Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ammonia removal from mixed dewatering liquors by three different deammonification technologies
Pascal
Ochs
ab,
Benjamin D.
Martin
b,
Eve
Germain
b,
Tom
Stephenson
a,
Mark C. M.
van Loosdrecht
 c and
Ana
Soares
c and
Ana
Soares
 *a
*a
aCranfield University, College Road, Cranfield, Bedford MK43 0AL, UK. E-mail: a.soares@cranfield.ac.uk
bThames Water, Reading STW, Island Road, RG2 0RP, Reading, UK
cTechnical University Delft, van der Maasweg 9, 2629 HZ, Delft, The Netherlands
First published on 3rd November 2020
Abstract
Deammonification is an established biological nitrogen removal process for dewatering liquors from anaerobic digestion. Different deammonification technologies are being commercialised varying in control philosophy, biomass structure and reactor design. In this study three different pilot scale deammonification technologies were investigated to assess total nitrogen removal from mixed (pre and post digestion) dewatering liquors originated from thermal hydrolysis based anaerobic digestion process. The technologies tested included a suspended sludge sequencing batch reactor (S-SBR), a moving bed biofilm reactor (MEDIA) and a granular sludge sequencing batch reactor (G-SBR). This is the first study to compare side-by-side, three different deammonification technologies. All tested technologies were operated according to the manufacturer guidelines and demonstrated suitable nitrogen removal at loads varying between 0.3–0.8 kgN m−3 d−1. During the operation of three technologies, periods of poor effluent quality due to disruptions or imbalances in the biological reactions were observed. The S-SBR had the lowest number of imbalances with 14 cases relating to free nitrous acid inhibition. Both S-SBR and MEDIA presented the highest nitrogen removal rate with 0.72 and 0.68 kgN m−3 d−1, respectively. The G-SBR achieved nitrogen removal rates of 0.31 kgN m−3 d−1 while presenting the highest number of imbalances that were related to inhibitive concentration of free ammonia or free nitrous acid of anammox. These inhibitions were caused by the control system relying on surrogate measurements for ammonia. Finally, only the S-SBR was operated at temperatures below 20 °C, caused by seasonal fluctuations, but still achieved nitrogen removal rates of 0.30 kgN m−3 d−1. The number of deammonification technologies is growing, however, only a few but contradictive comparison studies exist. This comparison study can provide support for selection of sidestream deammonification technologies, by identifying the critical parameters.
Water impactStudies comparing commercial deammonification technologies are limited and contradictory in places. This study demonstrated that the type of biomass form (biofilm, suspended, granular) had little impact on nitrogen removal. Nevertheless, it was the robustness and design of the instrumentation and control system based on ammonia, pH and nitrate, that was key to ensure stability of the deammonification chain of reactions. |
Introduction
Deammonification is a well-established process to treat ammonia from dewatering liquors originating from mesophilic anaerobic digestion (AD).1 The process is based on a two-step shortcut reaction of the nitrogen cycle. In the first step, the partial nitritation (PN), the ammonia oxidizing bacteria (AOB) consume ammonia and oxygen to produce nitrite. In the second step, anaerobic ammonia oxidation (anammox or A), anaerobic ammonia oxidizing bacteria (AMX) use ammonia and nitrite to produce nitrogen gas. The stoichiometry of the partial nitritation pathway is presented in (eqn (1)).2 The anammox stoichiometry was first described by Strous et al. (1997)3 and re-iterated by Lotti et al. (2014)2 (eqn (2)).| 1.00NH4+ + 1.383O2 + 0.09HCO3− → 0.0982NO2− + 1.036H2O + 0.018C5H7O2N + 1.982H+ | (1) |
| 1.00NH4+ + 1.146NO2− + 0.071HCO3− + 0.057H+ → 0.986N2 + 0.161NO3− + 0.071CH1.74O0.31N0.20 + 2.002H2O | (2) |
Different commercial deammonification technologies have been developed and applied successfully around the world. These vary in reactor configuration (e.g., sequencing batch reactor, continuous stirred tank reactor, plug-flow) and control strategy (e.g., nitrate produced to ammonia removed, pH change over time, etc.).1 Anammox are slow growing microorganisms.2 This led to the development of different strategies to maintain high AMX concentrations in the biological reactor, such as the use of granular sludge AMX,6,8 suspended sludge9,10 and biomass attached to plastic media.11,12 Today's most applied deammonification technology are based on single-stage reactors.1 Another difference between the technologies is the reactor design with sequencing batch reactors,9,10 continuous stirred tank reactors13,14 and plug-flow reactors.12,15
The benefits of deammonification include energy saving in relation to aeration, as well as the redundancy of carbon needed in denitrification and alkalinity needed in nitrification.9,16 Yet it's unclear how different deammonification technologies compare to each other, with only limited studies available. In an anoxic laboratory scale pure anammox reactor, Jin et al. (2008)17 compared a suspended sludge SBR to an biofilm up-flow biofilter (UBF) under controlled conditions treating synthetic wastewater. It was concluded that the SBR presented similar nitrogen removal rates (NRR) of 2.01 kgN m−3 d−1 compared to 1.99 kgN m−3 d−1 of the UBF, but the first required more time to recover from shock loads.17 Another study comparing performance and nitrous oxide emissions of two deammonification processes, investigated a suspended sludge SBR with a MBBR.18 The SBR and MBBR were operated as deammonification reactor with an additional pre-PN reactor.18 The MBBR achieved lower NRR's of 0.50 kgN m−3 d−1 compared to the SBR which achieved 0.60 kgN m−3 d−1.18 However, when the two technologies plus the pre-nitritation step were evaluated as a single-stage reactor, the MBBR proved to be superior over the SBR with NRR's of 0.39 kgN m−3 d−1 and 0.33 kgN m−3 d−1 respectively. In a further comparison, the MBBR outperformed again the suspended sludge SBR reactor when treating dewatering liquors from a bio-compost process.19 The authors report that the MBBR system achieved NRR's of 1.1–1.8 kgN m−3 d−1 and the SBR with NRR's of 0.3–0.6 kgN m−3 d−1.19 The authors associated the difference in performance with the biomass content which was 5410 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 mg L−1 for SBR and MBBR respectively.1 Additionally, Lackner and Horn (2013)19 reported the MBBR to be more robust by adapting faster to change of influent dewatering liquors as well as recovering 10 days faster from a cold temperature shock.19 In a full-scale deammonification process survey Lackner et al. (2014)1 discussed different operational challenges of existing deammonification processes. It was reported that the highest volumetric nitrogen loading rates (NLR) of 1.0–2.0 kgN m−3 d−1 were achieved by granular sludge based technologies.1 Furthermore, the study identified various operational issues and focused on their impact on the process' performance.1 The most severe operational issues were related to the aeration control, nitrate built up and solid separation.1
190 mg L−1 for SBR and MBBR respectively.1 Additionally, Lackner and Horn (2013)19 reported the MBBR to be more robust by adapting faster to change of influent dewatering liquors as well as recovering 10 days faster from a cold temperature shock.19 In a full-scale deammonification process survey Lackner et al. (2014)1 discussed different operational challenges of existing deammonification processes. It was reported that the highest volumetric nitrogen loading rates (NLR) of 1.0–2.0 kgN m−3 d−1 were achieved by granular sludge based technologies.1 Furthermore, the study identified various operational issues and focused on their impact on the process' performance.1 The most severe operational issues were related to the aeration control, nitrate built up and solid separation.1
The past comparison studies have not been performed under field conditions, side-by-side by comparing a wide range of loading rates and are not capable on evaluating the major commercial deammonification technologies side-by-side for their performance and robustness. Limited studies have compared different types of biomass and no studies were found to study the influence of systems instrumentation and control strategy combined with reactor designs. This controversy between the results of the studies makes it clear that there is a need for a study to compare reactor design, control system and biomass. Hence, the aim of this study was to provide a comparison of three different deammonification pilot plants, using the different biomass types of suspended sludge, granular sludge and biofilm on plastic media as well as different control strategies. This study investigated efficiency and robustness of different deammonification technologies to treat ammonia from mixed dewatering liquors.
