations of total gastrointestinal symptoms in the high dose L. casei group (3.5 d) and low dose L. casei group (5.8 d) were shorter than that in the control group (6.2 d). Regarding the specific gastrointestinal adverse events, a dose–response relationship was observed regarding the duration of vomiting, appetite loss, and constipation, which were lowered with higher L. casei doses (Table 3). When stratified by different durations of gastrointestinal adverse events, similar dose-dependent protective effects of L. casei were observed regarding the duration of vomiting and appetite loss (Table 4, Fig. 3b and e).| Group A (n = 126) | Group B (n = 133) | Control (n = 138) | p | |
|---|---|---|---|---|
| Data are median (min–max). GAE, gastrointestinal adverse events. Group A, anti-tuberculosis drugs plus 1 × 1010 colony-forming units of L. casei; group B: anti-tuberculosis drugs plus 2 × 1010 colony-forming units of L. casei; control, anti-tuberculosis drugs.a Kruskal–Wallis H test.b One patient in group A do not record the continuous days of diarrhea. | ||||
| GAE | 0 (0–135) | 0 (0–40) | 0.5 (0–180) | 0.005 |
| Nausea | 0 (0–60) | 0 (0–30) | 0 (0–20) | 0.762 |
| Vomiting | 0 (0–30) | 0 (0–0) | 0 (0–3) | 0.037 |
| Diarrheab | 0 (0–15) | 0 (0–10) | 0 (0–6) | 0.899 |
| Flatulence | 0 (0–15) | 0 (0–30) | 0 (0–60) | 0.871 |
| Appetite loss | 0 (0–60) | 0 (0–20) | 0 (0–20) | 0.013 |
| Constipation | 0 (0–60) | 0 (0–20) | 0 (0–60) | 0.040 |
| 0 daya | 1–7 days | 8–30 days | 31–60 days | p for trendb | |
|---|---|---|---|---|---|
| Data are % (n). Group A, anti-tuberculosis drugs plus 1 × 1010 colony-forming units of L. casei; group B: anti-tuberculosis drugs plus 2 × 1010 colony-forming units of L. casei; control, anti-tuberculosis drugs.a Duration of gastrointestinal symptoms throughout the trial period was classified into four groups (0 day, 1–7 days, 8–30 days and 31–60 days).b Wilcoxon-type test.c One patient in group A do not record the duration of diarrhea. | |||||
| Nausea | 0.848 | ||||
| Control (n = 138) | 87.7% (121/138) | 10.9% (15/138) | 1.5% (2/138) | 0 | |
| Group A (n = 126) | 90.4% (114/126) | 4.0% (5/126) | 3.2% (4/126) | 2.4% (3/126) | |
| Group B (n = 133) | 87.2% (116/133) | 8.3% (11/133) | 4.5% (6/133) | 0 | |
| Vomit | 0.011 | ||||
| Control (n = 138) | 95.7% (132/138) | 4.3% (6/138) | 0 | 0 | |
| Group A (n = 126) | 98.4% (124/126) | 0.7% (1/126) | 0.7% (1/126) | 0 | |
| Group B (n = 133) | 100% (133/133) | 0 | 0 | 0 | |
| Diarrhea | 0.935 | ||||
| Control (n = 138) | 94.9% (131/138) | 5.1% (7/138) | 0 | 0 | |
| Group A (n = 125)c | 96.0% (120/125) | 1.6% (2/125) | 2.4% (3/125) | 0 | |
| Group B (n = 133) | 94.7% (126/133) | 4.5% (6/133) | 0.8% (1/133) | 0 | |
| Flatulence | 0.626 | ||||
| Control (n = 138) | 98.6% (136/138) | 0 | 0.7% (1/138) | 0.7% (1/138) | |
| Group A (n = 126) | 98.4% (124/126) | 0 | 1.6% (2/126) | 0 | |
| Group B (n = 133) | 97.7% (130/133) | 0.8% (1/133) | 1.5% (2/133) | 0 | |
| Appetite loss | 0.004 | ||||
| Control (n = 138) | 86.2% (119/138) | 10.2% (14/138) | 3.6% (5/138) | 0 | |
| Group A (n = 126) | 92.0% (116/126) | 3.2% (4/126) | 2.4% (3/126) | 2.4% (3/126) | |
| Group B (n = 133) | 96.2% (128/133) | 3.0% (4/133) | 0.8% (1/133) | 0 | |
| Constipation | 0.181 | ||||
| Control (n = 138) | 73.9% (102/138) | 13.8% (19/138) | 11.6% (16/138) | 0.7% (1/138) | |
| Group A (n = 126) | 84.9% (107/126) | 11.9% (15/126) | 2.4% (3/126) | 0.8% (1/126) | |
| Group B (n = 133) | 79.7% (106/133) | 13.5% (18/133) | 6.8% (9/133) | 0 | |
Discussion
In this randomized, open-label, dose-ranging study, we investigated the effects of proprietary L. casei consumption on anti-TB-associated gastrointestinal adverse events. Our results showed that daily L. casei supplementation for patients undergoing intensive-phase anti-TB therapy significantly decreased the incidence and duration of anti-TB-associated gastrointestinal adverse events, particularly anti-TB-associated vomiting and appetite loss.To the best of our knowledge, this is the first study to investigate the preventive effect of a probiotic strain on anti-TB-associated gastrointestinal adverse events. Previous studies have primarily investigated the effects of probiotics on systemic antibiotics-induced adverse gastrointestinal reactions, especially diarrhea.25,26 Multiple studies have shown a positive effect of L. casei on antibiotics-induced gastrointestinal symptoms. Beausoleil et al.27 reported that patients taking a fermented milk containing Lactobacillus acidophilus and L. casei had a 15.9% antibiotics-associated diarrhea incidence compared with 35.6% in the placebo group. Gao et al.28 reported a