Open Access Article
Open Access ArticleChemical regeneration of granular activated carbon: preliminary evaluation of alternative regenerant solutions†
Amanda
Larasati
 *,
Geoffrey D.
Fowler
and
Nigel J. D.
Graham
*,
Geoffrey D.
Fowler
and
Nigel J. D.
Graham
Department of Civil and Environmental Engineering, Imperial College London, South Kensington Campus, London, SW7 2AZ, UK. E-mail: a.larasati16@imperial.ac.uk
First published on 15th June 2020
Abstract
Granular activated carbon (GAC) is used in drinking water treatment plants worldwide to remove micro-pollutants such as pesticides. Early breakthrough of problematic micro-pollutants leads to frequent and costly thermal regeneration off-site. A potential alternative approach is to chemically regenerate GAC on-site (possibly in situ) with an appropriate solution capable of desorbing organic contaminants, having a range of physico-chemical properties. In this study, four types of regenerant solution were evaluated in batch tests for their ability to desorb five target contaminants. The solutions were: high purity water, sodium hydroxide, ethanol, and a mixture of sodium hydroxide and ethanol. The contaminants included: phenol and nitrobenzene, as representative aromatic compounds; clopyralid and metaldehyde, as poorly-adsorbed pesticides; and isoproturon, a well-adsorbed pesticide. Among the properties of the contaminants, their hydrophobicity and aqueous solubility had the most significant influence on the desorption efficiency. NaOH/CH3CH2OH was found to be more effective than individual solutions in desorbing the target contaminants, indicating an ability to desorb both hydrophobic and hydrophilic compounds. The NaOH/CH3CH2OH regenerant solution yielded desorption efficiencies in the range of approximately 40–90%, with the efficiency dependent on the contaminant. A thermodynamic study provided valuable fundamental information regarding the adsorption and desorption mechanisms, and the existence of two binding sites involving a weak physisorption and a stronger chemisorption-like interaction between the contaminants and the GAC.
Water impactThe chemical regeneration of granular activated carbon (GAC) beds has been studied as a more cost-effective and flexible alternative to thermal regeneration. Chemical regeneration, using a novel regenerant solution, offers a low-energy and resource efficient process for the removal of poorly adsorbed organic micro-pollutants (e.g. pesticides), and to enhance the reliability of GAC operations in drinking water treatment practice. |
1. Introduction
Granular activated carbon (GAC) adsorption has found wide application as a treatment process for the removal of natural organic matter, pesticides, taste and odour in water over many years.1 During operation, these contaminants are adsorbed within the pores of GAC, which gradually become saturated with time. At saturation, or earlier if the filtrate quality is unacceptable, the GAC operation can no longer continue, and regeneration is carried out; this is a more cost-effective option to lengthen the carbon life rather than disposal and carbon replacement.2The presence of problematic, weakly adsorbable pesticides, such as metaldehyde3 and clopyralid4 (in the U.K.), causes early GAC bed exhaustion for these compounds, requiring more frequent GAC regeneration. The most common regeneration technique practised is thermal regeneration.1 This technique has a disadvantage that the spent GAC commonly needs to be transported off-site to a specialized facility to perform it. In addition, the more frequent regeneration increases operational costs due to high carbon usage and reduced carbon service. During thermal regeneration, usually 5–10% of the carbon is lost due to attrition and excessive burn-off,5 and the lost carbon must be made up with virgin carbon. For these reasons, several in situ or on-site regeneration studies have been reported in the last 30 years, as alternatives to thermal regeneration. One of these alternatives is chemical regeneration, which is a technique that can be performed in situ by exposing the spent GAC to chemical solvents to remove the contaminants,6,7 where the efficiency of chemical regeneration depends on the solvent used and the contaminants adsorbed.
Prior research on chemical regeneration has been limited in extent, but has considered both organic and inorganic solutions. For the former, organic solutions with solubilizing power and a lower molecular weight than the adsorbed contaminants were found to be the most effective regenerant solutions.8–10 A number of studies7,9,11 showed that alcohols such as ethanol and methanol were able to desorb phenol to a high degree. It was found that methanol was advantageous as a regenerant solution for activated carbon loaded by phenol,12 as it had a high regeneration efficiency, could be recovered and is easily rinsed by water. However, methanol carries health concerns, owing to the formation of formaldehyde if digested, which represents a major disadvantage to its application in drinking water treatment. As an alternative, ethanol had a similar performance to methanol as a regenerant7,13 and has much lower potential toxicity than methanol.
With regard to inorganic regenerants, several studies have considered acid and basic solutions to achieve a high regeneration efficiency for the carbon exhausted by aromatic compounds, such as aniline and benzoic acid. In these cases, the change of pH of the solution may affect the surface charge of the adsorbent, and chemical reactions between adsorbate contaminants and regenerant solutions may occur which later facilitate desorption.9,14 However, the changes to the GAC surface properties by the acid or base may affect the adsorption of the contaminant after the regeneration process, which may be beneficial or detrimental to contaminant removal. An advantage of using acid and/or basic solutions as a solvent is that these chemicals are commonly used in water treatment for pH correction or softening, and may only present minimum practical difficulties, including the possibility of changing or coating the materials used in the current tanks and pipe works. Among the various potential inorganic chemicals, sodium hydroxide was found to be a relatively effective regenerant solution.15,16
The effect of water at high temperatures was previously reported to be efficient in regenerating carbon exhausted by PFOS17 and other aromatic compounds.9 The high regeneration efficiency was attributed to the effect of elevated temperature on the thermodynamic equilibrium during the adsorption process.18 Not only does an increased temperature enhance the desorption process, it also increases the solubility of organic compounds in water9,18 by increasing their molecular kinetic energy. High temperature also reduces solution viscosity and consequently increases the diffusion rate of an adsorbate through the porous structure of the carbon during the desorption process.19
Despite a significant number of studies previously concerning chemical regeneration, the most suitable regenerant solutions for desorbing a wide range of aqueous contaminants have not been identified to-date. In particular, the occurrence of weakly adsorbable pesticides in raw waters presents different challenges in determining the most suitable solution for regeneration. The adsorption and desorption mechanisms for these pesticides with GAC also have not been investigated systematically. Some earlier studies9,14 have reported regeneration efficiencies but without mentioning the desorption ratio achieved during chemical regeneration. This makes it unclear whether a higher degree of regeneration efficiency achieved was because of the desorption of contaminants, or the result of changes in the GAC properties which increased the adsorbate uptake in the subsequent adsorption phase. In this paper, we summarize an extensive study of the adsorption and desorption behaviour of particular target contaminants, and their desorption performance using different regenerant solutions. Calorimetric analyses have been conducted to provide important information relating to the adsorption mechanisms of the target contaminants on GAC.
2. Materials and methods
2.1. Materials and reagents
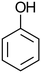
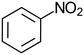
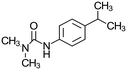
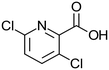
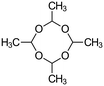
Virgin Filtrasorb 400 (Chemviron Carbon) GAC, with a granular size of 12 × 40 mesh (0.42–1.68 mm), effective size of 0.55–0.7 mm and maximum uniformity coefficient of 1.9,20 was used throughout the tests; Filtrasorb 400 is a commercial carbon that is widely used in drinking water treatment works in the UK. Prior to use, a conditioning step was conducted, as follows: the carbon was washed using laboratory-grade reverse osmosis (RO) water until its pH was stable, dried at 105 °C to constant weight, and stored in a desiccator.The target contaminants used in the tests were phenol (Fisher Scientific) and nitrobenzene (Honeywell) as representative aromatic compounds, and isoproturon (Sigma-Aldrich), clopyralid (Sigma-Aldrich) and metaldehyde (Acros Organics), which are pesticides that are commonly found in UK raw waters, where clopyralid and metaldehyde are often insufficiently removed by GAC (Table 1).
