Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Progress, challenges, and opportunities in enhancing NOM flocculation using chemically modified chitosan: a review towards future development
Paripurnanda
Loganathan
*a,
Michael
Gradzielski
 *b,
Heriberto
Bustamante
*c and
Saravanamuthu
Vigneswaran
*a
*b,
Heriberto
Bustamante
*c and
Saravanamuthu
Vigneswaran
*a
aFaculty of Engineering and Information Technology, University of Technology, Sydney, NSW 2007, Australia. E-mail: paripurnanda.loganathan@uts.edu.au; s.vigneswaran@uts.edu.au; Fax: +61 2 9514 2633; Tel: +61 2 9514 2641
bTechnische Universität Berlin, Stranski-Laboratorium für Physikalische und Theoretische Chemie, Institut für Chemie, Sekr., TC 7, Strasse des 17.Juni 124, D-10623 Berlin, Germany. E-mail: Michael.gradzielski@tu-berlin.de; Fax: +(0049) 30 314 26602; Tel: +(0049) 30 314 24934
cSydney Water, 1 Smith Street, Parramatta, NSW 2150, Australia. E-mail: heri.bustamante@sydneywater.com.au; Fax: 61 2 8849 3102; Tel: 61 2 8849 6817
First published on 12th November 2019
Abstract
Natural organic matter (NOM) occurs ubiquitously in water bodies and this can greatly affect feed or raw water quality (taste, colour, odour, bacterial growth). Furthermore, it reduces the performance of the coagulation-based water treatment process. Because the NOM content and its chemical complexity are increasing throughout the world, the removal of NOM from water has become a major challenge in supplying the required amounts of good quality water. The coagulation–flocculation process is widely used for purifying urban water supplies. However, it is not always sufficiently successful in removing the augmented NOM in the feed water, mostly because the polyelectrolytes currently used as coagulants/flocculants cannot effectively interact with all the NOM components consisting of different functional groups, molecular weights, charges, and hydrophobicity. Within the class of polyelectrolytes, chitosan (Cs), which is produced by the deacetylation of abundantly available chitin, has been tested in removing NOM. The effectiveness of Cs can be further improved by chemically modifying the abundant free amino and hydroxyl groups along the Cs chain backbone. This will provide new functional groups that can increase the positive charges, molecular weight, and allow for solubility over a wider pH range, as well as introduce tailored groups which interact in an optimised way with NOM, thereby reducing the solubility of the formed complexes. This paper critically reviews the chemistry of the formed polyelectrolyte/NOM complexes and provides information on how this can be taken advantage of, to identify modified chemical structures of Cs to improve NOM removal in water treatment strategies.
Water impactNatural organic matter (NOM) generally affects feed or raw water quality and increases complexity in water treatment processes. This paper critically reviews the current methods of enhancing NOM removal by the coagulation/flocculation process using chemically modified bio-flocculant, chitosan. Based on current knowledge in the colloid and surface chemistry field, this process is analysed, and we propose structural modifications of chitosan for optimising NOM removal. |
1. Introduction
The coagulation–flocculation process is a widely used method for purifying urban and industrial wastewaters because it can effectively remove contaminants, is simple to operate and low cost. Coagulation is the process of destabilisation of small colloidal particles by reducing the repulsion between similarly charged particles. They can form microflocs, whereas flocculation brings together the coagulated particles and microflocs to form larger macroscopic flocs which settle as a result of sedimentation and can be easily removed.1–4 In recent years, research has implemented the coagulation–flocculation process to wastewaters, mainly industrial waters containing suspended particles. These include, for example clay particles, metal oxy-hydroxides, dyes, and metals.1,2,5–9 It has not, however, dealt sufficiently enough with the removal of natural organic matter (NOM), especially from a fundamental colloid science point of view of complexing an anionic macromolecule by a polycation. Natural organic matter, which arises from the decomposition of plants and microorganisms, is ubiquitous in water bodies.10–12 It causes colour, taste and odour problems and acts as a substrate for bacterial growth. It is a major contributor to disinfection by-product (DBP) formation which is tightly regulated.Changes in the concentration and composition of the heterogeneous NOM in the feed attributed to climate change have impacted on coagulation-based water treatment plants' performance.13 This has often reduced the treatment capacity of direct filtration in the water treatment plants. Because NOM concentrations in raw water are continuously rising (e.g. in Australia14), its comprehensive removal has become increasingly more difficult to achieve. It has also been reported that numerous water utilities in the UK and USA have been experiencing operational difficulties due to the increase in dissolved organic carbon (DOC – a measure of NOM) levels during autumn and winter periods.15 Whitehead et al.16 reported that throughout the UK, NOM concentration in surface waters has increased progressively from the 1980's to 2003, in fact almost doubled, presumably due to climate change. Rising NOM concentrations in raw drinking waters of Nordic countries due to climate change were also reported.17 The raw water used in water treatment works in the UK had a strong seasonal variation in DOC concentration ranging from 4.3 mg L−1 in September to 14.5 mg L−1 in December, with this variation the DOC components ratio also changed.15 This variation was also observed in other temperate climate countries and it was generally ascribed to changes in temperature and hydrology.18 Yet nonetheless it is very important to retain high water quality and meet the stringent drinking water standards required by legislation and demanded in order to have safe water supplies. The topic of NOM in water has been comprehensively discussed in a recent book.19
As polyelectrolytes, polycations have mainly been employed as a coagulant–flocculant due to their high complexing tendency for opposite charged NOM.3–7 Naturally the strength and type of interaction between the NOM and polyelectrolytes will depend largely on the latter's molecular architecture and the strength of interaction. Accordingly, a number of different polyelectrolytes (both inorganics and organics) are employed here. Although inorganic coagulants (Al3+, Fe3+ and polymeric versions) have been used for a long time, they have some disadvantages.20 Firstly, a large amount of coagulant is required, and this generates large quantities of sludge which have disposal problems. Secondly, in the case of alum the process is very sensitive to pH. Thirdly, they are difficult to tailor for optimised removal, i.e. to adjust them specifically to various types of NOMs and thereby achieve as much NOM removal as possible. For these reasons, organic polyelectrolytes (e.g. poly diallyl dimethyl ammonium chloride (PDADMAC)) with enhanced abilities to flocculate NOM even when added in small quantities have been developed in the last few decades.10,11
Organic polyelectrolytes can be synthetic or natural. The advantage of using natural polyelectrolytes is that typically they are biodegradable, and mostly obtained from natural wastes or resources. Within the class of natural polyelectrolytes, chitosan (Cs), which is produced by the deacetylation of chitin, plays a leading role in effectively removing NOM.4,21 It has many uses in many fields including cosmetics, food, wine, pharmaceutical, wood and agrochemical industry as well as in water treatment.5,22 In water treatment the use of Cs is concentrated mostly on the removal of dyes, microorganic pollutants, heavy metals and inorganic colloidal particles and humic acid. The initial investigations concentrated on unmodified Cs, whose applicability is largely restricted to pH values below 6.5, as above it becomes insoluble. However, the effectiveness of Cs can be tuned and further improved by chemical modification to provide new functional groups, to modify its water solubility, and to enhance synergistic interaction with the NOM that needs to be removed. The modifications can be easily done due to the presence of abundant free amino and hydroxyl groups along the Cs chain backbone. A recent review article highlighted the easy chemical modifications of Cs resulting in a wide range of physico-chemical and biological properties of the modified Cs that have opened-up new opportunities for its application in many industries.22 Some modifications reported for Cs in the literature are:
(i) Enhancement of its solubility in a wider pH range.
(ii) Increasing the number of positive charges for stronger interaction with the negatively charged NOM.
(iii) Increasing molecular weight (Mw) for effective bridging with NOM.1,2,23–28
Such modifications have been made to improve the Cs′ adsorption capacity/flocculation properties to remove mainly pollutants such as heavy metals, clay minerals, microorganic compounds and dyes.1,2,5,9,27 Recent reviews have summarised the results obtained with chitosan-based flocculants for removing a variety of pollutants in water.2,22 However, the precise role of modified Cs (and also of other polycations) in the removal of NOM is still not currently well understood. As well, previous reviews did not comprehensively consider the necessary Cs modifications and possibility of removing all components of NOM, notably the more hydrophobic components. Furthermore, a central aspect here is the strength of interaction between the polyelectrolyte and NOM, which for the anionic NOM and cationic polyelectrolyte largely results from the electrostatics involved and the release of the counterions. However, other factors including hydrophobic interactions, van der Waals forces, and H-bonding may play an important role. In colloid science such systems of oppositely charged colloids/polymers have been studied to quite an extent over the last 30 years regarding thermodynamics, structures present and kinetics of their formation.29 Yet, only some aspects of the concept may have been transferred to the realm of water treatment.
This paper critically reviews the current methods and mechanisms of coagulation/flocculation used to remove NOM with a special emphasis on employing chemically modified Cs to promote the formation of polyelectrolyte complexes with NOM. New modifications based on the knowledge garnered from colloid stabilisation studies applied to industrial applications such as drug delivery, cosmetics, food and packaging, and paper manufacture that have the potential for the enhanced removal of NOM, are suggested.9,22,23,30–33 Some of the modifications suggested previously may not be applicable to the removal of NOM due to the removal mechanism being different from those for other chemical compounds. For example, it was reported that the molecular weight of Cs needs to be reduced by chemical modification so that Cs is better utilised in some commercial applications.22 In contrast, the molecular weight of Cs needs to be increased for the effective removal of NOM by the bridging mechanism. The novelty of this paper is: firstly, critically reviewing the mechanisms for removing NOM using polyelectrolytes and secondly, identifying the chemical modifications of Cs that are suitable for NOM removal based on the unique properties of NOM and its flocculation mechanisms.