Materials and methods
Influent characteristics
The liquors used in this study were a mixture of pre-THP and post THP/AD dewatering liquors at ratio between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as reported by operators on site. The dewatering liquors were collected from a UK wastewater treatment plant (WWTP) serving a population equivalent of 200
2, as reported by operators on site. The dewatering liquors were collected from a UK wastewater treatment plant (WWTP) serving a population equivalent of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The WWTP consists of primary clarifiers, a secondary MBBR and activated sludge process in parallel followed by secondary clarifiers. The pre-THP dewatering liquors were produced by two conventional belt presses operating on thickened sludge and the post-THP/AD dewatering liquors were produced by three hydraulic filter presses. The mixed liquors were collected in a 4 m3 balancing tank with residence time of 0.5–1 day before being distributed to the three deammonification technologies tested. The ammonia concentration varied between 98–1699 mg L−1 with an average of 587 mg L−1 (Table 1). The sCOD to ammonia ratio (sCOD/N) was around 3.3. The nitrite and nitrate concentration were always below detection range of 0.01 mgN L−1 and 0.20 mgN L−1, respectively. The pH and alkalinity were between 7.20–8.60 and 953–5883 mgCaCO3 L−1. The total suspended solid (TSS) concentration ranged from 50–600 mg L−1. The mixed dewatering liquors quality compared others reported at UK WWTPs.20,21
000. The WWTP consists of primary clarifiers, a secondary MBBR and activated sludge process in parallel followed by secondary clarifiers. The pre-THP dewatering liquors were produced by two conventional belt presses operating on thickened sludge and the post-THP/AD dewatering liquors were produced by three hydraulic filter presses. The mixed liquors were collected in a 4 m3 balancing tank with residence time of 0.5–1 day before being distributed to the three deammonification technologies tested. The ammonia concentration varied between 98–1699 mg L−1 with an average of 587 mg L−1 (Table 1). The sCOD to ammonia ratio (sCOD/N) was around 3.3. The nitrite and nitrate concentration were always below detection range of 0.01 mgN L−1 and 0.20 mgN L−1, respectively. The pH and alkalinity were between 7.20–8.60 and 953–5883 mgCaCO3 L−1. The total suspended solid (TSS) concentration ranged from 50–600 mg L−1. The mixed dewatering liquors quality compared others reported at UK WWTPs.20,21
| G-SBR | MEDIA | S-SBR | |||||
|---|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | Period 1 | Period 2 | Period 3 | |
| a NO3-N and NO2-N were measured in the influent and the concentrations were always below 0.20 and 0.01 mgN L−1 respectively. Hence ammonia was the only contributor to the influent nitrogen loading rate. | |||||||
| Nitrogen loading ratea (kgN m−3 d−1) | 0.29 ± 0.13 | 0.46 ± 0.22 | 0.30 ± 0.15 | 0.92 ± 0.39 | 0.38 ± 0.21 | 0.60 ± 0.25 | 1.01 ± 0.39 |
| Number of operational days (d) | 115 | 101 | 91 | 51 | 65 | 49 | 53 |
| Ammonia (NH4-N) (mgN L−1) | 504.1 ± 302.8 | 668.0 ± 252.4 | 516.7 ± 174.2 | 717.3 ± 318.1 | 504.0 ± 147.0 | 534.0 ± 219.0 | 794.0 ± 322.0 |
| pH | 7.83 ± 0.25 | 7.99 ± 0.24 | 7.85 ± 0.21 | 8.04 ± 0.25 | 7.83 ± 0.21 | 7.93 ± 0.19 | 8.06 ± 0.27 |
| Total suspended solids (TSS) (mg L−1) | 398 ± 232 | 430 ± 262 | 413 ± 277 | 414 ± 229 | 396 ± 279 | 426 ± 241 | 442 ± 236 |
| Soluble COD (sCOD) (mg L−1) | 1536 ± 830 | 1990 ± 705 | 1573 ± 546 | 2127 ± 830 | 1523 ± 468 | 1618 ± 673 | 2387 ± 780 |
| sCOD/NH4-N ratio (mg mgN−1) | 3.3 ± 1.5 | 3.2 ± 1.6 | 3.3 ± 1.8 | 3.1 ± 1.0 | 3.3 ± 1.7 | 3.4 ± 2.0 | 3.2 ± 1.0 |
| Alkalinity (CaCO3) (mg L−1) | 2241 ± 993 | 2754 ± 817 | 2282 ± 554 | 2911 ± 1054 | 2246 ± 464 | 2268 ± 723 | 3198 ± 1032 |
| Reactor temperature (°C) | 27.5 ± 2.8 | 27.3 ± 2.2 | 29.8 ± 0.8 | 27.5 ± 4.0 | 21.5 ± 4.6 | 17.4 ± 3.8 | 28.5 ± 4.1 |
| Inoculation volume to reactor volume | 25% | 13% | 54% | N/A | 50% | N/A | |
| Inoculation MLVSS (mg L−1) | 2500 | 1500 | 2000 | N/A | 1800 | N/A | |
| DO set point (mg L−1) | 1.20 | 0.80 | 0.30 | ||||
| pH set point | 7.50 | 7.20 | 6.80 | ||||
| Ammonia set-point (mgN L−1) | 100–150 | 150 | 150 | ||||
Technologies tested and configurations
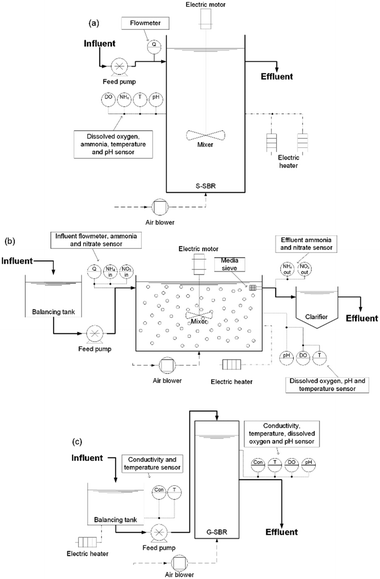
Three different deammonification pilot plants from manufactured from different commercial suppliers were started up at the same time and fed in parallel with the sludge dewatering liquors described above. The full deammonification technology characteristics and control philosophy set-points are described in Table 1 for the different operational periods tested and followed the specification provided by the commercial suppliers. In summary, the suspended sludge sequencing batch reactor (S-SBR) had a volume of 6 m3 (Fig. 1a) and a hydraulic retention time (HRT) of 31 h. The control philosophy measured ammonia and pH to actuate on feed flow and aeration. The setpoint for pH and ammonia were 6.8 and 150 mgN L−1 respectively. The S-SBR had intermittent aeration with a DO set-point of 0.3 mg L−1. During anoxic phases the biomass was kept in suspension with mechanical mixers operating at 12 RPM. The S-SBR was seeded with 3 m3 of sludge from a similar SBR technology in the UK. The seed sludge had a concentration of 1800 mg MLVSS L−1. The design solids retention time (SRT) was 3 days but in reality, it was between 3–7 days, as calculated from the solids mass balance. The S-SBR fill and reaction cycle was 5 h followed by 0.8 h of settling and 0.2 h of decanting.The MBBR pilot plant used plastic media carrier to support the biomass (MEDIA) (Fig. 1b). The MEDIA process had a volume of 1.2 m3, a media fill-ratio of 54% as specified by the commercial supplier and the plastic carriers had a surface area of 500 m2 m−3. The plastic carriers were discs with a diameter of 2.5 cm and a height of 0.2 cm manufactured from polyethylene. The MEDIA process was fed from a 0.5 m3 balancing tank. The HRT was 41 h. The MEDIA reactor was controlled by measuring nitrate production and ammonia conversion which actuated on influent flowrate and aeration. The reactor was continuously aerated at a DO setpoint of 0.8 mg L−1. Furthermore, the MEDIA reactor had a pH setpoint of 7.0 and an ammonia setpoint of 150 mgN L−1. The mixer in the MEDIA reactor operated at 20 RPM. The reactor was inoculated using pre-seeded plastic carriers from a biofarm in Sweden with a volume of 634 L.22 In period 2, the fill-ratio was changed to 37% by removing 200 L of plastic media from the reactor with the aim to enhance the mass-transfer and reactor hydraulics. The reduction in the fill ratio was due to the occurrence of frequent dead zones, accompanied by uneven aeration of the MEDIA reactor. The reactor temperature during period 1 and period 2 was maintained between 27–30 °C with an electric heater. The SRT of the suspended sludge in the MEDIA was left uncontrolled and was up to 30 d.
