Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of transformation, photodegradation and interaction with glutaraldehyde on the acute toxicity of the biocide DBNPA in cooling tower water†
Thomas V.
Wagner
 *ab,
Rick
Helmus
*ab,
Rick
Helmus
 a,
Elmar
Becker
a,
Huub H. M.
Rijnaarts
b,
Pim
de Voogt
a,
Elmar
Becker
a,
Huub H. M.
Rijnaarts
b,
Pim
de Voogt
 ab,
Alette A. M.
Langenhoff
b and
John R.
Parsons
a
ab,
Alette A. M.
Langenhoff
b and
John R.
Parsons
a
aInstitute for Biodiversity and Ecosystem Dynamics (IBED), University of Amsterdam, P.O. Box 94248, 1092 GE Amsterdam, The Netherlands
bDepartment of Environmental Technology, Wageningen University, P.O. Box 17, 6700 EV Wageningen, The Netherlands. E-mail: thomas.wagner@wur.nl; t.v.wagner@uva.nl; Tel: +31652654362
First published on 6th February 2020
Abstract
The reuse of cooling tower water (CTW) can substantially lower the freshwater footprint of cooling towers. CTW requires desalination before its reuse, and pre-treatment before desalination enhances the desalination efficiency. Constructed wetlands (CWs) have been shown to remove fractions from CTW that hamper physico-chemical desalination technologies, and thus provide adequate pre-treatment. However, CTW contains biocides, and these and their transformation products are potentially toxic to aquatic fauna in surface flow CWs. Therefore, we assessed the acute toxicity to daphnids of CTW which contained the biocide 2,2-dibromo-3-nitrilopropionamide (DBNPA), and the impact of photodegradation and the presence of biocide glutaraldehyde on its toxicity. It was observed that the toxicity of DBNPA in CTW was lower than that reported in the literature, and non-target screening showed that this was due to rapid DBNPA transformation, via 2-monobromo-3-nitrilopropionamide (MBNPA) and nitrilopropionamide (NPA). Photodegradation resulted in an increased CTW toxicity after 1 h of illumination as a result of the formation of new DBNPA transformation products, and subsequent decrease in toxicity after 48 h. The simultaneous presence of DBNPA and glutaraldehyde resulted in the formation of interaction products. Photodegradation did not result in unique interaction products, but increased the rate of interaction product formation, leading to a decrease in toxicity. Due to the toxicity of DBNPA in CTW and the effect of photodegradation on this toxicity, it is not recommended to use a surface flow CW as first treatment step in a multistage CW-system for the treatment of CTW.
Water impactThe reuse of discharged cooling tower significantly lowers the industrial fresh water footprint but requires desalination. A pre-treatment of the cooling tower water by constructed wetlands enhances the desalination efficiency. In this study, we determined the fate and toxic effect of biocides DBNPA and glutaraldehyde in cooling tower water in Daphnia magna that inhabit surface flow constructed wetlands. |
1. Introduction
In industrialized areas around the globe, the fresh water uptake for cooling towers accounts for a significant part of the total industrial fresh water uptake.1–3 After the cooling process, large volumes of cooling tower water (CTW) need to be discharged when the mineral concentration threshold is reached, for instance at an electric conductivity (EC) of >4500 μS cm−1.4 The reuse of brackish CTW after treatment in the cooling tower itself could significantly mitigate future fresh water scarcity problems.3 CTW has to be desalinated prior to its reuse in the cooling tower.4 However, physico-chemical desalination processes are hampered by CTW components, such as phosphate, nitrate and conditioning chemicals (CCs), that induce for instance fouling of reverse osmosis membranes.5 To prevent technical problems, these fractions have to be removed prior to physico-chemical desalination. Constructed wetlands (CWs) are a sustainable pre-treatment option for the removal of these CTW fractions.6CWs are man-made wetland systems that use natural removal processes, such as biodegradation, adsorption, plant uptake and photodegradation, for the removal of contaminants from waste water streams.7–9 These biological, physical and chemical removal pathways occur simultaneously in CWs, and the contribution of each removal pathway to the total removal efficiency of a CW depends on the CW design. Adsorption and biodegradation are relevant removal pathways for conditioning chemicals from CTW in subsurface flow CW designs.10,11 However, biodegradation of these chemicals by CW microorganisms is inhibited by the biocides 2,2-dibromo-3-nitrilopropionamide (DBNPA) and glutaraldehyde that are also present in CTW.10 Furthermore, if DBNPA and glutaraldehyde are both present, they form interaction products as a result of mutual reactions, and these, in turn, decrease the toxicity of the biocides on the CW microorganisms.10
A potential way to mitigate the toxic effect of biocides on the biodegradation in a multistage CW is to remove them before the CTW enters the biologically active stages of a CW. This could be achieved by combining abiotic removal in a surface flow CW with biologically more active CW designs, such as vertical- and/or horizontal subsurface flow CWs. A potential abiotic removal pathway in surface flow CWs is photodegradation.12–14 Photodegradation is the alteration of a chemical structure as a result of the exposure to sunlight. In the aquatic environment, both direct and indirect photodegradation occur. During direct photodegradation, the adsorption of sunlight results in a higher energy state and subsequent transformation of a chemical.15,16 Indirect photodegradation is the alteration of a chemical as a result of the reaction with photochemically excited photosensitizers present in the aquatic system, such as hydroxyl radicals (˙OH), singlet oxygen (1O2) and triplet excited dissolved organic matter (3DOM).13,16
The commonly used biocide DBNPA is known to be susceptible to photodegradation,17,18 and could potentially be removed from CTW in surface-flow CWs. Surface-flow CWs most closely resemble natural wetlands systems and are home to different aqueous and avian animal species. However, DBNPA photodegradation in surface-flow CWs could result in the production of various toxic transformation products.17 Hence, the objective of the present research is threefold: i) to identify whether the discharge of CTW containing DBNPA is toxic to organisms that live in surface flow CWs used for the treatment of CTW, such as Daphnia magna ii) to determine whether photodegradation of DBNPA in surface flow CWs affects the DBNPA toxicity to D. magna iii) to determine whether DBNPA photodegradation result in unique DBNPA transformation products (TPs) and interaction products formed between glutaraldehyde and DBNPA in CTW,10 and whether this has an influence on their combined toxicity to D. magna living in surface-flow CWs.