2. Natural organic matter (NOM) characteristics
Understanding the characteristics of NOM is essential in selecting the most suitable coagulation/flocculation process for its removal. However, it is difficult to assess the characteristics of NOM in thorough detail because of the heterogeneous size (wide molecular weight distribution), structure (different structural motifs contained), and the functional chemistry of its constituents. The NOM characteristics are broadly classified based on hydrophobicity/hydrophilicity, Mw, and charge of the functional groups, mainly using operationally defined procedures such as resin adsorption, size exclusion chromatography (SEC), nuclear magnetic resonance (NMR) spectroscopy, and fluorescence spectroscopy. One study reviewed the methods available for its characterisation several years ago, demonstrating the highly complex nature of NOM.34Natural organic matter in decreasing order of size consists of zooplankton, phytoplankton, bacteria, viruses, clay-humic acid complexes, humic acids, proteins, polysaccharides, fluvic acids, and very small molecules such as fatty acids, carbohydrates, amino acids, and hydrocarbons.35 Of these, humic acids and fulvic acids constitute the largest amounts in most NOM, typically accounting for 53–68% of the dissolved organic carbon (DOC) in natural water bodies.36 In one procedure called the rapid fractionation procedure, NOM is classified according to hydrophobicity, hydrophilicity, and charge, into four fractions: (i) very hydrophobic (VHA) or hydrophobic acid (HPA); (ii) slightly hydrophobic (SHA) or transphilic acid (TPA); (iii) hydrophilic charged (CHAR); and (iv) hydrophilic neutral (NEUT) by using adsorption of NOM on various ion exchange resins.35,37 The hydrophilic fractions are composed mostly of aliphatic carbon and nitrogenous compounds, for example carboxylic acids, carbohydrates, and proteins.10 The hydrophobic fractions are rich in aromatic carbon, phenolic structures and conjugated double bond.10,38 The various NOM fractions exhibit different properties in terms of treatability by coagulation, coagulant demand, and DBP formation potential.10
Another important aspect of the characterisation of NOM is the molecular weight distribution, which can be determined using high performance size exclusion chromatography (HPSEC) analysis.39–41 The chromatographic peaks correspond to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da (organometallic colloids and biological residues), 1900 Da (high Mw humic substances), 800–1200 Da (low molecular weight humics), 500 Da (building blocks), and 300 Da (low Mw acids and nitrogen containing aromatics).39,42 Coagulation studies with repeated applications of alum to a surface water revealed that the HPSEC profiles having high molecular weight peaks were readily removed with the first addition of alum. Conversely the low molecular weight peaks (800–1200 Da) were still present even after five applications of alum. Both the 500 Da and 300 Da remained unchanged.39 This then obviously constitutes a major problem since these lower Mw components are not removed in this treatment.
000 Da (organometallic colloids and biological residues), 1900 Da (high Mw humic substances), 800–1200 Da (low molecular weight humics), 500 Da (building blocks), and 300 Da (low Mw acids and nitrogen containing aromatics).39,42 Coagulation studies with repeated applications of alum to a surface water revealed that the HPSEC profiles having high molecular weight peaks were readily removed with the first addition of alum. Conversely the low molecular weight peaks (800–1200 Da) were still present even after five applications of alum. Both the 500 Da and 300 Da remained unchanged.39 This then obviously constitutes a major problem since these lower Mw components are not removed in this treatment.
In general, one can state that from a colloid science point of view the challenge is to achieve strong complexation of the NOM, which are in general anionic macromolecules comprising of carboxylic and phenolic groups and contain a relatively high percentage of aromatic groups, therefore being macromolecules with a relatively low degree of flexibility.
3. Mechanisms of organic polyelectrolyte coagulation/flocculation of NOM
In the coagulation/flocculation processes, the polyelectrolyte acts as a secondary coagulant, after the use of hydrolysed coagulants (Al3+ and Fe3+), via a charge neutralisation–precipitation mechanism (therefore working best at stoichiometric conditions),43 and is an agent for floc growth. The basic mechanism of this process is the binding of the oppositely charged cationic polyelectrolyte to the negatively charged NOM, where the main driving force is of an entropic nature and arises largely from the counterions' release.44–46 However, the counterions release is most effective for the case of counterions being condensed into the polylelectrolyte. According to Manning,47 counterion condensation takes place if the spacing between the polyelectrolyte and the counterion becomes smaller than the Bjerrum length LB and is controlled by the Manning parameter ξM = LB/b. The Bjerrum length is defined as: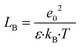 | (1) |
Such complex formation is generically observed for the interaction of polycations with oppositely charged colloids, for example micelles, nanoparticles, clays, proteins, other polyelectrolytes, etc., or in compaction in biological systems which leads to the formation of hierarchically structured complexes above a critical charge ratio29 (Fig. 1). Their size and stability depend on the colloid to be complexed, the effectiveness of the interaction with the polyelectrolyte, and then largely on the Mw of the polyelectrolyte employed. They increase in size as the Mw of the polyelectrolyte increases. Typically, around charge neutralisation, precipitation is observed but the range of mixing ratios where precipitation occurs depends for a given colloid largely on the type and Mw of the polyelectrolyte.48 Of course, the aim of enhanced NOM removal is to make the biphasic range as large as possible, and at the same time reduce the colloidal stability of such complexes as much as possible. This means the aim emphasises the thermodynamic aspect, so that strong attractive interactions will favour phase separation and at the same time in the range of colloidal kinetics to create conditions where this phase separation takes place quickly and efficiently. The waiting times in water treatment, due to the large quantities being treated, need to be kept in the range of some minutes. However, this may constitute a limitation to the polyelectrolytes applied as colloidally metastable complexes may be formed that precipitate only too slowly for a practical application.
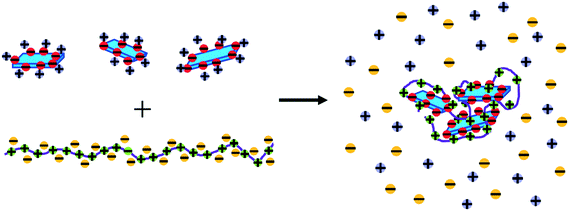 | ||
| Fig. 1 Complexation of NOM molecules (depicted as blue plates) by an oppositely charged polyelectrolyte, resulting in a compacted complex and released counterions. | ||
3.1. Complexation by polyelectrolyte – coagulation
Basically, it is possible to describe the coagulation process as depicted in Fig. 1 as one where the polyelectrolyte interacts with a multi-valent macroion (as for instance would be the case for humic acid). The many counterions released during binding as well as released water molecules drive the complexation process.49 However, this means that the main thermodynamic driving potential is already released within the initial complexation process, while further growth of these structures is not necessarily much favoured. In addition, one has to consider the fact that by complexation the macromolecules loose translational entropy, but this effect becomes less prominent with increasing Mw, therefore explaining why larger Mw both on the side of the NOM and on the side of the added polyelectrolyte facilitate precipitation. In addition, computer simulations,50 have shown that complexation is exothermic for weakly charged polyelectrolytes and endothermic for highly charged polyelectrolytes. Recent experiments by isothermal titration calorimetry (ITC) on complexes of carboxymethyl cellulose (CMC) and lentil protein isolate, confirmed these predictions. In addition, light scattering showed the formation of smaller aggregates with increasing charge density and decreasing Mw of the CMC.51 It is also important to note that the architecture of the charged macromolecules plays an important role with respect to the electrostatic contribution to the free energy, facilitating complexation for linear polyelectrolytes and suppressing it for comb-like ones.52 However, H-bonding will also be an important contributor to the free energy, either directly or water-mediated,53 as well as hydrophobic interactions.Studies have shown that the negative charge on NOM molecules can be effectively neutralised by the cationic polymer, facilitating agglomeration.54 The best removal occurs when the particles' zeta potential is close to zero and they carry approximately net zero charge. At this point, basically no electrostatic colloidal stabilisation is possible and phase separation is principally favoured. In jar tests on synthetic and natural waters of low turbidity and moderate to high colour, cationic polymers having high charge density can effectively remove the organics responsible for colour.55 Cationic polyelectrolytes have a distinct advantage over the metal salts which are commonly used in this procedure, because they affect charge neutralisation without the formation of additional solids in the form of metal hydroxide precipitate. This is quite logical since: firstly, polyelectrolytes naturally form bigger complexes than the low Mw inorganic compounds; and secondly, these bigger complexes are subsequently more likely to lead to faster and potentially more robust floc formation. The Mw of the complexes typically increases with the Mw of the polyelectrolyte, as similarly observed for complexing oppositely charged surfactant micelles.56
3.2. Flocculation
The properties of complexes of oppositely charged polyelectrolyte and colloid/macromolecule become further complicated as the process of precipitation (coagulation/flocculation) may depend largely on the detailed composition of the system. Sometimes it occurs immediately, while in other cases hours, days, or even weeks may be required before macroscopic phase separation takes place.57,58 For water treatment, one is, of course, aiming at fast precipitation in order make the process efficient. In any case, further growth of the initially formed nanometric complexes is required. The stability or metastability of these primary complexes (Fig. 1) is naturally linked to the remaining charge of the formed complexes (as measured by the zeta potential), but also largely to the question of how easily they can grow further (to larger flocs), provided there is a thermodynamic driving force and no kinetic hindrance. Here the polyelectrolyte, if not fully neutralized, may be counter-productive and function as a stabiliser, due to protruding uncomplexed chains that are highly hydrophilic. In that context, long chains (high Mw) typically lead to larger complexes but these may also invoke enhanced colloidal stability. Fig. 2 depicts the further growth (flocculation) process and this will largely depend on two things: firstly, attractive forces between the initially formed complexes; and secondly, how easily they can rearrange to form more compacted agglomerates (flocs). Here, especially hydrophobic interactions may play an important role, as typically simple electrostatic interactions were already largely reduced in the primary coagulation step.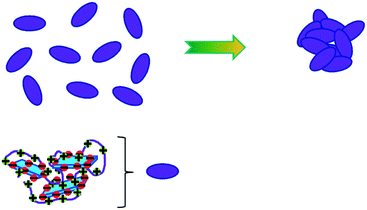 | ||
| Fig. 2 Flocculation of initially formed complexes of NOM/polyelectrolyte, to form larger agglomerates (flocs). | ||
In a review of the literature on flocculation and flocculants, Sharma et al.20 reported that flocculation of NOM with polyelectrolytes may occur through a bridging mechanism. Such a polyelectrolyte bridging mechanism of flocculation was similarly presented by Bolto and Gregory.11 Here segments of a polyelectrolyte chain adsorb on more than one particle, thereby linking them together. The polyelectrolyte adsorbed to NOM has portions of the molecule not bonded to the NOM extending into the solution as loops and tails; they also extend to some distance from the NOM surface into the solution. Their ends suspend and are adsorbed by another NOM molecule forming a bridge between them. The length of the polyelectrolyte chain is crucial for effective bridge formation with linear polyelectrolytes having long chains being the most effective. The bridging mechanism's relevance has also been confirmed due to the fact that intense flocculation takes place long before the isoelectric point is reached, as seen for the flocculation of bentonite and kaolin by polycations.59
If excess polyelectrolyte is added the particles are restabilised by surface saturation, i.e. an appropriate dosage is required as only in the range of compensating the NOM charges by polyelectrolyte precipitation can be expected (as pointed out above). From a practical point of view this dosage range should be as wide as possible, meaning that the addition of the polycation should render the solubility of formed complexes as low as possible. For instance, one model study on flocculating silica particles by cationic polymer of different Mw and charge density indicated that low charge density facilitates bridging, while high charge density favours the patch flocculation mechanism.60 This so-called ‘electrostatic patch’ mechanism is related to the bridging mechanism and makes it possible to explain flocculation of positively charged polyelectrolyte and negatively charged NOM. In the patch mechanism, a high charge density polyelectrolyte adsorbs on the negatively charged NOM having fairly low density of charges and neutralises the charges on NOM. However, because the distance between the charged sites in NOM is greater than the charged segments in the polyelectrolyte, positive charges between uncoated negative charges of NOM occur as patches. These positively charged patches are electrostatically attracted to the negatively charged areas in another particle and hence the particles become attached to form flocs.