The SBR with a granular sludge (G-SBR) had a volume of 0.2 m3 and was fed from a 1 m3 balancing tank (Fig. 1c). The HRT was around 43 h. The control philosophy was based on measuring conductivity and pH that actuated on feed flow. The conductivity difference was measured over the biological reaction of one cycle, taking into account the deammonification stoichiometry to adapt the NLR.8 The reactor was continuously aerated with a DO set-point of 1.2 mg L−1. The G-SBR was inoculated with 50 L of granular biomass originated from a similar full-scale granular SBR in Spain. The seed sludge had a MLVSS concentration of 2500 mg L−1. The G-SBR reactor temperature was maintained at 27 °C using an electric heater in the balancing tank. The G-SBR cycle length varied depending on the conductivity measurements with a fill length of 0.1–0.2 h, a reaction length of 3–8 h, a settling phase of 0.2–0.5 h and a decanting phase of 0.2–0.5 h. The SRT for the granular sludge is as long as the operation period whereas the SRT of the suspended phase was 3–6 d based on solids mass-balance.
Pilot plant operation
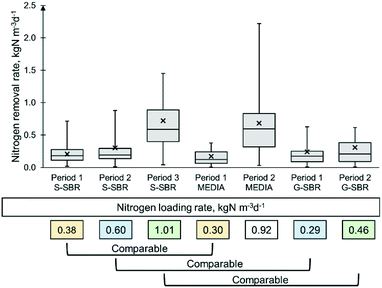
The three deammonification technologies were evaluated after the start-up. The technologies tested in this study were designed and manufactured by their respective commercial suppliers and then operated following their specifications. The three different deammonification technologies had different control strategies that actuated on the feed flow, leading consequently to a variety of ammonia loading rates to each reactor. In order to compare the different technologies tested, the data was divided into different operational periods based on the ammonia loading rates applied (Table 1). Since the influent nitrite and nitrate were always below detection limit, it was assumed that the ammonia loading rate was equal to the NLR. Period 1 and 2 for G-SBR had NLR's of 0.29 ± 0.13 and 0.46 ± 0.22 kgN m−3 d−1 respectively. For the MEDIA period 1, corresponded to an NLR of 0.30 ± 0.15 kgN m−3 d−1 and period 2 was 0.92 ± 0.39 kgN m−3 d−1. The S-SBR data set was divided into three periods, having NLR's of 0.38 ± 0.21, 0.60 ± 0.25 and 1.01 ± 0.39 kgN m−3 d−1, respectively. The temperature was varied in the S-SBR due to seasonal temperature change. In period 1 the reactor temperature was also grouped in operational periods at: 22 ± 5 °C, 17 ± 4 °C and 29 ± 4 °C. The G-SBR was re-seeded in period 2 with 25 L of seed sludge from a similar full-scale granular SBR from Spain with a MLVSS concentration of 1500 mg L−1.Technology evaluation
The different technologies were compared for similar operational periods (Table 1) by analysing NLR's using statistics (described in data collection and analysis). The statistical comparison of the different NLR periods indicated that G-SBR period 1 and MEDIA period 1, G-SBR period 2 and S-SBR period 2 as well as the S-SBR period 3 and MEDIA period 2 were comparable. The stable operations data of all other periods was too distinctive for a direct comparison.Stable operation was defined as the period without any disruptions. Disruptions included equipment failure and reactor operation that led to imbalance of the deammonification biological reactions. Root causes were determined for all individual imbalances following the schematic in Fig. 2. In summary, imbalances were related to:
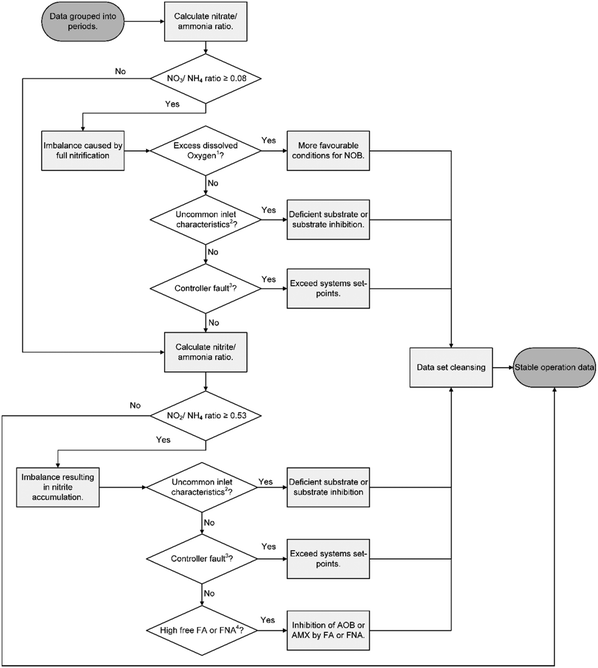 | ||
| Fig. 2 Flowchart for evaluation of process imbalances for the three technologies.1 Excess dissolved oxygen was when oxygen concentration exceeded reactor setpoints (G-SBR DO was 1.2 mg L−1, MEDIA was 0.8 mg L−1 and S-SBR was 0.3 mg L−1).2 Uncommon inlet characteristics were defined as either high or low influent concentration of sCOD, ammonia, pH or alkalinity but not being an outlier.3 Controller fault was when control setpoints were exceeded resulting in ammonia (>200 mgN L−1), nitrite (>50 mgN L−1) accumulation or high pH (>7.2).4 High free ammonia (FA) or free nitrous acid (FNA) was defined as inhibition by AOB or AMX. Free ammonia inhibition ranges were 8–120 mgN L−1 and 20–50 mgN L−1 for AOB and AMX respectively. Free nitrous acid inhibition ranges were 0.2–2.8 mgN L−1 and 0.01–0.2 mgN L−1 for AOB and AMX respectively.24,29 | ||
– Partial nitritation, the nitrate produced to ammonia removed ratio was used to evaluate the partial nitritation. Based on deammonification stoichiometry (eqn (1) and (2)) the ideal ratio is 0.08.
– Accumulation of nitrite, analysing the residual nitrite to ammonia ratio. Based on deammonification stoichiometry (eqn (1) and (2)) the ideal nitrite to ammonia ratio is 0.53.
For all processes imbalances and causalities were identified and analysed for root causes. After consideration of all the imbalances, the stable operation of the technologies was analysed, and these were compared based on their nitrogen removal rates (NRR).
