Open Access Article
Open Access ArticleLandfill leachate contributes per-/poly-fluoroalkyl substances (PFAS) and pharmaceuticals to municipal wastewater†
Jason R.
Masoner
 *a,
Dana W.
Kolpin
*a,
Dana W.
Kolpin
 b,
Isabelle M.
Cozzarelli
b,
Isabelle M.
Cozzarelli
 c,
Kelly L.
Smalling
c,
Kelly L.
Smalling
 d,
Stephanie C.
Bolyard
d,
Stephanie C.
Bolyard
 e,
Jennifer A.
Field
e,
Jennifer A.
Field
 f,
Edward T.
Furlong
f,
Edward T.
Furlong
 g,
James L.
Gray
g,
James L.
Gray
 g,
Duncan
Lozinski
g,
Duncan
Lozinski
 h,
Debra
Reinhart
h,
Debra
Reinhart
 i,
Alix
Rodowa
i,
Alix
Rodowa
 f and
Paul M.
Bradley
f and
Paul M.
Bradley
 j
j
aU.S. Geological Survey, Oklahoma City, Oklahoma 73116, USA. E-mail: jmasoner@usgsg.gov
bU.S. Geological Survey, Iowa City, Iowa 52240, USA
cU.S. Geological Survey, Reston, Virginia 20192, USA
dU.S. Geological Survey, Lawrenceville, New Jersey 08648, USA
eEnvironmental Research & Education Foundation, Raleigh, North Carolina 27609, USA
fOregon State University, Corvallis, Oregon 97331, USA
gU.S. Geological Survey, Lakewood, Colorado 80225, USA
hBrown and Caldwell, Maitland, Florida 32751, USA
iUniversity of Central Florida, Orlando, Florida 32816, USA
jU.S. Geological Survey, Columbia, South Carolina 29210, USA
First published on 13th March 2020
Abstract
Widespread disposal of landfill leachate to municipal sewer infrastructure in the United States calls for an improved understanding of the relative organic-chemical contributions to the wastewater treatment plant (WWTP) waste stream and associated surface-water discharge to receptors in the environment. Landfill leachate, WWTP influent, and WWTP effluent samples were collected from three landfill-WWTP systems and compared with analogous influent and effluent samples from two WWTPs that did not receive leachate. Samples were analyzed for 73 per-/poly-fluoroalkyl substances (PFAS), 109 pharmaceuticals, and 21 hormones and related compounds. PFAS were detected more frequently in leachate (92%) than in influent (55%). Total PFAS concentrations in leachate (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ng L−1) were more than 10 times higher than in influent (6950 ng L−1) and effluent samples (3730 ng L−1). Concentrations of bisphenol A; the nonprescription pharmaceuticals cotinine, lidocaine, nicotine; and the prescription pharmaceuticals amphetamine, carisoprodol, pentoxifylline, and thiabendazole were an order of magnitude higher in landfill leachate than WWTP influent. Leachate load contributions for PFAS (0.78 to 31 g d−1), bisphenol A (0.97 to 8.3 g d−1), and nonprescription (2.0 to 3.1 g d−1) and prescription (0.48 to 2.5 g d−1) pharmaceuticals to WWTP influent were generally low (<10 g d−1) for most compounds resulting from high influent-to-leachate volumetric ratios (0.983). No clear differences in concentrations were apparent between effluents from WWTPs receiving landfill leachate and those that did not receive landfill leachate.
100 ng L−1) were more than 10 times higher than in influent (6950 ng L−1) and effluent samples (3730 ng L−1). Concentrations of bisphenol A; the nonprescription pharmaceuticals cotinine, lidocaine, nicotine; and the prescription pharmaceuticals amphetamine, carisoprodol, pentoxifylline, and thiabendazole were an order of magnitude higher in landfill leachate than WWTP influent. Leachate load contributions for PFAS (0.78 to 31 g d−1), bisphenol A (0.97 to 8.3 g d−1), and nonprescription (2.0 to 3.1 g d−1) and prescription (0.48 to 2.5 g d−1) pharmaceuticals to WWTP influent were generally low (<10 g d−1) for most compounds resulting from high influent-to-leachate volumetric ratios (0.983). No clear differences in concentrations were apparent between effluents from WWTPs receiving landfill leachate and those that did not receive landfill leachate.
Water impactLandfill leachate in the U.S. is commonly disposed to wastewater treatment facilities. Leachate is known to contain a complex mixture of per-and-poly-fluoroalkyl substances and pharmaceutical chemicals. It is not known if this disposal practice has measurable effects on individual and total organic-chemical concentrations and loads in receiving treatment facilities and corresponding effluents discharged to surface waters. |
1 Introduction
In the United States (U.S.), landfill disposal of municipal solid and liquid waste from residential, commercial, and industrial sources continues to increase in response to population growth and to expanded manufacturing and availability of consumer products.1 Leachate is produced at landfills from the percolation of precipitation through solid waste and from liquid waste migrating downgradient. Complex mixtures of contaminants of concern for human and ecosystem health, including per- and poly-fluoroalkyl substances (PFAS),2–9 are increasingly detected in leachate due to the expanded availability of pharmaceuticals, personal-care products, and packaging of single-use items and containers.10,11 PFAS are used in a wide range of consumer products such as electronics, water-repellent textiles, food packaging materials, carpets, and upholsteries that are commonly discarded into landfills.9,12 PFAS are largely resistant to biotic transformations due to their extremely strong C–F bonds, have been shown to cause disruption to key cellular functions, and can cause negative biological effects in animals and humans exposed to PFAS at high levels.13–17 Exposure of PFAS even at low concentrations is of environmental concern as they exhibit long biological half-lives and bioaccumulation potential. The annual leachate load of PFAS from U.S. landfills to municipal wastewater treatment plant (WWTP) influent was estimated to be between 563 and 638 kg in 2013.6Likewise, the widespread occurrence of pharmaceuticals in environmental samples has become an increasingly important issue because they are bioactive chemicals designed to affect physiological or cellular functions.18 Landfills commonly receive unused pharmaceuticals both from household trash and from their presence in biosolids from WWTPs that are often disposed in landfills.19 Adverse environmental effects have been documented for some individual pharmaceuticals and biogenic hormones at low ng L−1 concentrations,20–22 but the environmental effects from exposure to complex contaminant mixtures, including PFAS and pharmaceuticals at low ng L−1 concentrations are currently unknown or inadequately characterized.23,24
In the United States, landfill leachate is primarily discharged to sewer infrastructure for co-treatment in WWTPs,5 which are well-documented sources of organic contaminants to the environment.25–35 Landfill leachate disposal rates (<0.1 to 2.0 million L d−1)5 are considerably lower (approximately 1% by volume) than WWTP influent rates (8 to 1300 million L d−1).29–32,35,36 However, leachate has been reported to contain substantially elevated concentrations of organic chemicals, such as PFAS (e.g., perfluorooctanoic acid (PFOA); perfluorohexanoic acid (PFHxA); perfluoroheptanoic acid (PFHpA); perfluorooctanesulfonate (PFOS); perfluorohexanesulfonate (PFHxS); and methyl perfluoropentane sulfonamido acetic acid (MeFPeSAA)), prescription pharmaceuticals (e.g., amphetamine, carbamazepine, carisoprodol, and pentoxifylline), nonprescription pharmaceuticals (e.g., cotinine, lidocaine, and nicotine), bisphenol A, and non-volatile dissolved organic carbon (NVDOC).2,4,5,25–28,32,35 PFAS such as PFOA and PFOS can be resistant to municipal wastewater treatment, and effluent concentrations can exceed influent levels due to transformation of precursor compounds during biological treatment.25–27,37 Potential negative effects of leachate on wastewater quality, including substantial decreases in treatment nitrification efficiency, may be improved by managing leachate dilution ratios at the WWTP throughout the daily treatment period.38,39 Such an approach, however, requires a better understanding of the effect of leachate disposal on organic-chemical compositions, concentrations, and loads to municipal wastewaters and associated surface-water receptors of treated WWTP effluent. To help fill this knowledge gap, a preliminary assessment of 203 target-organic chemicals and NVDOC was conducted on samples of landfill leachate, WWTP influent, and WWTP effluent from three paired landfill-WWTP systems and on influent and effluent samples from two WWTPs with no leachate input.
