Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Investigation of metaldehyde removal by powdered activated carbon from different water samples†
Zhuojun
Li
 a,
Juntao
Li
b,
Zhengxiao
Guo
bcd and
Luiza C.
Campos
a,
Juntao
Li
b,
Zhengxiao
Guo
bcd and
Luiza C.
Campos
 *a
*a
aDepartment of Civil, Environmental and Geomatic Engineering, University College London, Gower Street, London WC1E 6BT, UK. E-mail: zhuojun.li.09@ucl.ac.uk; l.campos@ucl.ac.uk; Tel: +44(0)207 679 4162
bDepartment of Chemistry, University College London, Gower Street, London WC1E 6BT, UK. E-mail: juntao.li.16@ucl.ac.uk
cDepartment of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong. E-mail: zxguo@hku.hk
dHKU Zhejiang Institute of Research and Innovation, The University of Hong Kong, Qingshanhu Scitech City, Hangzhou, China
First published on 19th March 2020
Abstract
Metaldehyde as a widely used pesticide has been detected in surface water and drinking water in the UK with concentrations higher than the EU and UK standard (0.1 μg L−1). Previous studies have shown that powdered activated carbon (PAC) can adsorb metaldehyde even with the presence of natural organic matter, suggesting a promising solution to the problem. This paper studies the adsorption of metaldehyde onto PAC using different water samples including synthetic water, natural surface water, and water samples taken at different treatment processes from a water treatment plant. Metaldehyde (5 μg L−1) was effectively removed by PAC (50 mg L−1) from all water samples in this study, regardless of the water quality (74.3% to 99.7%). A PAC dosage of 100 mg L−1 was considered appropriate to remove metaldehyde at 5 μg L−1 after the first treatment process of pre-ozone treatment with a maximum adsorption capacity (qm) of 0.25 μg mg−1 given by the data fitted to the Langmuir isotherm model. Removal of metaldehyde by PAC was found to be most effective when PAC was applied after the static flocculation treatment process (98.4%) with a qm of 0.29 μg mg−1. The low adsorption capacity of PAC for low initial concentrations of metaldehyde solution was observed due to the lower driving force for mass transfer in the process of adsorption and competition with water molecules for adsorption sites on PAC.
Water impactMetaldehyde is a persistent pesticide in surface water bodies which cannot be removed by traditional technologies in water treatment plants due to its physico-chemical properties. This research suggests that the application of powdered activated carbon after the ‘static flocculation’ treatment process in a water treatment plant can effectively remove metaldehyde. |
1. Introduction
There have been rising concerns about micropollutants, including pesticides, entering surface water bodies and endangering human and aquatic lives. Metaldehyde (C8H16O4) as a commonly used pesticide for molluscs has been detected by the UK Environment Agency in surface water (<8 μg L−1) and drinking water (<1 μg L−1) above the UK and EU standard of 0.1 μg L−1 for a single pesticide and 0.5 μg L−1 for total pesticides.1 This has been an issue for water companies in the UK, especially during autumn and winter when metaldehyde is largely applied in the field.2 Metaldehyde is a cyclic tetramer of acetaldehyde (CH3CHO); it has a molecular weight of 176.2 g mol−1 and a solubility of 0.188 g L−1 at 20 °C, with a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow value of 0.12 and log
Kow value of 0.12 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc value of 0.18–0.37, indicating that it is soluble at environmentally relevant temperatures and highly mobile in soil which makes it semi-persistent in the aquatic environment.2–4 With rainfall, metaldehyde can be washed down into streams and other surface water bodies which are one of the main water sources used by water treatment plants for drinking water. Therefore, it is important to develop an effective treatment method to lower the concentration of metaldehyde in the water to meet the UK and EU standard.
Koc value of 0.18–0.37, indicating that it is soluble at environmentally relevant temperatures and highly mobile in soil which makes it semi-persistent in the aquatic environment.2–4 With rainfall, metaldehyde can be washed down into streams and other surface water bodies which are one of the main water sources used by water treatment plants for drinking water. Therefore, it is important to develop an effective treatment method to lower the concentration of metaldehyde in the water to meet the UK and EU standard.
Granular activated carbon (GAC) filtration is the most widely used conventional water treatment method for removing organic pollutants, and has been proven to be effective in removing pharmaceutical and personal care products such as paracetamol, triclosan and caffeine.5,6 Chemical oxidation by ozone is another common treatment method that can be used to treat disinfection by-products (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs), as well as pesticides.7 However, oxidation by ozone and GAC are not effective in removing metaldehyde due to its physiochemical properties such as its stable ring structure and low log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow value, which suggests low sorption potential.8–10
Kow value, which suggests low sorption potential.8–10
There are many trending techniques regarding the removal of micropollutants from water, of which advanced oxidation processes (AOPs) are one. AOPs break down organic pollutants into benign sub-products by catalysed oxidation. For instance, Autin et al. have found that metaldehyde can be degraded by photocatalysis via UV/H2O2 and UV/TiO2.11 Another method is adsorption coupled with electrochemical organic destruction. The study of Nabeerasool et al. has shown that metaldehyde can be adsorbed and degraded by adsorbent Nyex™ with electrochemical regeneration without producing any wastes.12 However, these treatment methods require high energy input and are very difficult to apply in water treatment plants at the current stage. In fact, Autin et al. suggested that methods need to be established and tested to reduce the energy input before possible application at a large scale.11 Also, Nabeerasool et al. pointed out that large-scale continuous treatment and energy efficiency need to be determined before considering the viability of the method at the industrial scale.11,12
Recently, there has been a study of metaldehyde adsorption with the presence of serine, leucine, and resorcinol onto different filtration media including different sands, GAC, and biological activated carbon (BAC); results have shown that BAC is very effective for metaldehyde removal.13 This suggests that BAC filtration might be a feasible option for water treatment plants. However, there was observed desorption of metaldehyde from BAC back into the water, indicating that increased regeneration of BAC is required to maintain the effectiveness of metaldehyde removal which limits its economical application.13
Our previous research has shown that PAC, an alternative adsorbent to GAC, can remove metaldehyde very efficiently even in the presence of natural organic matter (NOM).14 In fact, it was reported that PAC has a much higher adsorption capacity (28 mg g−1) for metaldehyde14 compared to that of the GAC used by Busquets et al. which is 15 mg g−1 (ref. 15) and the BAC used by Rolph et al. which is 19 mg g−1.13 Busquets et al. explained that the adsorption of metaldehyde is affected by the pore size distribution of the carbon materials and a higher adsorption capacity was observed in microporous carbon materials.15 Since the pore size distribution of the PAC used in our previous research is dominated by micropores,14 the PAC may be an effective adsorbent for small molecular contaminants such as metaldehyde. Moreover, Rolph et al. argued that BAC could have low adsorption capacity for metaldehyde because of prior exposure and regeneration cycles.13 In comparison, the PAC is unused (virgin) when applied to water samples and its unoccupied adsorption sites are available for metaldehyde.