2. Materials & methods
2.1. Chemicals
DBNPA, glutaraldehyde (25% in H2O), methanol (UHPLC-grade), synthetic sea salt, NaHCO3, CaCl2, NaSeO3, formic acid (100%) and acetic acid (100%) were obtained from Sigma-Aldrich (Zwijndrecht, The Netherlands). NaNO3 (>99%), CaCl2 (>94%), Na2SO4 (>99%), KH2PO4 (>99%), humic acid (sodium salt) (45–70%), benzoic acid (>99.5%) and benzotriazole (>99%) for the preparation of synthetic CTW were obtained from Carl Roth GmbH (Karlsruhe, Germany).2.2. Photodegradation set-up
Photodegradation experiments were performed in 4 different aqueous solutions, with biocide concentrations that are representative for real CTW:19• Synthetic CTW, composed of NaNO3 (83 mg L−1), CaCl2 (830 mg L−1), Na2SO4 (1660 mg L−1), KH2PO4 (10 mg L−1), humic acid (50 mg L−1), benzoic acid (50 mg L−1) and benzotriazole (1 mg L−1) dissolved in tap water. Characteristics of the synthetic CTW are described in Wagner et al. (submitted).
• Synthetic CTW + DBNPA (2.5 mg L−1).
• Synthetic CTW + glutaraldehyde (2.5 mg L−1).
• Synthetic CTW + DBNPA (2.5 mg L−1) and glutaraldehyde (2.5 mg L−1).
The photodegradation experiments were performed in triplicate in cylindrical glass reactors with quartz glass covers to prevent evaporation. These reactors were filled with 25 ml of one of the 4 matrices, placed on a Variomag Telesystem (Thermo Scientific, Breda, The Netherlands) and stirred with a magnetic stirrer at 175 rpm. Sunlight was simulated with 4 35 W xenon lamps (Hella, Nieuwegein, The Netherlands) in a home-build aluminium construction. The wavelength intensity distribution of this lamps and solar light is provided in the supplementary info (ESI;† Fig. S1). Each photodegradation replicate had a corresponding dark control that was wrapped in aluminium foil. Samples for non-target analysis and toxicity analysis were taken after 1, 48 and 96 h.
2.3. Non-target screening for transformation and reaction products
The formation of transformation products of DBNPA and interaction products from the reaction between DBNPA and CTW, DBNPA and glutaraldehyde and DBNPA, glutaraldehyde and CTW was determined by non-target screening. Non-target screening was performed based on the procedures described by Wagner et al.10 on the four different matrices described in section 2.2 and their corresponding dark controls after 1, 48 and 96 h of illumination.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1
MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v:v) was introduced automatically for recalibration of the system. The column oven was kept at 40 °C. Mass spectrometry was performed with positive and negative ESI in separate runs with a resolving power typically of 30.000–60.000 FWHM.
1 v:v) was introduced automatically for recalibration of the system. The column oven was kept at 40 °C. Mass spectrometry was performed with positive and negative ESI in separate runs with a resolving power typically of 30.000–60.000 FWHM.
The workflow of ‘patRoon’ (Fig. S2†) starts with the import of UHPLC-q-ToF-MS/MS measurements. The analyses were converted to open MS data format mzML by DataAnalysis. Subsequently, the replicates were grouped according to the sample names in Table 2 and the blanks for blank subtraction were determined. Afterwards, features were extracted for each measurements with OpenMS using the FeatureFinderMetabo algorithm, aligned according to retention time using the MapAlignerPoseClustering algorithm and grouped using the FeatureLinkerUnlabeled algorithm. To clean up the data, an intensity threshold of 5000 was used, and the features intensity had to be at least five times as high as the blanks. In addition, the feature had to be present in all the replicates of a sample. In the next step, MS and MS/MS data were extracted using mzR. An average m/z window of 0.005 Da was used for clustering ions from multiple spectra along the chromatographic peak to average spectra. The maximum chromatographic peak window used for spectrum averaging was 5 s of the retention time. This was combined with a required minimal peak intensity of 500. The 50 highest MS and MS/MS peaks were kept. A default m/z window of 8 Da was used to find MS/MS spectra of a precursor ion. With GenForm, the MS and MS/MS data was used to predict molecular formulas. The elements used for molecular formula calculation were limited to C, H, N, O, P and Br to limit the calculation of improbable formulas and save calculation time. Any formula for a certain feature group was supposed to be detected in >75% of the measurements and the number of postulated formulas was limited to the 10 with the highest ranking. Subsequently, MetFrag was used for compound identification. A relative mass deviation of 5 ppm and an absolute mass deviation of 0.002 Da were used as thresholds. Potential candidates with the elements Cl, I, F, S and were excluded. The potential candidates were retrieved from PubChem and scored according to their similarity in fragmentation to in-silico generated MS/MS spectra and MassBank of North America (MoNA, http://mona.fiehnlab.ucdavis.edu/) derived mass spectra. The 25 candidates with the highest score were reported and considered for further interpretation. The next step was the generation of components. Isotopologues, multiple ionization adducts and in-source fragmentation can lead to different features with different m/z belonging to the same compounds. RAMclustR was used to cluster potential features in components that are likely to be the same compound. The last step of the ‘patRoon’ script consisted of the generation of a report of the results in CSV, PDF and HTML formats. These reports were manually checked for relevant features that could be compounds formed as result of the degradation of DBNPA or the interaction between glutaraldehyde and DBNPA. Subsequently, an identification confidence level was assigned according to Schymanski et al.23 This was done by examining MS/MS spectra, peak shape and intensity of extracted ion chromatograms in Data Analysis (Bruker Daltonics, Wormer, the Netherlands), excluding isotopologues, excluding adducts and, where possible, confirmation with standards.