In addition to the above electrostatic and bridging modes of coagulation/flocculation mechanisms, there are other mechanisms where the segments of polyelectrolyte are attached to NOM. They involve H-bonding, hydrophobic interaction, van der Waals force attraction and π–π stacking interactions11,24 and will depend largely on the detailed molecular architecture of the NOM. The nature of the flocculation process by polymers has been reviewed and discussed comprehensively some years ago.61
4. Natural polyelectrolyte (bioflocculant) – chitosan (Cs)
As already indicated most commercial organic polyelectrolytes are derived from petroleum-based raw materials, as is the case for the typically employed PDADMAC or alternatives like cationic polyacrylamides, ionenes, or epichlorohydrin/dimethylamine polymers in NOM removal. Compared with those polyelectrolytes, bioflocculants produced from natural materials are typically better biodegradable and mostly obtained from natural organic wastes. However, the overwhelming number of biopolyelectrolytes are polyanions, which are of limited use if one wants to precipitate/remove anionic NOM. As cationic biopolyelectrolytes that can act as primary flocculants, one mainly has cationic starch,62–64 which has been employed in paper modification,65 or Cs. Other alternatives are cationic polypeptides, such as polylysine or polyarginine, which for instance have proved to be quite effective in flocculation of cohesive clay suspensions.66For the application as bioflocculant, Cs appears to be the most promising one, since it is available in large quantities at low cost and has already been shown to be a versatile flocculant in water treatment.2 Applications of chitin and chitosan derivatives for the detoxification of water and wastewater were reviewed a decade ago.67 It was shown that they can remove a large range of contaminants, for example metal ions, dyes, phenol derivatives, and many other pollutants quite effectively from aqueous solution.67 Here modifications of Cs can improve the general performance substantially, as has been reviewed recently.5,22 However, literature on their use in the removal of NOM from water is scarce. Most of the available literature on this describes the removal of synthetic humic acid as a model representative of NOM. Utilising chitosan hydrogel beads for pollutant adsorption demonstrated that humic acid can become effectively bound to the beads by complexation in the pH 5 to 7.5 range.68 In general, Cs has emerged as an attractive option to other polyelectrolytes and therefore the literature on this natural polyelectrolyte that is relevant for water treatment will be discussed in this paper.
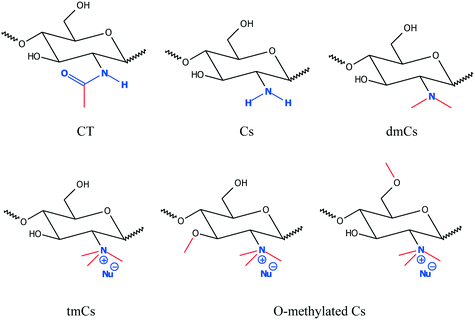
Cs is an amino-based polysaccharide consisting of a linear copolymer of D-glucosamine and N-acetyl-D-glucosamine (Fig. 3).4,5 It is a readily available biopolymer derived via deacetylation of chitin, the second most abundant polysaccharide in nature after cellulose. Chitin is present in many insects, diatoms, and marine animals. Steps involved in the extraction of chitin from fishery wastes (carapace of crustaceans and shellfish) were described elsewhere.22,33 It is positively charged in acidic conditions due to the protonation of the amino groups (Fig. 3).1,27 Naturally derived Cs is typically a linear copolymer, but its branched version has also been synthesised for the purpose of modifying and improving material properties.69
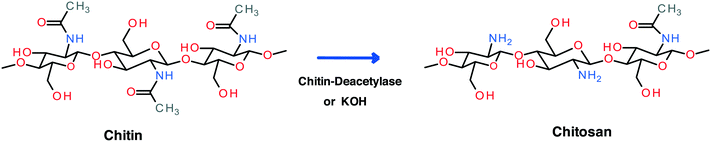 | ||
| Fig. 3 Process of forming chitosan from chitin; in the industrial process concentrated KOH solution is employed.4,5,22 | ||
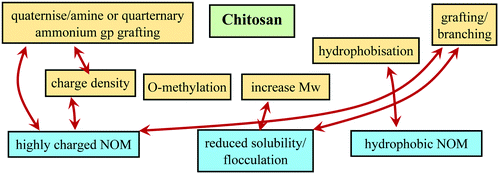
The structure and morphology of Cs depends on available functional groups, the degree of deacetylation, molecular weight, and the functional group's ionisation state.1 The effective pKa value of Cs is ∼6.5, depending on its degree of deacetylation. At pH values below ∼6.2–6.4, Cs is water-soluble but it becomes insoluble at higher pH,1,70 thereby restricting its potential applications in native form substantially. However, in addition to its high biodegradability, low cost, and non-polluting nature, the functional groups (hydroxylic, amino, and acetylic groups) can be easily modified to improve its molecular properties and overcome these limitations,71–73 for instance to address this solubility issue at higher pH. This can be done by introducing permanent charges, and also the control of hydrophobicity is interesting, which can be done via the amounts of acetylated groups contained and in addition by introducing additional hydrophobic moieties. Consequently, one controls the degree of hydrophobicity of the polymer.
Guibal et al.21 demonstrated that Cs can be used as a primary coagulant as well as a flocculant after coagulation: it has characteristics of both coagulants and flocculants. The addition of small amounts of Cs (for example 0.25 mg L−1) to kaolin + humic acid suspension was sufficient to diminish the amount of alum required to halve the original value (from 24 to 12 mg L−1). Interestingly, this study also indicated no effect of the Mw of the Cs on the coagulation/flocculation of humic acid. The mechanism of coagulation and flocculation of Cs was considered, to be charge-neutralisation (including electrostatic patch), inter-particle bridging, and precipitative coagulation (entrapment of NOM by excess of Cs). In treating drinking water, Cs was found to be very effective in removing turbidity but not very efficient in DOC removal.74 In removal of humic substances, Cs was found having a significantly higher maximum extent of flocculation in comparison to other conventional polycations like cationic polyacrylamide.75
As Cs is considered to have much potential for use as a coagulant/flocculant, much attention in recent years has focused on developing even more efficient products by chemical modification of Cs. The aim here is to have modified Cs tailor-made for a specific demand and in our study, this is NOM removal, and this requires a strong tendency for complexation and subsequent growth of strong flocs.
4.1. Chitosan modification
The three most important aspects of Cs which reduce its effectiveness as a coagulant/flocculant for more widespread use are:- Poor water solubility at high pH (low solubility at pH > 6), while water treatment typically takes place at higher pH.
- Often too low molecular weight.
- Low charge density at neutral and alkaline pHs.
Chemical modifications can be done to overcome these limitations, which mostly use the high chemical reactivity of the amino group that is obtained by deacetylation of chitin (Fig. 3).
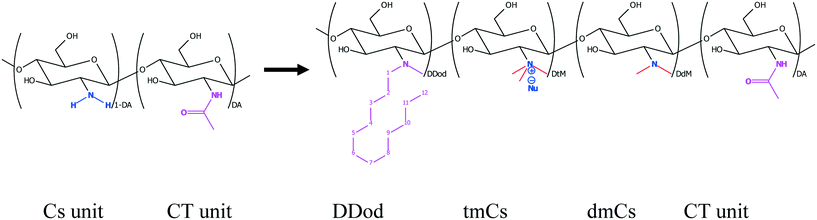
The simplest and most straightforward way to render chitosan soluble irrespective of pH is direct quaternisation of its amino group, thereby transforming the pH-dependent charge on the amine to a permanent one on a quaternary amine (see Fig. 4). Quarternisation of the amino groups to increase the solubility of Cs and its derivatives at higher pHs is also an advantage in cosmetics, medicine and food industries.33 Two other major methods of chemical modifications used to increase the number of positive charges of Cs at a wider pH range are grafting quaternary ammonium and amino acid groups. Along with increasing positive charges the solubility and molecular weight can also be increased. The quaternisation of the Cs amino group also allows for a parallel hydrophobic modification possible, which then leads to forming a markedly amphiphilic Cs derivative. These methods are discussed below in more detail and we focus here, only on modifications that can be considered to be interesting for enhanced NOM removal, which are mainly the control of:
- The charge.