Considering the potential application of PAC in water treatment plants, this paper investigates the removal of metaldehyde at a low initial concentration (5 μg L−1) by PAC using different water samples including synthetic water, surface water, and water collected from different treatment stages at Walton-on-Thames Water Treatment Works (WTWTW). The working concentration of metaldehyde solution in this study was 5 μg L−1 since it is close to the detected concentration of metaldehyde in surface water (8 μg L−1) by the UK Environment Agency and representative of the metaldehyde concentration in raw water during the peak season.1,13,16 This study investigates the effect of PAC dosage and water quality on the removal of low concentration of metaldehyde from water. It also identifies the best treatment stage to dose PAC for removing metaldehyde at WTWTW, illustrating the potential application of PAC in a real water treatment plant to remove metaldehyde with consideration of possible regeneration of PAC at low temperature.
2. Materials and method
2.1 Description of the site
The reservoir stored water feeding the Walton-on-Thames Water Treatment Works (WTWTW) is derived from the River Thames. The output of treated water from WTWTW varies from 50 to 135 million litres per day (MLD), depending on the season. There are six main treatment stages at WTWTW: 1) pre-ozone contactors – feed ozone to oxidize and break down organic pollutants; 2) static flocculation – use chemical dosing with ferric sulphate and polyelectrolyte as coagulant aids to trap natural organic matter as flocs; 3) counter-current dissolved air flotation (CoCoDAF) units with a bottom layer filter of 600 mm sand (effective size 0.7 mm) and 600 mm of anthracite – feed air to remove flocs formed at the previous stage and small particles in the water; 4) main ozone contactors – feed ozone to further break down any residual organic pollutants; 5) GAC adsorbers – to remove any small particles and pollutants that are difficult to remove by oxidation; 6) series of screens and a contact tank – to disinfect the water before its release to the mains. Fig. 1 illustrates the process of each main treatment stage and their contact time.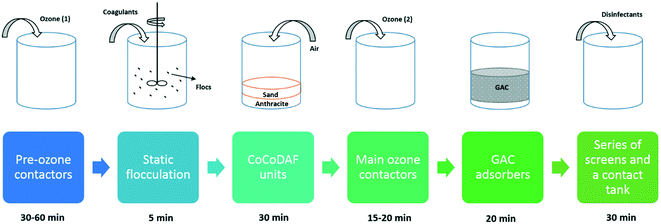 | ||
| Fig. 1 Illustration of the main treatment stages at Walton-on-Thames Water Treatment Works with their approximated contact time. | ||
Water samples were collected at the end of each stage and securely sealed into 1 L plastic bottles by professional personnel from Thames Water. Water samples were then immediately transported to the Environmental Engineering Lab at UCL and stored in a fridge at 4 °C. All water samples were collected on the same day in September 2018 with an overall treated water output of 50 million litres. Considering the water treatment processes installed at WTWTW and the removal of PAC from the water (possibly towards the end of the treatment processes), PAC could potentially be applied after any treatment stage before ‘GAC adsorbers’. Therefore, this study did not consider the dosing of PAC after ‘GAC adsorbers’ since it would require additional installations to remove PAC, leading to extra cost.
2.2 Materials
Different water samples such as MilliQ water, MilliQ water spiked with humic acid (HA), water collected from the Regent's Park lake, and water collected from the six different treatment stages at WTWTW were used to investigate the removal of metaldehyde by adsorption onto PAC. Metaldehyde PESTANAL, PAC (activated charcoal, DARCO®, 100 mesh size, powder) and humic acid sodium salt (technical grade H16752) were purchased from Sigma-Aldrich. HPLC grade methanol and dichloromethane (DCM) were purchased from Fisher Scientific.Metaldehyde stock solution in methanol (100 mL of 500 mg L−1), metaldehyde calibration stock solution in DCM (100 mL of 500 mg L−1), and HA stock solution in MilliQ water (500 mL of 1000 mg L−1) were prepared the same way following our previous research.14,17 0.2 mL of metaldehyde stock solution was added to MilliQ water to make 100 mL of 1 mg L−1 metaldehyde in MilliQ water. From there, different volumes of metaldehyde in MilliQ water (100 mL of 1 mg L−1) were added to different water samples to prepare working metaldehyde solutions with concentrations from 1 to 50 μg L−1. PAC was added to the solution as slurry. PAC stock solution was prepared by adding 1.5 g of PAC into 50 mL of MilliQ water (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1). Prior to the addition of PAC, the slurry was well shaken to ensure uniformity.
000 mg L−1). Prior to the addition of PAC, the slurry was well shaken to ensure uniformity.
2.3 Analytical methods
Detection of metaldehyde followed the method from our previous research using gas chromatography (Perkin Elmer precisely Clarus 500) with mass spectrometry (GC-MS).14,17 The concentration of HA in water was quantified using an Agilent Technologies Cary 60 UV-VIS at a wavelength of 254 nm.18 Since the concentrations of working metaldehyde solutions were low, a different solid phase extraction (SPE) loading method to our previous research was used.14,17 A sample solution of 500 mL was loaded into the SPE cartridge using a Dionex AutoTrace 280 at a rate of 5 mL min−1. The last stage of SPE is to evaporate the eluate to 1 mL; this means that the SPE process not only extracts metaldehyde from the aqueous phase to the organic phase in DCM, but also concentrates the metaldehyde solution 500 times, which allows the working metaldehyde solutions with low concentrations to be investigated. For instance, 500 mL of metaldehyde solution at 5 μg L−1 was loaded into the SPE cartridge; after SPE, 1 mL eluate was collected and analysed by GC-MS. This gives the concentration of the 1 mL eluate to be 2.5 mg L−1 with 100% recovery. The concentration of metaldehyde after SPE was then calibrated since it was concentrated 500 times during the process. This paper will only show the calibrated results (validation of the method is provided in Table S1†).Water characteristics including dissolved organic carbon (DOC), ions, dissolved oxygen (DO), pH, conductivity/total dissolved salts (TDS), turbidity, and UV absorbance at wavelength 254 (UV254) of different water samples were analysed using a Shimadzu total organic carbon analyser (TOC-L), Dionex ICS-1100, Jenway DO2 Meter 9200, pH/conductivity meter SevenMulti, Metter Toledo, HACH 2100AN IS turbidimeter (ISO method 7027), and Agilent Technologies Cary 60 UV-vis, respectively. These characteristics have been determined for the samples taken at different water sources without spiking metaldehyde (Table S2†), and they were measured in the Environmental Engineering Lab at UCL.