| Properties | Phenol | Nitrobenzene | Isoproturon | Clopyralid | Metaldehyde |
|---|---|---|---|---|---|
| a Notes: values reported by PubChem21 and CRC Handbook of Chemistry and Physics.22 NA = not applicable. | |||||
| Chemical structure |
|
|
|
|
|
| Molecular mass (g mol−1) | 94.1 | 123.1 | 206.3 | 191.9 | 176.2 |
| Solubility in water at 20 °C (g L−1) | 80 | 1.90 | 0.07 | 7.85 | 0.22 |
Hydrophobicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kow) kow) |
1.46 | 1.85 | 2.50 | 1.06 | 0.12 |
| Acid dissociation constant (pKa) | 9.99 (T = 25 °C) | 3.98 (T = 0 °C) | NA | 2.32 (T = 25 °C) | NA |
2.2. Target contaminant adsorption and desorption batch tests
The adsorption and desorption tests of the target contaminants were carried out in a batch system, as described in the following sections. Prior to the adsorption and desorption tests, the physico-chemical properties of the GAC used were investigated. The measurement of pH at the point of zero charge (pHpzc) of the carbon followed the procedures conducted in previous study.23 The surface area of carbon can be determined from nitrogen (N2) adsorption–desorption isotherm data. The N2 adsorption and desorption isotherms24 were measured using an automatic Quantachrome Autosorb iQ3 instrument. A standardized Boehm titration was performed to identify the surface functional groups of the carbon. The standardized procedures of the test followed those reported previously.25,26The pseudo-first order kinetic model proposed by Lagergren30 assumes that the rate of adsorption is proportional to the difference between the adsorbed quantity of the contaminant at equilibrium and the quantity adsorbed at a given time, as follows:
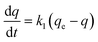 | (1) |
| q = qe(1 − e−k1t) | (2) |
The pseudo-second order kinetic model, as proposed by Ho and Mckay,31 assumes that the rate of adsorption is proportional to the square of the difference between the adsorbed quantity of the contaminant at equilibrium and the quantity adsorbed at a given time, and the model is expressed as:
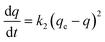 | (3) |
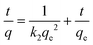 | (4) |
Adsorption isotherm tests were carried out based on a standard method.32 In summary, a varied amount of GAC F400 (0.01–1 g) and 100 mL of a target contaminant solution were transferred in to a borosilicate glass bottle and agitated on a platform shaker at 120 rpm for a predetermined time to reach equilibrium at room temperature (20 ± 1 °C). The solutions of target contaminant were prepared at initial concentrations of 2000, 400, 50, 100 and 100 mg L−1 for phenol, nitrobenzene, isoproturon, clopyralid and metaldehyde at pH 7.0 ± 0.1, respectively. The pH of the solutions was adjusted using either 0.01 M NaOH or 0.01 M HCl prior to adsorption tests. The target contaminant concentrations in the solution before and after adsorption were determined using the analytical methods described in section 2.3. The contaminant uptake per unit mass of carbon, q (mg g−1), was calculated by:
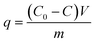 | (5) |
Both Langmuir and Freundlich isotherm models were fitted to the experimental data. These models are widely used to describe the adsorption equilibrium between an organic adsorbate and activated carbon.33,34 In the Langmuir isotherm model, the adsorbate is assumed to form a monolayer on the adsorbent surface.35 The Langmuir model is expressed as:
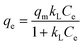 | (6) |
| qe = kFCe1/n | (7) |
Upon completion of the adsorption tests, 100 mL of a selected regenerant solution was added to the bottle, which contained the exhausted GAC, and agitated for a predetermined time to enable desorption. The solution was then decanted and filtered, and the pH adjusted to lie in the range of 6–8 using 0.1 M NaOH or 0.1 M H3PO4, prior to the detection and quantification of the desorbed contaminant. The desorption tests were conducted using four regenerant solutions, namely, RO water at three different temperatures of 20, 50 and 80 °C, sodium hydroxide (NaOH), ethanol (CH3CH2OH) and a mixture of sodium hydroxide and ethanol (NaOH/CH3CH2OH).
The efficiency of the chemical regeneration can be quantified based on the ability of a regenerant solution to recover the adsorbed contaminant, and calculated as desorption efficiency (DE), as follows:
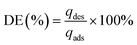 | (8) |
2.3. Detection and quantification of target contaminants
The concentrations of phenol, nitrobenzene, isoproturon and clopyralid were determined by UV-visible spectrophotometry (Shimadzu UV-2401PC UV-vis spectrophotometer), and metaldehyde concentrations were determined by ultra-high performance/pressure liquid chromatography (UHPLC) with quadruple-time of flight (Q-TOF) spectrometry (Waters Synapt G2-Si high definition mass spectrometry) using electrospray ionization (ESI) technique. The optimal detection wavelengths used for phenol, nitrobenzene, isoproturon and clopyralid analysis were 269, 268, 240 and 280 nm, respectively.Metaldehyde was detected and quantified in the ESI positive mode. An Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm particle size, Waters Ltd) was used and maintained at 40 °C for the analysis. The mobile phases used were A: LC-MS grade water and 0.1% of formic acid (VWR) and B: LC-MS grade acetonitrile (Honeywell). The gradient program was: 0–0.5 min isocratic of 30% B (v/v), 0.5–4 min linear from 30–80% B (v/v), 4–5 min isocratic of 80% B (v/v), 5–7 min linear from 80–30% B (v/v) and 7–10 isocratic of 30% B (v/v). Total analysis time was 10 min, the flow rate was 0.2 mL min−1 and the injection volume was 5 μl. Solid phase extraction (SPE) was carried out as a sample preparation using pre-made SPE cartridge (Waters Sep-Pak C18, 55–105 μm particle size) prior to injection. The instrument was set to detect metaldehyde ions of 199 m/z in MS/MS mode with a retention time of 2.45 min.
2.4. Investigation of reaction products during chemical regeneration
Possible reaction products arising from the regenerant solution and target contaminants were investigated by mass spectrometry, where identification was based on the structural information obtained from fragmentation and parent compound data.38 A direct analysis using an atmospheric pressure solid analysis probe (ASAP) technique using Q-TOF spectrometry (Waters Synapt G2-Si high definition mass spectrometry) was carried out for a rapid identification of products from phenol, nitrobenzene, isoproturon and clopyralid, while the ESI technique was applied for metaldehyde, as metaldehyde is easily degraded at the high temperature required to perform ASAP technique.The mass spectrometry parameter settings were as follows: the source and desolvation temperature were set at 120 and 150 °C, respectively; the desolvation and cone gas flows were 400 and 30 L h−1, respectively; the corona current was set at 5 μA, and the sampling cone voltage to 35 V. The instrument was operated in positive mode. Prior to identification, the capillary glass sample holder was rinsed using LC-MS grade methanol (VWR), and baked at 400 °C for 3 min.
2.5. Heat of adsorption using a calorimetric titration method
Calorimetric tests were carried out using a VP isothermal titration calorimeter (ITC) from Microcal, Inc. The heat exchange between the carbon and target contaminant was measured at 298.15 K (25 °C) to determine the thermodynamic parameters during the adsorption process. The ITC has been used widely to investigate interactions between molecules as it has the ability to accurately measure the Gibbs energy, enthalpy and entropy associated with the interactions.39,40 The target contaminants investigated were phenol, isoproturon and metaldehyde, as described subsequently.A well-dispersed suspension of F400 carbon in water (immersed for >24 h) at a concentration of 2 g L−1, and target contaminant solutions at a concentration of 0.25 mM, were thoroughly degassed for 5 min prior to each test to remove air bubbles from the samples. The suspension was placed in the reaction cell with a volume of 1.4 mL and a target contaminant was loaded into the 300 μl injection syringe. The titration was carried out with 1 injection of 4 μl, followed by 24 injections of 10 μl aliquots, at 180–300 s intervals between two injections, with a stirring speed of 300 rpm. The test procedure followed that used in previous studies of metal ions and methylene blue adsorption on carbon materials.41–43
The ITC data were automatically analysed using the Microcal Origin 7.0 software. All the data were blank corrected for the heat of dilution of target contaminant and water, and the heat of carbon and water interactions. The thermodynamic parameters, such as Ka (binding constant) and ΔadsH (enthalpy or heat of adsorption) were predicted as adjustable parameters in the fitting procedure of the Origin 7.0 software. Data were fitted using a non-linear least-squares (Levenberg–Marquardt) algorithm. The adsorption ΔadsG (Gibbs free energy) and ΔadsS (entropy) were calculated using the following thermodynamic relationship, where T is the temperature of the measurement (K).
