Editorial Perspectives: Scottish Water case study
Christine A.
Murray
*,
Nic
Booker
and
Simon A.
Parsons
Scottish Water, Edinburgh, Scotland, UK. E-mail: Christine.murray@scottishwater.co.uk
Background
Scottish Water abstracts water predominantly from surface water sources and over 80% of these are located in catchments with peat and organo-mineral soils. The levels of dissolved organic carbon (DOC) entering our treatment works can today exceed 20 mg l−1 and it is predicted that for many of these we will see further increases. One reason for this observed increase is the reduction in atmospheric sulfate deposition.1 There is also a risk that climate change and changes in land management will further increase DOC levels.2 DOC removal during water treatment remains a significant and growing challenge for Scottish Water.Our traditional coagulation based water treatment works (WTWs), representing 40% of all WTWs, supply over 90% of the potable water demand and can remove between 70–90% of the DOC. Our experience is that, even 1 mg l−1 of DOC can result in the formation of unacceptable concentrations of trihalomethane (THM) and of other disinfection by-products (DBPs), following chlorine based disinfection. The use of chlorine based disinfection is ubiquitous across the majority of Scottish Water's WTWs.
Nanofiltration (NF) membrane processes are also widely used for the removal of DOC at about 45% of (smaller) WTWs and account for about 7% of the potable supply in Scotland. Typically we use 2 kDa molecular weight cut off NF membranes which can remove 90 to 95% of the DOC, however, the residual DOC tends to be more reactive with chlorine and can form significant concentrations of DBPs.
Coupled with high concentrations of DOC, some of our coastal and island raw water sources have bromide concentrations in excess of 100 μg l−1, with a couple of sites experiencing bromide levels above 500 μg l−1. The current suite of water treatment technologies used in Scotland are ineffective at removing bromide and, even with high organic carbon removal efficiencies, unacceptably high concentrations of DBPs occur due to the formation of brominated THMs.
UK Water Quality Regulations require DBPs to be minimised. Recent research (UKWIR, Phase 2 – understanding DBP Formation: Interpretation of Laboratory experiments to operational conditions, dissemination event, 4th December 2019) and analysis by Scottish Water has confirmed that the presence of bromide can greatly increase the formation of brominated DBPs, even at low DOC concentrations. This is linked to the bromide to organic carbon ratios facilitating bromine substitution and Bougeard3 identified bromide threshold limits above which brominated DBPs tend to dominate. Brominated compounds also tend to form at a faster rate than chlorinated compounds. Therefore there is an increasing requirement for bromide removal in addition to organic carbon and further understanding of the bromide to carbon ratios at specific WTWs. This opens up the market for low whole life cost alternatives to reverse osmosis for bromide removal such as electrodialysis or ion exchange processes.
Examples
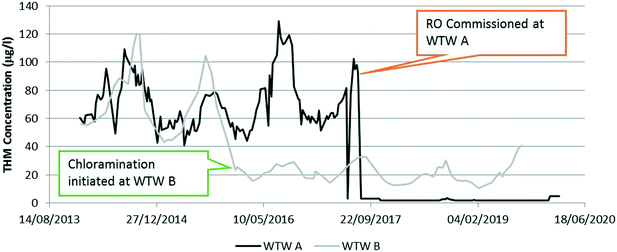
Recently a small island coagulation based WTWs (1 Ml d−1) was replaced with a nanofiltration and reverse osmosis (RO) WTW. RO was selected as the NF pilot plant results confirmed that although the final water TOC would average 1.2 mg l−1, the THM formation potential of the NF permeate was in excess of 100 μg l−1 indicating that the risk of THM failures was high and that DBPs were not minimised. Installation of RO yielded a significant improvement in the formation of THMs (Fig. 1) at WTW A.Another small island WTW had nanofiltration installed but there was still sufficient organic carbon passage that additional treatment was required. At this site, chloramination was installed to minimise DBP formation within distribution. Fig. 1 shows the impact of NF/RO on the formation of THMs compared to NF/chloramination.
Conclusions
From Fig. 1 it is clear that removal of DBP precursors (including DOC and bromide) is important to minimise the DBPs in potable water. Our experience is that NF membrane processes tend to be more cost effective for WTWs with a throughput of less than 1 MLD compared to coagulation based processes. We have also found that NF alone is often unable to sufficiently remove the DBP precursors. The need for additional treatment process stages to enhance the removal of organics and/or bromide significantly increases the treatment costs. The formation of brominated DBPs (in particular THMs) tends to dominate at lower DOC concentrations, due to the more rapid rate of their formation compared to chlorinated DBPs and the higher molecular weight of bromide.4 Given that brominated DBPs are more hazardous to health than chlorinated DBPs,5 there is potentially a balance to be struck between removal of organic carbon to improve microbiological stability and the formation of brominated disinfection by-products.To move water treatment forward in areas impacted by high (and changing) DOC and bromide, more research into understanding the impact of climate change on DOC levels and composition, and the impact this has on treatability, is needed. There is also a need for research into the development of small to medium scale processes for the removal of bromide from drinking water sources. There is also a need for more research and development into natural/biological treatment processes for DOC removal to help support the zero carbon agenda.
References
- D. T. Monteith, J. L. Stoddard, C. Evans, H. de Wit, M. Forsius, T. Hogasen, A. Wilander, B. L. Skelkvale, D. S. Jeffries, J. Vuorenmaa, B. Keller, J. Kopacek and J. Vesely, Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry, Nature, 2007, 450, 537–541 CrossRef CAS PubMed.
- K. Sawicka, E. C. Rowe, C. D. Evans, D. T. Monteith, E. I. Vanguelova, A. J. Wade and J. M. Clark, Modelling impacts of atmospheric deposition and temperature on long-term DOC trends, Sci. Total Environ., 2017, 578, 323–336, DOI:10.1016/j.scitotenv.2016.10.164.
- C. Bougeard, Haloacetic Acids and other Disinfection By-Products in UK Treated Waters: Occurrence, Fate and Precursor Investigation, PhD Thesis, Cranfield University, 2009 Search PubMed.
- C. Bougeard, E. Goslan, B. Jefferson and S. Parsons, Comparison of the disinfection by-product formation potential of treated waters exposed to chlorine and monochloramine, Water Res., 2009, 44, 729–740, DOI:10.1016/j.watres.2009.10.008.
- S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. DeMarini, Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research, Mutat. Res., 2007, 636(1–3), 178–242 CAS.
| This journal is © The Royal Society of Chemistry 2020 |