2.4. Acute toxicity tests with Daphnia magna
D. magna 48 h acute toxicity tests were conducted based on OECD guideline 202 (ref. 25) and protocols described in Hernandez-Leal et al.,27 Waaijers et al.28 and de Baat et al.29 The tests were performed in a test volume of 1 ml in 24-well plates that were covered by aluminium foil to prevent further photodegradation. Five D. magna neonates younger than 48 h were introduced to the wells by disposable transfer pipette, and the number of D. magna that were mobile after 24 h and 48 h were counted. The mobility of the D. magna was expressed as the percentage of mobility of the experimental control, and tests were considered valid with >80% mobility in the experimental controls.
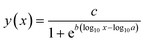 | (1) |
Treatment effects on the mobility of D. magna were analysed using a one-way analysis of variance (ANOVA) in SPSS.30 First, the D. magna mobility data was arcsine-transformed. Non-transformed data is shown in the figures. The homogeneity of variance was tested, and since the variance was not homogeneous, a Games–Howell post-hoc test was used to elucidate significant differences between treatments (p ≤ 0.05).
3. Results and discussion
3.1. DBNPA transformation in cooling tower water lowers the DBNPA toxicity
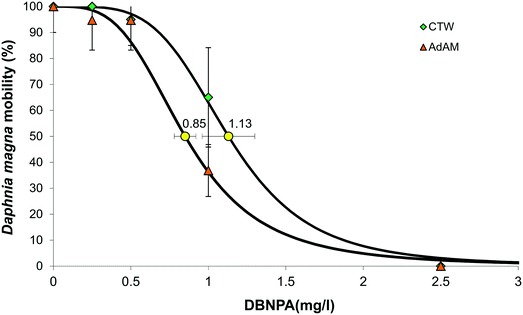
A 24 h LC50 experiment with reference toxicant K2Cr2O7 was performed according to our test protocol, and the LC50 of K2Cr2O7 for the Daphnia magna culture and test set-up used in this study was 0.8 mg L−1 (Fig. S3†), which is within the LC50 range of 0.6–2.1 mg L−1 as set by the OECD guidelines.25 The EC50 of DBNPA in CTW was 1.13 mg L−1, which is higher than the DBNPA EC50 of 0.85 mg L−1 in ADaM-medium, implying that DBNPA in CTW is less toxic than in ADaM-medium (Fig. 1). The DBNPA EC50 in CTW of 1.13 mg L−1 is also higher than the EC50 values reported in literature, that range from 0.54–1.05 mg L−1.32 We used non-target screening on the dark controls of the photodegradation experiments to elucidate the mechanisms behind the reduced toxicity of DBNPA in CTW in our study.Surprisingly, DBNPA itself was not identified with non-target screening, while the current non-target screening workflow can identify DBNPA.10 This can be an indication that DBNPA was transformed in CTW in between the addition of DBNPA, taking the sample after 1 h and UHPLC-q-ToF-MS/MS analysis of the sample within the following 6 h. Complete DBNPA transformation in the dark as a result of chemical degradation within hours has been observed earlier.18 A high pH of 8 increased the DBNPA transformation rate,18 and the pH of CTW of 7.9 in the dark controls is similar (Table S6†). Various transformation products (TPs) that corroborate this rapid transformation of DBNPA were identified by the non-target screening (Table 1).
| TP#a | m/z (ionization mode) | Detected adduct | RTb (min) | Name and molecular formula | m/z deviation (ppm) | Levelc | Test solutiond |
|---|---|---|---|---|---|---|---|
| a Transformation product number. In the text, this number is followed by the mass. b Retention time. c Identification level according to Schymanski et al. (2014).23 d Only the presence in test solutions with DBNPA is indicated. The number corresponds with the hours after which the test solutions were sampled. The -L samples correspond to illuminated test solutions, while the -C samples correspond to the dark controls. | |||||||
| 1 | 162.9500 (+) | [M + H]+ | 2.8 | 2-monobromo-3-nitrilopropionamide (MBNPA) | — | 1 | 1-DBNPA-C |
| [M − H]− | |||||||
| 160.9338 (−) | |||||||
| C3H3BrN2O | 1-DBNPA-L | ||||||
| 2 | 258.8714 (+) | [M + H]+ | 4.9 | 2,2-dibromopropanediamide | 0.7 | 1 | 1-DBNPA-C |
| C3H4Br2N2O2 | |||||||
| 1-DBNPA-L | |||||||
| 3 | 215.86482 (+) | [M + H]+ | 5.7 | 2,2-dibromoacetamide | 1.2 | 1 | 48-DBNPA-C |
| [M − H]− | C2H3Br2NO | ||||||
| 213.85049 (−) | |||||||
| 48-DBNPA-L | |||||||
| 96-DBNPA-C | |||||||
| 96-DBNPA-L | |||||||
| 4 | 460.5483 (+) | [M + H]+ | 10.3 | C19H37N7O6 | −2.9 | 4 | 1-DBNPA-C |