ΔadsG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = ΔadsH − TΔadsS Ka = ΔadsH − TΔadsS | (9) |
3. Results and discussion
The physico-chemical properties of the GAC F400 used throughout the tests are summarized in Table 2.| Properties | Value |
|---|---|
| pHpzc | 8.2 |
| Brunauer–Emmett–Teller (BET) surface area (m2 g−1) | 1222.6 ± 34.4 |
| Average pore width (nm) | 0.48 ± 0.01 |
| Surface chemistry: oxygen-containing functional groups (mmol g−1) | |
| Phenol | 0.119 ± 0.051 |
| Lactonic | 0.028 ± 0.023 |
| Carboxylic | 0.065 ± 0.027 |
| Carbonyl | 0.237 ± 0.028 |
| Total acid | 0.449 ± 0.018 |
| Total basic | 0.412 ± 0.029 |
3.1. Adsorption kinetics of target contaminants
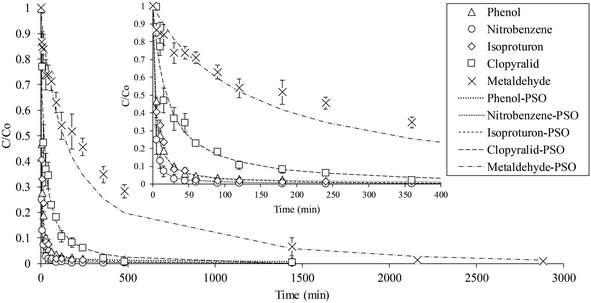
The results showed that the adsorption of the contaminants reached equilibrium after approximately 24 h (1440 min), except for metaldehyde, which required at least 48 h (2880 min). As can be seen in Fig. 1, the initial adsorption of all compounds was rapid, and for phenol, nitrobenzene and isoproturon, the adsorption occurred mostly in the first 60 min, owing to the strong affinity between the GAC and these contaminants. In all cases, the results were well described by a pseudo-second order kinetic model (R2 = 0.99–1.00), in comparison to a pseudo-first order model (Table 3 and Fig. 1), and indicated that the adsorption rate was related to the compound hydrophobicity and solubility. Thus, for isoproturon, clopyralid and metaldehyde, at the same initial concentration of 20 mg L−1, the calculated adsorption rate (k2) and adsorption capacity (qe) decreased in the order of isoproturon > clopyralid > metaldehyde, which corresponded directly to their decreasing hydrophobicity as indicated by their octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kow) (Table 1). A similar relationship between log
kow) (Table 1). A similar relationship between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kow and organic contaminant adsorption by carbon was also reported previously,36 and the low adsorption capacity of metaldehyde by activated carbon also reported in a number of previous studies.28,44,45
kow and organic contaminant adsorption by carbon was also reported previously,36 and the low adsorption capacity of metaldehyde by activated carbon also reported in a number of previous studies.28,44,45
| Target contaminant | C 0 (mg L−1) | Pseudo-first order (PFO) | Pseudo-second order (PSO) | ||||
|---|---|---|---|---|---|---|---|
| q e (mg g−1) | k 1 (min−1) | R 2 | q e (mg g−1) | k 2 (g mg−1 min−1) | R 2 | ||
| Phenol | 2000 | 3.3 | 0.010 | 0.71 | 101.0 | 0.003 | 1.00 |
| Nitrobenzene | 400 | 1.8 | 0.016 | 0.68 | 40.2 | 0.022 | 1.00 |
| Isoproturon | 20 | 0.6 | 0.011 | 0.71 | 2.2 | 0.147 | 1.00 |
| Clopyralid | 20 | 1.2 | 0.012 | 0.96 | 2.2 | 0.026 | 0.99 |
| Metaldehyde | 20 | 1.2 | 0.002 | 0.98 | 2.1 | 0.003 | 0.99 |
3.2. Adsorption isotherms of target contaminants
The adsorption equilibrium results for the target contaminants are shown in Fig. 2, and the corresponding model parameters are summarized in Table 4. Comparison with the experimental data indicated that the Langmuir model described the adsorption of the contaminants more closely, based on the correlation coefficient (R2) values. From the Langmuir model, a GAC monolayer uptake capacity of the target contaminants was derived, and the results indicated that the nitrobenzene and isoproturon had a higher qm value than phenol, clopyralid and metaldehyde. In the Freundlich model, the constant, kF, explains the favourability of the adsorption process of contaminants. As shown in Table 4, the kF values of the target contaminants show a similar trend to the qm values of the Langmuir model. The greater uptake of nitrobenzene and isoproturon by the carbon is attributed to their relatively greater hydrophobic nature, as indicated by their higher octanol–water partition coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kow), compared to the other contaminants.
kow), compared to the other contaminants.
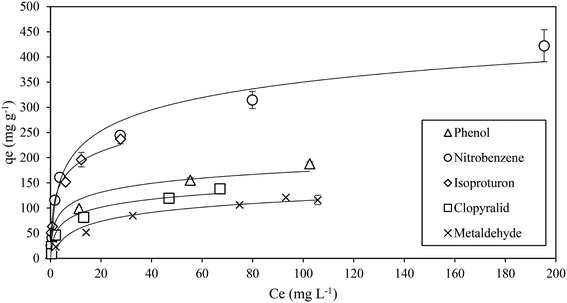 | ||
| Fig. 2 Comparison of experimental adsorption isotherm data among the target contaminants (error bars represent one standard deviation for duplicate measurements). | ||
| Target contaminant | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| q m (mg g−1) | k L (L mg−1) | R 2 | k F (mg g−1 (L mg−1)1/n) | n | R 2 | |
| Phenol | 192.3 | 0.16 | 0.99 | 33.5 | 2.49 | 0.94 |
| Nitrobenzene | 434.8 | 0.08 | 0.98 | 74.2 | 2.87 | 0.89 |
| Isoproturon | 250.0 | 0.51 | 0.99 | 69.5 | 2.50 | 0.97 |
| Clopyralid | 142.9 | 0.18 | 0.99 | 24.7 | 2.32 | 0.89 |
| Metaldehyde | 136.9 | 0.01 | 0.99 | 17.2 | 2.35 | 0.99 |
The qm value of 192 mg g−1 for phenol was similar to that reported previously.46 Nitrobenzene was found to have the highest qm value (435 mg g−1) of all the target contaminants, most likely because of the combination of a high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kow, low aqueous solubility and low molecular weight; the qm was slightly lower to that reported previously.47 For isoproturon, qm was 250 mg g−1, which was slightly lower than the value reported in an earlier study48 where the adsorption test was conducted at 30 °C. The adsorption performance of clopyralid has received little attention previously in scientific terms; however, like metaldehyde, a significant number of water quality compliance failures have been reported in the UK for clopyralid,4 which indicates a relatively low adsorption by activated carbon. In agreement with this, the GAC uptake of clopyralid in this study was relatively low (qm = 143 mg g−1), and similar to the value of qe of ∼150 mg g−1 (for Ce = 50 mg L−1) reported in a recent study.49 Lastly, for metaldehyde, the qm value of 137 mg g−1 found in this study was lower than the value of 320 mg g−1 reported by Salvestrini et al.,50 but greater than the values of 71 mg g−1 and 28.3 mg g−1 reported by Tao and Fletcher28 and Li et al.,45 respectively. These differences may be explained by differences in the properties of the GAC used in the studies, such as the carbon pore size distribution, particle size and surface chemistry. Busquets et al.44 previously reported that there was no correlation between the carbon surface area and metaldehyde adsorption, which suggested that the adsorption was governed by more complex effects than simply physisorption. However, isotherm data on their own are not sufficient to elucidate the adsorption mechanisms, and need to be complemented by additional tests, such as desorption and thermodynamics;37 these were carried out in this study and the results are summarized in subsequent sections of this paper.