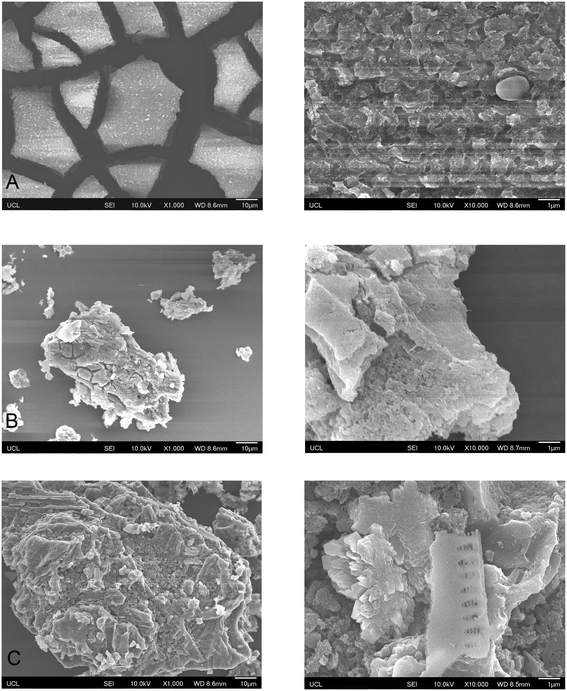
In this study, attenuated total reflection (ATR)-Fourier transform infrared (FTIR) (Bruker's Platinum ATR) and scanning electron microscopy (SEM, a JSM-6701F FESEM) were employed to analyse the flocs (from water collected after ‘static flocculation’) and the used PAC from both water collected after ‘static flocculation’ (PAC-SF) and from the Regent's Park lake (PAC-RP). The flocs and the used PAC samples were collected by filtering sample solutions through a 0.45 μm membrane and dried at room temperature for 24 hours. These analyses provide further insight into the relationships of flocs, PAC, and suspended solids in natural water.
2.4 Statistical analysis
ANOVA tests were carried out to assess the significant difference between concentrations of samples and p < 0.05 was considered statistically significant. Data processing was conducted using Microsoft Excel 2013.2.5 Adsorption experiments
To investigate the removal of metaldehyde from different water samples, four sets of experiments were carried out: 1) to identify the suitable PAC dosage to remove metaldehyde from the first treatment stage i.e. ‘after pre-ozone contactors’; 2) to compare the removal of metaldehyde by PAC at different treatment stages and identify the best treatment stage to dose PAC, as well as to determine the maximum adsorption capacity (qm) of PAC at that stage; 3) to study the effect of water quality on the removal of metaldehyde by PAC and the application of PAC on water quality; 4) to study the adsorption mechanism of metaldehyde onto PAC with different initial concentrations of working metaldehyde solutions.The percentage removal and the amount of metaldehyde adsorbed onto PAC were calculated using eqn (1) and (2), respectively, where C0 is the initial concentration of metaldehyde, Ce is the final concentration of metaldehyde after adsorption by PAC at equilibrium, V is the volume of metaldehyde solution, m is the mass of PAC, and qe has as unit of μg mg−1:14,19,20
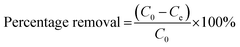 | (1) |
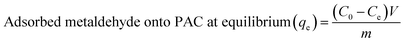 | (2) |
2.6 Regeneration of PAC
Since Rolph et al. discussed that metaldehyde can degrade thermally at 60 °C,13 hence, potential regeneration of used PAC for metaldehyde removal at the same temperature may be possible. This was therefore investigated. Two sets of 1 mg L−1 metaldehyde solutions (600 mL) were prepared using MilliQ water. And 30 mg of PAC (equivalent to a dosing concentration of 50 mg L−1) was dosed into each set of the metaldehyde solution for 30 min with constant stirring by a magnetic stirrer to ensure that PAC was well-mixed and evenly distributed in the solution. After that, PAC was separated from the solution by filtering through a 0.45 μm membrane and placed on a Petri dish. Meanwhile, the concentration of the filtered metaldehyde solutions (600 mL) after 30 mg PAC treatment was measured to calculate the amount of metaldehyde adsorbed onto the used PAC (qe). Then, one set of PAC was covered with foil and dried on the counter at room temperature for 24 hours. The other set of PAC was covered with foil and placed in the oven at 60 °C for 24 hours. After that, each set of PAC was dosed into 600 mL of MilliQ water with constant stirring to study the desorption of metaldehyde and possible regeneration of PAC after being heated at 60 °C in the oven for 24 hours. After 30 min, PAC was removed by filtering the solutions through a 0.45 μm membrane and the two sets of solutions were then analysed for the presence of metaldehyde.3. Results and discussion
3.1 PAC characterization
Brunauer–Emmett–Teller (BET) analysis was performed in our previous research for this PAC (unused, virgin), revealing that it is dominated by micropores and mesopores and has a specific surface area of 962 m2 g−1 and a total pore volume of 0.79 cm3 g−1.14 ATR-FTIR analysis (Fig. S1†) was carried out for flocs, PAC-SF, and PAC-RP based on other studies that have shown attachment of the adsorbate on the adsorbent surface; for example, the spectra of chitosan before and after adsorption of dyes from synthetic wastewater presented evidence of attachment of the dye on the chitosan polymer.21 The spectra of PAC samples did not show signature peaks due to the strong signal of carbon. SEM images of flocs, PAC-SF and PAC-RP are shown in Fig. 2. The angular, fractured pattern of flocs in Fig. 2A can be seen on the surface of PAC-SF in Fig. 2B which suggests that flocs can be adsorbed onto the surface of PAC. Suspended solids including minerals, micro plastics, plant fibres and microorganisms in the water from the Regent's Park lake can be seen in Fig. 2C. The SEM analysis of the PAC samples in this study can be compared with the analysis of virgin PAC in our previous research; SEM images of virgin PAC showed a porous surface without adsorbed flocs and suspended solids.143.2 Effect of PAC dosage on metaldehyde removal
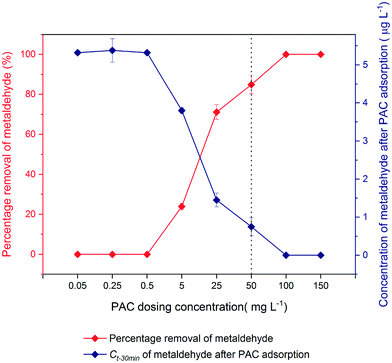
To identify the appropriate dosage of PAC to remove metaldehyde from water samples taken after ‘pre-ozone contactors’, different PAC dosages from 0.05 to 150 mg L−1 were applied to 600 mL of 5 μg L−1 metaldehyde solution for 30 min (Fig. 3).As the PAC dosage increased, the percentage removal of metaldehyde was enhanced; this agrees with the research of Anupam et al. who found higher removal of chromium(VI) from aqueous solutions with increasing dosage of PAC.22 There was no removal of metaldehyde for PAC dosage ≤ 0.5 mg L−1 and metaldehyde could not be detected with PAC dosage ≥ 100 mg L−1. The modified SPE loading method allows metaldehyde to be detected by GC-MS after SPE with a concentration 500 times lower than the limit of quantification of metaldehyde (5 ppb), around 0.01 ppb.17 Therefore, it indicates that PAC dosage ≥ 100 mg L−1 could ensure the concentration of metaldehyde solution after treatment to be below 0.01 μg L−1, which would be within the EU and UK standard of 0.1 μg L−1. The dotted line with a PAC dosage of 50 mg L−1 (30 mg of PAC in 600 mL metaldehyde solution) was the selected PAC dosage for the next set of experiments to make sure that metaldehyde is detected after adsorption.