2 Methods
2.1 Description of sites
Landfill leachate and WWTP influent and effluent samples were collected from July to October 2016, from three landfill-WWTP pairs (landfill A-WWTP A, landfill B-WWTP B, landfill C-WWTP C) in Florida during times when leachate was discharged into sewer infrastructure for WWTP co-treatment (Fig. 1). For comparison, additional influent and effluent samples were collected from two WWTPs (WWTP D and WWTP E) in Florida that did not receive leachate. WWTP field names are concatenated with “–INF” to indicate influent samples and with “–EF” to indicate effluent samples. A parcel-tracking sampling approach was employed to ensure that leachate is captured in raw influents as well as the treated effluents. Hydraulic retention time was determined based on the flow rate and volume of each unit process. Leachate was discharged to WWTPs through continuous and periodic discharge. Sample collection times were based on landfill-to-WWTP travel time and average WWTP average hydraulic-retention times based on previous research at these sites.40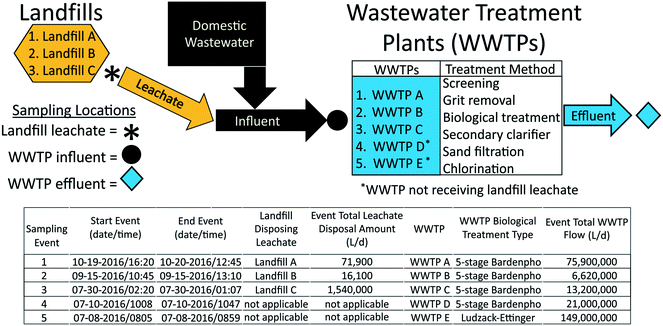 | ||
| Fig. 1 Sampling-event information, landfill leachate disposal volumes, and receiving wastewater treatment plant volumes. | ||
Sampled landfills were active municipal-owned facilities permitted to accept municipal and non-hazardous waste that varied in annual leachate volume produced (5.89 to 71.9 ML) and waste loads (147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 737
000 to 737![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons, Table 1). Landfill waste was generally municipal waste (54 to 71%) and construction debris (26 to 34%). Landfills were equipped with leachate-collection and recovery systems that discharged to municipal WWTPs. Untreated leachate was continuously discharged from landfill B, whereas leachate was treated prior to sewer disposal at landfills A and C using aerated tanks located on site. Sampled WWTPs were representative of other WWTPs across the United States in terms of populations served and treatment methods, used similar methods of wastewater treatment, and consisted of a wide range of WWTP sizes in terms of treatment volumes (6.62 to 149 million L d−1, Fig. 1). One leachate sample was collected at each landfill and one influent and effluent sample was collected at the WWTP facilities B, C, D, and E. Five samples were collected at 4 hour time increments at both the influent and effluent locations at WWTP A, with the median of these samples used to represent a single influent and effluent observation at this facility.
000 metric tons, Table 1). Landfill waste was generally municipal waste (54 to 71%) and construction debris (26 to 34%). Landfills were equipped with leachate-collection and recovery systems that discharged to municipal WWTPs. Untreated leachate was continuously discharged from landfill B, whereas leachate was treated prior to sewer disposal at landfills A and C using aerated tanks located on site. Sampled WWTPs were representative of other WWTPs across the United States in terms of populations served and treatment methods, used similar methods of wastewater treatment, and consisted of a wide range of WWTP sizes in terms of treatment volumes (6.62 to 149 million L d−1, Fig. 1). One leachate sample was collected at each landfill and one influent and effluent sample was collected at the WWTP facilities B, C, D, and E. Five samples were collected at 4 hour time increments at both the influent and effluent locations at WWTP A, with the median of these samples used to represent a single influent and effluent observation at this facility.
| Landfill A | Landfill B | Landfill C | |
|---|---|---|---|
| [WWTP, wastewater treatment plant]. | |||
| Landfill waste composition (%) | |||
| Municipal waste | 65 | 54 | 71 |
| Construction/debris | 34 | 26 | 26 |
| Other wastes (ash, biosolids, industrial waste) | <1 | 20 | 3 |
| Landfill/waste information | |||
| Municipal/private owned | Municipal | Municipal | Municipal |
| Average annual waste load 2010–2018 (metric tons) | 737![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
485![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Time period of received waste | 1972-Present | 1978-Present | 1972-Present |
| Annual leachate production (liters) | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 890![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Sampling source | Manhole | Manhole | Manhole |
| Leachate treatment | Aeration | No treatment | Aeration |
| Leachate disposal practice | WWTP | WWTP | WWTP |
2.2 Sampling methods
Landfill leachate grab samples were collected at the location of discharge to the sewer infrastructure using clean 3.5 L polyethylene containers. WWTP influent and WWTP effluent grab samples were collected at each WWTP using dedicated high-density polyethylene plastic dip-containers. Subsamples of the leachate, influent, and effluent samples were poured, unfiltered, directly from the grab container into a 1 L Nalgene bottle for PFAS analysis and a 500 mL polyethylene bottle for biogenic hormones analysis. Two additional subsamples were filtered using disposable syringe-filters for analysis of pharmaceuticals (0.7 μm nominal pore size glass fiber) and NVDOC (0.22 μm pore size) in baked amber-glass bottles. NVDOC samples were preserved with hydrochloric acid to pH <2. All samples were immediately chilled to 4 °C after collection and shipped overnight to the analytical laboratories. Upon receipt of samples, the Oregon State University Molecular Toxicology Field Laboratory, U.S. Geological (USGS) National Water Quality Laboratory, and USGS Biogeochemical Processes Laboratory also stored samples at 4 °C until analyses.2.3 Analytical methods
Three analytical methods were used to determine concentrations of 203 target-organic chemicals in leachate, influent, and effluent samples. A liquid chromatography (LC)-tandem mass spectrometry (MS/MS) method previously applied to leachate samples was used to determine the concentrations of 73 PFAS (Oregon State University Molecular Toxicology Field Laboratory).41 A gas chromatography (GC)-MS/MS method was used to determine the concentrations of 19 natural and synthetic hormones as well as bisphenol A and 4,4′-bisphenol F (USGS National Water Quality Laboratory).42 A direct aqueous injection LC-MS/MS method was used to determine the concentrations of 97 pharmaceuticals (prescription and nonprescription), 9 pharmaceutical degradates, and 3 other polar chemicals: atrazine, piperonyl butoxide, methyl–1H–benzotriazole (USGS National Water Quality Laboratory).43 Concentrations of NVDOC were determined by high-temperature combustion (USGS Biogeochemical Processes Laboratory).442.4 Quality assurance
Quality-assurance samples typically consisted of 10% laboratory reagent-water blanks and reagent-water spikes, four field equipment blanks, two laboratory blanks, and two field replicates. Blank samples were prepared in the field and laboratory by processing certified organic-free blank water through the sampling equipment in the same manner that field samples were collected, processed, and analyzed. In addition, isotope-dilution standard (IDS) and surrogate compounds were added to biogenic hormone and pharmaceutical samples prior to extraction or analysis, respectively. Chemical concentrations falling outside the calibration range (i.e., exceeding the analytical calibration curve or falling between the limit of quantitation [LOQ] and limit of detection [LOD] for each method) were used in interpretations, but coded “E” to indicate they were considered estimated detections with reduced precision and accuracy.Concentrations in field samples that were less than field blank sample concentrations were reported as non-detections and the LOQ was raised to the highest concentration in the blank sample. Only PFOA and 6:2 disubstituted polyfluoroalkyl phosphate (6:2 diPAP) had field blank concentrations exceeding their typical LOQs. Additional laboratory blanks (two samples) and field blanks (two samples) were collected and analyzed to assess the possible source of PFOA and 6:2 diPAP blank contamination but neither compound was detected in these additional blank samples. PFOA and 6:2 diPAP data in field samples were retained but the LOQs for PFOA and 6:2 diPAP were raised to the highest blank concentration. Accordingly, PFOA and 6:2 diPAP concentrations in field samples were retained if they exceeded raised LOQs of 740 and 50 ng L−1, respectively. In total there were 21 concentrations of PFOA and 3 concentrations of 6:2 diPAP that were <10 times the highest blank concentration and used in interpretations; these values are coded “V” to indicate that the concentration may be affected by blank contamination during collection and/or analysis of the samples. Median relative percent differences for replicate samples were 6% for LC-MS/MS pharmaceuticals, 27% for PFAS, and 49% for GC-MS/MS biogenic hormones (Table ESI-1†). Median recoveries for IDS and surrogate standards were 102% (interquartile range [IQR], 22%) for LC-MS/MS pharmaceuticals, 68% (IQR, 46%) for PFAS, and 68% (IQR, 32%) for GC-MS/MS biogenic hormones (Table ESI-2†).
3 Results and discussion
3.1 Potential landfill leachate contribution to WWTP influent concentrations
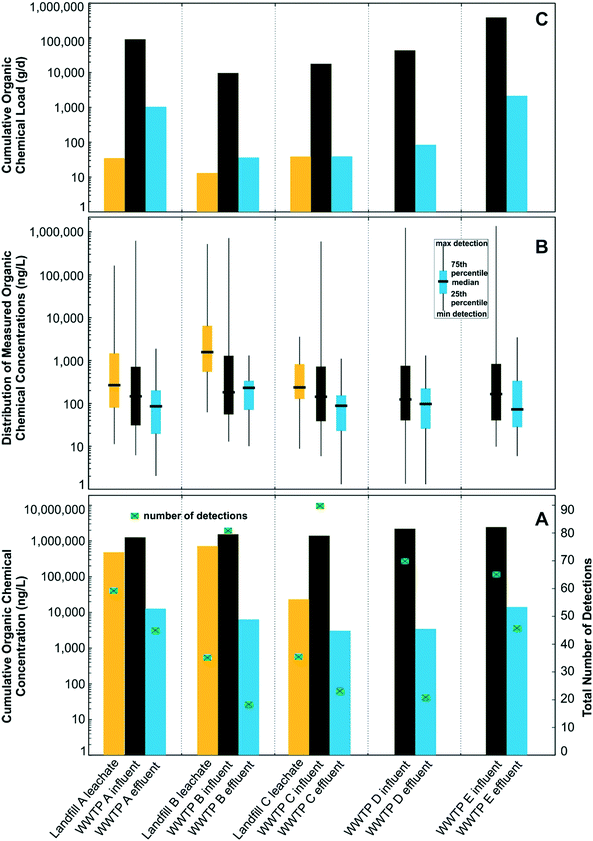
For the 203 target-organic chemicals, 121 (60%) were detected in at least one or more samples of landfill leachate (67 total) or WWTP influent (101 total; Table ESI-3†), with 82 (40%) not detected in any sample (Table ESI-4†). The number of chemicals detected in the three leachate samples ranged from 36 to 59, whereas the number of chemicals detected in the five influent samples ranged from 65 to 90 (Table 2). Total target-organic concentrations of detected chemicals were as much as 768![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 in leachate and 2
000 ng L−1 in leachate and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660
660![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 in influent (Fig. 2A, Table 2) and spanned 6 orders of magnitude (from low ng L−1 to low mg L−1; Fig. 2B). Bisphenol A accounted for 49% of the total target-organic concentration in leachate, followed by fecal indicator 3-beta-coprostanol (16.2%), 8 nonprescription pharmaceuticals (13.1%), cholesterol (10.5%), 35 PFAS (∼7.2%), and 16 prescription pharmaceuticals (∼3.9%). For influent, 89% of the total target-organic concentration consisted of 3-beta-coprostanol (47.3%) and cholesterol (41.4%), followed by 15 nonprescription pharmaceuticals (6.9%), 51 prescription pharmaceuticals (3.3%), bisphenol A (∼0.5%), 10 hormones (∼0.4%), and 21 PFASs (∼0.1%).