- The molecular weight.
- Hydrophobic modification.
- Solubility of the Cs backbone, e.g. controlled via O-methylation.
It should be noted that the chemistry of Cs modification is still much richer and has been discussed at length in the literature73 and this also determines their potential applications as biomaterials.76,77
Such q-Cs has already been prepared many years ago and is considered to have high potential for drug delivery purposes.32 The simplest version for its synthesis is the reaction with a methylating agent, such as methyl iodide and this reaction is well documented.22,73,78,79 However, any such electrophilic agent may not only react with the amino group but also have a propensity for reacting with the abundantly present OH-groups of the polysaccharide. The degree of O-methylation (see Fig. 4) as a side reaction will then have very significant relevance for the properties of the final q-Cs and its occurrence depends markedly on the conditions chosen for the methylation procedure. For instance, O-methylation reduces the water solubility.79 Direct quaternisation with a methylation agent like methyl iodide typically leads to a high degree of O-methylation of 0.1–0.6, depending on the degree of quaternisation utilised, which in turn depends on how much methylation agent is employed. However, the advantage of this reaction is that it is a “one-pot” synthesis and is consequently a simpler and more economical procedure. Yet, having good control over the degree of O-methylation is critical because it modifies the solution properties of the modified Cs, and this also affects its interaction with humic acid. In one study, an optimised solubility of modified Cs was claimed for an intermediate degree of quaternisation of ∼40%. However, for higher quaternisation also a higher degree of O-methylation was present, and this may have been responsible for this observation.80
By using a two-step reaction in which one first obtains the tertiary amine by an Eschweiler–Clarke reaction (reacting with an excess of formaldehyde and formic acid) and then in a second step quaternises the tertiarised Cs by a Menshutkin reaction, one can largely suppress O-methylation, while still achieving high levels of quaternisation.81 This final quaternisation stage may also be conducted with an alkyl iodide instead of methyl iodide, thereby allowing a hydrophobically modified Cs to form (hm-Cs, see sub-section 4.1.2). In addition, also a “one pot” synthesis of highly N-substituted trimethyl chitosan has been described.82
Being typically very biofriendly, Cs and its derivatives represent a very appealing choice for NOM removal. After quaternisation (yielding q-Cs) one is left with a rather complex polymer containing a variety of different monomeric units (see Fig. 4). Apart from the aimed for quaternised unit (tmCs) one will still have some units of chitin (CT) or Cs present, as well as a dimethylated product (dmCs). There may also be a smaller monomethylated product. Their presence may potentially even be desired as these units would then still show pH-dependence and have the capacity of H-bonding, while the tmCs has a pH-independent charge and no ability to form H-bonds. Furthermore, by not quaternising all of the amino units, the charge density along the backbone can still be varied, which is an additional control parameter for the electrostatic interactions with the NOMs. Of course, one always starts with a certain degree of acetylation (DA), which during the reaction is retained and determines the percentage of CT units in the final product, and thereby also the potential charge density along the Cs chain. The CT units themselves have some hydrophobic characteristics83 and will affect the properties of the q-Cs as well. This is also the case concerning the amount of O-methylated monomeric units.
The hydrophobic interactions in solution become pronounced once the alkyl chain used for hydrophobic modification of the free amino group of the Cs has a length of at least 6 C atoms, and then leads to a substantially higher viscosity for concentrations of hm-Cs about 1 g L−1 (ref. 85) and can even be tuned to gel-like behaviour.86 Based on the fluorescence experiments the presence of hydrophobic aggregates has been deduced both for hm-Cs and normal Cs, but for the hm-Cs an additional type of hydrophobic aggregation takes place.87 Accordingly, self-assembled aggregates will be formed that may be employed for instance for drug delivery purposes.88 hm-Cs will also associate with other amphiphilic moieties, as for instance shown for the case of linking surfactant vesicles and thereby forming a gel.89 Additionally, the hydrophobic modification makes the chitosan surface active.90 Referring to water treatment the hydrophobic modification is attractive because in addition to the already present electrostatic interaction, one can introduce additional hydrophobic interactions91 of the modified chitosan with the NOM. This may be important not only for interacting with the more hydrophobic parts of the NOM, but also help floc growth and building stronger flocs.
The most common approach for alkylation has been a two-step reaction in which first the free amino group of the Cs is reacted with an aldehyde and subsequent hydrogenation to yield a monoalkylated amino group, thereby yielding hm-Cs (Fig. 6).85 Then this hm-Cs (Fig. 7, left) can become quaternised by a methylation reaction, thereby yielding hm-q-Cs (Fig. 7, right). Here full quaternisation or only partial quaternisation can be considered, thereby retaining some protonable amino groups (and thus pH-sensitivity). An alternative is to produce a tertiarised Cs (N-dimethylchitosan) obtained by an Eschweiler–Clarke reaction, which then becomes in a next step alkylated and quaternised with a longer alkyl chain.81 The hydrophobic modification cannot only be done for introducing alkyl chains but similarly aromatic moieties can be introduced via a Schiff base. This is formed by the reaction of the 2-amino group of the chitosan with an aromatic aldehyde and subsequent reduction with cyanoborohydride.92 In a similar way, heterocyclic Cs derivatives have been produced.93
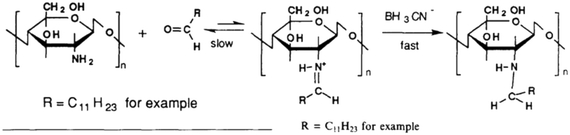 | ||
| Fig. 6 Alkylation of chitosan by reacting with an aldehyde to yield the imine, which subsequently becomes hydrogenated to yield the alklyated chitosan (taken from Desbrières et al.94). | ||
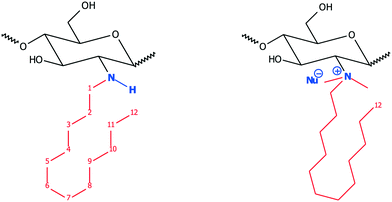 | ||
| Fig. 7 Structure of hydrophobically modified Cs (hm-Cs, left) and dimethylated-dodecylate Cs (hm-q-Cs, right). | ||
However, one can also achieve direct alkylation of the amino group by reaction with an ω-bromo-alkyl trimethyl ammonium salt in basic aqueous solution, which then leads to an additional cationic charge on the end of the added chain. Alternatively, one can combine both approaches to produce two different alkyl chains within one Cs derivative.95 Due to the increased hydrophobic interaction the hydrophobic modification of Cs should be advantageous for removing NOM which contains many hydrophobic fractions, for example very hydrophobic (VHA) or hydrophobic acid (HPA), and slightly hydrophobic (SHA) (see section 2).
| Chitosan (Cs) → CMCs → CMCs-g-PDMC Carboxymethylation Acrylamide copolymerisation |
Yang et al.26 compared the flocculation performance of CMCs-g-PDMC with poly-aluminium chloride (PACl) under acidic, neutral, and alkaline conditions on suspensions of kaolin and humic acid (HA), which are commonly found in real surface water. Zeta potential measurements explained the flocculation performance; floc size was found to be much higher at higher pH. They found that the zeta potential of Cs was positive at pH lower than 8.0 because amine groups were present.26 In contrast, CMCs indicated much lower zeta potential values with an isoelectric point of approximately 5.5. However, when PDMC was grafted onto CMCs, the CMCs-g-PDMC was found to be positively charged in the entire measured pH range and the amounts of positive charges were also higher than those of Cs.
The CMCs-g-PDMC had lower optimal dosage, superior humic acid removal efficiency, wider pH applicability range, and larger floc size, in comparison with inorganic PACl (Table 1). This difference in performance between flocculants was more prominent at the natural water pH of 7 rather than at the acidic pH of 4.
| pH | Polyelectrolyte | Optimum dose (mg L−1) | Residual humic acid (%) | Floc size (μm) |
|---|---|---|---|---|
| 4 | CMC-g-PDMC | 20 | 1.13 | 167 |
| PACl | 75 | 5.97 | 160 | |
| 7 | CMC-g-PDMC | 22 | 1.29 | 201 |
| PACl | 100 | 4.33 | 144 | |
| 11 | CMC-g-PDMC | 75 | 2.26 | 502 |
| PACl | — | — | — |
The CMCs-g-PDMC's better flocculation performance was ascribed to two structural advantages, specifically, increased number of positive charges and molecular weight.26 The increased number of positive charges was caused by the introduction of the positively charged quaternary NH4 group into the Cs. They did occur as seen in the increase in zeta potential values. The increased molecular weight resulted from the grafting of polyacrylamide branches to Cs in order to extend the chain length of Cs, provided more sites for bridging and patching with the humic acid molecules in solution. Charge neutralisation and patching were reported to be the predominant mechanisms of flocculation. If the net charge on the flocs (as indicated by the zeta potential) was close to zero at the optimal dosage, charge neutralisation contributed largely to the flocculation. If the net charge at the optimal dosage was still negative and away from zero as observed at high pHs, patching was reported to be dominant. Charge neutralisation resulted in flocs with relatively weak strength whereas patching produced strong ones.