| 5 | 153.0043 (−) | [M − H]− | 7.7 | C2N7O2 | 1.4 | 4 | 1-DBNPA-L |
| 48-DBNPA-L | |||||||
| 96-DBNPA-L | |||||||
| 6 | 162.9137 (−) | [M − H]− | 2.2 | C2HBrN2O2 | 1.7 | 4 | 1-DBNPA-L |
| 48-DBNPA-L | |||||||
| 96-DBNPA-L | |||||||
| 7 | 186.0409 (−) | [M − H]− | 10.9 | C8H5N5O | 0.4 | 4 | 1-DBNPA-L |
| 48-DBNPA-L | |||||||
| 96-DBNPA-L | |||||||
| 8 | 148.0146 (−) | [M − H]− | 7.1 | C6H3N3O2 | 2.7 | 4 | 48-DBNPA-L |
| 96-DBNPA-L | |||||||
| 9 | 169.0139 (−) | [M − H]− | 2.6 | C7H6O5 | 1.4 | 4 | 48-DBNPA-L |
| 96-DBNPA-L | |||||||
| 10 | 173.0239 (−) | [M − H]− | 10.3 | C10H6O3 | −2.6 | 4 | 48-DBNPA-L |
| 96-DBNPA-L | |||||||
| 11 | 206.0666 (+) | [M + H]+ | 8.3 | C8H7N5O2 | 2.7 | 4 | 48-DBNPA-L |
| 12 | 8.6 | ||||||
| 96-DBNPA-L | |||||||
| 13 | 344.1688 (+) | [M + H]+ | 5.7 | C12H21N7O5 | −3.1 | 4 | 48-DBNPA-L |
| 96-DBNPA-L | |||||||
| 14 | 170.0559 (+) | [M + H]+ | 6.1 | C6H7N3O3 | 0.6 | 4 | 96-DBNPA-L |
| [M − H]− | |||||||
| 168.0411 (−) | |||||||
Similarly to our earlier study,10 transformation products TP1-MBNPA (Table 1; Text S2†) and TP2-2,2-dibromopropanediamide (Table 1; Text S3†) were identified. However, the peak intensity of these TPs (Fig. S4†) was >5 times lower than those observed in that earlier study,10 although the starting concentration of the biocides in the present study was higher. In our previous study, MBNPA was produced over time.10 However, in the present study TP1-MBNPA is only detected at t = 1 h, indicating that this compound is further transformed. Blanchard et al.18 identified two competing DBNPA transformation pathways: one leading to nitrilopropionamide (NPA) via MBNPA and one leading to 2,2-dibromoacetamide via dibromoacetonitrile. Dibromoacetonitrile was also observed in DBNPA transformation studies by Sumner & Plata.33 An overview of known DBNPA degradation pathways is provided in Fig. S5.†
Comparison with an analytical standard showed that dibromoacetonitrile could not be detected with our chromatographical method. TP3-2,2-dibromoacetamide was identified using a commercially available standard after 48 h and 96 h (Table 1; Text S4†). The presence of TP3-2,2-dibromoacetamide can be an indication that the DBNPA in our CTW-matrix is transformed to dibromoacetonitrile and 2,2-dibromoacetamide within hours. This transformation to dibromoacetonitrile also explains the significant lower intensity of MBNPA than observed in our previous study,10 where 2,2-dibromoacetamide was not observed at all.
In addition to known TPs of DBNPA, the previously unreported TP4-mz461 with a relatively high molecular mass of 460.5483 corresponding to C19H38N7O6 was detected (Table 1; Text S5†). This TP had a high peak intensity in comparison with the other DBNPA TPs (Fig. S4†). Both GenForm and MetFrag could explain the fragmentation of this TP (Text S5†), however a unique molecular structure could not be proposed by the latter software. The formation of TPs with a high mass, such as TP4-mz461, is either the result of the interaction of DBNPA with the CTW-matrix or the bimolecular reaction of DBNPA itself. DBNPA has been shown to produce tribromoacetonitrile and trihalogenated methanes as a result of bimolecular reactions.17,33 The absence of bromine functional groups might be an indication that TP4-mz461 is indeed the result of the interaction of debrominated DBNPA TPs, either with each other or the CW-matrix. This could also be the reason that the peak intensity of TP1-MBNPA (Fig. S4†) is lower than that observed by Wagner et al.10
The toxicity of CTW containing ‘DBNPA’ will ultimately be determined by the toxicity of the mixture of DBNPA, TP1-MBNPA, TP2-dibromopropanediamide, TP3-2,2-dibromoacetamide, TP4-mz461 and potential TPs that were not identified, such as NPA or dibromoacetonitrile. The high peak intensity of TP4-mz461 in comparison with the other TPs (Fig. S4†) could be an indication that this TP contributes substantially to the lower than expected DBNPA toxicity to D. magna, if the toxicity of this compound is lower than DBNPA itself.