000 ng L−1 in influent (Fig. 2A, Table 2) and spanned 6 orders of magnitude (from low ng L−1 to low mg L−1; Fig. 2B). Bisphenol A accounted for 49% of the total target-organic concentration in leachate, followed by fecal indicator 3-beta-coprostanol (16.2%), 8 nonprescription pharmaceuticals (13.1%), cholesterol (10.5%), 35 PFAS (∼7.2%), and 16 prescription pharmaceuticals (∼3.9%). For influent, 89% of the total target-organic concentration consisted of 3-beta-coprostanol (47.3%) and cholesterol (41.4%), followed by 15 nonprescription pharmaceuticals (6.9%), 51 prescription pharmaceuticals (3.3%), bisphenol A (∼0.5%), 10 hormones (∼0.4%), and 21 PFASs (∼0.1%).
| Number of detections | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Landfill (LF) leachate | Wastewater influent | Wastewater effluent | |||||||||||
| a Treated leachate. b Non-treated leachate. c Based on the median concentration from five wastewater treatment plant (WWTP) influent (A-INF) or five WWTP effluent (A-EF) samples collected from 10-19-2016 @ 15:00 to 10-20-2016 @ 12:45, see Table ESI-3.† d No leachate discharge to WWTP; Italics indicate greater concentration in leachate compared to influent; nd, not detected; nc, not calculated due to non-detect. | |||||||||||||
| Group | LF Aa | LF Bb | LF Ca | WWTP A-INFc | WWTP B-INF | WWTP C-INF | WWTP D-INFd | WWTP E-INFd | WWTP A-EFc | WWTP B-EF | WWTP C-EF | WWTP D-EFd | WWTP E-EFd |
| PFAS | 31 | 20 | 21 | 13 | 17 | 16 | 4 | 3 | 11 | 6 | 5 | 7 | 7 |
| Prescription | 15 | 6 | 9 | 46 | 41 | 50 | 39 | 36 | 24 | 11 | 14 | 13 | 30 |
| Nonprescription | 6 | 4 | 4 | 15 | 12 | 14 | 15 | 13 | 6 | 1 | 2 | nd | 6 |
| Hormone | 3 | 2 | 0 | 9 | 7 | 6 | 8 | 9 | 2 | nd | nd | nd | 1 |
| Bisphenol A | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | nd | nd | nd | nd | nd |
| Cholesterol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | nd | nd | nd | 1 |
| 4,4′-Bisphenol F | 1 | 1 | nd | 1 | 1 | 1 | 1 | 1 | nd | nd | nd | nd | nd |
| 3-Beta-coprostanol | 1 | 1 | nd | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total number of detections | 59 | 36 | 36 | 87 | 81 | 90 | 70 | 65 | 45 | 19 | 22 | 21 | 46 |
| Concentrations (ng L−1) | |||||||||||||
| Bisphenol A |
115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 500 500
|
516![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 000
|
628 | 1190 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
258 | 618 | nd | nd | nd | nd | nd |
| 3-Beta-coprostanol | 176![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
nd | 486![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
738![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
527![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
768 | 494 | 288 | 442 | 1170 |
| Nonprescription | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1400 | 143![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2450 | 10.0 | 9.00 | nd | 2140 |
| Cholesterol | 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3260 | 1800 | 580![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
511![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
659![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
885![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1600 | nd | nd | nd | 1020 |
| PFAS |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 600 600
|
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 700 700
|
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 800 800
|
2220 | 3360 | 1380 | 1120 | 1030 | 1580 | 1820 | 330 | 2110 | 1420 |
| Prescription | 6700 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1620 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
6030 | 4010 | 2660 | 1400 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| 4,4′-Bisphenol F | 1100 | 1280 | nd | 36.0 | 53.0 | 175 | 44.0 | 37.0 | nd | nd | nd | nd | nd |
| Hormone | 86.0 | 341 | nd | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
4520 | 8520 | 6450 | 6380 | 5.20 | nd | nd | nd | 12 |
| Total concentration | 499![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
768![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660 660![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
6330 | 3290 | 3950 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| Loads (g d−1) | |||||||||||||
| PFAS | 1.77 | 0.783 | 30.5 | 174 | 22.6 | 18.2 | 23.6 | 153 | 120 | 12.0 | 4.36 | 44.3 | 211 |
| 3-Beta-coprostanol | 12.7 | 0.526 | nc | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
4890 | 6960 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
58.3 | 3.27 | 3.80 | 9.28 | 174 |
| Bisphenol A | 8.34 | 8.31 | 0.967 | 90.3 | 124 | 334 | 5.42 | 92.1 | nc | nc | nc | nc | nc |
| Cholesterol | 9.42 | 0.0525 | 2.77 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3380 | 8700 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
121 | nc | nc | nc | 152 |
| Nonprescription | 3.09 | 1.97 | 2.15 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
752 | 1980 | 1560 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
188 | 0.068 | 0.106 | nc | 319 |
| Prescription | 0.482 | 0.687 | 2.49 | 4020 | 593 | 756 | 482 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
489 | 26.6 | 35.2 | 29.4 | 1610 |
| 4,4′-Bisphenol F | 0.0755 | 0.0206 | nc | 2.73 | 0.351 | 2.31 | 0.924 | 5.51 | nc | nc | nc | nc | nc |
| Hormone | 0.00615 | 0.00549 | nc | 1110 | 29.9 | 112 | 136 | 950 | 0.448 | nc | nc | nc | 1.82 |
| Total load | 35.8 | 12.4 | 38.9 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
9790 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
395![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
978 | 41.9 | 43.4 | 83.0 | 2470 |
| Nonvolatile dissolved organic carbon (NVDOC) and volumetric flows | |||||||||||||
| NVDOC (mg L−1) | 581 | 1340 | 293 | 53.2 | 97.2 | 68.4 | 49.4 | 65.8 | 10.8 | 14.4 | 15.3 | 6.80 | 10.2 |
| NVDOC (g d−1) | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
451![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
643![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
903![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
819![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
143![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Flow volumes (L d−1) | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
PFAS and bisphenol A were detected more frequently and at higher concentrations in leachate than influent, whereas most of the nonprescription and prescription pharmaceuticals, sterols, and hormones were detected more frequently and at higher concentration in influent. Total PFAS concentrations were as much as 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 ng L−1 in leachate and 3360 ng L−1 in influent (Table 2, Fig. ESI-1A and B†). Previous studies have documented a similar range of total PFAS concentration in leachate (300 to 65
700 ng L−1 in leachate and 3360 ng L−1 in influent (Table 2, Fig. ESI-1A and B†). Previous studies have documented a similar range of total PFAS concentration in leachate (300 to 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 ng L−1)6,45,46 and influent (232 to 2450 ng L−1).47–50 Maximum PFAS concentrations occurred in leachate, as compared to WWTP influent, for 32 of the 36 PFAS detected in paired leachate-WWTP samples and were on average 23 times higher in leachate than in corresponding influent. Maximum concentrations in leachate for PFHxA (8300 ng L−1), MeFPeSAA (7600 ng L−1), PFHpA (6500 ng L−1), and PFOA (4800 ng L−1) were substantially larger than maximum concentrations in paired influent samples (470 ng L−1, 48 ng L−1, 350 ng L−1, and 1400 ng L−1, respectively, Fig. ESI-2 and ESI-3†). Twenty nine of the 36 PFAS measured in leachate accounted for >90% of the total concentration of individual PFAS chemicals in paired leachate-WWTP samples (Table ESI-5†). Of the 39 PFAS detected across all sample types, 35 (92%) were detected in leachate and 21 (55%) were detected in influent. There were 15 PFAS that were only detected in the three leachate samples with concentrations that ranged from 10 to 2400 ng L−1 (Fig. ESI-4†). NVDOC concentrations were over an order of magnitude higher in leachate (maximum 1340 mg L−1) than in influent (maximum 97.2 mg L−1, Table 2). Total NVDOC concentration in leachate samples accounted for 91% of the total NVDOC concentration in paired leachate and influent samples. Concentrations of bisphenol A were as much as 516
900 ng L−1)6,45,46 and influent (232 to 2450 ng L−1).47–50 Maximum PFAS concentrations occurred in leachate, as compared to WWTP influent, for 32 of the 36 PFAS detected in paired leachate-WWTP samples and were on average 23 times higher in leachate than in corresponding influent. Maximum concentrations in leachate for PFHxA (8300 ng L−1), MeFPeSAA (7600 ng L−1), PFHpA (6500 ng L−1), and PFOA (4800 ng L−1) were substantially larger than maximum concentrations in paired influent samples (470 ng L−1, 48 ng L−1, 350 ng L−1, and 1400 ng L−1, respectively, Fig. ESI-2 and ESI-3†). Twenty nine of the 36 PFAS measured in leachate accounted for >90% of the total concentration of individual PFAS chemicals in paired leachate-WWTP samples (Table ESI-5†). Of the 39 PFAS detected across all sample types, 35 (92%) were detected in leachate and 21 (55%) were detected in influent. There were 15 PFAS that were only detected in the three leachate samples with concentrations that ranged from 10 to 2400 ng L−1 (Fig. ESI-4†). NVDOC concentrations were over an order of magnitude higher in leachate (maximum 1340 mg L−1) than in influent (maximum 97.2 mg L−1, Table 2). Total NVDOC concentration in leachate samples accounted for 91% of the total NVDOC concentration in paired leachate and influent samples. Concentrations of bisphenol A were as much as 516![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 in leachate and 25
000 ng L−1 in leachate and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ng L−1 in influent samples.