kow, low aqueous solubility and low molecular weight; the qm was slightly lower to that reported previously.47 For isoproturon, qm was 250 mg g−1, which was slightly lower than the value reported in an earlier study48 where the adsorption test was conducted at 30 °C. The adsorption performance of clopyralid has received little attention previously in scientific terms; however, like metaldehyde, a significant number of water quality compliance failures have been reported in the UK for clopyralid,4 which indicates a relatively low adsorption by activated carbon. In agreement with this, the GAC uptake of clopyralid in this study was relatively low (qm = 143 mg g−1), and similar to the value of qe of ∼150 mg g−1 (for Ce = 50 mg L−1) reported in a recent study.49 Lastly, for metaldehyde, the qm value of 137 mg g−1 found in this study was lower than the value of 320 mg g−1 reported by Salvestrini et al.,50 but greater than the values of 71 mg g−1 and 28.3 mg g−1 reported by Tao and Fletcher28 and Li et al.,45 respectively. These differences may be explained by differences in the properties of the GAC used in the studies, such as the carbon pore size distribution, particle size and surface chemistry. Busquets et al.44 previously reported that there was no correlation between the carbon surface area and metaldehyde adsorption, which suggested that the adsorption was governed by more complex effects than simply physisorption. However, isotherm data on their own are not sufficient to elucidate the adsorption mechanisms, and need to be complemented by additional tests, such as desorption and thermodynamics;37 these were carried out in this study and the results are summarized in subsequent sections of this paper.
3.3. Desorption of single contaminants
The five target contaminants and four alternative regenerant solutions were used to investigate the performance of chemical regeneration, and the influence of pH, compound solubility and interactions between the contaminant, regenerant and activated carbon during the regeneration process. The maximum desorption efficiency (DE) using each regenerant solution was achieved within 120 min; details of the desorption kinetics are given in Fig. S1–S3, in ESI.†| Temperature (°C) | DE (%) for the carbon exhausted with target compound | ||||
|---|---|---|---|---|---|
| Phenol | Nitrobenzene | Isoproturon | Clopyralid | Metaldehyde | |
| a The DE values for duplicate measurements have a standard deviation less than 0.01%. | |||||
| 20 | 3.3–3.9 | 0.1a | 1.3–2.2 | 2.5–2.6 | 2.3–3.1 |
| 50 | 18.2–18.6 | 0.2a | 0.9–1.4 | 2.8–3.1 | 2.4–2.5 |
| 80 | 21.1–23.6 | 0.4a | 5.1–5.4 | 14.0–14.3 | 4.7–5.9 |
The low desorption efficiency by water of nitrobenzene and isoproturon was attributed to their limited water solubility and relatively high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kow. Clopyralid and metaldehyde, which were only weakly adsorbed by carbon, also had a low desorption efficiency, suggesting that the potential energy of adsorption was strong enough to prevent desorption. Although Tao and Fletcher28 reported a modest amount (26%) of metaldehyde leaching from an exhausted GAC column during a water washing process, no significant leaching was observed in this study. A low desorption (<9%) of metaldehyde using water at room temperature in a batch system was also reported by Li et al.53 A plausible explanation is that the tests in this study were conducted in a batch system where pore blockage may affect the desorption efficiency.54
kow. Clopyralid and metaldehyde, which were only weakly adsorbed by carbon, also had a low desorption efficiency, suggesting that the potential energy of adsorption was strong enough to prevent desorption. Although Tao and Fletcher28 reported a modest amount (26%) of metaldehyde leaching from an exhausted GAC column during a water washing process, no significant leaching was observed in this study. A low desorption (<9%) of metaldehyde using water at room temperature in a batch system was also reported by Li et al.53 A plausible explanation is that the tests in this study were conducted in a batch system where pore blockage may affect the desorption efficiency.54
Previously, it has been reported that boiling water performed effectively in desorbing phenol from exhausted carbon,19 as the high temperature increases phenol diffusivity in water. Similarly, in this study the results showed an increasing desorption efficiency for phenol with increasing water temperature, but this was still modest (<24%) even at 80 °C (Table 5). For metaldehyde, the desorption efficiency was less than 6% at 80 °C and little mineralization was observed, suggesting that high water temperature did not have a significant impact on the desorption or degradation of adsorbed metaldehyde. A recent study by Rolph et al.55 reported that metaldehyde degraded in water at temperatures >60 °C and the DEs yielded in this study suggested that metaldehyde was more stable when adsorbed to the carbon and not degraded at high temperature (80 °C). The low DE of the other target contaminants, nitrobenzene, isoproturon and clopyralid, in water at 80 °C (Table 5) demonstrated that these contaminants were strongly adsorbed to the carbon, which suggested that their solubility in water greatly affected the DE values.
Overall, the results indicated that water was unable to achieve sufficient desorption of the target contaminants from exhausted carbon, and may only be effective for desorbing highly water-soluble contaminants.
| Concentration (M) | DE (%) for the carbon exhausted with target compound | ||||
|---|---|---|---|---|---|
| Phenol | Nitrobenzene | Isoproturon | Clopyralid | Metaldehyde | |
| a The DE values for duplicate measurements have a standard deviation less than 0.01%. | |||||
| 0.1 | 46.8–48.6 | 0.1–0.2 | 4.2–4.2 | 3.2–3.6 | 4.2–4.8 |
| 0.5 | 47.4–51.7 | 0.1–0.2 | 2.6–4.0 | 3.1–3.9 | 3.0–3.4 |
| 1 | 50.7–54.5 | 0.2a | 3.2–5.3 | 4.2–4.3 | 3.2–6.7 |
| 2 | 42.5–49.1 | 0.3a | 4.1–5.3 | 4.8–5.3 | 2.9–4.8 |
For nitrobenzene, isoproturon, clopyralid and metaldehyde, the DE of the NaOH solutions was no greater than water at room temperature. Nitrobenzene is known to have a strong affinity for activated carbon (Fig. 2) and predominantly in its neutral form in the pH range of 2–14; an almost negligible DE of nitrobenzene with NaOH was also reported in a previous study.58 Isoproturon showed a similar affinity for activated carbon as nitrobenzene, and does not dissociate in water at any pH; increasing the NaOH concentration did not enhance the desorption of either nitrobenzene or isoproturon to any significant extent.
Clopyralid exists almost entirely in a dissociated, anionic form in neutral and high pH solutions and therefore a substantial desorption of clopyralid in high pH solution was anticipated, owing to electrostatic repulsion between the dissociated compound and the negatively charged carbon surface. However, desorption was found to be poor suggesting the importance of other factors. Nevertheless, the effect of charge interactions was indicated by the small increasing in DE with increasing NaOH concentration (Table 6).
The very low desorption efficiency for metaldehyde using NaOH was most likely because of the limited solubility of the compound in the regenerant solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kow > 1.4), namely, phenol, nitrobenzene and isoproturon, had a high DE, compared to clopyralid and metaldehyde, which had lower hydrophobicity (log
kow > 1.4), namely, phenol, nitrobenzene and isoproturon, had a high DE, compared to clopyralid and metaldehyde, which had lower hydrophobicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kow < 1.4) and a DE less than 15%. In addition to hydrophobicity, it was speculated that the DE using ethanol was related to the solubility of the target contaminant in ethanol. However, even though the solubility of clopyralid and metaldehyde in ethanol was greater than in water, this did not result in a significant DE, indicating that the two contaminants preferred to remain adsorbed on the activated carbon rather than solvate in ethanol.
kow < 1.4) and a DE less than 15%. In addition to hydrophobicity, it was speculated that the DE using ethanol was related to the solubility of the target contaminant in ethanol. However, even though the solubility of clopyralid and metaldehyde in ethanol was greater than in water, this did not result in a significant DE, indicating that the two contaminants preferred to remain adsorbed on the activated carbon rather than solvate in ethanol.