3.3 Effect of the treatment process on metaldehyde removal
The removal of metaldehyde at different treatment processes including after ‘pre-ozone contactors’, after ‘static flocculation’, ‘CoCoDAF units’, and ‘main ozone contactors’ was investigated by adding 50 mg L−1 PAC into 600 mL of metaldehyde solution at 5 μg L−1 prepared using water collected after these treatment stages (Fig. 4).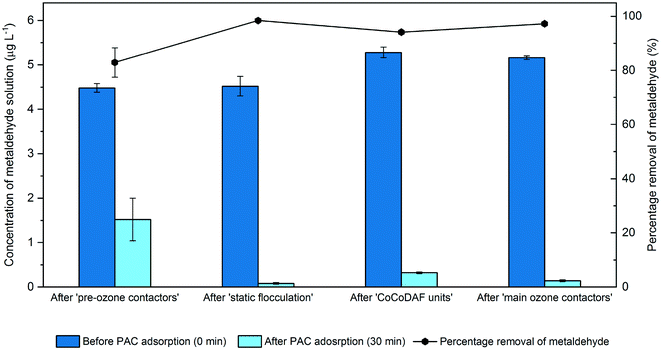 | ||
| Fig. 4 Concentrations of metaldehyde solution before and after adsorption using 50 mg L−1 PAC in water samples taken from the different treatment stages. | ||
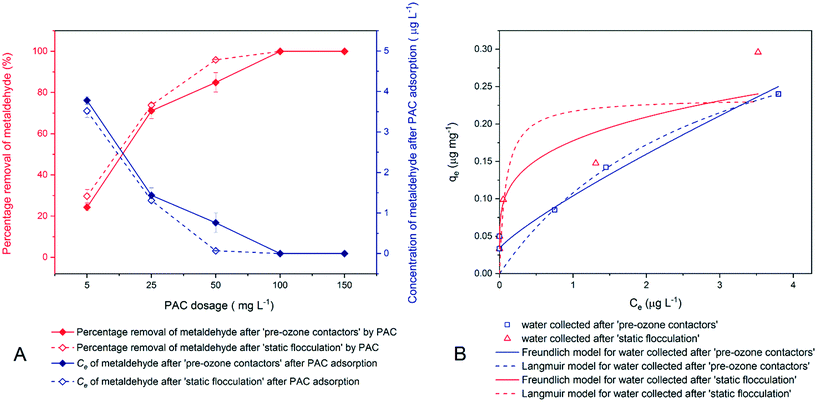
The removal of metaldehyde was expected to be higher at the later treatment stages such as after ‘CoCoDAF units’ and after ‘main ozone contactors’. This is because the water quality is expected to be better (i.e. with fewer pollutants, and hence less competitive adsorption) in the later treatment stages compared to earlier stages such as after ‘static flocculation’. However, the highest removal of metaldehyde (98.4%) was found in water collected after ‘static flocculation’, while 94.1% and 97.2% of metaldehyde were removed from water collected after ‘CoCoDAF units’ and after ‘main ozone contactors’, respectively. The existence of flocs in water collected after ‘static flocculation’ distinguishes this water sample from all others; it is therefore suggested that flocs might play an important role in assisting the adsorption of metaldehyde onto PAC. This agrees with the finding of Jiang et al. who showed that there is a high degree of removal of salicylic acid, ibuprofen and diclofenac from water using super PAC with the presence of flocs, due to neutralization of charge and possible adsorption on the flocs.23 Cook and Newcombe explained that flocs in the system may have an open structure which enables the adsorbate to diffuse easily to the PAC particle.24
Since the removal of metaldehyde was the highest using water collected after ‘static flocculation’, another set of experiments was performed using this water sample with PAC dosages ranging from 5 to 150 mg L−1 to determine the maximum adsorption capacity of PAC. The percentage removals of metaldehyde between the most effective PAC dosing stage (after ‘static flocculation’) and the least effective PAC dosing stage (after ‘pre-ozone contactors’) were compared (Fig. 5A). The removal of metaldehyde from water collected after ‘static flocculation’ was slightly higher than that from the water collected after ‘pre-ozone contactors’ at all PAC dosages but relatively more distinctive at 50 mg L−1. This suggests that as the PAC dosage increased, the removal of metaldehyde was more effective when PAC was dosed in water collected after ‘static flocculation’. This finding agrees with Zhou et al. who suggested that a higher dosage of PAC achieved a higher removal of DOC from water with the presence of flocs and Li et al. who found that there was a higher removal of chemical oxygen demand and lead ions using a higher PAC dosage from wastewater with the presence of flocs.25,26 This is explained by Serpa et al. and Jiang et al. in that there is adsorption on both PAC-embedded flocs and PAC.23,27 In this case, a higher dosage of PAC provides more adsorption sites for removing metaldehyde; therefore, a relatively more distinctive increase in percentage removal of metaldehyde from water collected after ‘static flocculation’ was observed at a PAC dosage of 50 mg L−1.