Based on CMCs, one can also produce amphoteric Cs, which has been done by reacting with 3-chloro-2-hydroxypropyl trimethyl ammonium chloride (CTA), thereby yielding CMCs-CTA. The amphoteric CMCs-CTA demonstrated excellent flocculation performance in water treatment and this occurred in a wider pH range than Cs and with a lower optimal dosage than that of traditional flocculants.96 Modification with polyacrylamide (PAM) allows for the formation of CMCs-PAM which was tested for flocculating kaolin. Here patching was claimed to be the main mechanism at acidic conditions and bridging the dominant mechanism for neutral and alkaline conditions.28
Li et al.25 prepared a Cs-based cationic polyacrylamide flocculant (Cs-g-PAMA) with enhanced function of sterilisation and flocculation by copolymerisation of a cationic quaternary ammonium salt (methacrylamide propyl trimethyl ammonium chloride, MAPTAC) and acrylamide monomer and tested it on the treatment of a series of simulated wastewater containing Escherichia coli. The Cs dissolved in water only below pH 3.0, whereas Cs-g-PAMA was soluble at pHs up to 13, which indicated that the largely improved solubility resulting from the modification should enhance its flocculation performance and broaden the pH range of its application. Li et al.25 compared the flocculation and sterilisation performance of Cs-g-PAMA, PAMA and PAM using Escherichia coli suspension. Their analysis showed that Cs-g-PAMA led to a relatively better flocculation and sterilisation outcome and was less affected by pH compared to PAMA and PAM. The positively charged Cs-g-PAMA was reported to be adsorbed on the negatively charged cytoderm of Escherichia coli, reducing the repulsion between cells and causing cell destabilisation. The unstable cells were reported to have formed large and dense bacteria flocs through the bridging and netting effect. Simultaneously, excess Cs-g-PAMA was said to have destroyed cell membranes through the quaternary ammonium group, thereby helping sterilisation.
Algae and its metabolites in water sources can seriously damage the quality of drinking water, for example by producing unpleasant tastes and odours, formation of DBD and toxins from cyanobacteria. Dong et al.97 prepared a quaternised carboxymethyl chitosan (q-CMCs) and determined the flocculation properties and mechanisms of coagulation/flocculation of algal turbid water (turbidity of 10–40 NTU). They also examined the formation, breakage and regrowth of algal flocs using q-CMCs in comparison with aluminium sulphate (Al2(SO4)3). Cs had the narrowest solubility pH region (pH < 5), indicating its poor solubility. Although CMCs elicited better solubility than Cs, it was still insoluble or partially soluble in slightly acidic water (pH = 4–7). Compared with Cs and CMCs, q-CMCs demonstrated remarkably improved solubility and are soluble almost in the whole pH range and only showed partial insolubility near its isoelectric point at around pH 9.2.
The flocculation performance of modified and unmodified Cs and several conventional coagulants/flocculants, such as Al2(SO4)3, FeCl3 and PAM, were investigated by Dong et al.97 at different pH values for comparison. q-CMCs consistently revealed the best flocculation performance amongst all five coagulants/flocculants and indicated both the lowest optimal dosage (OD) and highest turbidity removal efficiency (TRE) at both pH 5 and 9 (Table 2). This is due to the higher molecular weight of q-CMC and larger number of positive charges on q-CMC as shown by the higher positive zeta potential than the other coagulants/flocculants in the study. The optimum doses of the inorganic coagulants were 40–200 times higher than that of q-CMC. The floc strength for q-CMCs was also stronger than that for Al2(SO4)3 under each flocculation condition, because the algal cells adsorbed onto q-CMCs molecule chains with much stronger physical or chemical forces (charge neutralisation, hydrogen bonding, and extensive strong bridging due to higher molecular weight).
| Flocculant/coagulant | Price (US $ per kg) | pH 5, OD (mg L−1) | pH 5, TRE (%) | Cost (US $ per m3) | pH 9, OD (mg L−1) | pH 9, TRE (%) | Cost (US $ per m3) |
|---|---|---|---|---|---|---|---|
| Chitosan | 51.2 | 0.4 | 94.7 | 0.02 | 5.5 | 85.3 | 0.28 |
| QCMCs | 368.2 | 0.1 | 96.3 | 0.04 | 0.6 | 95.8 | 0.22 |
| PAM | 22.1 | 3.0 | 94.1 | 0.07 | 8.0 | 86.5 | 0.18 |
| Al2(SO4)3 | 5.8 | 20.0 | 89.2 | 0.12 | 35.0 | 88.2 | 0.20 |
| FeCl3 | 3.7 | 15.0 | 93.4 | 0.06 | 25.0 | 91.2 | 0.09 |
The coexistence of kaolin particles and HA (both negatively charged at slightly acidic pH) plays a positive role in the removal of antibiotics, as parts of antibiotic molecules (with positively charged amine groups (R3N+)) were reported to be adsorbed onto kaolin and the NOM by electrostatic attraction, hydrogen bonds (antibiotic–kaolin/HA), π–π stacking (two of the antibiotics–HA) or charge attraction (two of the antibiotics–HA/kaolin). In contrast, kaolin and HA alone without adding flocculants did not remove much of the antibiotics.
Bhalkaran and Wilson1 reported the following modified Cs: glycidyl-trimethyl ammonium chloride, poly(2-methacryloyloxyethyl) trimethyl ammonium chloride-grafted Cs (Cs-g-PDMC), Cs sulphate, Cs-graft-polyacrylamide, 3-chloro-2-hydroxypropyl trimethyl ammonium chloride-modified carboxymethyl Cs; to have higher flocculation efficiency than unmodified Cs in removing turbidity, clay mineral suspension, biological cells, or anionic and cationic dyes. The potential of these modifications in flocculating NOM, still has to be explored more thoroughly in future studies.
4.2. Perspectives of using modified Cs in NOM removal
When looking at the above abundant options for chemically modifying Cs, Cs emerges as an interesting starting molecule to construct optimised modified versions for NOM removal and water treatment in general. Since the backbone is retained, good biodegradability and biocompatibility is given and by being able to play with the charge density, hydrophobic modification, and ability for H-bonding and specific interactions, there is a wide field for optimising its ability to strongly interact with NOMs. Looking at Fig. 5 and 7, one may think in particular about the following parameters to be varied:- Molecular weight (Mw) of the Cs to be achieved by a higher degree of polymerisation of its backbone or grafting of side chains where especially grafting appears interesting as for instance for starch it was shown to be rendering it much more efficient in removing humic acid by flocculation.99
- Degree of acetylation (DA).
- Degree of quaternisation (tm-Cs, or hm-q-Cs) and thereby also the amount of primary, secondary, and tertiary amines still contained.
- Type of hydrophobic modification.
- Percentage of hydrophobic modification.
This is a rather large parameter space and one that cannot easily be explored in its fullest extent. However, in general, it has been found that: firstly, high Mw of the flocculating polyelectrolyte is preferred; and secondly, relatively high charge densities of the polyelectrolyte are expected to be required for strong binding to humic and fluvic acids. For instance, bentonite was more effectively coagulated and flocculated by Cs of higher Mw.100 Much less clear and not really in great depth is the importance of specific binding via H-bonds, aromatic groups, or hydrophobic interactions. This may determine whether one prefers to have a completely quaternised Cs or still retain some amino groups. In this respect, additionally, the degree of O-methylation as well as the degree of acetylation (DA) might be important parameters.
Accordingly, the first and principal parameter to be explored is the effect of the charge density on the quaternised Cs in conjunction with using Cs having different DA. For binding and especially flocculation then an optimised hydrophobic modification (or a series thereof) could be important; here one can have electrostatic binding and binding via hydrophobic interactions at the same time. In that context the relevance of hydrophobic interactions is often underestimated, but the transfer of a dodecyl group from aqueous to hydrophobic environment releases about 15 kT in free energy, while coupling to elementary charges in aqueous solutions typically just releases about 3–5 kT. The latter especially may also be important for building up flocs. With reference to the type of hydrophobic modification one can be flexible in terms of chemistry, as that can be straight or branched aliphatic chains, or also contain aromatic moieties. In principle one is also not restricted to having just one type of hydrophobic modification on the amino group but could also react a second or third hydrophobic group on it (or on different amine groups along the Cs backbone). Furthermore, their content in the modified Cs can be varied, where most likely values up to 10 mol% modification are most likely to be relevant for NOM removal. In general, modified Cs is an interesting type of flocculant, as at the same time it offers the opposite charge to humic/fluvic acid and hydrophobic chains that may allow for additional interaction. On this basis it may be speculated to be most effective in flocculation.
4.3. Cost effectiveness, secondary water pollution and sludge management
One of the major impediments restricting the use of modified Cs in practical application is their cost. Even the cost of unmodified chitosan is higher than that of the inorganic coagulants/flocculants. The cost of modified Cs would further increase because expensive chemicals are used for modification purposes. Dong et al.97 reported that the cost of the modified Cs (US$368 per kg) was much higher than that of the inorganic Al/Fe coagulant (US$4–6 per kg) and unmodified Cs (US$51) (Table 2). However, the optimum dose for the removal of algal turbidity was much lower, turning the modified Cs into a competitive and feasible coagulant in terms of cost per unit volume of water treated.Furthermore, the smaller dose of modified Cs necessary to coagulate NOM implies that a smaller amount of sludge will be produced for disposal. This benefits water treatment plants because it can greatly reduce sludge handling costs.26 Also, the flocs produced have larger size and denser structure with rapid settling velocity.26,97 This too is beneficial to water treatment plants leading to reduction in operational cost.4,97
Although the beneficial effects of modified Cs have been demonstrated in laboratory studies, no large-scale production of these coagulants/flocculants has been reported for practical use in water treatment plants. However, the global production of the raw material, chitin for chitosan production is increasing. Brasselet et al.22 reported that globally 1010 tons of chitin are produced annually, and the global market is very highly valued at US$1205 million in 2015 and this was predicted to more than double by 2022. Such developments make it likely that the cost of modified Cs will become substantially reduced due to larger scale production of chitin.
Secondary water pollution is a concern when polyelectrolytes are used. Chitosan is a bio-organic polyelectrolyte and therefore it is not expected to be toxic to humans and animals. Chemically modified chitosan compounds are used in wine and food industries, but no toxicity has been reported.2,4,22 Yang et al.26 conducted acute toxicity assays on water treated with the chemically modified CMC-g-PDMC (see section 4.13) and PACl using Daphnia magna. They found that the former had no effect on the immobilisation of Daphnia magna, whereas PACl produced a certain degree of toxicity. This would also indicate that the sludge produced after coagulation/flocculation using Cs based materials could be disposed of with less impact on the environment, which is important because legislation in this field is expected to become much more punitive over time.