| Concentration (% v/v) | DE (%) for the carbon exhausted with target compound | ||||
|---|---|---|---|---|---|
| Phenol | Nitrobenzene | Isoproturon | Clopyralid | Metaldehyde | |
| a The DE values for duplicate measurements have a standard deviation less than 0.01%. | |||||
| 99 | 62.1–62.6 | 43.6–44.6 | 67.9a | 8.8–11.1 | 9.5–14.6 |
| 80 | 58.9–60.1 | 35.0–37.1 | 39.0–39.4 | 3.7–3.8 | 6.8–9.9 |
As expected, the DE values for the compounds decreased with the concentration of ethanol in water (Table 7). As ethanol is highly soluble in water, the presence of water adversely affected the ability of ethanol to desorb the target contaminants9 as the water molecules will form a hydration shell around ethanol, making it too large to enter the carbon pores.
| Concentration (M of NaOH and % v/v of CH3CH2OH) | DE (%) for the carbon exhausted with target compound | ||||
|---|---|---|---|---|---|
| Phenol | Nitrobenzene | Isoproturon | Clopyralid | Metaldehyde | |
| a The DE values for duplicate measurements have a standard deviation less than 0.01%. | |||||
| 0.1 in 99 | 84.0–88.2 | 35.4–46.8 | 44.6–45.0 | 75.9–79.4 | 50.0–53.4 |
| 0.1 in 80 | 82.1–89.0 | 34.3–35.5 | 35.2a | 73.8–77.1 | 60.0–68.6 |
| 0.5 in 80 | 78.6–82.8 | 31.4–32.1 | 37.6–37.7 | 80.5–83.0 | 77.6–82.4 |
When NaOH is dissolved in ethanol, both hydroxide and ethoxide ions are present in solution.59 Ethoxide has been shown to be able to neutralize acidic functional groups on carbon surfaces using the Boehm titration method, which is employed widely to identify the surface chemistry of any carbon materials.60,61 As the adsorption of organic contaminants on GAC can be through interactions with the surface functional groups, the presence of ethoxide may disturb these interactions and facilitate contaminant desorption. As shown previously, hydroxide alone also has an ability to interact with the surface functional groups on the carbon,62 but ethoxide is a stronger base and more aggressive than hydroxide as atoms in the alkane chain donate electron density to oxygen, allowing ethoxide to be more polarizable. The aggressive nature of ethoxide thus resulted in the greater DE of clopyralid and metaldehyde observed with NaOH/CH3CH2OH compared to only NaOH. Additionally, the use of ethanol as a co-solvent is more advantageous as the contaminants are more soluble in ethanol than in water. It is well known that organic contaminants adsorb through different mechanisms on the carbon, such as through donor acceptor complex interactions,63,64 where carbonyl groups on the surface act as an electron donor and the contaminant as an electron acceptor. Ethoxide, which has a high nucleophilic property, is strong enough to neutralise the carbonyl groups on the carbon surface through hemiacetal reactions65,66 and thereby promote the desorption of the adsorbed contaminants.
The results for the NaOH/CH3CH2OH combination are very promising in terms of chemical desorption of adsorbed contaminants and recovery of surface sites for subsequent adsorption, thereby increasing the duration of the GAC bed life prior to full thermal regeneration or carbon disposal.
3.4. Fate of the contaminants
A possible reaction between the NaOH/CH3CH2OH mixture and adsorbed contaminants was investigated using mass spectrometry to further clarify the desorption mechanisms involved. It was found that only phenol transformed upon exposure to a mixture of NaOH/CH3CH2OH (results are given in Table S1, in ESI†). In this case phenolate ion was formed as phenol has a pKa value of 9.99 as shown in Table 1, supporting the previous notion that phenol desorbed from the carbon surface because of the formation of a water-soluble phenolate ion. The absence of any detectible transformation or chemical reaction for the other target contaminants suggested that the desorption of these contaminants was affected by their solubility in the mixture, and/or a reduced affinity for the carbon surface as a result of changes to the properties of the carbon caused by the mixture. A similar finding was observed with aromatic contaminants, such as nitrobenzene, which is strongly adsorbed to the carbon, but where its desorption was driven by the decrease of carbon affinity.143.5. Heat of adsorption
In order to provide more information regarding the mechanisms of adsorption and desorption of the contaminants, a calorimetric study was conducted involving three of the contaminants: phenol to represent general organic compounds, isoproturon to represent hydrophobic compounds and metaldehyde to represent hydrophilic compounds. The results of the tests are summarized in Fig. 3 and Table 9, which show that the adsorption of the target contaminants occurs at two types of binding sites (denoted as 1 and 2) on the carbon and these binding sites might be identical or dependent. In all cases it was found that the magnitude of the respective binding constants was Ka2 > Ka1, which meant that the binding to the first site energetically favoured the subsequent binding to the other site.67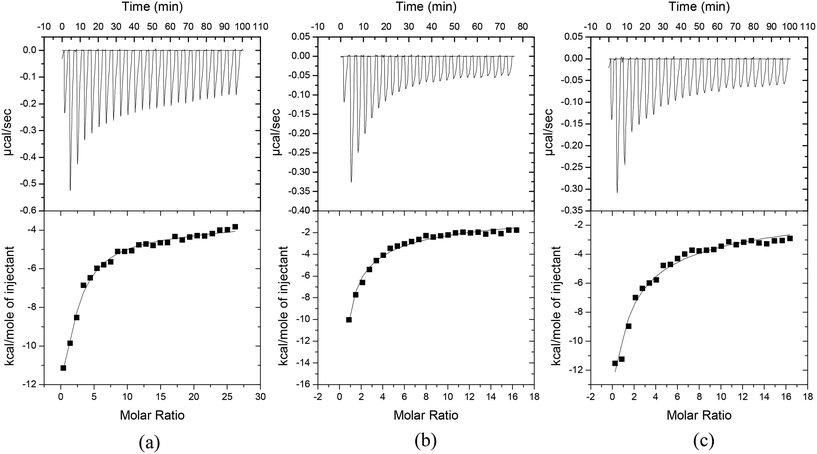 | ||
| Fig. 3 Real-time thermograms (top) and binding isotherms after blank subtraction (bottom) for the titration of (a) phenol, (b) isoproturon and (c) metaldehyde against activated carbon F400. | ||
| Target contaminant | Site | K a (M−1) | ΔadsG (kJ mol−1) | ΔadsH (kJ mol−1) | TΔadsS (kJ mol−1) |
|---|---|---|---|---|---|
| Phenol | (1) | (7.5 ± 0.9) × 103 | −22.1 ± 0.3 | −53.2 ± 0.4 | −31.3 ± 0.7 |
| (2) | (8.0 ± 0.7) × 105 | −33.7 ± 0.2 | 36.9 ± 1.8 | 70.7 ± 1.6 | |
| Isoproturon | (1) | (4.4 ± 0.2) × 102 | −14.9 ± 0.4 | −123.2 ± 7.9 | −108.3 ± 7.6 |
| (2) | (5.9 ± 0.2) × 105 | −32.7 ± 0.2 | 146.2 ± 14.5 | 171.4 ± 3.6 | |
| Metaldehyde | (1) | (2.0 ± 0.8) × 102 | −13.1 ± 0.1 | −186.7 ± 1.9 | −173.5 ± 2.1 |
| (2) | (4.3 ± 0.5) × 105 | −32.2 ± 0.3 | 179.2 ± 7.2 | 211.3 ± 6.9 |
For the first site (site 1), which is a relatively weak binding site (ΔadsG = −22.1 kJ mol−1 for phenol, −14.9 kJ mol−1 for isoproturon and −13.1 kJ mol−1 for metaldehyde), the values of all of the thermodynamic parameters were negative, indicating that the adsorption at this site is spontaneous and thermodynamically favourable. This site may be where the target contaminants physically adsorb onto the carbon through electrostatic interactions, or involving Van der Waals forces, or where the contaminants, hydrated by water molecules, interact via the water molecules with the hydrophilic carbon surface.60 The adsorption at this site is where the carbon surface is covered with a monolayer of the contaminant molecules. A higher negative value of phenol adsorption (ΔadsG) reflects a more energetically favourable adsorption compared to isoproturon and metaldehyde,68 which is possibly because the smaller molecular size of phenol enables it to penetrate to, and adsorb in, deeper pores of the carbon. Isoproturon has a higher negative value of ΔadsG than metaldehyde which may reflect its greater hydrophobicity, and thus the adsorption of isoproturon at site 1 was more thermodynamically favourable compared to metaldehyde. Phenol had the highest desorption efficiency using water at 80 °C compared to the other contaminants as it is the most water-soluble, and this suggested that physisorption is an important adsorption mechanism of phenol but not a dominant mechanism for isoproturon and metaldehyde adsorption. The negative value of the entropy (TΔadsS) of the system describes the transfer of the contaminant from the solution bulk phase to the solid–liquid interface, which decreases the entropy of the system and the degree of freedom of the contaminant.69