Fig. 5(B) shows the fittings of the Freundlich isotherm model and the Langmuir isotherm model for metaldehyde adsorbed onto PAC in water collected after ‘pre-ozone contactors’ and after ‘static flocculation’. These two models were used in this study since they are the two most common isotherm models which give the maximum adsorption capacity (qm) of PAC for metaldehyde, hence providing indicative information for the potential application of PAC at WTWTW. The Freundlich isotherm model assumes multilayer sorption28 and it is generally used for heterogeneous surfaces. This model predicts that an increase of the adsorbate in the liquid will lead to an increase of the adsorbate concentrations on the adsorbent.14,19Eqn (3) and (4) describe the Freundlich isotherm model, with 1/n being the heterogeneity factor (adsorption intensity) and KF being the Freundlich constant (adsorption capacity).19,29
| qe = KFCe1/n | (3) |
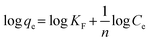 | (4) |
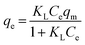 | (5) |
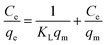 | (6) |
3.4 Effect of water quality on metaldehyde removal and effect of application of PAC on water quality
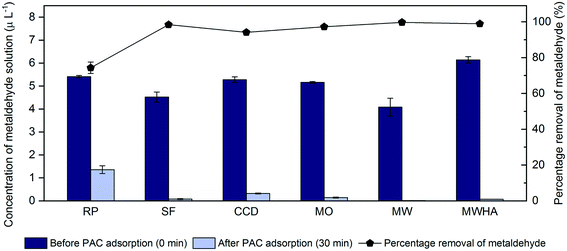
Water quality parameters such as different pH values and concentrations of ions and DOC of water samples can affect the removal of organic pollutants by adsorption. For example, Li et al. argued that the removal of metaldehyde from MilliQ water by PAC was slightly more effective under alkaline conditions.14 Moreover, according to Mukherjee et al., the removal of phenol from water by three carbon materials, including activated carbon, decreased with increasing concentrations of nitrate and chloride, due to the competition for adsorption sites between the ions and the adsorbate.33 This suggests that different concentrations of ions in water samples may affect the adsorption of metaldehyde onto PAC. Additionally, Altmann et al. pointed out that PAC can adsorb DOC fractions, especially small fractions with low molecular-weight,34 which indicates that DOC in water samples can affect the adsorption of metaldehyde onto PAC. In this study, the chloride, nitrate, NPOC and turbidity levels of water samples taken at different treatment stages at WTWTW were significantly different (p < 0.05), which suggests that their water quality were different. Hence, the effect of water quality on metaldehyde removal was studied.The percentage removals of metaldehyde in Regent's Park lake water, MilliQ water, and MilliQ water spiked with HA were compared with the percentage removal of metaldehyde in water samples collected from different treatment stages at WTWTW (Fig. 6), and the water characteristics of the different water samples before and after PAC adsorption are presented (Fig. S2†).
Metaldehyde was effectively removed from all water samples, suggesting that adsorption of metaldehyde onto the PAC used in this study was not significantly affected by water quality such as the presence of organic matter and negative ions (Fig. 6). The presence of humic acid did not affect the removal of metaldehyde by the PAC as well, which agrees with our previous research.14 Removal of metaldehyde from water collected from the Regent's Park lake was the lowest (74.3%). The most distinctive characteristic of this water sample is that it has the highest NPOC of 11.47 mg L−1 among all the water samples (Fig. S2†). It is almost twice as high compared to the water samples collected at WTWTW (6.01–6.7 mg L−1). However, the high NPOC in this water sample was expected because the Regent's Park lake is inhabited by aquatic animals. The PAC used in this study is characterized by abundant micropores and mesopores which is favoured by adsorption of metaldehyde.14,15 However, this characteristic would also allow other organic compounds with small molecules to adsorb onto this PAC. In fact, Altmann et al. argued that small dissolved organic matter constituents are more effectively reduced by adsorption onto PAC, compared to high molecular-weight organics such as humic substances.35 Therefore, the relatively lower removal of metaldehyde from water collected from the Regent's Park lake, compared to the other water samples, could result from its relatively high NPOC; this water sample possibly contains organic matter with smaller fractions than humic acid and can adsorb onto PAC,35 as its NPOC decreased from 11.47 to 8.37 mg L−1 after PAC treatment.
The conductivity, pH and TDS (Fig. S2†) increased for all the water samples after adsorption by PAC due to the point of zero charge of PAC being 7.35.14 In fact, the conductivity and TDS slightly increased after adsorption by PAC for almost all the water samples. This may be explained by the fact that there are inorganic impurities from the making of activated carbon such as salts of alkali and iron from coconut shells.36 These impurities could detach from activated carbon, dissolve in water, and therefore increase the conductivity and TDS of the water. It is suggested by Song et al. and Cooney et al. that PAC can be washed with weak acid and deionized water to remove inorganic impurities prior to application.37,38
The removal of NPOC varied from 2% for water collected after ‘static flocculation’ to 41.6% for water collected after ‘CoCoDAF units’ (Fig. S2†), and there was a significant difference in the NPOC level before and after PAC treatment for all the water samples (p < 0.05), except for water collected after ‘static flocculation’. It is worth noting that NPOC increased after PAC treatment for the metaldehyde solution prepared using MilliQ water and MilliQ water spiked with HA. This suggests that the addition of PAC increased the DOC content in the solutions prepared using MilliQ water. A control test by adding 30 mg of PAC into 600 mL of MilliQ water with 30 min of constant stirring was performed to confirm that NPOC increased from 0.158 mg L−1 to 1.086 mg L−1 after PAC treatment. This was caused by the release of DOC from the surface of PAC due to the high concentration gradient between PAC and MilliQ water. The relatively low removal of NPOC may also be explained by the fact that organic matter in most of these water samples has large molecules and does not favour adsorption by the PAC used.35
Considering the potential application of PAC in WTWTW, the optimal location found in the laboratory tests to dose PAC is suggested to be after ‘static flocculation’. This is not only because the highest removal of metaldehyde (98.4%) was found in the water sample taken after this treatment stage (Fig. 4 and 6), but also because the majority of PAC in water can potentially be removed in the subsequent treatment stages of ‘CoCoDAF units’ and ‘GAC adsorbers’. However, dosing PAC after ‘static flocculation’ may affect the performance of ‘CoCoDAF units’ since a large amount of suspended solids would be present in the system. If the suspended PAC in water cannot be completely removed by the ‘CoCoDAF units’, it will then be filtered and retained by the ‘GAC adsorber’, which suggests that more frequent backwashing of the ‘GAC adsorber’ may be required. For example, the appropriate PAC dosage to treat 600 mL of 5 μg L−1 metaldehyde solution was larger than or equal to 60 mg to ensure that the treated water would meet the UK and EU standard. Considering the daily output of WTWTW (50 to 135 million litres per day), 5 to 13.5 tons of PAC might be needed to treat water with a metaldehyde concentration of 5 μg L−1 every day. And this could have a potential impact on the CoCoDAF units depending on their solid loading capacity which is normally 4–15 kg dry solids per hour per m2.39 Therefore, although the optimum dosing position of PAC at WTWTW to remove metaldehyde was found to be after the ‘static flocculation’, it is suggested that the removal of PAC from water in WTWTW needs to be investigated in situ.