5. Conclusions and outlook
Coagulation–flocculation followed by sedimentation is a widely used process to remove NOM. This treatment process currently provides healthy drinking water. However, the effectiveness of this process depends on the characteristics of the NOM and whether the selected coagulant and flocculant are efficient, and cost-effective.Organic polyelectrolytes (synthetic and natural) that have marked abilities to flocculate NOM have been developed and employed in the last few decades. Polyelectrolytes have a distinct advantage over the Al and Fe salts which are commonly used in this process. This is because they affect coagulation/flocculation without the formation of additional solids in the form of metal hydroxide precipitate. Also, much smaller dosages of polyelectrolytes can be used to produce stronger flocs and are less pH dependent. However, there is the danger that when overdosed, they may restabilise a dispersion of NOMs.
As NOMs are anionic in nature, cationic polyelectrolytes are frequently used as primary coagulants or coagulation aids. Anionic and non-ionic polyelectrolytes are mainly used as either coagulation aids, flocculants, flocculant aids, or filter aids, but they have shown synergistic effects when employed together with a polycation. In particular, the mechanisms of these cationic polyelectrolytes' coagulation/flocculation are assumed to be charge neutralisation, electrostatic patch formation, and bridging. Charge neutralisation was reported to create flocs with relatively weak strength whereas patching and bridging of polyelectrolytes produced strong ones. A detailed mechanistic understanding in terms of molecular and mesoscopic changes is still largely missing in this field – and challenging due to the complex chemical nature of NOM. In principle, the interaction of NOM with polycations is just one version of complexes of polyelectrolytes with colloids, which have been much studied in colloid science but mostly with the focus on forming soluble complexes.49,101–103 However, in contrast to the many studies of polyelectrolyte/colloid complexes, for the case of polyelectrolyte/NOM complexes information about the thermodynamics of complexation (for example, obtainable by isothermal titration calorimetry)104,105 as well as a detailed characterisation of the mesoscopic structure, for instance to be done by scattering methods,23 is largely missing. This is, despite the fact, that this information is very important for understanding their properties. It includes for example, designing conditions with polyelectrolytes like Cs for a tailored removal of organic compounds.106 Finally, also theoretical modelling and computer simulations may give additional insights into this topic. All such fundamental studies are still largely missing for the case of NOM and these are very important for gaining a thorough understanding needed to optimise NOM removal by polycations.
Bioflocculants produced from natural materials are safe, biodegradable and less expensive. Of the possible bioflocculants, chitosan (Cs) has become a promising and attractive option to other polyelectrolytes. The strength of the Cs as a scaffold for an optimised polycation for water treatment is the relatively high flexibility in chemical modification. Its functional groups (especially the amino group, but also the hydroxylic and acetylic groups) can be easily modified in order to increase its solubility over a wider pH range, increase its molecular weight, provide additional positive charges, and one may also include additional hydrophobic moieties that may enhance binding of some NOM components via hydrophobic interactions. Accordingly, one can tune the extent of electrostatic and hydrophobic interaction but also the ability to form H-bonds. Due to the complex chemical nature of the NOMs one can expect that such high flexibility in terms of being able to interact with the NOMs is required in order to facilitate effective binding of the Cs to the NOMs. In addition, the intrinsically high extent of chain–chain interactions in Cs solutions (as evidenced by the rather high viscosity of its aqueous solution) is another advantage. Such interactions are certainly helpful in floc growth and forming strong flocs.
Intrinsically Cs has a rather poor solubility in water (as evidenced by its chain–chain attraction), which would be good for coagulation/flocculation. However, it also limits its usage to only low pH and therefore chemical modifications are needed to enhance its solubility. A straightforward modification of Cs is the direct quaternisation of the amino groups in Cs that introduces a permanent positive charge to the polymer. This can be coupled to hydrophobic modification of Cs which may improve the coagulation/flocculation properties of NOM. Two other major methods of chemical modifications used to increase the number of positive charges at wider pH range are grafting quaternary ammonium groups and amino acids. Chitosan modifications used in other processes such as adsorptive removal of pollutants and surfactants interactions can be explored for use in improved coagulation/flocculation processes. One aspect that is little explored with reference to Cs is the chain architecture. For cationic starch it has been found that the removal of HA improved substantially by having strongly cationic branch chains present in the molecule.99 This has been ascribed to more efficient charge neutralisation and bridging flocculation,107 and one may expect this to be similar for Cs and its derivatives. In general, the chemical architecture of an optimised Cs should be such that it has a strong interaction with humic substances, while at the same time not having been rendered too water soluble. Key parameters in that respect should be the charge density and O-methylation, which largely control the solubility. The different options for modification of Cs are summarised in Fig. 8 together with their main effects in terms of interaction with NOM and the coagulation/flocculation process.
With this “construction kit” depicted in Fig. 8 and ongoing synthetic advances in modification of Cs it is to be expected that further research on Cs-based flocculants is required. Doing so, should especially take into account all relevant aspects of fundamental colloid science, from mesoscopic structure and thermodynamics of interactions. These should make a substantial impact on a much-improved NOM removal in the water purification process.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Michael Gradzielski is grateful to Bin Dai (TU Berlin) for informative discussions and drawing of Fig. 4, 5 and 7. We also thank Dr. Roobavannan (University of Technology Sydney, Australia) for help in preparing the graphical abstract. We are grateful to University of Technology Sydney for funding towards Paripurnanda Loganathan's employment in this review.References
- S. Bhalkaran and L. D. Wilson, Investigation of self-assembly processes for chitosan-based coagulant-flocculant systems: a mini-review, Int. J. Mol. Sci., 2016, 17, 1662 CrossRef PubMed.
- R. Yang, H. Li, M. Huang, H. Yang and A. Li, A review on chitosan-based flocculants and their applications in water treatment, Water Res., 2016, 95, 59–89 CrossRef CAS.
- B. A. Bolto, Soluble polymers in water purification, Prog. Polym. Sci., 1995, 20, 987–1041 CrossRef CAS.
- F. Renault, B. Sancey, P. M. Badot and G. Crini, Chitosan for coagulation/flocculation processes – An eco-friendly approach, Eur. Polym. J., 2009, 45, 1337–1348 CrossRef CAS.
- G. Z. Kyzas and D. N. Bikiaris, Recent modifications of chitosan for adsorption applications: a critical and systematic review, Mar. Drugs, 2015, 13, 312–337 CrossRef PubMed.
- C. S. Lee, J. Robinson and M. F. Chong, A review on application of flocculants in wastewater treatment, Process Saf. Environ. Prot., 2014, 92, 489–508 CrossRef CAS.
- G. Petzold and S. Schwarz, Polyelectrolyte complexes in flocculation applications, Adv. Polym. Sci., 2014, 256, 25–66 CrossRef CAS.
- C. Y. Teh, P. M. Budiman, K. P. Y. Shak and T. Y. Wu, Recent advancement of coagulation−flocculation and its application in wastewater treatment, Ind. Eng. Chem. Res., 2016, 55, 4363–4389 CrossRef CAS.
- J. Ji, L. Wang, H. Yu, Y. Chen, Y. Zhao, H. Zhang, W. A. Amer, Y. Sun, L. Huang and M. Saleem, Chemical modifications of chitosan and its applications, Polym.-Plast. Technol. Eng., 2014, 53, 1494–1505 CrossRef CAS.
- A. Matilainen, M. Vepsäläinen and M. Sillanpää, Natural organic matter removal by coagulation during drinking water treatment: A review, Adv. Colloid Interface Sci., 2010, 159, 189–197 CrossRef CAS.
- B. Bolto and J. Gregory, Organic polyelectrolytes in water treatment, Water Res., 2007, 41, 2301–2324 CrossRef CAS.
- S. M. Korotta-Gamage and A. Sathasivan, A review: potential and challenges of biologically activated carbon to remove natural organic matter in drinking water purification process, Chemosphere, 2017, 167, 120–138 CrossRef CAS.
- A. Mohiuddin, C. Rajanayagam and C. Kearney, Optimisation of non-ionic polymer to address production issues with high-colour low-turbidity raw water. A report of five events at Sydney Water's Nepean Water Filtration Plant to compare plant performance before and after optimisation, Water, 2014, 41, 58–63 CAS.
- Sydney Water. Blueprint/Facility Plan. Assessment of processes capability of treatment facilities. Nepean water filtration plant WFP008, 2015, p. 69.
- E. L. Sharp, S. A. Parsons and B. Jefferson, Seasonal variations in natural organic matter and its impact on coagulation in water treatment, Sci. Total Environ., 2006, 363, 183–194 CrossRef CAS PubMed.
- P. G. Whitehead, R. L. Wilby, R. W. Battarbee, M. Kernan and A. J. Wade, A review of the potential impacts of climate change on surface water quality, Hydrol. Sci. J., 2009, 54, 101–123 CrossRef.
- N. Moona, K. R. Murphy, M. Bondelind, O. Bergstedt and T. J. R. Pettersson, Partial renewal of granulated activated carbon biofilters for improved drinking water treatment, Environ. Sci.: Water Res. Technol., 2018, 4, 529–538 RSC.
- S. K. Oni, M. N. Futter, L. A. Molot and P. J. Dillon, Modelling the long term impact of climate change on the carbon budget of Lake Simcoe, Ontario using INCA-C, Sci. Total Environ., 2012, 414, 387–403 CrossRef CAS PubMed.
- M. Sillanpää, Natural Organic Matter in Water. Characterization and Treatment Methods. Elsevier, 2015 Search PubMed.
- B. R. Sharma, N. C. Dhuldhoya and U. C. Merchant, Flocculants-an ecofriendly approach, J. Polym. Environ., 2006, 14, 195–202 CrossRef CAS.