Data collection and analysis
Autosamplers (Hach Lange AS900, Loveland, Colorado, USA) were used to collect 24 h influent and effluent composite samples. All samples were analysed for total COD, soluble COD, ammonia, nitrite, nitrate, alkalinity, TSS, VSS using standard methods.23 The pH was measured using a Hach Lange HQ11D portable pH meter (Hach Lange, Loveland, Colorado, USA). Free ammonia (FA) and free nitrous acid (FNA) were calculated following Anthonisen (1976).24 Once per week mixed liquor suspended solids (MLSS) and mixed liquor volatile suspended solids (MLVSS) were collected from the liquid phase of each technology and analysed using standard methods.23T-Tests, ANOVA tests and Tukey's honestly significance difference (HSD) were used to compare the periods during stable operation.Results and discussion
Technology evaluation
All three technologies were able to achieve ammonia removal from mixed sludge dewatering liquors via deammonification. The total NRR's varied between 0.10 kgN m−3 d−1 and 0.70 kgN m−3 d−1. This is in agreement with a full-scale deammonification process survey on 14 installations that identified that deammonification technologies had NLR's between 0.30–1.00 kgN m−3 d−1 achieving nitrogen removal efficiency (NRE) of 70–85%,1 which corresponded to NRR's of 0.21–0.85 kgN m−3 d−1.The first data analysis focused on identifying disruptions on the normal reactor operation that included equipment failure and imbalances in the biological deammonification reactions. The nitrate to ammonia ratio and nitrite to ammonia ratio for the nitrate production rate are described in Fig. 3. The G-SBR and MEDIA reactor exceeded the ideal stoichiometric ratios in some occasions, indicating unwanted nitrite oxidation to nitrate (Fig. 2). The disruptions in operation that caused imbalances in deammonification are represented in Table 2. The G-SBR had the highest number of imbalances in biological reactions. One of the causalities was related to the dissolved oxygen concentration exceeding the operational set-point of 1.2 mg L−1. This subsequently led to full nitrification with nitrate production, which was reflected in an elevated nitrate to ammonia ratio. Control system faults were caused by issues with the conductivity measurements as surrogate for ammonia. This conductivity difference was not reflecting the actual ammonia conversion in the reactor (e.g., day 210, 267 and 357), leading to accumulation of ammonia or nitrite.
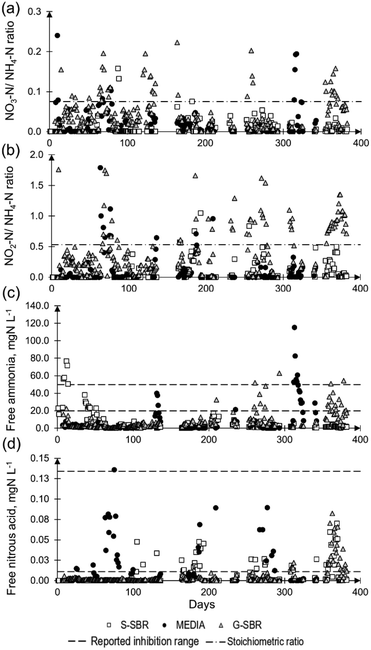 | ||
| Fig. 3 a) Nitrate/ammonia ratio and b) nitrite/ammonia ratio representing the excess of substrates and products for the biological pathways. The stoichiometric ratio (dash-dotted line) for nitrate/ammonia ratio was 0.08 and for nitrite/ammonia ratio 0.53. c) Free ammonia and d) free nitrous acid concentration for the tree technologies. The reported inhibition ranges for AMX (dashed line) for free ammonia and free nitrous acid were 20–50 mgN L−1 and 0.01 and 0.2 mgN L−1 respectively.24,29 | ||
| G-SBR | MEDIA | S-SBR | |||||
|---|---|---|---|---|---|---|---|
| Period | Period | Period | Period | Period | Period | Period | |
| 1 | 2 | 1 | 2 | 1 | 2 | 3 | |
| Nitrogen loading rate (kgN m−3 d−1) | 0.29 | 0.46 | 0.30 | 0.92 | 0.38 | 0.60 | 1.01 |
| Imbalance resulting in nitrite accumulation NO2/NH4 ratio > 0.53 | |||||||
|---|---|---|---|---|---|---|---|
| a Excess dissolved oxygen was when oxygen concentration exceeded reactor set-points (G-SBR DO was 1.2 mg L−1, MEDIA was 0.8 mg L−1 and S-SBR was 0.3 mg L−1). b Uncommon inlet characteristics was defined as either high or low influent concentration of sCOD, ammonia, pH or alkalinity but not being an outlier. c Controller fault was when control set-points were exceeded resulting in ammonia (>200 mgN L−1), nitrite (>50 mgN L−1) accumulation or high pH (>7.2). d High free ammonia (FA) or free nitrous acid (FNA) was defined as inhibition of AOB or AMX. Free ammonia inhibition ranges were 8–120 mgN L−1 and 20–50 mgN L−1 for AOB and AMX respectively. Free nitrous acid inhibition ranges were 0.2–2.8 mgN L−1 and 0.01–0.2 mg L−1 for AOB and AMX respectively.24,29 | |||||||
| Total sample number | 115 | 101 | 91 | 51 | 65 | 49 | 53 |
| Number of samples with NO2/NH4 > 0.53 | 10 | 53 | 8 | 2 | 8 | 3 | 0 |
| Uncommon inlet characteristicsb | 10 | 11 | 1 | ||||
| Controller faultc | 8 | ||||||
| High free ammonia or free nitrous acidd | 22 | 10 | 2 | 8 | 3 | ||
Great variability of influent characteristics led to limitation in substrate being fed to the reactors. For example, on days 62, 208 and 253, low influent ammonia concentration of <250 mgN L−1, with sufficient alkalinity, resulted in the conversion of ammonia to nitrite by AOB, leaving little ammonia for AMX, leading to nitrite accumulation. On days 209 and 356, high soluble COD concentration of 3000–4000 mg L−1 promoted growth of heterotrophic bacteria converting the biodegradable part of the COD to CO2 and competing with AOB for oxygen. It has been documented that a high soluble COD concentration can lead to the activation of denitrification bacteria, converting the available nitrite and nitrate to nitrogen gas.25 In period 2 of the G-SBR operation, the most frequent type of imbalance was related to inhibition of AMX by FA and FNA. The effect of FA and FNA on AOB and NOB is well understood and discussed in literature (Anthonisen et al., 1976; Vadivelu et al., 2007).24,26 The inhibition of AMX by FA was reported to be between 20–50 mgN L−1 (ref. 27–29) and is caused by the unprotonated ions of NH3 rather than ammonium (NH4+).30 Similar is the inhibition of AMX by FNA which is reported to be between 0.01–0.2 mgN L−1.29,31 Free ammonia and FNA are effected by pH and can be calculated using the method described by Anthonisen et al., (1976).24
It was identified that the suppression of AMX by FNA was either an effect of the uncontrolled pH (e.g., pH > 7.5) or the use of conductivity measurements as a surrogate for ammonia. This contradicts with the results of Lotti et al. (2012)32 who was reporting that nitrite rather than FNA would be the main inhibitor. However, the inhibitive nitrite concentration of 400 mgN L−1 (ref. 32) was only reached on 5 occasions following a prolonged period of FNA inhibition at pH values between 7.5–8.3. Imbalance of the biological reaction in the MEDIA reactor were due to high influent ammonia concentrations of >1000 mgN L−1. This led to an accumulation of ammonia in the reactor reaching inhibitive concentration of FA, and suppressing AMX. The S-SBR presented the lowest number of operational issues, in comparison with the other two technologies. The reactor reached inhibitive FNA of 0.02 and 0.04 mgN L−1 on days 166, 193 and 256. This could be related to an accumulation of nitrite >50 mgN L−1 in the reactor. In summary, it was identified that high FA and FNA concentrations of 62–115 mgN L−1 and 0.1–0.3 mgN L−1 respectively were the most frequent cause for unbalanced biological reactions of the three technologies. The inhibition of FA and FNA in the biological reactors originated from a combined effect of pH and accumulation of ammonia or nitrite, respectively. In the G-SBR the imbalances were mainly caused by issues with the control system where conductivity was used as surrogate measure for ammonia. An offset between conductivity measurements and reactor ammonia concentration led to frequent accumulation of ammonia in the biological reactor. The overall higher DO concentration of 0.8–1.4 mg L−1 (Table 3) and more ammonia substrate available resulting in greater conversion to nitrite by AOB which subsequently led to nitrite accumulation in the reactor. In a deammonification process application survey Lackner et al. (2014)1 gave an overview of typical process stability issues identifying ammonia, nitrite and nitrate build-up as some of the most frequent full-scale operational issues. It was reported in previous studies that the inhibition of FNA was greater on suspended sludge anammox processes compared to biofilm on plastic media processes systems.31 That effect was not observed in this study, since imbalances caused by inhibition were picked up by the control system and limiting the feed when excess substrate was available in the S-SBR, recovering within 1–2 days.