dose–response effect, in which patients treated with probiotics capsules containing 100 billion CFU of live organisms (Lactobacillus acidophilus CL1285, L. casei LBC80R Bio-K, and CL1285) showed reduced antibiotics-associated and Clostridium difficile-associated gastrointestinal events (diarrhea, abdominal pain, flatulence, loose stool, and constipation), compared with those taking a 50 billion CFU capsule or the placebo group. While these studies always reported the effects of a mixture of probiotic strains and could not interpret whether these positive effects were caused by synergistic interactions between strains or due to the high dose of individual probiotic species used, in this study, our results showed the efficacy of a specific single strain of L. casei Shirota on preventing anti-TB-associated adverse gastrointestinal reactions. Similarly, Koebnick et al.29 studied the effect of L. casei Shirota on patients with symptoms of chronic constipation and showed a significant decrease of gastrointestinal symptoms compared with placebo (89% vs. 56%).
Our study showed that the positive effect of L. casei on anti-TB-associated adverse gastrointestinal reactions was focused on vomiting and appetite loss. Gastric intolerance frequently occurs with anti-TB treatment, especially pyrazinamide.30 Namasivayam et al.,31 recently reported a distinct and long-lasting dysbiosis due to anti-TB therapy and identified rifampin as the major driver in M. tuberculosis-infected mice. Fetissov et al.32 reported that the gut microbiota might regulate host appetite via directly activating central appetite pathways (e.g. ATP/AMP ratio) or modulating intestinal release of satiety hormones (e.g. peptide tyrosine tyrosine and ghrelin). Furthermore, gut microbiota might play an important role in regulating intestinal bile acids and serotonin metabolism, which could contribute to the association between gut microbiota and gastrointestinal motility.33 Therefore, we speculated that dysbiosis could be a cause of anti-TB-associated loss of appetite and vomiting. Our results showed dose–response protective effects of L. casei on anti-TB-associated vomiting and appetite loss, which indicated that these positive effects might be partly attributed to the higher doses of L. casei used. The higher L. casei load likely adequately overwhelms the gastrointestinal tract and protects the gastrointestinal mucosa, in addition to shifting immune responses against other antigens.27
Study limitations
This study had several limitations. First, the open-label design did not allow us to control for a placebo effect, which may have led to potential bias. Second, we could not completely rule out the impact of dietary habits on the results of the study. Third, due to the highest dose group of L. casei being 20 billion in this study, it was impossible to judge the effect of higher doses of L. casei intervention, especially potential side effects. Finally, the patients of this study were solely of Chinese background; further studies with populations from other ethnic origins are needed before applying these study outcomes worldwide.Study strong points
A strength of our study was that we first identified a specific strain of L. casei that had a positive effect on anti-TB therapy-associated adverse gastrointestinal reactions. Another strength is that our results showed a dose–response of the protective effects of L. casei on anti-TB-associated adverse gastrointestinal reactions, especially with regard to vomiting and appetite loss.Conclusions
Considering its efficacy, safety, and feasibility, preventative L. casei therapy is particularly appealing for patients who received either intensive-phase anti-TB therapy or prolonged treatment. For example, according to previous studies, 0.9 adverse gastrointestinal cases occurred per 100 person-months of anti-TB treatment.34 Given that there are an estimated 10.4 million new cases of TB worldwide,35 we speculate that preventive L. casei therapy has potential to prevent approximately 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 adverse gastrointestinal cases and 94
000 adverse gastrointestinal cases and 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 adverse gastrointestinal days during just the 2 months of intensive period treatment worldwide each year. Given the long cycle of anti-TB treatment, preventative L. casei therapy could increase patient welfare and quality of life, which may directly feedback to better adherence to the anti-TB therapy. In conclusion, L. casei prophylaxis was safe and effective for reducing the risk of anti-TB-associated gastrointestinal adverse events.