3.2. Impact of photodegradation on the acute toxicity of DBNPA
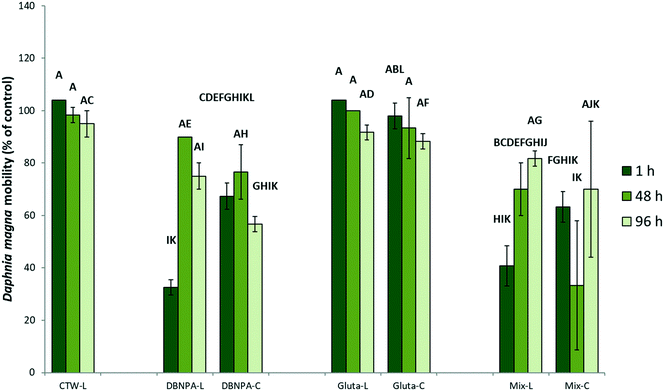
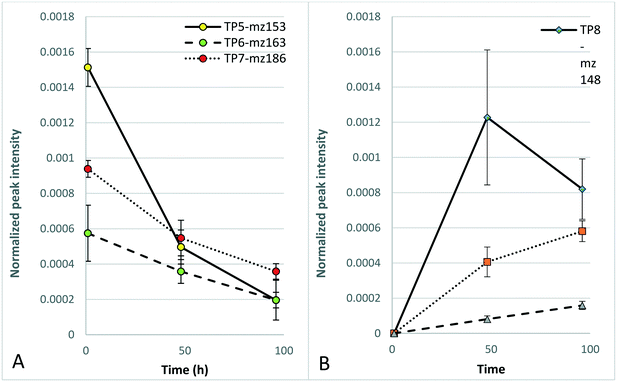
To test the applicability of surface-flow CWs for the photodegradation of DBNPA and the potential influence of photodegradation on the toxicity of DBNPA, photodegradation experiments with simulated sunlight and subsequent D. magna acute 48 h toxicity tests were performed. The toxicity of CTW containing DBNPA after 1 h of illumination is significantly higher than that of the dark control (Fig. 2), evidenced by the reduced D. magna mobility, implying that photodegradation increases the acute toxicity of DBNPA. The retention time of surface-flow CWs is higher than 1 h, and therefore the fauna in surface-flow CWs will be exposed to CTBD with an increased toxicity. This increase in toxicity cannot be attributed to changes in the CTW-matrix as a result of photodegradation, since illumination of the CTW-matrix did not result in a significant change of the D. magna mobility compared to the dark CTW-matrix control after 1 h (Fig. 2).The non-target screening showed the presence of various products that were only detected in illuminated test solutions, confirming the previously described photodegradability of DBNPA.17,34 These photodegradation products could not be identified, probably because the interaction between DBNPA and the CTW-matrix resulted in compounds that are not present in databases. However, a tentative molecular formula of these TPs could be determined. TP5-mz153 (Table 1; Text S6†), TP6-mz163 (Table 1; Text S7†) and TP7-mz186 (Table 1; Text S8†) were detected after 1 h, indicating that rapid formation of TPs occurred as result of photodegradation. After 48 h of photodegradation, new TPs were detected, such as TP8-mz148 (Table 1; Text S9†), TP9-mz169 (Table 1; Text S10†), TP10-mz173 (Table 1; Text S11†), TP11-mz206 and TP12-mz206 (Table 1; Text S12†) and TP13-mz344 (Table 1; Text S13†). TP13-mz344 is another indication that new TPs with a larger m/z than DBNPA itself are formed. The formation of TP8-TP13 (Fig. 3B coincided with a decline in peak intensity of the TPs that already formed after 1 h (Fig. 3A), indicating possible transformation of TPs formed after 1 h to new TPs.
The TPs that were detected first after 48 h behaved differently; TP8-mz148 was detected with a high peak intensity compared to TP9-mz169 and TP13mz344 but decreased in peak intensity at 96 h (Fig. 3B). In contrast, TP9-mz169 and TP13-mz344 further increased in peak intensity at 96 h (Fig. 3B). At this time point, TP14-mz170 (Table 1; Text S14†) was first detected.
The non-significant difference in the toxicity after 1 h between illuminated DBNPA solutions and DBNPA dark controls is likely a result of the formation of photodegradation products that are more toxic than the products formed in the dark (section 3.1). In addition, TP4-mz461 was not present in the illuminated test solutions, while it was considered to be responsible for the reduced toxicity of DBNPA in CTW (section 3.1). The decrease in toxicity of the illuminated DBNPA test solutions after 48 h might coincide with the further photodegradation of the relatively toxic TPs initially formed after 1 h (Fig. 3A) and the formation of less toxic TPs between 1 h and 96 h (Fig. 3B).
3.3. Formation of glutaraldehyde interaction products in cooling tower water
To determine the influence of photodegradation on the formation of glutaraldehyde interaction products (GIPs) and their corresponding toxicity in CTW, non-target screening and D. magna acute 48 h toxicity tests were performed on CTW solutions in which both biocides were spiked.GIP1 (Table 2; Text S15†), with a retention time of 5.1 min, was not observed in the Wagner study.10 GIP1 could either be an interaction product between DBNPA and glutaraldehyde itself, or a fragment of a larger GIP. Four more GIPs with a higher m/z than that of GP1 were found, that were also observed by Wagner et al.10 GIP2 (Table 2; Text S16†), GIP3 (Table 2; Text S17†), and GIP4 (Table 2; Text S18†) are likely to be the result of the interaction between glutaraldehyde and the debrominated DBNPA transformation product NPA.10 In addition, TP13-mz344 (Table 1) was observed with a high peak intensity, and GIP5 (Table 2; Text S19†) could be an indication that reaction products were formed from the interaction between this TP and glutaraldehyde.
| GIP#a | m/z (ionization) | Detected adduct | RTb (min) | Molecular formula | m/z deviation (ppm) | Levelc | Test solutiond |
|---|---|---|---|---|---|---|---|
| a Glutaraldehyde interaction product number. b Retention time. c Identification level according to Schymanski et al. (2014).23 d The number corresponds with the hours after which the test solutions were sampled. The -L samples correspond to illuminated test solutions, while the -C samples correspond to the dark controls. | |||||||
| 1 | 128.07079 (+) | [M + H]+ | 5.1 | C6H9NO2 | −3.1 | 5 | 1-mix-C |
| 1-mix-L | |||||||
| 48-mix-C | |||||||
| 48-mix-L | |||||||
| 2 | 181.06119 (−) | [M − H]− | 7.2 | C8H10N2O3 | 0.6 | 3 | 1-mix-C |
| 183.07633 (+) | [M + H]+ | 0.1 | 1-mix-L | ||||
| 48-mix-C | |||||||
| 48-mix-L | |||||||
| 96-mix-C | |||||||
| 96-mix-L | |||||||
| 3 | 183.07678 (−) | [M − H]− | 3.8 | C8H12N2O3 | −0.3 | 5 | 1-mix-C |
| 185.09193 (+) | [M + H]+ | 0.7 | 1-mix-L | ||||
| 48-mix-C | |||||||
| 48-mix-L | |||||||
| 96-mix-C | |||||||
| 96-mix-L | |||||||
| 4 | 263.0777 (−) | [M − H]− | 7.9 | C11H12N4O4 | 1.6 | 3 | 1-mix-C |
| 265.0931 (+) | [M + H]+ | 0.7 | 1-mix-L | ||||
| 48-mix-C | |||||||
| 48-mix-L | |||||||
| 96-mix-C | |||||||
| 96-mix-L | |||||||
| 5 | 385.20753 (−) | [M − H]− | 9.1 | C17H30N4O6 | 3.4 | 5 | 1-mix-C |
| 6 | 264.06206 (−) | [M − H]− | 6.5 | C11H11N3O5 | 1.8 | 5 | 48-mix-C |
| 48-mix-L | |||||||
| 96-mix-C | |||||||
| 96-mix-L | |||||||
GIP2 was present with a substantially higher peak intensity (Fig. S6†) than in the study by Wagner et al.,10 indicating that DBNPA must have been present and was transformed within hours. In addition, this might indicate that MBNPA is also quickly transformed to NPA, which would explain the low peak intensity and sole presence at timepoint t = 1 of MBNPA. Another indication that debromination occurred within hours was the absence of a brominated TP with m/z 280 that was observed in the study by Wagner et al.10 This TP resulted from the interaction of MBNPA with glutaraldehyde,10 and its absence in the present study corresponds with the substantially lower peak intensity of MBNPA (section 3.1). The peak intensity of GIP2 and absence of a GIP with m/z 280 provide evidence that DBNPA transformation via MBNPA and NPA is the most likely option of the two DBNPA transformation pathways discussed in section 3.1.