The second site (site 2) is a stronger binding site than site 1 (ΔadsG = −33.7 kJ mol−1 for phenol, −32.7 kJ mol−1 for isoproturon and −32.2 kJ mol−1 for metaldehyde). There were complex interactions between the carbon and the target contaminants evident at this binding site. This site can be where a dynamic equilibrium occurred. The positive value of the TΔadsS suggested that the contaminant molecules on the carbon surface adsorbed in a multilayer arrangement. This arrangement commonly involves a weaker strength of intermolecular force, such as van der Waals, dispersion and electrostatic interactions,60 thus the molecules that are furthest from the carbon surface or that have lower adsorption potential energy become less stable and may desorb, becoming less arranged. The formation of multilayers is expected before the contaminants fill the higher energy adsorption sites (high ΔadsH or low −ΔadsH).70 Taking into account that the ΔadsH values of isoproturon and metaldehyde exceeded 100 kJ mol−1, and the process was endothermic at this site, the adsorption mechanisms of isoproturon and metaldehyde most likely involved chemisorption-like interactions. Bansal and Goyal60 reported that the heat of chemisorption is usually in the range of 40–400 kJ mol−1 and Tong et al.71 suggested that a strong H-bond between carbon and a micropollutant could occur with an enthalpy value of ∼100 kJ mol−1. As previously reported,63,64 organic contaminants are able to form strong donor-acceptor complexes with oxygen functional groups on the carbon surface, such as carbonyl groups, which has a large dipole moment compared to the carboxylic groups. This kind of interaction requires activation energy and the extent of the adsorption depends upon the temperature of adsorption. Furthermore, Ferro-García et al.72 suggested that surface carbonyl groups play an important role for chemisorbed organic contaminants. In the evaluation of the desorbability of target contaminants in this study, a higher degree of desorption was achieved using the mixed NaOH/CH3CH2OH solution, which was attributed to its ability to interact with, and weaken, the carbonyl functional group on the carbon.
A study conducted by Busquets et al.44 showed that metaldehyde adsorption onto activated carbon was independent of the carbon's specific surface area, which indicated that the adsorption mechanism was more complex than just physisorption. The results from this study also support those reported previously, including both a theoretical study73 and a laboratory investigation28 which indicated that metaldehyde adsorption involves hydrogen bonding with carbon surface functional groups, with or without mediation by water molecules. Other phenomena such as van der Waals interactions and the thermodynamic gradients may also be contributory mechanisms for metaldehyde adsorption. The heat-consuming processes during the contaminant adsorption can also be attributed to the energy required to break hydrogen bonds between the contaminant and water in the bulk phase, and between the surface functional groups of the carbon and water. To interact with these functional groups, the contaminants have to displace possibly more than one water molecule from their adsorption site, resulting in the endothermic nature of the process. The endothermic nature of the adsorption process was also reported for phenol and metaldehyde28,74 whereby the contaminant uptake increased with the solution temperature.
Additionally, the large and positive value of ΔadsH is most likely due to the release of water molecules from the displacement of the contaminants at the adsorption sites.43,69 The high entropy of isoproturon adsorption onto carbon also might be due to the hydrophobic interaction involved.39 The unfavourable nature of positive ΔadsH value for the second site, which would make a reaction non-spontaneous, is counteracted by the positive ΔadsS values and negative value of ΔadsG, indicating that the contaminant adsorption process is spontaneous.75
In summary, the calorimetric investigation suggested that isoproturon and metaldehyde were more strongly bonded compared to phenol. This is consistent with the results of the desorption tests using different types of regenerant solution, which showed it was more difficult to desorb isoproturon and metaldehyde from GAC, compared to phenol.
4. Conclusions
To evaluate the feasibility of a novel on-site chemical regeneration of GAC, an adsorption and desorption study has been conducted using five organic compounds (known contaminants) of different physico-chemical properties (phenol, nitrobenzene, isoproturon, clopyralid and metaldehyde) and four regenerant solutions (laboratory grade RO water at 20, 50 and 80 °C, NaOH, ethanol and a mixture of NaOH and ethanol – NaOH/CH3CH2OH). Thermodynamic parameters were also determined to help explain the adsorption and desorption mechanisms involved. The main conclusions can be summarized as follows:• The affinity of adsorption of the compounds for the carbon F400 was in the following order: nitrobenzene > isoproturon > phenol > clopyralid > metaldehyde. The comparative affinity was related to the aqueous solubility and hydrophobicity (represented by the log kow value) of the compounds.
• RO water was unable to achieve any significant desorption (<5%) of the compounds at room temperature, while greater desorption was evident at higher temperatures for phenol (∼24% at 80 °C) and clopyralid (∼14% at 80 °C). These findings indicated the existence of stronger interactions between the target contaminants and GAC, than might be explained by physisorption only.
• With the exception of phenol, strong solutions of NaOH (<2 M) were unable to achieve any significant desorption (<5%) of the other four compounds. For phenol, a DE of 40–50% was evident for a wide range of NaOH concentrations (0.1–2 M). This was partly attributed to the transformation of phenol to the phenolate ion and electrostatic charge repulsion from the negatively charged carbon surface.
• The hydrophobicity of the target contaminant was observed to influence the effectiveness of ethanol as a regenerant solution. Ethanol effectively desorbed phenol (∼60%), nitrobenzene (35–45%) and isoproturon (40–70%), which are readily soluble in alcohol owing to their hydrophobicity.
• The NaOH/CH3CH2OH mixture was generally effective in desorbing all of the target contaminants due to the aggressiveness of ethoxide. Ethoxide interacts with the surface functional groups of the carbon and weakens the interactions between the adsorbed contaminants and the GAC, causing them to desorb.
• The NaOH/CH3CH2OH mixture did not transform or react with the target contaminants, except phenol, indicating that desorption process was governed by changes in the carbon surface properties.
• The results of calorimetry suggested that the selected contaminants, phenol, isoproturon and metaldehyde, adsorbed at two binding sites: a relatively weak binding site (site 1) where the contaminants are physisorbed to the carbon, and a second site (site 2) where a dynamic equilibrium occurs, and the contaminants bind with the carbon under chemisorption-like interactions, as indicated by a high energy of adsorption.
This study has demonstrated that a combined NaOH/CH3CH2OH solution was effective as a chemical regenerant by achieving the substantial desorption of a range of organic contaminants. Future research will consider the performance of repeated adsorption/desorption cycles in column tests, and the possible effects of the regenerant solution on the GAC properties, in order to confirm the potential value of chemical regeneration as a means of increasing the GAC bed life prior to full thermal regeneration and carbon disposal.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from Indonesia Endowment Fund for Education (LPDP), Severn Trent Water, Thames Water, Yorkshire Water, Northumbrian Water, Anglian Water, and the EPSRC under the STREAM IDC Programme.References
- UK Water Industry Research, GAC management (and alternative technologies) to meet future water quality standards (Final Report), London, 2017 Search PubMed.
- P. C. Chiang and J. S. Wu, Evaluation of chemical and thermal regeneration of activated carbon, Water Sci. Technol., 1989, 21, 1697–1700 CrossRef CAS.
- G. D. Castle, G. A. Mills, A. Gravell, L. Jones, I. Townsend, D. G. Cameron and G. R. Fones, Review of the molluscicide metaldehyde in the environment, Environ. Sci.: Water Res. Technol., 2017, 3, 415–428 RSC.