| G-SBR | MEDIA | S-SBR | ||||||
|---|---|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | Period 1 | Period 2 | Period 3 | ||
| Nitrogen removal rate (kgN m−3 d−1) | Mean | 0.24 | 0.31 | 0.17 | 0.68 | 0.21 | 0.30 | 0.72 |
| Min | 0.06 | 0.07 | 0.02 | 0.03 | 0.01 | 0.07 | 0.09 | |
| Max | 0.69 | 0.68 | 0.40 | 2.25 | 0.72 | 0.95 | 1.54 | |
| Ammonia (NH4-N) (mgN L−1) | Mean | 58.5 | 148.5 | 170.8 | 191.2 | 238.9 | 219.8 | 177.8 |
| Min | 0.3 | 35.2 | 8.5 | 18.1 | 47.0 | 75.5 | 57.6 | |
| Max | 308.4 | 530.1 | 662.9 | 550.8 | 555.0 | 565.1 | 603.8 | |
| Nitrate (NO3-N) (mgN L−1) | Mean | 16.8 | 8.3 | 6.8 | 10.8 | 2.1 | 5.5 | 7.3 |
| Min | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | |
| Max | 42.1 | 21.3 | 57.2 | 51.7 | 29.0 | 24.0 | 22.2 | |
| Nitrite (NO2-N) (mgN L−1) | Mean | 8.3 | 19.0 | 7.9 | 10.3 | 3.3 | 14.4 | 18.3 |
| Min | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| Max | 21.8 | 90.7 | 75.0 | 127.1 | 44.6 | 79.6 | 83.0 | |
| Nitrogen removal efficiency (%) | Mean | 82.2 | 74.7 | 60.5 | 70.4 | 51.0 | 52.9 | 69.3 |
| Min | 28.1 | 16.3 | 7.1 | 8.8 | 10.0 | 22.6 | 19.5 | |
| Max | 95.1 | 95.8 | 98.5 | 94.8 | 87.1 | 83.2 | 94.5 | |
| Ammonia removal efficiency (%) | Mean | 88.1 | 77.1 | 65.2 | 75.5 | 53.6 | 57.5 | 72.9 |
| Min | 30.6 | 19.5 | 7.1 | 8.8 | 10.5 | 2.1 | 20.6 | |
| Max | 99.0 | 96.8 | 98.6 | 96.6 | 90.4 | 86.8 | 95.8 | |
| Organic removal efficiency (%) | Mean | 79.5 | 71.6 | 60.8 | 66.3 | 50.1 | 57.5 | 58.8 |
| Min | 49.1 | 16.0 | 0.7 | 19.5 | 2.0 | 9.0 | 38.0 | |
| Max | 91.4 | 91.1 | 82.7 | 93.7 | 79.5 | 81.5 | 77.7 | |
| NO3/NH4 ratio | Mean | 0.03 | 0.01 | 0.01 | 0.02 | 0.47 | 0.01 | 0.01 |
| Min | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 | 0.003 | 0.001 | |
| Max | 0.07 | 0.05 | 0.07 | 0.07 | 0.06 | 0.04 | 0.05 | |
| NO2/NH4 ratio | Mean | 0.19 | 0.21 | 0.05 | 0.07 | 0.03 | 0.09 | 0.12 |
| Min | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Max | 0.50 | 0.52 | 0.46 | 0.52 | 0.37 | 0.44 | 0.50 | |
| pH | Mean | 7.6 | 7.8 | 8.0 | 8.0 | 8.0 | 7.9 | 8.1 |
| Min | 6.9 | 7.3 | 7.5 | 7.3 | 7.7 | 7.6 | 7.7 | |
| Max | 8.3 | 8.8 | 8.4 | 8.6 | 8.5 | 8.3 | 8.4 | |
| Soluble COD (sCOD) (mg L−1) | Mean | 299 | 566 | 782 | 1283 | 801 | 1017 | 1107 |
| Min | 182 | 114 | 124 | 234 | 349 | 400 | 431 | |
| Max | 748 | 5500 | 6159 | 8023 | 1773 | 6953 | 8933 | |
| Total suspend solids (TSS) (mg L−1) | Mean | 82 | 130 | 335 | 456 | 245 | 228 | 199 |
| Min | 12 | 20 | 40 | 34 | 47 | 19 | 38 | |
| Max | 1366 | 1680 | 1910 | 1780 | 875 | 920 | 850 | |
| Free nitrous acid (FNA) (μg L−1) | Mean | 0.4 | 0.5 | 7.1 | 4.4 | 2.6 | 6.3 | 12.9 |
| Min | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Max | 1.2 | 1.5 | 89.5 | 68.8 | 47.6 | 47.7 | 70.1 | |
| Free ammonia (FA) (mg L−1) | Mean | 5.7 | 3.6 | 4.4 | 11.5 | 11.8 | 4.5 | 1.9 |
| Min | 0.3 | 0.3 | 0.02 | 0.03 | 0.1 | 0.6 | 0.4 | |
| Max | 22.5 | 62.9 | 39.9 | 115.1 | 76.6 | 16.9 | 5.3 | |
| Alkalinity (CaCO3) (mg L−1) | Mean | 411 | 573 | 905 | 905 | 1213 | 1037 | 882 |
| Min | 167 | 230 | 312 | 288 | 627 | 344 | 443 | |
| Max | 1344 | 3317 | 2433 | 1523 | 2398 | 2414 | 2328 | |
| Mixed volatile suspended solids (MLVSS) in suspension (mg L−1) | Mean | 1825 | 1602 | 1812 | 2001 | 3776 | 2868 | 3918 |
| Min | 820 | 1080 | 300 | 260 | 976 | 1350 | 1080 | |
| Max | 4224 | 2680 | 5616 | 4644 | 5520 | 5400 | 6460 | |
It was determined that the control system selection played a crucial role in robustness of deammonification systems. The control system of the G-SBR that was based on conductivity measurement instead of ammonia and had the highest number of imbalances of the biological reaction. The control system of the MEDIA and the S-SBR had the least imbalances in biological reactions by relying on pH, ammonia and nitrate measurements. In a full-scale deammonification technology study it was proposed, that robust online measurement of ammonia, nitrite and nitrate are needed to early detect accumulation by nitrite and nitrate and to balance the biological reactions.33 This indicates that a robust control strategy with well-maintained sensors should be one of the key considerations in selecting a sidestream deammonification technology.