300 ng L−1 in influent samples.
For the 79 nonprescription and prescription pharmaceuticals, sterols, and hormones detected across all leachate and influent samples, 32 (41%) were detected in one or more leachate samples and all (100%) were detected in one or more influent samples. Total nonprescription pharmaceutical concentrations were as much as 123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 in leachate and 150
000 ng L−1 in leachate and 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 in influent, whereas total prescription pharmaceutical concentrations were as much as 42
000 ng L−1 in influent, whereas total prescription pharmaceutical concentrations were as much as 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 ng L−1 in leachate and 90
700 ng L−1 in leachate and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 in influent (Table 2). Although most pharmaceuticals were detected at higher concentrations in influent than in leachate, maximum concentrations for three nonprescription pharmaceuticals (nicotine, cotinine, lidocaine) and five prescription pharmaceuticals (amphetamine, thiabendazole, carisoprodol, fluconazole, pentoxifylline) were over an order of magnitude higher in leachate than in influent (Fig. ESI-5 and ESI-6†). Previous research showed that these nonprescription and prescription pharmaceuticals are commonly detected in landfill leachates across the United States at concentrations similar to those in this study.5
000 ng L−1 in influent (Table 2). Although most pharmaceuticals were detected at higher concentrations in influent than in leachate, maximum concentrations for three nonprescription pharmaceuticals (nicotine, cotinine, lidocaine) and five prescription pharmaceuticals (amphetamine, thiabendazole, carisoprodol, fluconazole, pentoxifylline) were over an order of magnitude higher in leachate than in influent (Fig. ESI-5 and ESI-6†). Previous research showed that these nonprescription and prescription pharmaceuticals are commonly detected in landfill leachates across the United States at concentrations similar to those in this study.5
To evaluate the effects of leachate compositions and concentrations on the WWTP waste stream, individual mean chemical concentrations in influent were calculated and compared between two groups, WWTPs that received leachate (WWTP A, B, and C) and WWTPs with no leachate inputs (WWTP D and E). There were 101 chemicals with concentrations allowing comparisons between these groups, of which 86 target-organic chemicals (85%) had higher mean concentrations in influents that received leachate and only 15 chemicals (15%) that had higher mean concentrations in influents that did not receive leachate. NVDOC concentrations were as high as 97.2 mg L−1 in influent that received leachate and 65.8 mg L−1 in influent that did not receive leachate (Table 2). Of all the chemical classes, PFAS were detected more frequently in influent that received leachate than in influent that did not receive leachate (Fig. ESI-1A†). For the 39 PFAS detected across all samples, 21 PFAS (54%) were detected in influent that received leachate, whereas only 6 PFAS (15%) were detected in influent that did not receive leachate. The maximum PFAS concentration was over three times greater in influent that received leachate (3360 ng L−1) than maximum PFAS concentration in influent that did not receive leachate (1120 ng L−1). Bisphenol A concentrations were three orders of magnitude higher in influent that received leachate (range was from 1190 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ng L−1) than concentrations in influent that did not receive leachate (range was from 258 to 618 ng L−1) (Table 2).
300 ng L−1) than concentrations in influent that did not receive leachate (range was from 258 to 618 ng L−1) (Table 2).
Our results agree with previous research showing that landfill leachates and WWTP influents consist of an extensive mixture of organic chemicals that are of concern to human and ecosystem health.2–9 Our study indicates that disposal of landfill leachate into WWTPs contributes substantially to concentrations of numerous PFAS (e.g. PFOA, PFOS, perfluorodecanoic acid (PFDA), PFHxA, methyl perfluorobutane sulfonamido acetic acid (MeFBSAA), ethyl perfluoropentane sulfonamido acetic acid (EtFPeSAA), 3-perfluoropentyl propanoic acid (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (FPePA), and 2-perfluorooctylethanoic acid (FOEA)), NVDOC, bisphenol A, and some pharmaceuticals (e.g. amphetamine, thiabendazole, carbamazepine, carisoprodol, fluconazole, pentoxifylline, nicotine, cotinine, and lidocaine) in WWTP influents. Many of the organic chemicals (e.g. PFOA, perfluoropentanoic acid, and perfluorobutanesulfonate, carbamazepine, carisoprodol, fluconazole, phenytoin) observed in high concentrations in leachate in this study are resistant to biotic transformation and, thus, potentially undergo little reduction from biological treatment in a WWTP. Whereas this is the first such study to measure the inputs of PFAS and pharmaceutical compositions and concentrations to municipal wastewaters from disposal of landfill leachate, some limitations exist, including (1) a small sample size and sampling network (three landfills and five WWTPs) confined to a small region in Florida; (2) the WWTPs varied in size, daily treatment volumes, and leachate volumes; (3) concentrations of pharmaceuticals were determined from filtered samples, thus the total pharmaceutical concentrations and loads in influent are likely biased low; and (4) two of the three sampled landfills used aeration as a leachate treatment method prior to disposal to WWTP. Previous research has shown that leachate production and strength (in terms of organic-chemical detections and concentrations) can vary considerably based on location, climate region, and leachate handling practices.5 In addition, untreated leachate can have significantly greater organic-chemical concentrations than those in treated leachate.4
3) (FPePA), and 2-perfluorooctylethanoic acid (FOEA)), NVDOC, bisphenol A, and some pharmaceuticals (e.g. amphetamine, thiabendazole, carbamazepine, carisoprodol, fluconazole, pentoxifylline, nicotine, cotinine, and lidocaine) in WWTP influents. Many of the organic chemicals (e.g. PFOA, perfluoropentanoic acid, and perfluorobutanesulfonate, carbamazepine, carisoprodol, fluconazole, phenytoin) observed in high concentrations in leachate in this study are resistant to biotic transformation and, thus, potentially undergo little reduction from biological treatment in a WWTP. Whereas this is the first such study to measure the inputs of PFAS and pharmaceutical compositions and concentrations to municipal wastewaters from disposal of landfill leachate, some limitations exist, including (1) a small sample size and sampling network (three landfills and five WWTPs) confined to a small region in Florida; (2) the WWTPs varied in size, daily treatment volumes, and leachate volumes; (3) concentrations of pharmaceuticals were determined from filtered samples, thus the total pharmaceutical concentrations and loads in influent are likely biased low; and (4) two of the three sampled landfills used aeration as a leachate treatment method prior to disposal to WWTP. Previous research has shown that leachate production and strength (in terms of organic-chemical detections and concentrations) can vary considerably based on location, climate region, and leachate handling practices.5 In addition, untreated leachate can have significantly greater organic-chemical concentrations than those in treated leachate.4
3.2 Potential landfill leachate contribution to WWTP influent organic loads
Total target-organic-chemical loads in leachate (12.4 to 38.9 g d−1) were substantially less than loads in influent (9790 to 395![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g d−1), mostly due to larger flow volumes and large concentrations of 3-beta-coprostanol and cholesterol, which are common in sewer influents (Fig. 1 and Table 2). Whereas the total volume of landfill leachate disposed (sum of all 3 sites: 1.6 million L d−1) only accounted for 1.7% of the total daily flow into the paired WWTP (95.7 Million L d−1), the contribution of total PFAS (33.1 g d−1) and NVDOC (514
000 g d−1), mostly due to larger flow volumes and large concentrations of 3-beta-coprostanol and cholesterol, which are common in sewer influents (Fig. 1 and Table 2). Whereas the total volume of landfill leachate disposed (sum of all 3 sites: 1.6 million L d−1) only accounted for 1.7% of the total daily flow into the paired WWTP (95.7 Million L d−1), the contribution of total PFAS (33.1 g d−1) and NVDOC (514![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g d−1) load in leachate accounted for 18% and 10%, respectively, of the total PFAS (215 g d−1) and NVDOC (5
000 g d−1) load in leachate accounted for 18% and 10%, respectively, of the total PFAS (215 g d−1) and NVDOC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590
590![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g d−1) load in paired influent samples [method of % leachate load (LL) contribution to influent load (IL) = ∑ LL/(∑ IL − ∑ LL) × 100; Table ESI-5†]. Total PFAS loads across all samples ranged from 0.783 to 30.5 g d−1 in leachate and 18.2 to 174 g d−1 in influent (Fig. ESI-1C† and Table 2). NVDOC loads were as large as 451
000 g d−1) load in paired influent samples [method of % leachate load (LL) contribution to influent load (IL) = ∑ LL/(∑ IL − ∑ LL) × 100; Table ESI-5†]. Total PFAS loads across all samples ranged from 0.783 to 30.5 g d−1 in leachate and 18.2 to 174 g d−1 in influent (Fig. ESI-1C† and Table 2). NVDOC loads were as large as 451![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g d−1 in leachate and 9
000 g d−1 in leachate and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g d−1 in influent. Total loads in leachate samples for perfluorobutanesulfonate (PFBS, 3.53 g d−1), MeFPeSAA (1.39 g d−1), PFOS (1.37 g d−1), and methyl perfluorohexane sulfonamido acetic acid (MeFHxSAA, 0.530 g d−1) were larger than corresponding loads in paired influent samples. These PFAS were not detected in the two WWTP influent samples that did not receive leachate (Table ESI-6†).