000 adverse gastrointestinal days during just the 2 months of intensive period treatment worldwide each year. Given the long cycle of anti-TB treatment, preventative L. casei therapy could increase patient welfare and quality of life, which may directly feedback to better adherence to the anti-TB therapy. In conclusion, L. casei prophylaxis was safe and effective for reducing the risk of anti-TB-associated gastrointestinal adverse events.
Abbreviations
| TB | Tuberculosis |
| Lactobacillus casei | L. casei |
| Colony-forming unites | CFU |
Author contributions
AM, SZ and JL designed the study; JZ, CZ, HH, YS, YY, YM and YL conducted the research; SL, JC and JW performed the statistical analysis; SL and AM drafted the manuscript. All authors contributed to the final submitted version and agree to be responsible for all the work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant no. 81673160 to Aiguo Ma). Yakult Honsha Co., Ltd provided L. casei preparation used in this study in sponsorship character. The sponsoring company did not exert any influence on the study design, data collection and analysis, as well as writing of the manuscript.References
- P. Glaziou, K. Floyd and M. C. Raviglione, Global epidemiology of tuberculosis, Semin. Respir. Crit. Care Med., 2018, 39, 271–285 CrossRef PubMed.
- W. H. Organization, Global tuberculosis report 2018, Geneva, Switzerland, World Health Organization, 2018.
- W. H. Organization, Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 Search PubMed.
- X. Lv, S. Tang, Y. Xia, X. Wang, Y. Yuan, D. Hu, F. Liu, S. Wu, Y. Zhang and Z. Yang, Adverse reactions due to directly observed treatment strategy therapy in Chinese tuberculosis patients: a prospective study, PLoS One, 2013, 8, e65037 CrossRef CAS PubMed.
- T. Schaberg, K. Rebhan and H. Lode, Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis, Eur. Respir. J., 1996, 9, 2026–2030 CrossRef CAS PubMed.
- P. Shang, Y. Xia, F. Liu, X. Wang, Y. Yuan, D. Hu, D. Tu, Y. Chen, P. Deng and S. Cheng, Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (ATLI) in China, PLoS One, 2011, 6, e21836 CrossRef CAS PubMed.
- E. J. Forget and D. Menzies, Adverse reactions to first-line antituberculosis drugs, Expert Opin. Drug Saf., 2006, 5, 231–249 CrossRef CAS PubMed.
- Z. Chen and F. Shi, Observation on the meidication complicance and adverse reactions of different fixed-dose combination of anti-tuberculosis drug regimens (in Chinese), J. Chin. Physician, 2015, 17, 757–759 Search PubMed.
- S. Wu, Y. Xia, X. Lv, S. Tang, Z. Yang, Y. Zhang, X. Wang, D. Hu, F. Liu, Y. Yuan, D. Tu, F. Sun, L. Zhou and S. Zhan, Preventive use of hepatoprotectors yields limited efficacy on the liver toxicity of anti-tuberculosis agents in a large cohort of Chinese patients, J. Gastroenterol. Hepatol., 2015, 30, 540–545 CrossRef CAS PubMed.
- K. Skrypnik, P. Bogdanski, M. Sobieska and J. Suliburska, The effect of multistrain probiotic supplementation in two doses on iron metabolism in obese postmenopausal women: a randomized trial, Food Funct., 2019, 10, 5228–5238 RSC.
- K. Skrypnik and J. Suliburska, Association between the gut microbiota and mineral metabolism, J. Sci. Food Agric., 2018, 98, 2449–2460 CrossRef CAS PubMed.
- Y.-Y. Ma, L. Li, C.-H. Yu, Z. Shen, L.-H. Chen and Y.-M. Li, Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis, World J. Gastroenterol., 2013, 19, 6911 CrossRef PubMed.
- K. Yao, L. Zeng, Q. He, W. Wang, J. Lei and X. Zou, Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials, Med. Sci. Monit., 2017, 23, 3044 CrossRef PubMed.
- M. Szulinska, I. Loniewski and K. Skrypnik, Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women-A 12-Week Placebo-Controlled and Randomized Clinical Study, Nutrients, 2018, 10(11), 1672 CrossRef PubMed.
- E. Nikbakht, S. Khalesi, I. Singh, L. T. Williams, N. P. West and N. Colson, Effect of probiotics and synbiotics on blood glucose: a systematic review and meta-analysis of controlled trials, Eur. J. Nutr., 2018, 57, 95–106 CrossRef PubMed.
- S. Hempel, S. J. Newberry, A. R. Maher, Z. Wang, J. N. Miles, R. Shanman, B. Johnsen and P. G. Shekelle, Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis, J. Am. Med. Assoc., 2012, 307, 1959–1969 CrossRef CAS PubMed.