Stable operation
During stable operation (periods without imbalances in the biological reactions) (Table 3), the G-SBR achieved average NRR of 0.24 kg N m−3 d−1 in period 1 and 0.31 kgN m−3 d−1 in period 2 (Fig. 4). Similar G-SBR technologies reached higher average NRR of 0.80 kgN m−3 d−1 when treating AD dewatering liquors in pilot scale.8 The difference in performance can be explained by the different MLVSS concentration of the studies, whereas the authors maintained a concentration of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 (ref. 8) and the G-SBR of this study was kept at 1602–1825 mg L−1 (Table 3). The MEDIA process achieved NRR of 0.17 and 0.68 kgN m−3 d−1 in period 1 and period 2, respectively. In a pilot scale comparison of a deammonification MBBR and integrated fixed film activated sludge process (IFAS) treating AD dewatering liquors, the MBBR achieved an average NRR of 0.5 kgN m−3 d−1,11 which was lower than 0.68 kgN m−3 d−1 obtained in period 2, in the current study. However, another study compared different full-scale deammonification MBBR reactors reported NRR of 1.0–1.2 kgN m−3 d−1.13 The S-SBR pilot plant presented removal rates of 0.21 kgN m−3 d−1 for period 1, 0.30 kgN m−3 d−1 for period 2 and 0.72 kgN m−3 d−1 for period 3. Similar S-SBR technologies reported NRR's of 0.50 kgN m−3 d−1 treating AD dewatering liquors,9,34 which were much lower than the 0.72 kgN m−3 d−1 achieved in period 3. The effluent solids concentration ranged between 199–245 mg L−1 for the S-SBR, 355–456 mg L−1 for the MEDIA and 82–130 mg L−1 for the G-SBR. It is known that the suspended sludge AMX biomass is more susceptive to wash-out compared to MEDIA and G-SBR.35 However, excessive wash-out was not observed in this study by maintaining long sedimentation and decanting periods of 1 h.
000 mg L−1 (ref. 8) and the G-SBR of this study was kept at 1602–1825 mg L−1 (Table 3). The MEDIA process achieved NRR of 0.17 and 0.68 kgN m−3 d−1 in period 1 and period 2, respectively. In a pilot scale comparison of a deammonification MBBR and integrated fixed film activated sludge process (IFAS) treating AD dewatering liquors, the MBBR achieved an average NRR of 0.5 kgN m−3 d−1,11 which was lower than 0.68 kgN m−3 d−1 obtained in period 2, in the current study. However, another study compared different full-scale deammonification MBBR reactors reported NRR of 1.0–1.2 kgN m−3 d−1.13 The S-SBR pilot plant presented removal rates of 0.21 kgN m−3 d−1 for period 1, 0.30 kgN m−3 d−1 for period 2 and 0.72 kgN m−3 d−1 for period 3. Similar S-SBR technologies reported NRR's of 0.50 kgN m−3 d−1 treating AD dewatering liquors,9,34 which were much lower than the 0.72 kgN m−3 d−1 achieved in period 3. The effluent solids concentration ranged between 199–245 mg L−1 for the S-SBR, 355–456 mg L−1 for the MEDIA and 82–130 mg L−1 for the G-SBR. It is known that the suspended sludge AMX biomass is more susceptive to wash-out compared to MEDIA and G-SBR.35 However, excessive wash-out was not observed in this study by maintaining long sedimentation and decanting periods of 1 h.
When performing a statistical comparison between NRR obtained for period 1 (NLR of 0.29 and 0.30 kgN m−3 d−1 respectively) for both G-SBR and MEDIA, it was found that the G-SBR achieved a greater NRR (0.24 kgN m−3 d−1) than the MEDIA technology (0.17 kgN m−3 d−1). The DO concentration of the G-SBR was 1.4 mg L−1 and of the MEDIA 0.8 mg L−1. This was accompanied by nitrite concentrations of 8.3 mgN L−1 for G-SBR and 7.9 mgN L−1 for MEDIA (Table 3). Additionally, the alkalinity for period 1 was 411 mg L−1 for G-SBR and 905 mg L−1 for MEDIA. This could indicate that the AOB conversion was slightly more prevailing in the G-SBR. It has been reported that granular deammonification technologies operated at dissolved oxygen concentration >1.0 mg L−1 to allow diffusion of oxygen into the granular.36,37 Studies comparing granular and media deammonification technologies could not be found to the best of our knowledge. However, in an application survey of various full-scale deammonification technologies Lackner et al., (2014)1 identified that granular sludge technologies had higher loading rates (1.0–2.0 kgN m−3 d−1) than other full-scale processes. But the comparison of this is limited since the loading rates of G-SBR and MEDIA in period 1 were low with 0.29 and 0.30 kgN m−3 d−1, respectively.
When comparing G-SBR and S-SBR during period 2 (0.46 kgN m−3 d−1 and 0.60 kgN m−3 d−1 respectively) it was found that the NRR's of 0.31 kgN m−3 d−1 for G-SBR and 0.30 kgN m−3 d−1 for S-SBR were similar (Table 3). The ammonia and nitrite concentration in the reactor were 148.5 mgN L−1 and 19.0 mgN L−1 for G-SBR and 219.9 mgN L−1 and 14.4 mgN L−1 for the S-SBR. The alkalinity concentration in the G-SBR was 573 mg L−1 and for the S-SBR was 1037 mg L−1. In the S-SBR the ammonia and alkalinity concentration in the reactor indicated that there still was capacity to convert ammonia via AOB. On the contrary, the reactor nitrite concentration of 14.0 mgN L−1 led to the conclusion that the S-SBR was limited by AMX conversion, with plenty of ammonia and nitrite as substrate available. This was an effect of the lower temperature of 17 °C in the S-SBR that impacted the anammox removal negatively. Other studies reported that anammox activities decreased when the temperature declined below 20 °C (Lotti et al., 2015).38 In the period 2 of G-SBR it was observed that the organic removal efficiency (ORE) of 71.6% was much higher than the one of the S-SBR of 57.5% respectively. The DO concentration in G-SBR and S-SBR were 1.4 and 0.4 mg L−1 respectively. In the G-SBR the heterotrophic growth could be considered aerobic, since the reactor was continuously aerated. In the S-SBR this organic removal was related to denitrification activity, as for the intermittent aeration with anoxic periods. However, it is understood that the overall contribution to total nitrogen removal of denitrifying heterotrophic bacteria in sidestream is minimal due to a very limited biodegradable COD fraction.13,39 Furthermore, heterotrophic growth was observed on the granular surface in the G-SBR where the colour changed from reddish to grey-blackish from period 1 to period 2. In biofilm systems heterotrophic bacteria are known to build in the outside layer of the biofilm and are considered to be fast growing microorganisms (van Loosdrecht et al., 1995;40 Kindaichi et al., 2004).41 It would be expected, that the heterotrophic bacteria compete with AOB for oxygen in the G-SBR but the average nitrite concentration of 19.0 mgN L−1 was the highest for all deammonification (Table 3). This could indicate that the G-SBR was limited by AMX rather than AOB. Furthermore, The MLVSS concentration in the G-SBR was 1462 mg L−1, which was much lower than the MLVSS of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–15
000–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 of G-SBR system that achieved high NRR's of up to 1.00 kgN m−3 d−1.8 This implies that the AMX growth and granular formation needs to be maximized by adapting a control philosophy that keeps the deammonification reaction chain more balanced.
000 mg L−1 of G-SBR system that achieved high NRR's of up to 1.00 kgN m−3 d−1.8 This implies that the AMX growth and granular formation needs to be maximized by adapting a control philosophy that keeps the deammonification reaction chain more balanced.