- S. Cosgrove, B. Jefferson and P. Jarvis, Pesticide removal from drinking water sources by adsorption: a review, Environ. Technol. Rev., 2019, 8, 1–24 CrossRef CAS.
- F. J. Guymont, The effect of capital and operating costs on GAC adsorption system design, in Activated Carbon Adsorption of Organics from the Aqueous Phase, ed. M. J. Mcguire and I. H. Suffet, Ann Arbor Science, Michigan, 1980, pp. 531–538 Search PubMed.
- S. Kanpirom, Chemical Regeneration of Spent Granular Activated Carbon, PhD thesis, Univeristy of Hawaii, 2005 Search PubMed.
- J. T. Matheickal, Q. Yu and J. Linden, In-situ regeneration of phenol-saturated activated carbon using ethanol, Dev. Chem. Eng. Miner. Process., 1998, 6, 263–272 CrossRef.
- M. Streat and L. A. Sweetland, Removal of pesticides from water using hypercrosslinked polymer phases: part 1 - physical and chemical characterization of adsorbents, Process Saf. Environ. Prot., 1998, 76, 115–126 CrossRef CAS.
- R. J. Martin and W. J. Ng, Chemical regeneration of exhausted activated carbon - I, Water Res., 1984, 18, 59–73 CrossRef CAS.
- H. Tamon, T. Saito, M. Kishimura, M. Okazaki and R. Toei, Solvent regeneration of spent activated carbon in wastewater treatment, J. Chem. Eng. Jpn., 1990, 23, 426–432 CrossRef CAS.
- P. C. Chiang, E. E. Chang and J. S. Wu, Comparison of chemical and thermal regeneration of aromatic compounds on exhausted activated carbon, Water Sci. Technol., 1997, 35, 279–285 CrossRef CAS.
- D. O. Cooney, A. Nagerl and A. L. Hines, Solvent regeneration of activated carbon, Water Res., 1983, 17, 403–410 CrossRef CAS.
- P. Magne and P. L. Walker, Phenol adsorption on activated carbons: Application to the regeneration of activated carbons polluted with phenol, Carbon, 1986, 24, 101–107 CrossRef CAS.
- C.-C. Leng and N. G. Pinto, An investigation of the mechanisms of chemical regeneration of activated carbon, Ind. Eng. Chem. Res., 1996, 35, 2024–2031 CrossRef CAS.
- K. J. Himmelstein, Regeneration of Activated Carbon with A Solution of Sorbed Species in A Solvent, US Pat., 3965036, 1976 Search PubMed.
- R. J. Martin and W. J. Ng, Chemical regeneration of exhausted activated carbon - II, Water Res., 1985, 18, 1527–1535 CrossRef.
- W. Wang, Z. Du, S. Deng, M. Vakili, L. Ren, P. Meng, A. Maimaiti, B. Wang, J. Huang, Y. Wang and G. Yu, Regeneration of PFOS loaded activated carbon by hot water and subsequent aeration enrichment of PFOS from eluent, Carbon, 2018, 134, 199–206 CrossRef CAS.
- F. Salvador, N. Martin-Sanchez, R. Sanchez-Hernandez, M. J. Sanchez-Montero and C. Izquierdo, Regeneration of carbonaceous adsorbents. Part II: chemical, microbiological and vacuum regeneration, Microporous Mesoporous Mater., 2015, 202, 277–296 CrossRef CAS.
- S. K. Bhatia, A. Kalam, H. S. Joglekar and J. B. Joshi, Effective diffusivity of phenol in activated carbon, Chem. Eng. Commun., 1990, 98, 139–154 CrossRef CAS.
- Corporation Calgon Carbon, Data Sheet: Filtrasorb® 400, 2015 Search PubMed.
- PubChem: National Library of Medicine, National Center for Biotechnology Information, https://pubchem.ncbi.nlm.nih.gov/, (accessed 25 May 2020).
- CRC Handbook of Chemistry and Physics (National Institute of Standards and Technology), ed. D. R. Lide, CRC Press LLC, Boca Raton, 84th edn, 2003 Search PubMed.
- M. Franz, H. A. Arafat and N. G. Pinto, Effect of chemical surface heterogeneity on the adsorption mechanism of dissolved aromatics on activated carbon, Carbon, 2000, 38, 1807–1819 CrossRef CAS.
- ASTM International, Standard Test Method for Carbon Black - Total and External Surface Area by Nitrogen Adsorption: D6556 – 17 Standard, 2012 Search PubMed.
- S. L. Goertzen, K. D. Thriault, A. M. Oickle, A. C. Tarasuk and H. A. Andreas, Standardization of the Boehm titration: Part I. CO2 expulsion and endpoint determination, Carbon, 2009, 48, 1252–1261 CrossRef.
- A. M. Oickle, S. L. Goertzen, K. R. Hopper, Y. O. Abdalla and H. A. Andreas, Standardization of the Boehm titration: Part II. Method of agitation, effect of filtering and dilute titrant, Carbon, 2010, 48, 3313–3322 CrossRef CAS.
- J. M. Salman and B. H. Hameed, Adsorption of 2,4-dichlorophenoxyacetic acid and carbofuran pesticides onto granular activated carbon, Desalination, 2010, 256, 129–135 CrossRef CAS.
- B. Tao and A. J. Fletcher, Metaldehyde removal from aqueous solution by adsorption and ion exchange mechanisms onto activated carbon and polymeric sorbents, J. Hazard. Mater., 2013, 244–245, 240–250 CrossRef CAS PubMed.
- K. Tan and B. Hameed, Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions, J. Taiwan Inst. Chem. Eng., 2017, 74, 25–48 CrossRef CAS.
- S. Lagergren, About the theory of so-called adsorption of soluble substances, K. Sven. Vetenskapsakad. Handl., 1898, 24, 1–39 Search PubMed.
- Y. S. Ho and G. Mckay, Pseudo-second order model for sorption processes, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- ASTM International, Standard Practice for Determination of Adsorptive Capacity of Activated Carbon by Aqueous Phase Isotherm Technique: D3860–98 (Reapproved 2014), 2004, vol. 15 Search PubMed.
- C. H. Bolster and G. M. Hornberger, On the use of linearized Langmuir equations, Soil Sci. Soc. Am. J., 2008, 72, 1848 CrossRef CAS.
- I. Efremenko and M. Sheintuch, Predicting solute adsorption on activated carbon: Phenol, Langmuir, 2006, 22, 3614–3621 CrossRef CAS.
- Z. Aksu, Application of biosorption for the removal of organic pollutants : a review, Process Biochem., 2005, 40, 997–1026 CrossRef CAS.
- H. Kaur, A. Bansiwal, G. Hippargi and G. R. Pophali, Effect of hydrophobicity of pharmaceuticals and personal care products for adsorption on activated carbon: Adsorption isotherms, kinetics and mechanism, Environ. Sci. Pollut. Res., 2018, 25, 20473–20485 CrossRef CAS PubMed.
- H. N. Tran, S. J. You, A. Hosseini-Bandegharaei and H. P. Chao, Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review, Water Res., 2017, 120, 88–116 CrossRef CAS PubMed.
- A. E. F. Nassar and A. Lopez-Anaya, Strategies for dealing with reactive intermediates in drug discovery and development, Curr. Opin. Drug Discovery Dev., 2004, 7, 126–136 CAS.
- K. Chiad, Adsorption of Macromolecules on Interfaces Studied by Isothermal Titration Calorimetry, PhD thesis, Johannes Gutenberg-Universität Mainz, 2011 Search PubMed.
- E. A. Lewis and K. P. Murphy, Isothermal Titration Calorimetry, in Protein-Ligand Interactions, ed. G. U. Nienhaus, Humana Press, Totowa, New Jersey, 2005, pp. 1–16 Search PubMed.
- Y. Huang and A. A. Keller, Isothermal titration microcalorimetry to determine the thermodynamics of metal ion removal by magnetic nanoparticle sorbents, Environ. Sci.: Nano, 2016, 3, 1206–1214 RSC.