3.5 Adsorption mechanism of metaldehyde onto PAC regarding different initial concentrations
1 mg L−1 was selected as the studied initial concentration of the working metaldehyde solution in our previous research,14 while 5 μg L−1 was selected as the studied initial concentration in this study. The results from our previous research and section 3.3 in this study have shown that the adsorption capacity (qm) of metaldehyde onto PAC was affected by the initial concentration of the working metaldehyde solution.14,17Table 1 summarises the maximum adsorption capacity and the Langmuir constants obtained by fitting these experimental data to the Langmuir model.| Water samples | q m (μg mg−1) | K L (L mg−1) | Experimental conditions |
|---|---|---|---|
| MilliQ | 28.3 | 88.3 | 5 to 500 mg of PAC into 500 mL of 1 mg L−1 metaldehyde solution for 2 hours14 |
| Water collected after ‘pre-ozone contactors’ | 0.25 | 1860 | 0.05 to 150 mg of PAC to 600 mL of 5 μg L−1 metaldehyde solution for 30 min |
| Water collected after ‘static flocculation’ | 0.29 | 4200 | 5 to 150 mg of PAC to 600 mL of 5 μg L−1 metaldehyde solution for 30 min |
From Table 1, the qm and KL of metaldehyde adsorption by PAC varied significantly between different experimental conditions with a high initial concentration of 1 mg L−1 and low initial concentration of 5 μg L−1.14 Therefore, it suggests that the adsorption mechanism of metaldehyde onto PAC is different for metaldehyde solutions with different initial concentrations. Hence, to compare with the qm and KL obtained by our previous study in Table 1, MilliQ water was used for the study of the effect of the initial concentration of metaldehyde solution on metaldehyde adsorption onto PAC.14 30 mg of PAC was added into 600 mL of metaldehyde solutions prepared using MilliQ water with different initial concentrations (1 μg L−1 to 1000 μg L−1) for a contact time of 30 min to investigate the effect of the initial concentration on metaldehyde adsorption in a single adsorption system (Fig. 7).
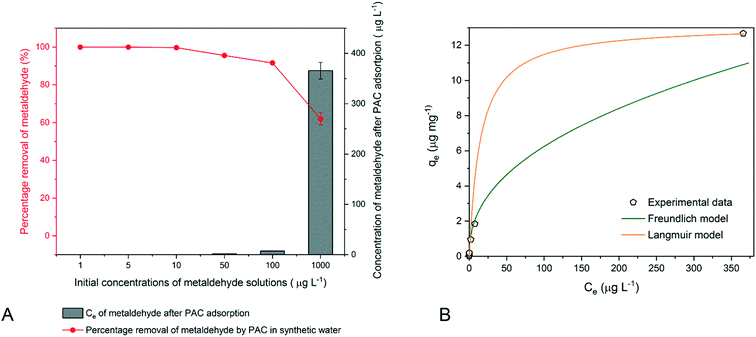
As the initial concentration increased, the percentage removal of metaldehyde decreased (Fig. 7A). A similar decrease in removal of Alizarin Red S dye by a mustard husk adsorbent with increasing initial concentration was observed.32 The UK and EU standard of 0.1 g L−1 can be met by adding 30 mg of PAC into 600 mL metaldehyde solution prepared using MilliQ water with an initial concentration ≤10 μg L−1.
Adsorption isotherm models with experimental data points are presented (Fig. 7B). Data were quite well fitted with the Freundlich model (R2 = 0.9894) and the Langmuir model (R2 = 0.9857). The 1/n obtained from the Freundlich model is 0.43 (n = 2.33), suggesting moderate affinity, similar to that of water collected after ‘pre-ozone contactors’ but suggesting a slightly more homogeneous adsorption system.19 The KF is 0.87 μg mg−1 (μg−1 L)1/n, higher than that of the water collected after ‘pre-ozone contactors’. However, KF as an indicator for adsorption capacity is still low in this case; as discussed in section 3.3, a KF as low as 0.87 cannot explain the experimental results of effective removal of metaldehyde. Hence, the Freundlich model is not suitable for explaining the adsorption mechanism. In terms of fitting with the Langmuir model, the maximum adsorption capacity (qm) is 13.16 μg mg−1 and the Langmuir constant (KL) is 0.07 L μg−1. Although they are lower than those of our previous study (28.3 μg mg−1 and 88.3 L mg−1), they could confirm the experimental results of effective removal of metaldehyde. Hence, the Langmuir model is considered a better model for adsorption of metaldehyde in MilliQ water onto PAC with low initial concentrations.
Since the qm of 13.16 μg mg−1 and KL of 70 L mg−1 are lower than those obtained in our previous study using MilliQ water,14 it suggests that the adsorption capacity of PAC is higher with a higher initial concentration of metaldehyde. This behaviour may be explained by the fact that the driving force to overcome the resistance to the mass transfer from the adsorbate in the aqueous phase to the solid phase on the adsorbent was initiated by the initial concentration of the adsorbate, and the increase in initial concentration would enhance the interaction between them.32 Therefore, the increase in initial concentration would increase the driving force of the concentration gradient and result in enhanced uptake of the adsorbate. This was also confirmed by the research of Gautam et al. who found that the adsorption capacity of activated carbon for metal ions increased with increasing initial concentration.40 The low adsorption capacity of PAC for metaldehyde at low initial concentrations can be explained from another aspect. The water molecule can be considered as a significant factor in explaining the mechanism of adsorption of metaldehyde by PAC as well. A low initial concentration of metaldehyde solution suggests that more water molecules are present in the system. The research of Ferino-Pérez et al. showed that water is like an intermediary between activated carbon and metaldehyde and it competes with metaldehyde for adsorption sites on PAC.41 Busquets et al. also argued that there is a possible competition between water and metaldehyde molecules for adsorption onto activated carbon.15 Therefore, less metaldehyde may be adsorbed onto PAC when the initial concentration of metaldehyde is low and there are more water molecules present in the system.
3.6 Desorption and regeneration of PAC
After removing the used PAC from the two sets of 600 mL of metaldehyde solutions at 1 mg L−1 after 30 min contact time, the concentrations of metaldehyde solutions decreased from 1.16 ± 0.05 mg L−1 to 0.24 ± 0.02 mg L−1. Therefore, according to eqn (2), the qe of the used PAC in this case is 18.35 μg mg−1. Hence, the two used sets of 30 mg PAC each had 550.58 μg of metaldehyde adsorbed on them. Table 2 shows the results of experiments on these two used sets of PAC.| C metaldehyde in water after dosing used PAC | M metaldehyde desorbed back to water | |
|---|---|---|
| PAC dried at room temperature | 79.07 ± 7.17 μg L−1 | 47.44 ± 4.3 μg |
| PAC dried in an oven at 60 °C | 6.41 ± 0.83 μg L−1 | 3.85 ± 0.5 μg |
From Table 2, it is suggested that a small amount of metaldehyde (i.e. 8.6 ± 0.8%) would desorb from PAC back to water which was due to the high concentration gradient of metaldehyde between MilliQ water and PAC. Desorption is very common for activated carbon and it is reported that desorption of metaldehyde from GAC back to water occurs when the inlet concentration of metaldehyde decreases.13,42 However, after heating the used PAC in the oven at 60 °C for 24 hours, the amount of metaldehyde found in water was 3.85 ± 0.5 μg which was only 0.7 ± 0.1% of the total 550.58 μg metaldehyde adsorbed, significantly less than the PAC dried at room temperature. This implies that the majority of adsorbed metaldehyde on PAC might have been thermally degraded in the oven at 60 °C. Therefore, although there was desorption of metaldehyde from PAC back to water, the degree of desorption was relatively small. Degradation of metaldehyde at 60 °C suggested that the used PAC for metaldehyde removal could be potentially regenerated at low temperature. Therefore, it is recommended to further investigate the regeneration of used PAC by thermal treatments using a wide range of temperatures. Also, it is suggested to repeat the tests using natural water to investigate the effects of combined adsorption and desorption of metaldehyde on PAC with the presence of other organic compounds and analyse the adsorption capacity of regenerated PAC regarding metaldehyde removal.