The loading rates of S-SBR period 3 and MEDIA period 2 of 1.01 and 0.92 kgN m−3 d−1 respectively were comparable (Table 1). It was found that the NRR's of 0.72 kg N m−3 d−1 for S-SBR and 0.68 kg N m−3 d−1 for the MEDIA were similar (Table 3). The reactor ammonia concentration of the S-SBR in period 3 was 177 mgN L−1 and 191 mgN L−1 for the MEDIA period 2. The MEDIA reactor had a nitrite concentration of 10.3 mgN L−1 and a DO concentration of 1.0 mg L−1. The nitrite concentration in the S-SBR was slightly higher with 18.33 mgN L−1 and the DO concentration was 0.2 mg L−1. This indicated, that the S-SBR had higher nitrite accumulation rates of 0.12 compared to the MEDIA with 0.1 (NO2/NH4 ratio in Table 3). The alkalinity for both deammonification technologies was similar with 905 and 882 mg L−1 for MEDIA and S-SBR respectively. Which could indicate that the S-SBR was limited by AMX rather than AOB. Past studies discussed that the anammox conversion could be enhanced when using an external selector such as a sieve or a hydrocyclone.42 In the MEDIA, it was observed that the biofilm on the plastic media was coloured blackish, similar to the G-SBR, indicating heterotrophic growth. This is being supported by an ORE in the MEDIA for period 2 of 66.3%. Furthermore, the MLVSS concentration of the MEDIA was 2001 mg L−1. Deammonification MBBR systems are reported to have biomass concentrations of 5000–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1.11,19 The results of the present study contradict results from the study by Lackner and Horn (2013).19 Which indicated that the MBBR technology outperformed the suspended SBR. In Lackner and Horn (2013)19 the MBBR reactor achieved NRR's of 1.1–1.8 kgN m−3 d−1 while the SBR with suspended biomass achieved only 0.3–0.6 kgN m−3 d−1. The difference in performance was related to the difference in biomass concentration of 5410 and 12
000 mg L−1.11,19 The results of the present study contradict results from the study by Lackner and Horn (2013).19 Which indicated that the MBBR technology outperformed the suspended SBR. In Lackner and Horn (2013)19 the MBBR reactor achieved NRR's of 1.1–1.8 kgN m−3 d−1 while the SBR with suspended biomass achieved only 0.3–0.6 kgN m−3 d−1. The difference in performance was related to the difference in biomass concentration of 5410 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 mg L−1 for SBR and MBBR respectively.19 In a study by Leix et al. (2016)18 the MBBR achieved a lower NRR of 0.50 kgN m−3 d−1 compared to a SBR with 0.60 kgN m−3 d−1. Both reactors operated with a pre-nitritation reactor feeding higher nitrite to ammonia ratios to the SBR which was believed to have caused the higher performance of the reactor.18 However, when the two reactors plus the pre-nitritation step were evaluated as a single-stage process it was found that the MBBR achieved ammonia removal rates greater than the SBR with 0.39 and 0.33 kgN m−3 d−1 respectively. Another study, comparing a SBR with a biofilter for the anammox pathway, reported that the SBR had similar removal of 2.01 kgN m−3 d−1 compared to the biofilter 1.99 kgN m−3 d−1.17 The past studies comparing different deammonification reactors paint a rather unclear picture to which technology provides higher biological nitrogen removal performance. This study demonstrated that the S-SBR and MEDIA reactor achieved highest NRR with 0.72 and 0.68 kgN m−3 d−1 respectively (Fig. 4), making it the best fit for the application for mixed dewatering liquors.
190 mg L−1 for SBR and MBBR respectively.19 In a study by Leix et al. (2016)18 the MBBR achieved a lower NRR of 0.50 kgN m−3 d−1 compared to a SBR with 0.60 kgN m−3 d−1. Both reactors operated with a pre-nitritation reactor feeding higher nitrite to ammonia ratios to the SBR which was believed to have caused the higher performance of the reactor.18 However, when the two reactors plus the pre-nitritation step were evaluated as a single-stage process it was found that the MBBR achieved ammonia removal rates greater than the SBR with 0.39 and 0.33 kgN m−3 d−1 respectively. Another study, comparing a SBR with a biofilter for the anammox pathway, reported that the SBR had similar removal of 2.01 kgN m−3 d−1 compared to the biofilter 1.99 kgN m−3 d−1.17 The past studies comparing different deammonification reactors paint a rather unclear picture to which technology provides higher biological nitrogen removal performance. This study demonstrated that the S-SBR and MEDIA reactor achieved highest NRR with 0.72 and 0.68 kgN m−3 d−1 respectively (Fig. 4), making it the best fit for the application for mixed dewatering liquors.
Impact of seasonal temperature on S-SBR
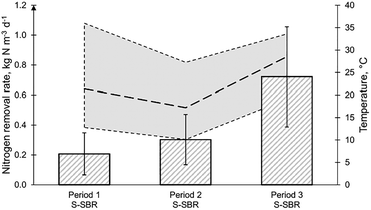
The temperature of the S-SBR changed due to normal seasonal variations. The NLR for period 1, period 2 and period 3 was 0.38, 0.60 and 1.01 kgN m−3 d−1 (Fig. 5). The ammonia concentration in period 1 was 238.9 mgN L−1, in period 2 was 219.8 mgN L−1 and in period 3 was 177.8 mgN L−1 (Table 3). It was reported that the operation at temperatures below 20 °C would result in nitrite accumulation.19,38 During period 2, the elevated nitrite concentration of 14.4 mgN L−1 and low temperature of 17 °C could indicate the suppression of anammox. However, it should be considered that the MLVSS concentration during this period was 2868 mg L−1 which was lower than period 1 and period 2 with 4160 and 3904 mg L−1, respectively. It is well acknowledged that the temperature optimum for AMX is between 30–35 °C and a decreasing temperature negatively impacts the activity (Lotti et al., 2015).38 In a study, investigating temperature impact on robustness of two deammonification reactors, the authors reported a complete loss of removal capacity in a suspended sludge SBR after the temperature was decreased below 20 °C.19 This effect could not be observed in this study, maintaining NRR of 0.30 kgN m−3 d−1 at temperatures of 17 °C (Fig. 5). In period 3 the NRR reached 0.72 kgN m−3 d−1 at a temperature of 27 °C. The nitrite concentration in the reactor in period 2 was 14.4 mgN L−1 but increased even further in period 3 to 18.3 mgN L−1 (Table 3). This could imply that the performance could be enhanced in the S-SBR by improving the conversion by AMX. With the AMX biomass in small flocs in suspension, biomass retention in the biological reactor was only achieved by gravity. It has been suggested that an external selector could enhance the removal efficiency of suspended sludge deammonification technologies by either using a hydrocyclone or a sieve.42 Overall, it was found that an NRR of 0.30 kgN m−3 d−1 could be maintained at an average temperature of 17 °C, implying that the deammonification technologies are robust enough to cope with sudden heat-loss or seasonal temperature fluctuations.Conclusion
Three sidestream deammonification technologies (S-SBR, MEDIA and G-SBR) were tested for their ability to remove ammonia from mixed dewatering liquors. The different technologies were evaluated based on the disruptions caused by imbalances in the two biological reactions, leading to poor effluent quality. The S-SBR had the lowest number of disruptions with 14 occasions that led to imbalances in the biological reactions. The G-SBR had the highest number disruptions with 92 occasions relating to inhibition caused by FA and FNA. It was identified that the process control focusing on pH, ammonia, nitrite and nitrate is essential for stable operation of deammonification technologies and to treat mixed dewatering liquors. In a performance comparison of similar NLR periods, it was found that there was no difference in NRR between S-SBR and MEDIA unlike previously reported in the literature. The S-SBR and MEDIA reactor achieved the highest NRR in this study with 0.72 and 0.68 kgN m−3 d−1, respectively. The results of this study imply that the evaluation of the process control should be given more weight than the reactor selection. Furthermore, it was found that during a seasonal temperature change in the S-SBR an average NRR of 0.30 kgN m−3 d−1 could be maintained at temperatures of 10–25 °C. The results of the study provide support in selecting sidestream deammonification technologies for mixed dewatering liquors by evaluating robustness and performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the funding provided by Thames Water Utilities Ltd. and the Engineering and Physical Sciences Research Council (EPSRC) through their funding of the STREAM Industrial Doctoral Centre (IDC) EP/L015412/1. We would like to express our thanks to the Laboratory of Thames Water Utilities Ltd. for analysing the composite samples. We would also like to thank Adrian Steele and Steve Perry for their support with the pilot plants.Notes and references
- S. Lackner, E. M. Gilbert, S. E. Vlaeminck, A. Joss, H. Horn, M. C. M. van Loosdrecht, C. M. Van Loosdrecht and M. C. M. van Loosdrecht, Water Res., 2014, 55, 292–303 CrossRef CAS.