- P. F. R. Ortega, J. P. C. Trigueiro, M. R. Santos, Â. M. L. Denadai, L. C. A. Oliveira, A. P. C. Teixeira, G. G. Silva and R. L. Lavall, Thermodynamic Study of Methylene Blue Adsorption on Carbon Nanotubes Using Isothermal Titration Calorimetry: A Simple and Rigorous Approach, J. Chem. Eng. Data, 2017, 62, 729–737 CrossRef CAS.
- V. Karlsen, E. Bævre and M. Sørlie, The use of isothermal titration calorimetry to determine the thermodynamics of metal ion binding to low-cost sorbents, Thermochim. Acta, 2010, 501, 119–121 CrossRef CAS.
- R. Busquets, O. P. Kozynchenko, R. L. D. Whitby, S. R. Tennison and A. B. Cundy, Phenolic carbon tailored for the removal of polar organic contaminants from water: A solution to the metaldehyde problem?, Water Res., 2014, 61, 46–56 CrossRef CAS PubMed.
- Z. Li, Y. Yang, U. Jauregui-Haza, Z. Guo and L. C. Campos, The impact of humic acid on metaldehyde adsorption onto powdered activated carbon in aqueous solution, RSC Adv., 2019, 9, 11–22 RSC.
- P. Cañizares, M. Carmona, O. Baraza, A. Delgado and M. A. Rodrigo, Adsorption equilibrium of phenol onto chemically modified activated carbon F400, J. Hazard. Mater., 2006, 131, 243–248 CrossRef PubMed.
- L. R. Radovic, I. F. Silva, J. I. Ume, J. A. Menéndez, C. A. L. Y. Leon and A. W. Scaroni, An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons, Carbon, 1997, 35, 1339–1348 CrossRef CAS.
- J. L. Sotelo, G. Ovejero, A. Rodríguez, S. Álvarez and J. García, Removal of atenolol and isoproturon in aqueous solutions by adsorption in a fixed-bed column, Ind. Eng. Chem. Res., 2012, 51, 5045–5055 CrossRef CAS.
- M. Muñoz-Morales, C. Sáez, P. Cañizares and M. A. Rodrigo, A new strategy for the electrolytic removal of organics based on adsorption onto granular activated carbon, Electrochem. Commun., 2018, 90, 47–50 CrossRef.
- S. Salvestrini, P. Vanore, A. Bogush, S. Mayadevi and L. C. Campos, Sorption of metaldehyde using granular activated carbon, J. Water Reuse Desalin., 2017, 7, 280–287 CrossRef.
- R. D. Vidic, M. T. Suldan and R. C. Brenner, Oxidative coupling of phenols on activated carbon: Impact on adsorption equilibrium, Environ. Sci. Technol., 1993, 27, 2079–2085 CrossRef CAS.
- R. H. Pahl, K. G. Mayhan and G. L. Bertrand, Organic desorption from carbon - II. The effect of solvent in the desorption of phenol from wet carbon, Water Res., 1973, 7, 1309–1322 CrossRef.
- Z. Li, J. Lo, Z. Guo and L. C. Campos, Investigation of metaldehyde removal by powdered activated carbon from different water samples, Environ. Sci.: Water Res. Technol., 2020, 6, 1432–1444 RSC.
- S. G. J. Heijman and R. Hopman, Activated carbon filtration in drinking water production : model prediction and new concepts, Colloids Surf., A, 1999, 151, 303–310 CrossRef CAS.
- C. A. Rolph, B. Jefferson, F. Hassard and R. Villa, Metaldehyde removal from drinking water by adsorption onto filtration media: Mechanisms and optimisation, Environ. Sci.: Water Res. Technol., 2018, 4, 1543–1552 RSC.
- B. Özkaya, Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models, J. Hazard. Mater., 2006, 129, 158–163 CrossRef PubMed.
- V. Bernal, L. Giraldo, J. C. Moreno-Piraján, M. Balsamo and A. Erto, Mechanisms of methylparaben adsorption onto activated carbons: Removal tests supported by a calorimetric study of the adsorbent–adsorbate interactions, Molecules, 2019, 24, 1–21 Search PubMed.
- C. Moreno-castilla, Adsorption of organic molecules from aqueous solutions on carbon materials, Carbon, 2004, 42, 83–94 CrossRef CAS.
- E. F. Caldin and G. Long, The equilibrium between ethoxide and hydroxide ions in ethanol and in ethanol-water mixtures, J. Chem. Soc., 1954, 3737–3742 RSC.
- R. C. Bansal and M. Goyal, Activated Carbon Adsorption, Activated Carbon Adsorption, CRC Press, New York, 2005 Search PubMed.
- H. P. Boehm, Chemical identification of surface groups, Adv. Catal., 1966, 16, 179–274 CAS.
- D. R. U. Knappe, Chapter 9: Surface chemistry effects in activated carbon adsorption of industrial pollutants, in Interface Science in Drinking Water Treatment, ed. G. Newcombe and D. Dixo, Elsevier Ltd, 2006, pp. 155–177 Search PubMed.
- J. A. Mattson, H. B. Mark Jr, M. D. Malbin, W. J. Weber and J. C. Crittenden, Surface chemistry of active carbon: Specific adsorption of phenols, J. Colloid Interface Sci., 1969, 31, 116–130 CrossRef CAS.
- E. Castillejos-López, D. M. Nevskaia, V. Muñoz and A. Guerrero-Ruiz, On the interactions of phenol, aniline and p-nitrophenol on activated carbon surfaces as detected by TPD, Carbon, 2008, 46, 870–875 CrossRef.
- H. P. Boehm, Surface oxides on carbon and their analysis: A critical assessment, Carbon, 2002, 40, 145–149 CrossRef CAS.
- H. P. Boehm, Review article: Some aspects of the surface chemistry of carbon blacks and other carbons, Carbon, 1994, 32, 759–769 CrossRef CAS.
- S. J. Darby, L. Platts, M. S. Daniel, A. J. Cowieson and R. J. Falconer, An isothermal titration calorimetry study of phytate binding to lysozyme: A multisite electrostatic binding reaction, J. Therm. Anal. Calorim., 2017, 127, 1201–1208 CrossRef CAS.
- Y. Liu, Is the Free Energy Change of Adsorption Correctly Calculated ?, J. Chem. Eng. Data, 2009, 54, 1981–1985 CrossRef CAS.
- G. M. D. Ferreira, G. M. D. Ferreira, M. C. Hespanhol, J. de Paula Rezende, A. C. dos Santos Pires, L. V. A. Gurgel and L. H. M. da Silva, Adsorption of red azo dyes on multi-walled carbon nanotubes and activated carbon: A thermodynamic study, Colloids Surf., A, 2017, 529, 531–540 CrossRef CAS.
- B. Y. F. C. Tompkins, Adsorption isotherm for non-uniform surfaces, Trans. Faraday Soc., 1950, 46, 504–586 RSC.
- Y. Tong, P. J. Mcnamara and B. K. Mayer, Adsorption of organic micropollutants onto biochar: a review of relevant kinetics, mechanisms and equilibrium, Environ. Sci.: Water Res. Technol., 2019, 5, 821–838 RSC.
- M. A. Ferro-García, E. Utrera-Hidalgo, J. Rivera-Utrilla, C. Moreno-Castilla and J. P. Joly, Regeneration of activated carbons exhausted with chlorophenols, Carbon, 1993, 31, 857–863 CrossRef.
- A. Ferino-Perez, J. J. Gamboa-Carballo, Z. Li, L. C. Campos and U. Jauregui-Haza, Explaining the interactions between metaldehyde and acidic surface groups of activated carbon under different pH conditions, J. Mol. Graphics Modell., 2019, 90, 94–103 CrossRef CAS PubMed.
- V. C. Srivastava, M. M. Swamy, I. D. Mall, B. Prasad and I. M. Mishra, Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics, Colloids Surf., A, 2006, 272, 89–104 CrossRef CAS.
- Y. H. Li, Z. Di, J. Ding, D. Wu, Z. Luan and Y. Zhu, Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes, Water Res., 2005, 39, 605–609 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ew00328j |
| This journal is © The Royal Society of Chemistry 2020 |