4. Conclusions
Powdered activated carbon can effectively remove metaldehyde from the different water samples collected from Walton-on-Thames Water Treatment Works and MilliQ water, giving a qm of 0.25 μg mg−1 for water collected after ‘pre-ozone contactors’, 0.29 μg mg−1 for water collected after ‘static flocculation’, and 13.16 μg mg−1 for MilliQ water. In all the illustrative feasible dosing points at Walton-on-Thames Water Treatment Works, the addition of PAC into water collected after ‘static flocculation’ achieved the highest percentage removal of metaldehyde (98.4%) due to the presence of flocs that assist the adsorption of metaldehyde. PAC could effectively remove metaldehyde from the studied water samples regardless of their water quality. Furthermore, the increase in initial concentration of metaldehyde would enhance the adsorption of metaldehyde onto PAC due to the high driving force and enhanced mass transfer from metaldehyde in the aqueous phase to the solid phase onto PAC. Therefore, the qm of metaldehyde onto PAC would vary depending on the initial concentration range of the metaldehyde solution. This needs to be taken into account when treating metaldehyde using PAC in water treatment plants and the dosing of PAC needs to be adjusted based on the concentration of metaldehyde detected in water via constant monitoring. It is also essential to monitor the desorption of metaldehyde from PAC back to water during the treatment processes which identifies the specific time to replace the PAC that might have been exhausted, as well as considering the regeneration of the used PAC at the same time, possibly at a low temperature. Since the described method in this study was effective in removing metaldehyde from the studied water samples, it is suggested to further investigate the removal of pesticides such as glyphosate, propyzamide, and 2-methyl-4-chlorophenoxyacetic acid (MCPA) from water using PAC.Disclaimer
The views and opinions expressed in this paper are those of the authors and are the product of ongoing research. They do not represent the position or opinion of Thames Water Utilities Ltd.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Jenny O'Reilly and Dr Michael Chipps for their kind help with the description of Walton-on-Thames Water Treatment Works and providing the water samples, and the Royal Parks for authorising water sampling at the Regent's Park lake.References
- The determination of metaldehyde in waters using chromatography with mass spectrometric detection, UK Environment Agency, 2009.
- G. D. Castle, G. A. Mills, A. Gravell, L. Jones, I. Townsend and D. G. Cameron, et al., Review of the molluscicide metaldehyde in the environment, Environ. Sci.: Water Res. Technol., 2017, 3(3), 415–428 RSC.
- P. Kay and R. Grayson, Using water industry data to assess the metaldehyde pollution problem, Water Environ. J., 2014, 28(3), 410–417 CAS.
- PPDB: Pesticide Properties DataBase 2017 [Available from: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/446.htm.
- J. Li, Q. Zhou and L. C. Campos, The application of GAC sandwich slow sand filtration to remove pharmaceutical and personal care products, Sci. Total Environ., 2018, 635, 1182–1190 CrossRef CAS PubMed.
- J. Li, X. Han, B. W. Brandt, Q. Zhou, L. Ciric and L. C. Campos, Physico-chemical and biological aspects of a serially connected lab-scale constructed wetland-stabilization tank-GAC slow sand filtration system during removal of selected PPCPs, Chem. Eng. J., 2019, 369, 1109–1118 CrossRef CAS.
- K. L. Raknes, Ozone in Drinking Water Treatment: Process Design, Operation, and Optimization, American Water Works Association, Denver, 2005 Search PubMed.
- Y. Luo, W. Guo, H. H. Ngo, L. D. Nghiem, F. I. Hai and J. Zhang, et al., A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment, Sci. Total Environ., 2014, 473–474, 619–641 CrossRef CAS PubMed.
- Briefing paper on metaldehyde: Water UK, 2013 [updated 13th August 2013, Available from: https://www.water.org.uk/wp-content/uploads/2018/11/Metaldehyde-Water-UK-Policy-Briefing-Paper-2013.pdf.
- L. V. Nguyen, R. Busquets, S. Ray and A. B. Cundy, Graphene oxide-based degradation of metaldehyde: Effective oxidation through a modified Fenton's process, Chem. Eng. J., 2017, 307, 159–167 CrossRef CAS.
- O. Autin, J. Hart, P. Jarvis, J. MacAdam, S. A. Parsons and B. Jefferson, Comparison of UV/H2O2 and UV/TiO2 for the degradation of metaldehyde: Kinetics and the impact of background organics, Water Res., 2012, 46(17), 5655–5662 CrossRef CAS PubMed.
- M. Nabeerasool, A. Campen, D. Polya, N. Brown and B. van Dongen, Removal of Metaldehyde from Water Using a Novel Coupled Adsorption and Electrochemical Destruction Technique, Water, 2015, 7(6), 3057–3071 CrossRef CAS.
- C. A. Rolph, B. Jefferson, F. Hassard and R. Villa, Metaldehyde removal from drinking water by adsorption onto filtration media: mechanisms and optimisation, Environ. Sci.: Water Res. Technol., 2018, 4(10), 1543–1552 RSC.
- Z. Li, Y. Yang, U. Jáuregui-Haza, Z. Guo and L. C. Campos, The impact of humic acid on metaldehyde adsorption onto powdered activated carbon in aqueous solution, RSC Adv., 2019, 9(1), 11–22 RSC.
- R. Busquets, O. P. Kozynchenko, R. L. Whitby, S. R. Tennison and A. B. Cundy, Phenolic carbon tailored for the removal of polar organic contaminants from water: a solution to the metaldehyde problem?, Water Res., 2014, 61, 46–56 CrossRef CAS PubMed.
- C. A. Rolph, B. Jefferson and R. Villa, Switching on Pesticide Degraders in Biological Filters used in Drinking Water Production, in Progress in Slow Sand and Alternative Biofiltration, ed. N. G. Nobutada Nakamoto, M. R. Collins and R. Gimbel, IWA, 2014, p. 195 Search PubMed.