000 g d−1 in influent. Total loads in leachate samples for perfluorobutanesulfonate (PFBS, 3.53 g d−1), MeFPeSAA (1.39 g d−1), PFOS (1.37 g d−1), and methyl perfluorohexane sulfonamido acetic acid (MeFHxSAA, 0.530 g d−1) were larger than corresponding loads in paired influent samples. These PFAS were not detected in the two WWTP influent samples that did not receive leachate (Table ESI-6†).
Loads for nonprescription and prescription pharmaceuticals were substantially lower in leachate (1.97 to 3.09 g d−1 and 0.482 to 2.49 g d−1, respectively) than in influent (752 to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g d−1 and 482 to 10
200 g d−1 and 482 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g d−1, respectively; Table 2). Even though leachate provided small pharmaceutical load to influents (<1% of the total load contribution to influents), there were 9 individual pharmaceuticals that had total loads that contributed a range of 1 to 25% to paired influents (Table ESI-5†). The total carisoprodol load (0.997 g d−1, Table ESI-6†) in leachate contributed 25% of the carisoprodol load (4.08 g d−1) in paired influents, thiabendazole (0.456 g d−1, 19%), metaxalone (0.338 g d−1, 8%), and lidocaine (2.74 g d−1, 5%). Maximum total loads for hormones were substantially less in leachate (cis-androsterone <0.01 g d−1) than loads in influent (cis-androsterone 1050 g d−1).
700 g d−1, respectively; Table 2). Even though leachate provided small pharmaceutical load to influents (<1% of the total load contribution to influents), there were 9 individual pharmaceuticals that had total loads that contributed a range of 1 to 25% to paired influents (Table ESI-5†). The total carisoprodol load (0.997 g d−1, Table ESI-6†) in leachate contributed 25% of the carisoprodol load (4.08 g d−1) in paired influents, thiabendazole (0.456 g d−1, 19%), metaxalone (0.338 g d−1, 8%), and lidocaine (2.74 g d−1, 5%). Maximum total loads for hormones were substantially less in leachate (cis-androsterone <0.01 g d−1) than loads in influent (cis-androsterone 1050 g d−1).
These results indicate that even though the total volume of leachate disposed was 1.7% of the daily flow into the receiving WWTP, leachate contributed substantially to loads of select PFAS (e.g., ethyl perfluorooctane sulfonamide acetic acid (EtFOSAA), 2H-perfluoro-2-hexenoic acid (FBUEA), MeFHxSAA, MeFPeSAA, PFBS, PFHxS, PFOS), NVDOC, carisoprodol, thiabendazole, metaxalone, and lidocaine to influent. Landfill C disposed the largest volume of leachate (1.54 million L d−1), accounting for 11.6% of the daily flow (13.2 million L d−1) into paired WWTP C and had a larger PFAS load in leachate (30.5 g d−1) than influent (18.2 g d−1). In addition, the NVDOC load in leachate (450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g d−1) was approximately half of the corresponding NVDOC load in influent at the receiving WWTP C. This nearly two times greater PFAS load in leachate and the NVDOC load in leachate accounting for half of NVDOC load in receiving influent, indicates that relatively higher volumetric leachate-to-wastewater influent ratios >0.10 can be one important factor for overall contaminant contribution into WWTPs. In addition, these results show there is much variability in contaminant contribution to influents from disposal of leachate from widely different individual chemical concentrations in leachates as well as different leachate management, treatment, and disposal volumes.
000 g d−1) was approximately half of the corresponding NVDOC load in influent at the receiving WWTP C. This nearly two times greater PFAS load in leachate and the NVDOC load in leachate accounting for half of NVDOC load in receiving influent, indicates that relatively higher volumetric leachate-to-wastewater influent ratios >0.10 can be one important factor for overall contaminant contribution into WWTPs. In addition, these results show there is much variability in contaminant contribution to influents from disposal of leachate from widely different individual chemical concentrations in leachates as well as different leachate management, treatment, and disposal volumes.
3.3 Landfill leachate contribution to WWTP effluent organic concentrations and loads
For the 203 analyzed target-organic chemicals, 56 (28%) were detected overall in effluents and were approximately half of those detected overall in the corresponding influent samples. The number of target-organic chemicals detected within each of the five effluent samples ranged from 19 to 46 (Table 2). Total target-organic concentration in effluent ranged from 3290 to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 ng L−1 (Fig. 2A). Total target-organic-chemical loads in effluent ranged from 41.9 to 2470 g d−1 and were ∼2-orders of magnitude less than influent loads. Prescription pharmaceuticals accounted for 59% of the total target-organic chemical concentration in effluent samples, followed by PFASs (17%), nonprescription (11%), 3-beta-coprostanol (7%), and cholesterol (7%). NVDOC concentrations in effluent ranged from 6.8 to 15.3 mg L−1. NVDOC loads ranged from 95
600 ng L−1 (Fig. 2A). Total target-organic-chemical loads in effluent ranged from 41.9 to 2470 g d−1 and were ∼2-orders of magnitude less than influent loads. Prescription pharmaceuticals accounted for 59% of the total target-organic chemical concentration in effluent samples, followed by PFASs (17%), nonprescription (11%), 3-beta-coprostanol (7%), and cholesterol (7%). NVDOC concentrations in effluent ranged from 6.8 to 15.3 mg L−1. NVDOC loads ranged from 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 to 1
300 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520
520![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g d−1 in effluent and were ∼83% less than influent NVDOC loads. The NVDOC discharged in effluent could have important implications to the transport of co-contaminants by forming contaminant-NVDOC complexes. Previous studies have documented that dissolved organic matter can facilitate the fate and transport of co-contaminants such as arsenic, mercury, pharmaceuticals, and personal care products.9,51–53 Although characterizing the composition of the NVDOC from the landfills, and in influent and effluent, was beyond the scope of this study, the substantial concentrations of NVDOC in effluent warrant consideration as potentially important co-contamination.
000 g d−1 in effluent and were ∼83% less than influent NVDOC loads. The NVDOC discharged in effluent could have important implications to the transport of co-contaminants by forming contaminant-NVDOC complexes. Previous studies have documented that dissolved organic matter can facilitate the fate and transport of co-contaminants such as arsenic, mercury, pharmaceuticals, and personal care products.9,51–53 Although characterizing the composition of the NVDOC from the landfills, and in influent and effluent, was beyond the scope of this study, the substantial concentrations of NVDOC in effluent warrant consideration as potentially important co-contamination.