- E. Guibal, M. V. Vooren, B. A. Dempsey and J. Roussy, A review of the use of chitosan for the removal of particulate and dissolved contaminants, Sep. Sci. Technol., 2006, 41, 2487–2514 CrossRef CAS.
- C. Brasselet, G. Pierre, P. Dubessay, M. Dols-Lafargue, J. Coulon, J. Maupeu, A. Vallet-Courbin, H. de Baynast, T. Doco, P. Michaud and C. Delattre, Modification of chitosan for the generation of functional derivatives, Appl. Sci., 2019, 9, 1321–1333 CrossRef.
- L. Chiappisi, S. Prevost, I. Grillo and M. Gradzielski, Chitosan/alkylethoxy carboxylates: a surprising variety of structures, Langmuir, 2014, 30, 1778–1787 CrossRef CAS.
- S. Jia, Z. Yang, K. Ren, Z. Tian, C. Dong, R. Ma, G. Yu and W. Yang, Removal of antibiotics from water in the coexistence of suspended particles and natural organic matters using amino-acid-modified-chitosan flocculants: A combined experimental and theoretical study, J. Hazard. Mater., 2016, 317, 593–601 CrossRef CAS.
- X. Li, H. Zheng, Y. Wang, Y. Sun, B. Xu and C. Zhao, Fabricating enhanced sterilization chitosan-based flocculants: Synthesis, characterization, evaluation of sterilization and flocculation, Chem. Eng. J., 2017, 319, 119–130 CrossRef CAS.
- Z. Yang, H. Li, H. Yan, H. Wu, Q. Yang, H. Wu, A. Li, A. Li and R. Cheng, Evaluation of a novel chitosan-based flocculant with high flocculation performance, low toxicity and good floc properties, J. Hazard. Mater., 2014, 276, 480–488 CrossRef CAS PubMed.
- J. Wang and S. Zhuang, Removal of various pollutants from water and wastewater by modified chitosan adsorbents, Crit. Rev. Environ. Sci. Technol., 2018, 47, 1–57 Search PubMed.
- Z. Yang, B. Yuan, X. Huang, J. Zhou, J. Cai, H. Yang, A. Li and R. Cheng, Evaluation of the flocculation performance of carboxymethyl chitosan- graft-polyacrylamide, a novel amphoteric chemically bonded composite flocculant, Water Res., 2012, 46, 107–114 CrossRef CAS PubMed.
- J. F. Berret, Controlling electrostatic co-assembly using ion-containing copolymers: From surfactants to nanoparticles, Adv. Colloid Interface Sci., 2011, 167, 38–48 CrossRef CAS.
- L. Chiappisi and M. Gradzielski, Co-assembly in chitosan-surfactant mixtures: thermodynamics, structures, interfacial properties and applications, Adv. Colloid Interface Sci., 2015, 220, 92–107 CrossRef CAS.
- J. H. Hamman, Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems, Mar. Drugs, 2010, 8, 1305–1322 CrossRef CAS PubMed.
- V. K. Mourya and N. N. Inamdar, Trimethyl chitosan and its applications in drug delivery, J. Mater. Sci.: Mater. Med., 2009, 20, 1057–1079 CrossRef CAS.
- S. Ahmed and S. Ikram, Chitosan and its derivatives: a review in recent innovations, Int. J. Pharma Sci. Res., 2015, 6, 14–30 Search PubMed.
- A. Matilainen, E. T. Gjessing, T. Lahtinen, L. Hed, A. Bhatnagar and M. Sillanpää, An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment, Chemosphere, 2011, 83, 1431–1442 CrossRef CAS.
- B. Bolto, D. Dixon and R. Eldridge, Ion exchange for the removal of natural organic matter, React. Funct. Polym., 2004, 60, 171–182 CrossRef CAS.
- K. Kalbitz, J. Schmerwitz, D. Schwesig and E. Matzner, Biodegredation of soil-derived dissolved organic matter as related to its properties, Geoderma, 2003, 113, 273–291 CrossRef CAS.
- C. W. K. Chow, R. Fabris and M. Drikas, A rapid fractionation technique to characterise natural organic matter for the optimisation of water treatment processes, J. Water Supply: Res. Technol.--AQUA, 2004, 53, 85–92 CrossRef CAS.
- J. Swietlik, A. Dabrowska, U. Raczyk-Stanilawiak and J. Nawrocki, Reactivity of natural organic matter fractions with chlorine dioxide and ozone, Water Res., 2004, 38, 547–558 CrossRef CAS.
- C. W. K. Chow, R. Fabris, J. V. Leeuwen, D. Wang and M. Drikas, Assessing natural organic matter treatability sing high performance size exclusion chromatography, Environ. Sci. Technol., 2008, 42, 6683–6689 CrossRef CAS.
- S. A. Huber, A. Balz, M. Albert and W. Pronk, Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography – organic carbon detection – organic nitrogen detection (LC-OCD-OND), Water Res., 2011, 45, 879–885 CrossRef CAS PubMed.
- S. Velten, D. R. U. Knappe, J. Traber, H. P. Kaiser, U. von Gunten, M. Boller and S. Meylan, Characterization of natural organic matter adsorption in granular activated carbon adsorbers, Water Res., 2011, 45, 3951–3959 CrossRef CAS PubMed.
- B. P. Allpike, A. Heitz, C. A. Joll, R. I. Kagi, G. Abbt-Braun, F. H. Frimmel, T. Brinkmann, N. Her and G. Amy, Size exclusion chromatography to characterise DOC removal in drinking water treatment, Environ. Sci. Technol., 2005, 39, 2334–2342 CrossRef CAS PubMed.
- N. Narkis and M. Rebhun, Stoichiometric relationship between humic and fulvic acids and flocculants, J. - Am. Water Works Assoc., 1977, 69, 325–328 CrossRef CAS.
- C. B. Bucur, Z. Sui and J. B. Schlenoff, Ideal Mixing in Polyelectrolyte Complexes and Multilayers: Entropy Driven Assembly, J. Am. Chem. Soc., 2006, 128, 13690–13691 CrossRef CAS PubMed.
- J. Gummel, F. Cousin and F. Boué, Counterions release from electrostatic complexes of polyelectrolytes and proteins of opposite charge: A direct measurement, J. Am. Chem. Soc., 2007, 129, 5806–5807 CrossRef CAS PubMed.
- M. T. Record, C. F. Anderson and T. M. Lohman, Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity, Q. Rev. Biophys., 1978, 11, 103–178 CrossRef CAS PubMed.
- G. S. Manning, Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions I. Colligative Properties, J. Chem. Phys., 1969, 51, 924–933 CrossRef CAS.
- Y. Wang, K. Kimura, P. L. Dubin and W. Jaeger, Polyelectrolyte−micelle coacervation: Effects of micelle surface charge density, polymer molecular weight, and polymer/surfactant ratio, Macromolecules, 2000, 33, 3324–3331 CrossRef CAS.
- J. van der Gucht, E. Spruijt, M. Lemmers and M. A. Cohen Stuart, Polyelectrolyte complexes: Bulk phases and colloidal systems, J. Colloid Interface Sci., 2011, 361, 407–422 CrossRef CAS PubMed.
- Z. Y. Ou and M. Muthukumar, Entropy and enthalpy of polyelectrolyte complexation: Langevin dynamics simulations, J. Chem. Phys., 2006, 124, 154902 CrossRef PubMed.
- Y. Wang, P. K. S. Pillai and M. T. Nickerson, Effect of molecular mass and degree of substitution of carboxymethyl cellulose on the formation electrostatic complexes with lentil protein isolate, Food Res. Int., 2019, 126, 108652 CrossRef CAS PubMed.
- B. M. Johnston, C. W. Johnston, R. A. Letteri, T. K. Lytle, C. E. Sing, T. Emrick and S. L. Perry, The effect of comb architecture on complex coacervation, Org. Biomol. Chem., 2017, 15, 7630–7642 RSC.
- P. Batys, S. Kivistö, S. M. Lalwani, J. L. Lutkenhaus and M. Sammalkorpi, Comparing water-mediated hydrogen-bonding in different polyelectrolyte complexes, Soft Matter, 2019, 15, 7823–7831 RSC.
- J. K. Edzwald, Conventional water treatment and direct filtration: treatment and removal of total organic carbon and trihalomethane precursors. in Organic Carcinogens in Drinking Water, ed. N. M. Ram, E. J. Calabrese and R. F. Christman, Wiley, New York, 1986, vol. 208, pp. 199–236 Search PubMed.
- H. T. Glazer and J. K. Edzwald, Coagulation and direct filtration of humic substances with polyethylenimine, Environ. Sci. Technol., 1979, 13, 299–305 CrossRef.
- Y. Li, P. L. Dubin, H. Dautzenberg, U. Lück, J. Hartmann and Z. Tuzar, Dependence of structure of polyelectrolyte/micelle complexes upon polyelectrolyte chain length and micelle size, Macromolecules, 1995, 28, 6795–6798 CrossRef CAS.
- C. G. de Kruif, F. Weinbreck and R. de Vries, Complex coacervation of proteins and anionic polysaccharides, Curr. Opin. Colloid Interface Sci., 2004, 9, 340–349 CrossRef CAS.
- S. L. Turgeon, M. Beaulieu, C. Schmitt and C. Sanchez, Protein–polysaccharide interactions: phase-ordering kinetics, thermodynamic and structural aspects, Curr. Opin. Colloid Interface Sci., 2003, 8, 401–414 CrossRef CAS.
- S. Barany, R. Meszaros, I. Kozakova and I. Skvarla, Kinetics and mechanism of flocculation of bentonite and kaolin suspensions with polyelectrolytes and the strength of flocc, Colloid J., 2009, 71, 285–292 CrossRef CAS.
- Y. Zhou and G. V. Frank, Flocculation mechanism induced by cationic polymers investigated by light scattering, Langmuir, 2006, 22, 6775–6786 CrossRef CAS PubMed.