Photodegradation resulted in a D. magna mobility in the DBNPA plus glutaraldehyde mixture that was not statistically different to the mobility in the illuminated DBNPA test solutions after 1 h (Fig. 2). This indicates that the toxicity of the mixture of biocides is mainly determined by the toxicity of the DBNPA photodegradation TPs (section 3.2). In contrast to the dark controls (section 3.3.2), photodegradation resulted in a significant decrease of the toxicity of the mixture of biocides between 1 h and 96 h (Fig. 2). This trend is also similar to the one observed in the illuminated DBNPA test solutions (Fig. 2).
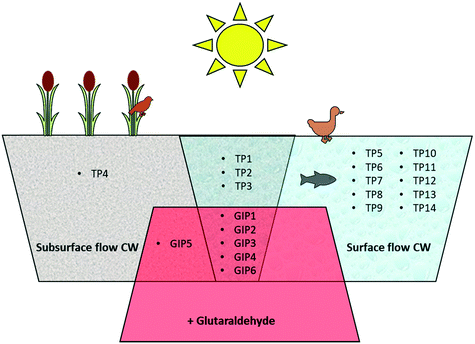
Photodegradation did not result in the formation of unique reaction products formed as result of the interaction between DBNPA and glutaraldehyde in the light. Fig. 4 provides an overview of the TPs and GIPs of DBNPA that were identified in the various experimental setups of the present study. Photodegradation of DBNPA results in various TPs that are only formed in surface-flow CWs (Fig. 4). The interaction with glutaraldehyde results in the formation of GIPs, but none of these is formed exclusively as a result of photodegradation in surface flow CWs, and can thus also be formed in subsurface-flow CWs.
3.4. Implications of DBNPA photodegradation on cooling tower water treatment in full-scale constructed wetlands
The toxicity of biocide DBNPA in CTW to D. magna was assessed to evaluate the potential toxicity of DBNPA-containing CTW to fauna living in surface-flow CWs used for the treatment of CTW. DBNPA was less toxic to D. magna in CTW than in ADaM medium. Non-target screening showed that this is likely due to the rapid transformation of DBNPA via the MBNPA–NPA pathway. Photodegradation of DBNPA in CTW resulted in the formation of various direct TPs of DBNPA that significantly increased the toxicity of the DBNPA mixture to D. magna after 1 h of illumination. This increased toxicity of CTW introduced into the CW can have detrimental effects on the fauna living in surface-flow CWs. To determine the impact of DBNPA on the fauna in surface flow CWs, it is recommended to test the effect of DBNPA in CTW on both aquatic species as well as birds and mammals. An overview of DBNPA 96 h LC50 and 24 h LC50 values for different aquatic species, such as different fish species, shrimp, oyster and crab, is provided by Klaine et al.,32 and these values range from 0.3 mg L−1 to 14 mg L−1. However, since the transformation of DBNPA has shown to be dependent on the water matrix composition by the present study as well as a previous one,10 it is recommended to repeat these tests in genuine CTW to predict the impact on a full-scale surface flow CW.In addition to aquatic fauna, avian fauna can enter surface flow CWs and be affected by toxic substances. In subacute dietary toxicity tests, DBNPA LC50 values of >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 for mallard ducks (Anas platyrhynchos) and >5620 mg L−1 for bobwhite quail (Colinus virginianus) were observed,36 and thus no effect of DBNPA in CTW for avian species entering surface flow CWs can be expected because of the much lower actual concentrations of DBNPA in CTW.
000 mg L−1 for mallard ducks (Anas platyrhynchos) and >5620 mg L−1 for bobwhite quail (Colinus virginianus) were observed,36 and thus no effect of DBNPA in CTW for avian species entering surface flow CWs can be expected because of the much lower actual concentrations of DBNPA in CTW.
The abovementioned studies determined the acute toxicity of DBNPA, which would resemble a single exposure event to DBNPA. However, biocides are generally continuously dosed to cooling systems to prevent microbial fouling.3 Therefore, chronic toxicity studies with DBNPA in CTW are needed. Cheng37 showed that chronic toxicity tests with DBNPA in mineral water resulted in lower 21 d EC50 values for D. magna, with 0.072 mg L−1 for longevity, 0.053 mg L−1 for reproduction and 0.055 mg L−1 for brood size. These values are substantially lower than the acute 48 h EC50 value for mobility observed in the present study (section 3.1). Nevertheless, it is common practice in cooling towers to shock-dose DBNPA, when the microbial fouling in the cooling system reaches a threshold value.32 This results in short episodes of discharge of CTW with DBNPA into surface flow CWs. Hence the concentration of the mixture of toxic TPs of DBNPA will be diluted if residual DBNPA TPs from a previous shock-dose episode are removed in the CW, and thus the exposure to DBNPA TPs will be non-chronic.