Of the 39 PFAS detected across all samples, only 12 (31%) were detected in effluent and a 35% PFAS load reduction was observed between influent and effluent samples. Total PFAS concentrations in effluent ranged from 330 to 2110 ng L−1 (Table 2, Fig. ESI-1A and B†) and loads ranged from 4.36 to 211 g d−1 (Fig. ESI-1C†). For some PFAS such as PFOA, effluent loads can exceed influent loads due to transformation of precursor compounds during biological treatment.54,55 Total effluent loads for PFOA (300 g d−1), PFHxA (27.9 g d−1), perfluoropentanoic acid (PFPeA; 19.3 g d−1), PFHpA (18.6 g d−1), MeFBSAA (13.3 g d−1), PFBS (4.27 g d−1), and PFHxS (2.36 g d−1) were larger in corresponding influent loads (Table ESI-6†), and consistent with load trends previously reported.56,57
For the 79 nonprescription and prescription pharmaceuticals and hormones detected across all samples, 44 (56%) were detected in effluent. Total concentrations for detected nonprescription pharmaceuticals in effluent ranged from 9.00 to 2450 ng L−1, whereas prescription pharmaceutical concentrations ranged from 1400 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ng L−1 (Table 2). Loads for detected total nonprescription pharmaceuticals in effluent ranged from 0.068 to 319 g d−1, whereas loads for prescription pharmaceuticals were larger and ranged from 26.6 to 1610 g d−1. There was 99% load reduction between influent and effluent samples for hormones, 98% for nonprescription pharmaceuticals, and 90% reduction for prescription pharmaceuticals. Total loads in effluent samples for carbamazepine (17.2 g d−1), fluconazole (20.8 g d−1), phenytoin (8.75 g d−1), and metaxalone (4.16 g d−1) indicated the most resistance to treatment, with <15% load reduction between paired influent and effluent samples. In addition, the load reductions for carisoprodol and thiabendazole, both common in leachate, were 24% and 38%, respectively, between paired influent and effluent samples. Other chemicals common in leachate that resulted in larger reductions between paired influent and effluent samples included lidocaine (85% load reduction), while 4,4′-bisphenol F, amphetamine, bisphenol A, cotinine, nicotine, and pentoxifylline had even greater reductions as they were not detected in any effluent samples. Two potential mechanisms for reductions in organic concentration between influent and effluent samples include chemical sorption to particles and subsequent particle removal from the waste stream and biodegradation during biological treatment.58
800 ng L−1 (Table 2). Loads for detected total nonprescription pharmaceuticals in effluent ranged from 0.068 to 319 g d−1, whereas loads for prescription pharmaceuticals were larger and ranged from 26.6 to 1610 g d−1. There was 99% load reduction between influent and effluent samples for hormones, 98% for nonprescription pharmaceuticals, and 90% reduction for prescription pharmaceuticals. Total loads in effluent samples for carbamazepine (17.2 g d−1), fluconazole (20.8 g d−1), phenytoin (8.75 g d−1), and metaxalone (4.16 g d−1) indicated the most resistance to treatment, with <15% load reduction between paired influent and effluent samples. In addition, the load reductions for carisoprodol and thiabendazole, both common in leachate, were 24% and 38%, respectively, between paired influent and effluent samples. Other chemicals common in leachate that resulted in larger reductions between paired influent and effluent samples included lidocaine (85% load reduction), while 4,4′-bisphenol F, amphetamine, bisphenol A, cotinine, nicotine, and pentoxifylline had even greater reductions as they were not detected in any effluent samples. Two potential mechanisms for reductions in organic concentration between influent and effluent samples include chemical sorption to particles and subsequent particle removal from the waste stream and biodegradation during biological treatment.58
To evaluate the effects of leachate compositions and concentrations on the WWTP waste stream, individual mean chemical concentrations in influent were calculated and compared between two groups: WWTP that received leachate (WWTP A, B, and C) and WWTPs with no leachate inputs (WWTP D and E). In total, there were 56 paired target-organic chemical concentrations of which only 25 (45%) had larger mean concentrations in effluent that had received leachate and 31 (55%) that had larger mean concentrations in effluent that had not received leachate. Whereas the disposal of landfill leachate to influent contributed noticeable and substantial increases to influent compositions and concentrations for some organic chemicals, no differences in compositions and concentrations were apparent in effluents from WWTPs that received leachate compared to those that did not receive leachate.
Conclusions
Even though landfills and WWTPs are known to contain a complex mixture of organic contaminants,4–6,59 they serve as critical and necessary infrastructure to reduce exposure of humans and aquatic organisms to known and emerging contaminants. Landfill leachate is commonly discharged to WWTPs for treatment, yet limited research has been conducted to understand if this disposal practice has measurable effects on many organic-chemical concentrations and loads in receiving WWTPs and in corresponding treated effluents discharged to surface waters. Our study presents important first insights on the understanding of the effects on organic-contaminant concentrations in the untreated and treated stages of municipal waters from disposal of leachate from landfills. The presented data indicates that the disposal of leachate into WWTPs can contribute substantially to the concentrations in WWTP influents for some pharmaceutical and many PFAS chemicals, but no clear differences were observed between effluent concentrations for WWTPs with or without landfill leachate input. Although this study employed a comprehensive suite of 203 target-organic chemicals for characterization in leachate, that is probably a small fraction of the total contaminants and complex mixtures in leachate. These contaminants and contaminant mixtures could be resistant to biological treatment and could potentially have negative health effects to receptors in the environment. Further study is needed that includes both non-target analysis60 and bioassays or ecotoxicity tools61,62 to generate bulk toxicity data for comparison of treated effluents between WWTPs that receive leachate and WWTPs that do not receive leachate.Although the total volume of leachate disposed was just 1.7% of the total daily flow into the paired WWTP, there was substantial load contribution of PFAS, NVDOC, and select pharmaceuticals to WWTP influents from the disposal of landfill leachate. Consistent with load trends previously reported,55–57 total effluent loads for PFOA, PFHxA, PFPeA, PFHpA, MeFBSAA, PFBS, and PFHxS were larger than corresponding influent loads, indicating microbial degradation of transformation precursor compounds during the treatment process can result in highly persistent and even toxic compounds.63,64 Due to the well-documented role of NVDOC in facilitating transport of co-contaminants, the potential role of leachate-derived NVDOC to the persistence and bioavailability of these PFAS compounds into the waste stream warrants further study. Total load reductions observed between influent and effluent samples were at 99% for hormones, 98% for nonprescription pharmaceuticals, 90% for prescription pharmaceuticals, and 35% for PFAS. Due to the increased practice of land application of biosolids as cost-effective and useful method of disposal, additional research is needed to determine if the source of reductions in organic concentrations between influent and effluent samples is from biodegradation during biological treatment or chemical sorption to particles and subsequent particle removal from the treatment and into biosolids.
Notes
All analytical data are available in the associated data release at https://doi.org/10.5066/P97LMTKZ (Romanok et al., 2020).65Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The research was supported by U.S. Geological Survey (USGS) Toxic Substances Hydrology Program, USGS Contaminant Biology Program, USGS Water Mission Area, National Science Foundation [CBET#1438416], and by the Environmental Research & Education Foundation doctoral scholarship program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The authors would like to thank many staff from the USGS; Jeanne Jaeschke for analyses of NVDOC samples, the National Water Quality Laboratory for analyses of samples for pharmaceutical and hormone chemicals, Windy Brace and Sandra Kinnaman for establishing sites in USGS national database, and William Foreman, John Gordon, and anonymous reviewers for review of manuscript.References
- U.S. Environmental Protection Agency, Advancing Sustainable Materials Management: 2015 Fact Sheet, https://www.epa.gov/sites/production/files/2018-07/documents/2015_smm_msw_factsheet_07242018_fnl_508_002.pdf (accessed Jun 26, 2019) Search PubMed.
- H. Hamid, L. Y. Li and J. R. Grace, Review of the Fate and Transformation of Per- and Polyfluoroalkyl Substances (PFASs) in Landfills, Environ. Pollut., 2018, 235, 74–84, DOI:10.1016/j.envpol.2017.12.030.
- B. O. Clarke, T. Anumol, M. Barlaz and S. A. Snyder, Investigating Landfill Leachate as a Source of Trace Organic Pollutants, Chemosphere, 2015, 127, 269–275, DOI:10.1016/j.chemosphere.2015.02.030.
- J. R. Masoner, D. W. Kolpin, E. T. Furlong, I. M. Cozzarelli and J. L. Gray, Landfill Leachate as a Mirror of Today's Disposable Society: Pharmaceuticals and Other Contaminants of Emerging Concern in Final Leachate from Landfills in the Conterminous United States, Environ. Toxicol. Chem., 2016, 35(4), 906–918, DOI:10.1002/etc.3219.
- J. R. Masoner, D. W. Kolpin, E. T. Furlong, I. M. Cozzarelli, J. L. Gray and E. A. Schwab, Contaminants of Emerging Concern in Fresh Leachate from Landfills in the Conterminous United States, Environ. Sci.: Processes Impacts, 2014, 16(10), 2335–2354, 10.1039/c4em00124a.
- J. R. Lang, B. M. Allred, J. A. Field, J. W. Levis and M. A. Barlaz, National Estimate of Per- and Polyfluoroalkyl Substance (PFAS) Release to U.S. Municipal Landfill Leachate, Environ. Sci. Technol., 2017, 51(4), 2197–2205, DOI:10.1021/acs.est.6b05005.
- T. Eggen, M. Moeder and A. Arukwe, Municipal Landfill Leachates: A Significant Source for New and Emerging Pollutants, Sci. Total Environ., 2010, 408(21), 5147–5157, DOI:10.1016/j.scitotenv.2010.07.049.
- W. J. Andrews, J. R. Masoner and I. M. Cozzarelli, Emerging Contaminants at a Closed and an Operating Landfill in Oklahoma, Groundwater Monit. Rem., 2012, 32(1), 120–130, DOI:10.1111/j.1745-6592.2011.01373.x.
- Z. Wei, T. Xu and D. Zhao, Treatment of Per- and Polyfluoroalkyl Substances in Landfill Leachate: Status, Chemistry and Prospects, Environ. Sci.: Water Res. Technol., 2019, 5(11), 1814–1835, 10.1039/C9EW00645A.
- A. Vellinga, S. Cormican, J. Driscoll, M. Furey, M. O'Sullivan and M. Cormican, Public Practice Regarding Disposal of Unused Medicines in Ireland, Sci. Total Environ., 2014, 478, 98–102, DOI:10.1016/j.scitotenv.2014.01.085.