- Z. Li, J. K. Kim, V. Chaudhari, S. Mayadevi and L. C. Campos, Degradation of metaldehyde in water by nanoparticle catalysts and powdered activated carbon, Environ. Sci. Pollut. Res., 2017, 24(21), 17861–17873 CrossRef CAS PubMed.
- A. Radian and Y. Mishael, Effect of humic acid on pyrene removal from water by polycation-clay mineral composites and activated carbon, Environ. Sci. Technol., 2012, 46(11), 6228–6235 CrossRef CAS PubMed.
- A. Kumar, B. Prasad and I. M. Mishra, Adsorptive removal of acrylonitrile by commercial grade activated carbon: kinetics, equilibrium and thermodynamics, J. Hazard. Mater., 2008, 152(2), 589–600 CrossRef CAS PubMed.
- Q. Wang, N. Cissoko, M. Zhou and X. Xu, Effects and mechanism of humic acid on chromium(VI) removal by zero-valent iron (Fe0) nanoparticles, Phys. Chem. Earth, 2011, 36(9–11), 442–446 CrossRef.
- N. Sakkayawong, P. Thiravetyan and W. Nakbanpote, Adsorption mechanism of synthetic reactive dye wastewater by chitosan, J. Colloid Interface Sci., 2005, 286(1), 36–42 CrossRef CAS PubMed.
- K. Anupam, S. Dutta, C. Bhattacharjee and S. Datta, Adsorptive removal of chromium (VI) from aqueous solution over powdered activated carbon: Optimisation through response surface methodology, Chem. Eng. J., 2011, 173(1), 135–143 CrossRef CAS.
- W. Jiang, F. Xiao, D. S. Wang, Z. C. Wang and Y. H. Cai, Removal of emerging contaminants by pre-mixed PACl and carbonaceous materials, RSC Adv., 2015, 5(45), 35461–35468 RSC.
- D. Cook and G. Newcombe, Comparison and modeling of the adsorption of two microcystin analogues onto powdered activated carbon, Environ. Technol., 2008, 29(5), 525–534 CrossRef CAS PubMed.
- Z. Zhou, Y. Yang, X. Li, W. Gao, H. Liang and G. Li, Coagulation efficiency and flocs characteristics of recycling sludge during treatment of low temperature and micro-polluted water, J. Environ. Sci., 2012, 24(6), 1014–1020 CrossRef CAS.
- W. Li, T. Hua, Q. Zhou, S. Zhang and F. Li, Treatment of stabilized landfill leachate by the combined process of coagulation/flocculation and powder activated carbon adsorption, Desalination, 2010, 264(1–2), 56–62 CrossRef CAS.
- A. L. Serpa, I. A. H. Schneider and J. Rubio, Adsorption onto Fluidized Powdered Activated Carbon Flocs-PACF, Environ. Sci. Technol., 2005, 39(3), 885–888 CrossRef CAS PubMed.
- A. de Sá, A. S. Abreu, I. Moura and A. V. Machado, 8 - Polymeric materials for metal sorption from hydric resources, in Water Purification, ed. A. M. Grumezescu, Academic Press, 2017, pp. 289–322 Search PubMed.
- Y. C. Wong, Y. S. Szeto, W. H. Cheung and G. McKay, Pseudo-first-order kinetic studies of the sorption of acid dyes onto chitosan, J. Appl. Polym. Sci., 2004, 92(3), 1633–1645 CrossRef CAS.
- I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc., 1918, 40(9), 1361–1403 CrossRef CAS.
- E. Ajenifuja, J. A. Ajao and E. O. B. Ajayi, Adsorption isotherm studies of Cu(II) and Co(II) in high concentration aqueous solutions on photocatalytically modified diatomaceous ceramic adsorbents, Appl. Water Sci., 2017, 7(7), 3793–3801 CrossRef CAS.
- R. K. Gautam, A. Mudhoo and M. C. Chattopadhyaya, Kinetic, equilibrium, thermodynamic studies and spectroscopic analysis of Alizarin Red S removal by mustard husk, J. Environ. Chem. Eng., 2013, 1(4), 1283–1291 CrossRef CAS.
- S. Mukherjee, S. Kumar, A. K. Misra and M. Fan, Removal of phenols from water environment by activated carbon, bagasse ash and wood charcoal, Chem. Eng. J., 2007, 129(1–3), 133–142 CrossRef CAS.
- J. Altmann, A. S. Ruhl, F. Zietzschmann and M. Jekel, Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment, Water Res., 2014, 55, 185–193 CrossRef CAS PubMed.
- J. Altmann, F. Zietzschmann, E. L. Geiling, A. S. Ruhl, A. Sperlich and M. Jekel, Impacts of coagulation on the adsorption of organic micropollutants onto powdered activated carbon in treated domestic wastewater, Chemosphere, 2015, 125, 198–204 CrossRef CAS PubMed.
- M. A. Kononova, N. V. Vorob'ev-Desyatovskii, R. I. Ibragimova and S. A. Kubyshkin, Effect of inorganic compounds in the activated carbon phase and in solution on the adsorption of gold(I) cyanide complex, Russ. J. Appl. Chem., 2009, 82(2), 173–182 CrossRef CAS.
- K.-Y. Song, P.-K. Park, J.-H. Kim, C.-H. Lee and S. Lee, Coupling effect of 17β-estradiol and natural organic matter on the performance of a PAC adsorption/membrane filtration hybrid system, Desalination, 2009, 237(1–3), 392–399 CrossRef CAS.
- D. O. Cooney, A. Nagerl and A. L. Hines, Solvent regeneration of activated carbon, Water Res., 1983, 17(4), 403–410 CrossRef CAS.
- D. D. Ratnayaka, M. J. Brandt and M. Johnson, Twort's Water Supply, Butterworth-Heinemann, 2009, 6th edn, p. 744 Search PubMed.
- R. K. Gautam, A. Mudhoo, G. Lofrano and M. C. Chattopadhyaya, Biomass-derived biosorbents for metal ions sequestration: Adsorbent modification and activation methods and adsorbent regeneration, J. Environ. Chem. Eng., 2014, 2(1), 239–259 CrossRef CAS.
- A. Ferino-Pérez, J. J. Gamboa-Carballo, Z. Li, L. C. Campos and U. Jauregui-Haza, Explaining the interactions between metaldehyde and acidic surface groups of activated carbon under different pH conditions, J. Mol. Graphics Modell., 2019, 90, 94–103 CrossRef PubMed.
- UKWIR, DW14: Treatment for new and emerging pesticides Final, London, 2011 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00962k |
| This journal is © The Royal Society of Chemistry 2020 |