In a preceding study,38 we proposed a system of sequential CWs as a suitable pre-treatment option for CTW prior to desalination. However, the toxicity to aquatic fauna in surface-flow CWs was not assessed in the latter study. When a surface flow CWs is deemed necessary in a hybrid-CW system for the treatment of CTW, for instance for the storage of large volumes of CTW, the results from the present study suggest to not use the surface CW as a first treatment step in a hybrid-CW and to start the hybrid-CW with a subsurface flow to prevent unwanted toxic effects to aquatic fauna in subsequent surface flow CWs. DBNPA concentrations of 1 and 10 mg L−1 did not negatively affect the biodegradation in these subsurface-flow CWs. Furthermore, no detectable DBNPA concentrations were observed in the effluent of the subsurface-flow CWs,38 and thus no DBNPA would enter a subsequent surface-flow CW.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research is financed by the Netherlands Organisation for Scientific Research (NWO), which is partly funded by the Ministry of Economic Affairs, and co-financed by the Netherlands Ministry of Infrastructure and Environment and partners of the Dutch Water Nexus consortium (Project nr. STW 14302 Water Nexus 3). Milo de Baat and Michiel Kraak are acknowledged for their help in designing the toxicity tests. Taymaz Azarno and Titus Rombouts are acknowledged for maintaining the Daphnia magna cultures. Samira Absalah is acknowledged for her analytical support. Vinnie de Wilde, Bert Willemsen, Joke Westerveld and Merijn Schuurmans are acknowledged for technical support.References
- J. Macknick, R. Newmark, G. Heath and K. C. Hallett, Operational water consumption and withdrawal factors for electricity generating technologies: a review of existing literature, Environ. Res. Lett., 2012, 7, 045802 CrossRef.
- C. Zhang, L. Zhong, X. Fu, J. Wang and Z. Wu, Revealing water stress by the thermal power industry in China based on a high spatial resolution water withdrawal and consumption inventory, Environ. Sci. Technol., 2016, 50, 1642–1652 CrossRef CAS PubMed.
- S. Pan, S. W. Snyder, A. I. Packman, Y. J. Lin and P. Chiang, Cooling water use in thermoelectric power generation and its associated challenges for addressing water-energy nexus, Water-Energy Nexus, 2018, 1, 26–41 CrossRef.
- C. K. Groot, W. B. P. van den Broek, J. Loewenberg, N. Koeman-Stein, M. Heidekamp and W. de Schepper, Mild desalination of various raw water streams, Water Sci. Technol., 2015, 72, 371–376 CrossRef CAS PubMed.
- J. Löwenberg, J. A. Baum, Y. Zimmermann, C. Groot, W. van den Broek and T. Wintgens, Comparison of pre-treatment technologies towards improving reverse osmosis desalination of cooling tower blow down, Desalination, 2015, 357, 140–149 CrossRef.
- T. V. Wagner, J. R. Parsons, H. H. M. Rijnaarts, P. de Voogt and A. A. M. Langenhoff, A review on the removal of conditioning chemicals from cooling tower water in constructed wetlands, Crit. Rev. Environ. Sci. Technol., 2018, 48, 1094–1125 CrossRef CAS.
- G. Imfeld, M. Braeckevelt, P. Kuschk and H. H. Richnow, Monitoring and assessing processes of organic chemicals removal in constructed wetlands, Chemosphere, 2009, 74, 349–362 CrossRef CAS PubMed.
- J. Garcia, D. P. L. Rousseau, J. Morato, E. Lesage, V. Matamoros and J. M. Bayona, Contaminant removal processes in subsurface-flow constructed wetlands: A review, Crit. Rev. Environ. Sci. Technol., 2009, 40, 561–661 CrossRef.
- Y. He, N. B. Sutton, Y. Lei, H. H. M. Rijnaarts and A. A. M. Langenhoff, Fate and distribution of pharmaceutically active compounds in mesocosm constructed wetlands, J. Hazard. Mater., 2018, 357, 198–206 CrossRef CAS PubMed.
- T. V. Wagner, R. Helmus, S. Quiton Tapia, J. R. Parsons, P. de Voogt, H. H. M. Rijnaarts and A. A. M. Langenhoff, Non-target screening reveals the mechanisms responsible for the antagonistic inhibiting effect of the biocides DBNPA and glutaraldehyde on benzoic acid biodegradation, J. Hazard. Mater., 2020, 386, 121661 CrossRef PubMed.
- T. V. Wagner, J. R. Parsons, H. H. M. Rijnaarts, P. de Voogt and A. A. M. Langenhoff, Benzotriazole removal mechanisms in pilot-scale constructed wetlands treating cooling tower water, J. Hazard. Mater., 2020, 384, 121314 CrossRef CAS PubMed.
- J. T. Jasper and D. L. Sedlak, Phototransformation of wastewater-derived trace organic contaminants in open-water unit process treatment wetlands, Environ. Sci. Technol., 2013, 47, 10781–10790 CrossRef CAS PubMed.
- C. Prasse, J. Wenk, J. T. Jasper, T. A. Ternes and D. L. Sedlak, Co-occurence of photochemical and microbiological transformation processes in open-water unit process wetlands, Environ. Sci. Technol., 2015, 49, 14136–14145 CrossRef CAS PubMed.
- Y. He, N. B. Sutton, H. H. M. Rijnaarts and A. A. M. Langenhoff, Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation, Appl. Catal., B, 2016, 182, 132–141 CrossRef CAS.