- T. Lotti, R. Kleerebezem, C. Lubello and M. C. M. van Loosdrecht, Water Res., 2014, 60, 1–14 CrossRef CAS.
- M. Strous, E. Van Gerven, P. Zheng, J. G. Kuenen and M. S. M. Jetten, Water Res., 1997, 31, 1955–1962 CrossRef CAS.
- H. Ødegaard, A road-map for energy-neutral wastewater treatment plants of the future based on compact technologies (including MBBR), Front. Environ. Sci. Eng., 2016, 10(4) DOI:10.1007/s11783-016-0835-0.
- W. P. F. Barber, Water Res., 2016, 104, 53–71 CrossRef CAS.
- W. R. L. van der Star, W. R. Abma, D. Blommers, J. W. Mulder, T. Tokutomi, M. Strous, C. Picioreanu and M. C. M. van Loosdrecht, Water Res., 2007, 41, 4149–4163 CrossRef CAS.
- H. Bozkurt, M. C. M. van Loosdrecht, K. V. Gernaey and G. Sin, Chem. Eng. J., 2016, 286, 447–458 CrossRef CAS.
- J. R. Vázquez-Padín, N. Morales, R. Gutiérrez, R. Fernández, F. Rogalla, J. P. Barrio, J. L. Campos, A. Mosquera-Corral and R. Méndez, Water Sci. Technol., 2014, 69, 1151–1158 CrossRef.
- B. Wett, Water Sci. Technol., 2007, 56, 81–88 CrossRef CAS.
- I. Mouilleron, K. Hyde, K. Reid, A. Keegan, S. Rinck-Pfeiffer, J. Krampe and B. van den Akker, Water, 2014, 1–5 Search PubMed.
- F. Veuillet, S. Lacroix, A. Bausseron, E. Gonidec, J. Ochoa, M. Christensson and R. Lemaire, Water Sci. Technol., 2014, 69, 915–922 CrossRef CAS.
- K. H. Rosenwinkel and A. Cornelius, Chem. Eng. Technol., 2005, 28, 49–52 CrossRef CAS.
- M. Christensson, S. Ekström, A. A. Chan, E. Le Vaillant and R. Lemaire, Water Sci. Technol., 2013, 67, 2677–2684 CrossRef CAS.
- W. Driessen, G. Reitsma and T. Hülsen, in 16th European Biosolids and Organic Resources Conference, Aquaenviro, Manchester, 2011, pp. 1–10 Search PubMed.
- J. R. Vázquez-Padín, M. Figueroa, I. Fernández, A. Mosquera-Corral, J. L. Campos and R. Méndez, Water Sci. Technol., 2009, 60, 1135–1143 CrossRef.
- G. T. Daigger, Water Environ. Res., 2014, 86, 204–209 CrossRef CAS.
- R. C. Jin, P. Zheng, A. H. Hu, Q. Mahmood, B. L. Hu and G. Jilani, Chem. Eng. J., 2008, 138, 224–230 CrossRef CAS.
- C. Leix, R. Hartl, C. Zeh, F. Beer, J. E. Drewes and K. Koch, Water, 2016, 8, 578, DOI:10.3390/w8120578.
- S. Lackner and H. Horn, Environ. Technol., 2013, 34, 1319–1328 CrossRef CAS.
- P. Winter, B. Ng, S. R. Smith, G. Strange, S. Jarvis, F. Macedo and X. Shen, in IWA Specialist Conference On SLudge Management 17, IWA Publishing, 2017, pp. 1–17 Search PubMed.
- F. Simoes, R. Colston, C. Rosa-fernandes, P. Vale, T. Stephenson and A. Soares, Environmental Science and Ecotechnology, 2020, 3, 100052, DOI:10.1016/j.ese.2020.100052.
- M. Christensson, S. Ekström, R. Lemaire, E. Le Vaillant, E. Bundgaard, J. Chauzy, L. Stålhandske, Z. Hong and M. Ekenberg, Proc. Water Environ. Fed., 2012, 2011, 265–282 CrossRef.
- APHA, Standard methods for the examination of water and wastewater, American Public Health Association, Washington D.C., 21st edn, 2012 Search PubMed.
- A. C. Anthonisen, E. G. Srinath, R. C. Loehr and T. B. S. Prakasam, J. - Water Pollut. Control Fed., 1976, 48, 835–852 CAS.
- H. Chen, S. Liu, F. Yang, Y. Xue and T. Wang, Bioresour. Technol., 2009, 100, 1548–1554 CrossRef CAS.
- V. M. Vadivelu, J. Keller and Z. Yuan, Water Sci. Technol., 2007, 56, 89–97 CrossRef CAS.
- A. Dapena-Mora, I. Fernández, J. L. Campos, A. Mosquera-Corral, R. Méndez and M. S. M. Jetten, Enzyme Microb. Technol., 2007, 40, 859–865 CrossRef CAS.
- C. J. Tang, P. Zheng, Q. Mahmood and J. W. Chen, J. Cent. South Univ. Technol., 2010, 17, 444–449 Search PubMed.
- R. C. Jin, G. F. Yang, J. J. Yu and P. Zheng, Chem. Eng. J., 2012, 197, 67–79 CrossRef CAS.
- P. C. Kadam and D. R. Boone, Appl. Environ. Microbiol., 1996, 62, 4486–4492 CrossRef CAS.
- I. Fernández, J. Dosta, C. Fajardo, J. L. Campos, A. Mosquera-Corral and R. Méndez, J. Environ. Manage., 2012, 95, S170–S174 CrossRef.
- T. Lotti, W. R. L. Van Der Star, R. Kleerebezem, C. Lubello and M. C. M. van Loosdrecht, Water Res., 2012, 46, 2559–2569 CrossRef CAS.
- A. Joss, N. Derlon, C. Cyprien, S. Burger, I. Szivak, J. Traber, H. Siegrist and E. Morgenroth, Environ. Sci. Technol., 2011, 45, 9735–9742 CrossRef CAS.
- B. Figdore, B. Wett, M. Hell and S. Murthy, Proc. Water Environ. Fed., 2012, 1037–1052 Search PubMed.
- J. Desloover, H. De Clippeleir, P. Boeckx, G. Du Laing, J. Colsen, W. Verstraete and S. E. Vlaeminck, Water Res., 2011, 45, 2811–2821 CrossRef CAS.
- N. Morales, A. Val Del Río, J. R. Vázquez-Padín, R. Gutiérrez, R. Fernández-González, P. Icaran, F. Rogalla, J. L. Campos, R. Méndez and A. Mosquera-Corral, Water Sci. Technol., 2015, 72, 520–527 CrossRef CAS.
- E. I. P. P. Volcke, C. Picioreanu, B. De Baets and M. C. M. M. Van Loosdrecht, Environ. Technol., 2010, 31, 1271–1280 CrossRef CAS.
- T. Lotti, R. Kleerebezem and M. C. M. van Loosdrecht, Biotechnol. Bioeng., 2015, 112, 98–103 CrossRef CAS.
- B. Wett, M. Hell, G. Nyhuis, T. Puempel, I. Takacs and S. Murthy, Water Sci. Technol., 2010, 61, 1915–1922 CrossRef CAS.
- M. C. M. van Loosdrecht, L. Tijhuis, A. M. S. Wijdieks and J. J. Heijnen, Water Sci. Technol., 1995, 31, 163–171 CrossRef CAS.
- T. Kindaichi, T. Ito and S. Okabe, Appl. Environ. Microbiol., 2004, 70, 1641–1650 CrossRef CAS.
- T. Van Winckel, S. E. Vlaeminck, A. Al-Omari, B. Bachmann, B. Sturm, B. Wett, I. Takács, C. Bott, S. N. Murthy and H. De Clippeleir, Environ. Sci.: Water Res. Technol., 2019, 5, 1769–1781 RSC.
| This journal is © The Royal Society of Chemistry 2020 |