Adsorption performance and mechanism of antibiotics from aqueous solutions on porous boron nitride–carbon nanosheets†
Abstract
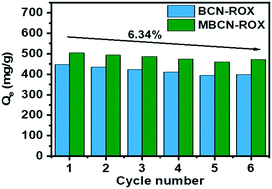
Antibiotics are a class of emerging contaminants with a potential threat to human and animal health. Chloramphenicol (CAP) and roxithromycin (ROX), which are extensively used, have attracted attention recently. In this work, two kinds of porous boron nitride–carbon nanosheets (BCNs and MBCNs) with different pore distributions were synthesized for the effective adsorption of antibiotics. The maximum adsorption capacity for CAP was 546.15 mg g−1, and the adsorption capacity for ROX was 575.68 mg g−1. It is worth noting that with the increase of molecular weight of antibiotics, huge molecules are blocked from entering micropores, and the adsorption capacity of BCNs decreases, indicating that a micropore-filling effect is the main mechanism of antibiotic adsorption on BCNs. In addition, hydrophobic interaction in chemical adsorption and electrostatic interaction were also important factors affecting the adsorption capacity of MBCN and BCN. The adsorbents were regenerated by simple high temperature calcination and excellent reusability was achieved after 6 cycles. Overall, the boron nitride–carbon nanosheets should be a good choice for the next generation of high performance adsorption materials.
- This article is part of the themed collection: Environmental Science: Water Research & Technology Cover Art


 Please wait while we load your content...
Please wait while we load your content...