- S. Blaabjerg, D. M. Artzi and R. Aabenhus, Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients-A Systematic Review and Meta-Analysis, Antibiotics, 2017, 6(4), 21 CrossRef CAS PubMed.
- G. Barbara, L. Zecchi, R. Barbaro, C. Cremon, L. Bellacosa, M. Marcellini, R. De Giorgio, R. Corinaldesi and V. Stanghellini, Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome, J. Clin. Gastroenterol., 2012, 46, S52–S55 CrossRef PubMed.
- M. Hickson, A. L. D'Souza, N. Muthu, T. R. Rogers, S. Want, C. Rajkumar and C. J. Bulpitt, Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial, Br. Med. J. (Clin. Res. Ed.), 2007, 335, 80 CrossRef PubMed.
- E. Isolauri, P. Kirjavainen and S. Salminen, Probiotics: a role in the treatment of intestinal infection and inflammation?, Gut, 2002, 50, iii54–iii59 CrossRef PubMed.
- Chinese Ministry of Health, Guidelines for diagnosis and management of tuberculosis (2012 version), Chin. J. Front. Med. Sci., 2013, 5, 73–75 Search PubMed.
- S. Jackson, A. Sleigh, G. Wang and X. Liu, Poverty and the economic effects of TB in rural China, Int. J. Tuberc. Lung Dis., 2006, 10, 1104–1110 CAS.
- J. Tack, N. J. Talley, M. Camilleri, G. Holtmann, P. Hu, J.-R. Malagelada and V. Stanghellini, Functional gastroduodenal disorders, Gastroenterology, 2006, 130, 1466–1479 CrossRef PubMed.
- F. Cremonini, S. Di Caro, M. Covino, A. Armuzzi, M. Gabrielli, L. Santarelli, E. C. Nista, G. Cammarota, G. Gasbarrini and A. Gasbarrini, Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study, Am. J. Gastroenterol., 2002, 97, 2744–2749 CrossRef PubMed.
- J. Cai, C. Zhao, Y. Du, Y. Zhang, M. Zhao and Q. Zhao, Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: Systematic review with network meta-analysis, United Eur. Gastroenterol. J., 2018, 6, 169–180 CrossRef PubMed.
- A. C. Ouwehand, C. DongLian, X. Weijian, M. Stewart, J. Ni, T. Stewart and L. E. Miller, Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study, Vaccine, 2014, 32, 458–463 CrossRef PubMed.
- M. Beausoleil, N. Fortier, S. Guenette, A. L'Ecuyer, M. Savoie, M. Franco, J. Lachaine and K. Weiss, Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial, Can. J. Gastroenterol. Hepatol., 2007, 21, 732–736 CAS.
- X. W. Gao, M. Mubasher, C. Y. Fang, C. Reifer and L. E. Miller, Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients, Am. J. Gastroenterol., 2010, 105, 1636–1641 CrossRef PubMed.
- C. Koebnick, I. Wagner, P. Leitzmann, U. Stern and H. J. Zunft, Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation, Can. J. Gastroenterol. Hepatol., 2003, 17, 655–659 Search PubMed.
- A. Abbara, S. Chitty, J. K. Roe, R. Ghani, S. M. Collin, A. Ritchie, O. M. Kon, J. Dzvova, H. Davidson, T. E. Edwards, C. Hateley, M. Routledge, J. Buckley, R. N. Davidson and L. John, Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK, BMC Infect. Dis., 2017, 17, 231 CrossRef PubMed.
- S. Namasivayam, M. Maiga, W. Yuan, V. Thovarai, D. L. Costa, L. R. Mittereder, M. F. Wipperman, M. S. Glickman, A. Dzutsev, G. Trinchieri and A. Sher, Longitudinal profiling reveals a persistent intestinal dysbiosis triggered by conventional anti-tuberculosis therapy, Microbiome, 2017, 5, 71 CrossRef PubMed.
- S. O. Fetissov, Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour, Nat. Rev. Endocrinol., 2017, 13, 11–25 CrossRef CAS PubMed.
- X. Ge, C. Ding, W. Zhao, L. Xu, H. Tian, J. Gong, M. Zhu, J. Li and N. Li, Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility, J. Transl. Med., 2017, 15, 13 CrossRef PubMed.
- T. Zhang, J. Du, X. Yin, F. Xue, Y. Liu, R. Li, C. Luo, L. Li and X. Li, Adverse events in treating smear-positive tuberculosis patients in China, Int. J. Environ. Res. Public Health, 2016, 13, 86 CrossRef PubMed.
- K. Floyd, P. Glaziou, A. Zumla and M. Raviglione, The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era, Lancet Respir. Med., 2018, 6, 299–314 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2020 |