- E. De Laurentiis, C. Prasse, T. A. Ternes, M. Minella, V. Maurino, C. Minero, M. Sarakha, M. Brigante and D. Vione, Assessing the photochemical transformation pathways of acetaminophen relevant to surface waters: Transformation kinetics, intermediates and modelling, Water Res., 2014, 53, 235–248 CrossRef CAS PubMed.
- L. C. Bodhipaksha, C. M. Sharpless, Y. Chin and A. A. Mackay, Role of effluent organic matter in the photochemical degradation of compounds of wastewater origin, Water Res., 2017, 110, 170–179 CrossRef CAS PubMed.
- J. H. Exner, G. A. Burk and D. Kyriacou, Rates and products of decomposition of 2,2-dibromo-3-nitrilopropionamide, J. Agric. Food Chem., 1973, 21, 838–842 CrossRef CAS PubMed.
- F. A. Blanchard, S. J. Gonsoir and D. L. Hopkins, 2,2-dibromo-3-nitrilopropionamide (DBNPA) chemical degradation in natural waters: Experimental evaluation and modeling of competitive pathways, Water Res., 1987, 21, 801–807 CrossRef CAS.
- D. Benelux, personal communication, 2019.
- V. Albergamo, R. Helmus and P. de Voogt, Direct injection analysis of polar micropollutants in natural drinking water sources with biphenyl liquid chromatography coupled to high-resolution time-of-flight mass spectrometry, J. Chromatogr. A, 2018, 1569, 53–61 CrossRef CAS PubMed.
- R Core Team, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, 2013, http://www.R-project.org/ Search PubMed.
- R. Helmus, patRoon, 2018, http://github.com/rhelmus Search PubMed.
- E. L. Schymanski, J. Jeon, R. Gulde, K. Fenner, M. Ruff, H. P. Singer and J. Hollender, Identifying small molecules via high resolution mass spectrometry: Communicating confidence, Environ. Sci. Technol., 2014, 48, 2097–2098 CrossRef CAS PubMed.
- D. M. M. Adema, Daphnia magna as a test animal in acute and chronic toxicity tests, Hydrobiologia, 1978, 59, 125–134 CrossRef CAS.
- Organisation for Economic Co-operation and Development (OECD), OECD guideline for testing of chemicals – Daphnia sp., acute immobilisation test 202, 2004 Search PubMed.
- B. Klüttgen, U. Dülmer, M. Engels and H. T. Ratte, ADaM, an artificial freshwater for the culture of zooplankton, Water Res., 1994, 28, 743–746 CrossRef.
- L. Hernandez Leal, A. M. Soeter, S. A. E. Kools, M. H. S. Kraak, J. R. Parsons, H. Temmink, G. Zeeman and C. J. N. Buisman, Ecotoxicological assessment of grey water treatment systems with Daphnia magna and Chironomus riparius, Water Res., 2012, 46, 1038–1044 CrossRef CAS PubMed.
- S. L. Waaijers, J. Hartmann, A. M. Soeter, R. Helmus, S. A. E. Kools, P. de Voogt, W. Admiraal, J. R. Parsons and M. H. S. Kraak, Toxicity of new generation flame retardants to Daphnia magna, Sci. Total Environ., 2013, 463–464, 1042–1048 CrossRef CAS PubMed.
- M. L. de Baat, M. H. S. Kraak, R. van der Oost, P. de Voogt and P. F. M. Verdonschot, Effect-based nationwide surface water quality assessment to identify ecotoxicological risks, Water Res., 2019, 159, 434–443 CrossRef CAS PubMed.
- C. R. IBM, IBM SPSS Statistics for Windows, Version 24.0, IBM Corp, Armonk, NY, 2016 Search PubMed.
- L. Haanstra, P. Doelman and J. H. O. Voshaar, The use of sigmoidal dose-response curves in soil ecotoxicological research, Plant Soil, 1985, 84, 293–297 CrossRef CAS.
- S. J. Klaine, G. P. Cobb, R. L. Dickerson, K. R. Dixon, R. J. Kendall, E. E. Smith and K. R. Solomon, An ecological risk assessment for the use of the biocide dibromonitrilopropionamide (DBNPA) in industrial cooling systems, Environ. Toxicol. Chem., 1996, 15, 21–30 CrossRef CAS.
- A. J. Sumner and D. J. Plata, Halogenation chemistry of hydraulic fracturing additives under highly saline simulated subsurface conditions, Environ. Sci. Technol., 2018, 52, 9097–9107 CrossRef CAS PubMed.
- G. A. Kahrilas, J. Blotevogel, P. S. Stewart and T. Borch, T. Biocides in hydraulic fracturing fluids: A critical review of their usage, mobility, degradation, and toxicity, Environ. Sci. Technol., 2015, 49, 16–32 CrossRef CAS PubMed.
- G. Ganzer, J. Summerfield, M. Freid, J. Soost and G. Regginni, The use of glutaraldehyde/DBNPA combination treatment program for effective control of microbial growth in cooling water systems, Corrosion 2002, NACE International Search PubMed.
- E. P. A. US, Reregistration eligibility decision (RED), 2,2-dibromo-3-nitrilopropionamide (DBNPA), United States Environmental Protection Agency, 1994 Search PubMed.
- F. Cheng, The chronic aquatic toxicity of a microbiocide dibromonitrilopropionamide, Toxicol. Ind. Health, 2011, 28, 181–185 CrossRef PubMed.
- T. V. Wagner, V. de Wilde, B. Willemsen, M. Mutaqin, G. Putri, J. Opdam, J. R. Parsons, H. H. M. Rijnaarts, P. de Voogt and A. A. M. Langenhoff, Pilot-scale hybrid constructed wetlands for the treatment of cooling tower water prior to desalination, J. Environ. Manage. Search PubMed submitted.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew01018a |
| This journal is © The Royal Society of Chemistry 2020 |