- G. Hawkins, The Performativity of Food Packaging: Market Devices, Waste Crisis and Recycling, Sociol. Rev., 2012, 60(S2), 66–83, DOI:10.1111/1467-954X.12038.
- M. Kotthoff, J. Müller, H. Jürling, M. Schlummer and D. Fiedler, Perfluoroalkyl and Polyfluoroalkyl Substances in Consumer Products, Environ. Sci. Pollut. Res., 2015, 22(19), 14546–14559, DOI:10.1007/s11356-015-4202-7.
- B. D. Key, R. D. Howell and C. S. Criddle, Fluorinated Organics in the Biosphere, Environ. Sci. Technol., 1997, 31(9), 2445–2454, DOI:10.1021/es961007c.
- K. Li, P. Gao, P. Xiang, X. Zhang, X. Cui and L. Q. Ma, Molecular Mechanisms of PFOA-Induced Toxicity in Animals and Humans: Implications for Health Risks, Environ. Int., 2017, 99, 43–54, DOI:10.1016/j.envint.2016.11.014.
- R. Crebelli, S. Caiola, L. Conti, E. Cordelli, G. De Luca, E. Dellatte, P. Eleuteri, N. Iacovella, P. Leopardi and F. Marcon, et al. Can Sustained Exposure to PFAS Trigger a Genotoxic Response? A Comprehensive Genotoxicity Assessment in Mice after Subacute Oral Administration of PFOA and PFBA, Regul. Toxicol. Pharmacol., 2019, 106, 169–177, DOI:10.1016/j.yrtph.2019.05.005.
- Agency for Toxic Substances and Disease Registry, PFAS and Their Health Effects https://www.atsdr.cdc.gov/pfas/PFAS-health-effects.html (accessed Oct 10, 2019) Search PubMed.
- K. Winkens, R. Vestergren, U. Berger and I. T. Cousins, Early Life Exposure to Per- and Polyfluoroalkyl Substances (PFASs): A Critical Review, Emerg. Contam., 2017, 3(2), 55–68, DOI:10.1016/j.emcon.2017.05.001.
- J. Corcoran, M. J. Winter and C. R. Tyler, Pharmaceuticals in the Aquatic Environment: A Critical Review of the Evidence for Health Effects in Fish, Crit. Rev. Toxicol., 2010, 40(4), 287–304, DOI:10.3109/10408440903373590.
- S. E. Musson and T. G. Townsend, Pharmaceutical Compound Content of Municipal Solid Waste, J. Hazard. Mater., 2009, 162(2), 730–735, DOI:10.1016/j.jhazmat.2008.05.089.
- N. J. Niemuth and R. D. Klaper, Emerging Wastewater Contaminant Metformin Causes Intersex and Reduced Fecundity in Fish, Chemosphere, 2015, 135, 38–45, DOI:10.1016/j.chemosphere.2015.03.060.
- K. A. Kidd, P. J. Blanchfield, K. H. Mills, V. P. Palace, R. E. Evans, J. M. Lazorchak and R. W. Flick, Collapse of a Fish Population after Exposure to a Synthetic Estrogen, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(21), 8897–8901, DOI:10.1073/pnas.0609568104.
- K. A. Kidd, M. J. Paterson, M. D. Rennie, C. L. Podemski, D. L. Findlay, P. J. Blanchfield and K. Liber, Direct and Indirect Responses of a Freshwater Food Web to a Potent Synthetic Oestrogen, Philos. Trans. R. Soc., B, 2014, 369, 1656, DOI:10.1098/rstb.2013.0578.
- M. I. Vasquez, A. Lambrianides, M. Schneider, K. Kümmerer and D. Fatta-Kassinos, Environmental Side Effects of Pharmaceutical Cocktails: What We Know and What We Should Know, J. Hazard. Mater., 2014, 279, 169–189, DOI:10.1016/j.jhazmat.2014.06.069.
- V. Futran Fuhrman, A. Tal and S. Arnon, Why Endocrine Disrupting Chemicals (EDCs) Challenge Traditional Risk Assessment and How to Respond, J. Hazard. Mater., 2015, 286, 589–611, DOI:10.1016/j.jhazmat.2014.12.012.
- B. G. Loganathan, K. S. Sajwan, E. Sinclair, K. Senthil Kumar and K. Kannan, Perfluoroalkyl Sulfonates and Perfluorocarboxylates in Two Wastewater Treatment Facilities in Kentucky and Georgia, Water Res., 2007, 41(20), 4611–4620, DOI:10.1016/j.watres.2007.06.045.
- M. M. Schultz, C. P. Higgins, C. A. Huset, R. G. Luthy, D. F. Barofsky and J. A. Field, Fluorochemical Mass Flows in a Municipal Wastewater Treatment Facility, Environ. Sci. Technol., 2006, 40(23), 7350–7357, DOI:10.1021/es061025m.
- X. Dauchy, V. Boiteux, C. Bach, A. Colin, J. Hemard, C. Rosin and J.-F. Munoz, Mass Flows and Fate of Per- and Polyfluoroalkyl Substances (PFASs) in the Wastewater Treatment Plant of a Fluorochemical Manufacturing Facility, Sci. Total Environ., 2017, 576, 549–558, DOI:10.1016/j.scitotenv.2016.10.130.
- R. Guo, W.-J. Sim, E.-S. Lee, J.-H. Lee and J.-E. Oh, Evaluation of the Fate of Perfluoroalkyl Compounds in Wastewater Treatment Plants, Water Res., 2010, 44(11), 3476–3486, DOI:10.1016/j.watres.2010.03.028.
- T.-T. Pham and S. Proulx, PCBs and PAHs in the Montreal Urban Community (Quebec, Canada) Wastewater Treatment Plant and in the Effluent Plume in the St Lawrence River, Water Res., 1997, 31(8), 1887–1896, DOI:10.1016/S0043-1354(97)00025-0.
- S. Sun, L. Jia, B. Li, A. Yuan, L. Kong, H. Qi, W. Ma, A. Zhang and Y. Wu, The Occurrence and Fate of PAHs over Multiple Years in a Wastewater Treatment Plant of Harbin, Northeast China, Sci. Total Environ., 2018, 624, 491–498, DOI:10.1016/j.scitotenv.2017.12.029.
- A. M. Sadaria, S. D. Supowit and R. U. Halden, Mass Balance Assessment for Six Neonicotinoid Insecticides During Conventional Wastewater and Wetland Treatment: Nationwide Reconnaissance in United States Wastewater, Environ. Sci. Technol., 2016, 50(12), 6199–6206, DOI:10.1021/acs.est.6b01032.
- J. Sánchez-Avila, J. Bonet, G. Velasco and S. Lacorte, Determination and Occurrence of Phthalates, Alkylphenols, Bisphenol A, PBDEs, PCBs and PAHs in an Industrial Sewage Grid Discharging to a Municipal Wastewater Treatment Plant, Sci. Total Environ., 2009, 407(13), 4157–4167, DOI:10.1016/j.scitotenv.2009.03.016.
- P. Gago-Ferrero, M. Gros, L. Ahrens and K. Wiberg, Impact of On-Site, Small and Large Scale Wastewater Treatment Facilities on Levels and Fate of Pharmaceuticals, Personal Care Products, Artificial Sweeteners, Pesticides, and Perfluoroalkyl Substances in Recipient Waters, Sci. Total Environ., 2017, 601–602, 1289–1297, DOI:10.1016/j.scitotenv.2017.05.258.
- W. Zhang, Y. Zhang, S. Taniyasu, L. W. Y. Yeung, P. K. S. Lam, J. Wang, X. Li, N. Yamashita and J. Dai, Distribution and Fate of Perfluoroalkyl Substances in Municipal Wastewater Treatment Plants in Economically Developed Areas of China, Environ. Pollut., 2013, 176, 10–17, DOI:10.1016/j.envpol.2012.12.019.
- Y. Yang, Y. S. Ok, K.-H. Kim, E. E. Kwon and Y. F. Tsang, Occurrences and Removal of Pharmaceuticals and Personal Care Products (PPCPs) in Drinking Water and Water/Sewage Treatment Plants: A Review, Sci. Total Environ., 2017, 596–597, 303–320, DOI:10.1016/j.scitotenv.2017.04.102.
- Florida Department of Environmental Protection, General Facts and Statistics about Wastewater in Florida https://floridadep.gov/water/domestic-wastewater/content/general-facts-and-statistics-about-wastewater-florida Search PubMed.
- P. Guerra, M. Kim, L. Kinsman, T. Ng, M. Alaee and S. A. Smyth, Parameters Affecting the Formation of Perfluoroalkyl Acids during Wastewater Treatment, J. Hazard. Mater., 2014, 272, 148–154, DOI:10.1016/j.jhazmat.2014.03.016.
- R. B. Brennan, E. Clifford, C. Devroedt, L. Morrison and M. G. Healy, Treatment of Landfill Leachate in Municipal Wastewater Treatment Plants and Impacts on Effluent Ammonium Concentrations, J. Environ. Manage., 2017, 188, 64–72, DOI:10.1016/j.jenvman.2016.11.055.
- R. Zhao, A. Gupta, J. T. Novak, C. D. Goldsmith and N. Driskill, Characterization and Treatment of Organic Constituents in Landfill Leachates That Influence the UV Disinfection in the Publicly Owned Treatment Works (POTWs), J. Hazard. Mater., 2013, 258–259, 1–9, DOI:10.1016/j.jhazmat.2013.04.026.
- S. C. Bolyard and D. R. Reinhart, Evaluation of Leachate Dissolved Organic Nitrogen Discharge Effect on Wastewater Effluent Quality, Waste Manage., 2017, 65, 47–53, DOI:10.1016/j.wasman.2017.03.025.
- B. M. Allred, J. R. Lang, M. A. Barlaz and J. A. Field, Orthogonal Zirconium Diol/C18 Liquid Chromatography–Tandem Mass Spectrometry Analysis of Poly and Perfluoroalkyl Substances in Landfill Leachate, J. Chromatogr. A, 2014, 1359, 202–211, DOI:10.1016/j.chroma.2014.07.056.
- W. T. Foreman, J. L. Gray, R. C. ReVello, C. E. Lindley, S. A. Losche and L. B. Barber, Determination of Steroid Hormones and Related Compounds in Filtered and Unfiltered Water by Solid-Phase Extraction, Derivatization, and Gas Chromatography with Tandem Mass Spectrometry, U.S. Geological Survey, 2012 Search PubMed.
- E. T. Furlong, C. J. Kanagy, L. K. Kanagy, L. J. Coffey and M. R. Burkhardt, Determination of Human-Use Pharmaceuticals in Filtered Water by Direct Aqueous Injection–High-Performance Liquid Chromatography/Tandem Mass Spectrometry, U.S. Geological Survey, 2014 Search PubMed.
- I. M. Cozzarelli, J. K. Bohlke, J. R. Masoner, G. N. Breit, M. M. Lorah, M. L. W. Tuttle and J. B. Jaeschke, Biogeochemical Evolution of a Landfill Leachate Plume, Norman, Oklahoma, Groundwater, 2011, 49(5), 663–687, DOI:10.1111/j.1745-6584.2010.00792.x.
- H. Yan, I. T. Cousins, C. Zhang and Q. Zhou, Perfluoroalkyl Acids in Municipal Landfill Leachates from China: Occurrence, Fate during Leachate Treatment and Potential Impact on Groundwater, Sci. Total Environ., 2015, 524–525, 23–31, DOI:10.1016/j.scitotenv.2015.03.111.
- J. P. Benskin, B. Li, M. G. Ikonomou, J. R. Grace and L. Y. Li, Per- and Polyfluoroalkyl Substances in Landfill Leachate: Patterns, Time Trends, and Sources, Environ. Sci. Technol., 2012, 46(21), 11532–11540, DOI:10.1021/es302471n.
- A. Y.-C. Lin, S. C. Panchangam and P.-S. Ciou, High Levels of Perfluorochemicals in Taiwan's Wastewater Treatment Plants and Downstream Rivers Pose Great Risk to Local Aquatic Ecosystems, Chemosphere, 2010, 80(10), 1167–1174, DOI:10.1016/j.chemosphere.2010.06.018.
- C. G. Pan, Y. S. Liu and G. G. Ying, Perfluoroalkyl Substances (PFASs) in Wastewater Treatment Plants and Drinking Water Treatment Plants: Removal Efficiency and Exposure Risk, Water Res., 2016, 106, 562–570, DOI:10.1016/j.watres.2016.10.045.
- U. Eriksson, P. Haglund and A. Kärrman, Contribution of Precursor Compounds to the Release of Per- and Polyfluoroalkyl Substances (PFASs) from Waste Water Treatment Plants (WWTPs), J. Environ. Sci., 2017, 61, 80–90, DOI:10.1016/j.jes.2017.05.004.
- C. Zhang, H. Yan, F. Li and Q. Zhou, Occurrence and Fate of Perfluorinated Acids in Two Wastewater Treatment Plants in Shanghai, China, Environ. Sci. Pollut. Res., 2015, 22(3), 1804–1811, DOI:10.1007/s11356-013-2044-8.
- S. Hernandez-Ruiz, L. Abrell, S. Wickramasekara, B. Chefetz and J. Chorover, Quantifying PPCP Interaction with Dissolved Organic Matter in Aqueous Solution: Combined Use of Fluorescence Quenching and Tandem Mass Spectrometry, Water Res., 2012, 46(4), 943–954, DOI:10.1016/j.watres.2011.11.061.
- Y. Yamashita and R. Jaffé, Characterizing the Interactions between Trace Metals and Dissolved Organic Matter Using Excitation-Emission Matrix and Parallel Factor Analysis, Environ. Sci. Technol., 2008, 42(19), 7374–7379, DOI:10.1021/es801357h.
- Y. Zhang, B. Zhang, Y. He, O. Lev, G. Yu, G. Shen and S. Hu, DOM as an Indicator of Occurrence and Risks of Antibiotics in a City-River-Reservoir System with Multiple Pollution Sources, Sci. Total Environ., 2019, 686, 276–289, DOI:10.1016/j.scitotenv.2019.05.439.
- E. Sinclair and K. Kannan, Mass Loading and Fate of Perfluoroalkyl Surfactants in Wastewater Treatment Plants, Environ. Sci. Technol., 2006, 40(5), 1408–1414, DOI:10.1021/es051798v.
- J. Liu and S. Mejia Avendaño, Microbial Degradation of Polyfluoroalkyl Chemicals in the Environment: A Review, Environ. Int., 2013, 61, 98–114, DOI:10.1016/j.envint.2013.08.022.
- T. L. Coggan, D. Moodie, A. Kolobaric, D. Szabo, J. Shimeta, N. D. Crosbie, E. Lee, M. Fernandes and B. O. Clarke, An Investigation into Per- and Polyfluoroalkyl Substances (PFAS) in Nineteen Australian Wastewater Treatment Plants (WWTPs), Heliyon, 2019, 5(8), e02316, DOI:10.1016/j.heliyon.2019.e02316.
- H. T. Nguyen, S. L. Kaserzon, P. K. Thai, S. Vijayasarathy, J. Bräunig, N. D. Crosbie, A. Bignert and J. F. Mueller, Temporal Trends of Per- and Polyfluoroalkyl Substances (PFAS) in the Influent of Two of the Largest Wastewater Treatment Plants in Australia, Emerg. Contam., 2019, 5, 211–218, DOI:10.1016/j.emcon.2019.05.006.
- T. A. Ternes, A. Joss and H. Siegrist, Peer Reviewed: Scrutinizing Pharmaceuticals and Personal Care Products in Wastewater Treatment, Environ. Sci. Technol., 2004, 38(20), 392A–399A, DOI:10.1021/es040639t.
- L. Ahrens, M. Shoeib, T. Harner, S. C. Lee, R. Guo and E. J. Reiner, Wastewater Treatment Plant and Landfills as Sources of Polyfluoroalkyl Compounds to the Atmosphere, Environ. Sci. Technol., 2011, 45(19), 8098–8105, DOI:10.1021/es1036173.
- J. Hollender, B. van Bavel, V. Dulio, E. Farmen, K. Furtmann, J. Koschorreck, U. Kunkel, M. Krauss, J. Munthe and M. Schlabach, et al. High Resolution Mass Spectrometry-Based Non-Target Screening Can Support Regulatory Environmental Monitoring and Chemicals Management, Environ. Sci. Eur., 2019, 31(1), 42, DOI:10.1186/s12302-019-0225-x.
- J. M. Conley, N. Evans, M. C. Cardon, L. Rosenblum, L. R. Iwanowicz, P. C. Hartig, K. M. Schenck, P. M. Bradley and V. S. Wilson, Occurrence and In Vitro Bioactivity of Estrogen, Androgen, and Glucocorticoid Compounds in a Nationwide Screen of United States Stream Waters, Environ. Sci. Technol., 2017, 51(9), 4781–4791, DOI:10.1021/acs.est.6b06515.
- E. K. Medlock Kakaley, B. R. Blackwell, M. C. Cardon, J. M. Conley, N. Evans, D. J. Feifarek, E. T. Furlong, S. T. Glassmeyer, L. E. Gray and P. C. Hartig, et al. De Facto Water Reuse: Bioassay Suite Approach Delivers Depth and Breadth in Endocrine Active Compound Detection, Sci. Total Environ., 2020, 699, 134297, DOI:10.1016/j.scitotenv.2019.134297.
- S. Nakayama, K. Harada, K. Inoue, K. Sasaki, B. Seery, N. Saito and A. Koizumi, Distributions of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) in Japan and Their Toxicities, Environ. Sci., 2005, 12(6), 293–313 CAS.
- M. Wielsøe, M. Long, M. Ghisari and E. C. Bonefeld-Jørgensen, Perfluoroalkylated Substances (PFAS) Affect Oxidative Stress Biomarkers in Vitro, Chemosphere, 2015, 129, 239–245, DOI:10.1016/j.chemosphere.2014.10.014.
- K. M. Romanok, J. R. Masoner and P. M. Bradley, Target-chemical concentrations in landfill leachate and wastewater treatment influent and effluent, U.S. Geological Survey, 2020, DOI:10.5066/P97LMTKZ.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI:10.1039/d0ew00045k |
| This journal is © The Royal Society of Chemistry 2020 |