- J. Gregory and S. Barany, Adsorption and flocculation by polymers and polymer mixtures, Adv. Colloid Interface Sci., 2011, 169, 1–12 CrossRef CAS PubMed.
- M. Huang, Y. Wang, J. Cai, J. Bai, H. Yang and A. Li, Preparation of dual-function starch-based flocculants for the simultaneous removal of turbidity and inhibition of Escherichia coli in water, Water Res., 2016, 98, 128–137 CrossRef CAS PubMed.
- D. B. Solarek, Cationic Starches, in Modified Starches: Properties and Uses ed. O. B. Wurzburg, CRC Press, Inc., Boca Raton, FL, 1986, pp. 113–130 Search PubMed.
- J. P. Wang, S. J. Yuan, Y. Wang and H. Q. Yu, Synthesis, characterization and application of a novel starch-based flocculant with high flocculation and dewatering properties, Water Res., 2013, 47, 2643–2648 CrossRef CAS PubMed.
- R. C. Howard and C. J. Jowsey, The effect of cationic starch on the tensile strength of paper, J. Pulp Pap. Sci., 1989, 15, J225–J229 CAS.
- Y. M. Kwon, J. Im, I. Chang and G. C. Cho, ε-polylysine biopolymer for coagulation of clay suspensions, Geomech. Geoeng., 2017, 12, 753–770 CrossRef.
- A. Bhatnagar and M. Sillanpää, Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater — A short review, Adv. Colloid Interface Sci., 2009, 152, 26–38 CrossRef CAS PubMed.
- W. L. Yan and R. Bai, Adsorption of lead and humic acid on chitosan hydrogel beads, Water Res., 2005, 39, 688–698 CrossRef CAS.
- D. Aggarwal and H. W. T. Matthew, Branched chitosans: Effects of branching parameters on rheological and mechanical properties, J. Biomed. Mater. Res., 2007, 82A, 201–213 CrossRef CAS.
- K. M. Varum, M. H. Ottoy and O. I. Smidsrod, Water-solubility of partially N-acetylated chitosans as a function of pH: effect of chemical composition and depolymerisation, Carbohydr. Polym., 1994, 25, 65–70 CrossRef CAS.
- N. M. Alves and J. F. Mano, Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications, Int. J. Biol. Macromol., 2008, 43, 401–414 CrossRef CAS.
- B. E. Benediktsdóttir, Ó. Baldursson and M. Másson, Challenges in evaluation of chitosan and trimethylated chitosan (TMC) as mucosal permeation enhancers: From synthesis to in vitro application, J. Controlled Release, 2014, 173, 18–31 CrossRef.
- V. K. Mourya and N. N. Inamdar, Chitosan-modifications and applications: Opportunities galore, React. Funct. Polym., 2008, 68, 1013–1052 CrossRef CAS.
- R. Fabris, C. W. K. Chow and M. Drikas, Evaluation of chitosan as a natural coagulant for drinking water treatment, Water Sci. Technol., 2010, 61, 2119–2128 CrossRef CAS PubMed.
- T. Kvinnesland and H. Odegaard, The effects of polymer characteristics on nano particle separation in humic substances removal by cationic polymer coagulation, Water Sci. Technol., 2004, 50, 185–191 CrossRef CAS.
- H. Sashiwa and S. I. Aiba, Chemically modified chitin and chitosan as biomaterials, Prog. Polym. Sci., 2004, 29, 887–908 CrossRef CAS.
- C. Choi, J. P. Nam and J. W. Nah, Application of chitosan and chitosan derivatives as biomaterials, J. Ind. Eng. Chem., 2016, 33, 1–10 CrossRef CAS.
- Z. Jia, D. Shen and W. Xu, Synthesis and antibacterial activities of quaternary ammonium salt of chitosan, Carbohydr. Res., 2001, 333, 1–6 CrossRef CAS.
- A. Polnok, G. Borchard, J. C. Verhoef, N. Sarisuta and H. E. Junginger, Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride, Eur. J. Pharm. Biopharm., 2004, 57, 77–83 CrossRef CAS PubMed.
- A. Jintapattanakit, S. Mao, T. Kissel and V. B. Junyaprasert, Physicochemical properties and biocompatibility of N-trimethyl chitosan: Effect of quaternization and dimethylation, React. Funct. Polym., 2008, 70, 563–571 CAS.
- R. J. Verheul, M. Amidi, S. van der Wal, E. van Riet, W. Jiskoot and W. E. Hennink, Synthesis, characterization and in vitro biological properties of O-methyl free N,N,N-trimethylated chitosan, Biomaterials, 2008, 29, 3642–3649 CrossRef CAS PubMed.
- Ö. V. Rúnarsson, J. Holappa, S. Jónsdóttir, H. Steinsson and M. Másson, N-selective ‘one pot’ synthesis of highly N-substituted trimethyl chitosan (TMC), Carbohydr. Polym., 2008, 74, 740–744 CrossRef.
- M. Rinaudo, Chitin and chitosan: Properties and applications, Prog. Polym. Sci., 2006, 31, 603–632 CrossRef CAS.
- J. Wang, L. Wang, H. Yu, Zain-Ul-Abdin, Y. Chen, Q. Chen, W. Zhou, H. Zhang and X. Chen, Recent progress on synthesis, property and application of modified chitosan: An overview, Int. J. Biol. Macromol., 2016, 88, 333–344 CrossRef CAS PubMed.
- J. Desbrières, C. Martinez and M. Rinaudo, Hydrophobic derivatives of chitosan: Characterization and rheological behaviour, Int. J. Biol. Macromol., 1996, 19, 21–28 CrossRef.
- M. Rinaudo and J. Desbrieres, Thermally induced gels obtained with some amphiphilic polysaccharide derivatives: Synthesis, mechanism and properties. in Hydrocolloids – Part I, ed. K. Nishinari, Elsevier Sci. BV 2000, pp. 111–123 Search PubMed.
- O. E. Philippova, E. V. Volkov, N. L. Sitnikova, A. R. Khokhlov, J. Desbrieres and M. Rinaudo, Two types of hydrophobic aggregates in aqueous solutions of chitosan and its hydrophobic derivative, Biomacromolecules, 2001, 2, 483–490 CrossRef CAS.
- J. Zhang, C. Guang, Y. Y. Li and C. S. Liu, Self-assembled nanoparticles based on hydrophobically modified chitosan as carriers for doxorubicin, Nanomedicine, 2007, 3, 258–265 CrossRef CAS.
- J. H. Lee, J. P. Gustin, T. Chen, G. F. Payne and S. R. Raghavan, Vesicle-biopolymer gels: networks of surfactant vesicles connected by associating biopolymers, Langmuir, 2005, 21, 26–33 CrossRef CAS.
- J. Desbrières, M. Rinaudo, V. Babak and G. Vikhoreva, Surface activity of water soluble amphiphilic chitin derivatives, Polym. Bull., 1997, 39, 209–215 CrossRef.
- C. Tanford, The Hydrophobic Effect: Formation of Micelles and Biological Membranes, Wiley, Brisbane, Chichester, New York, Toronto, 1980, p. 233 Search PubMed.
- W. Sajomsang, S. Tantayanon, V. Tangpasuthadol, M. Thatte and W. H. Daly, Synthesis and characterization of N-aryl chitosan derivatives, Int. J. Biol. Macromol., 2008, 43, 79–87 CrossRef CAS PubMed.
- M. El Badawy, Chemical modification of chitosan: synthesis and biological activity of new heterocyclic chitosan derivatives, Polym. Int., 2008, 57, 254–261 CrossRef CAS.
- J. Desbriéres, C. Martinez and M. Rinaudo, Hydrophobic derivatives of chitosan: characterization and rheological behaviour, Int. J. Biol. Macromol., 1996, 19, 21–28 CrossRef.
- R. de Oliveira Pedro, C. C. Schmitt and M. G. Neumann, Syntheses and characterization of amphiphilic quaternary ammonium chitosan derivatives, Carbohydr. Polym., 2016, 147, 97–103 CrossRef CAS PubMed.
- Z. Yang, Y. Shang, Y. Lu, Y. Chen, X. Huang, A. Chen, Y. Jiang, W. Gu, X. Qian, H. Yang and R. Cheng, Flocculation properties of biodegradable amphoteric chitosan-based flocculants, Chem. Eng. J., 2011, 172, 287–295 CrossRef CAS.
- C. Dong, W. Chen and C. Liu, Flocculation of algal cells by amphoteric chitosan-based flocculant, Bioresour. Technol., 2014, 170, 239–247 CrossRef CAS PubMed.
- G. Kumar, P. J. Smith and G. F. Payne, Enzymatic grafting of a natural product onto chitosan to confer water solubility under basic conditions, Biotechnol. Bioeng., 1999, 63, 154–165 CrossRef CAS PubMed.
- H. Wu, Z. Liu, H. Yang and A. Li, Evaluation of chain architectures and charge properties of various starch-based flocculants for flocculation of humic acid from water, Water Res., 2016, 96, 126–135 CrossRef CAS.
- J. Roussy, M. Van Vooren, B. A. Dempsey and E. Guibal, Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions, Water Res., 2005, 39, 3247–3258 CrossRef CAS.
- V. A. Kabanov and A. B. Zezin, Soluble interpolymeric complexes as a new class of synthetic polyelectrolytes, Pure Appl. Chem., 1984, 56, 343–354 CAS.
- V. A. Kabanov, Polyelectrolyte complexes in solution and in bulk, Russ. Chem. Rev., 2005, 74, 3–20 CrossRef CAS.
- A. V. Il'ina and V. P. Varlamov, Chitosan-Based Polyelectrolyte Complexes: A Review, Appl. Biochem. Microbiol., 2005, 41, 5–11 CrossRef.
- A. B. Kayitmazer, Thermodynamics of complex coacervation, Adv. Colloid Interface Sci., 2017, 239, 169–177 CrossRef CAS PubMed.
- S. Yu, X. Xu, C. Yigit, M. van der Giet, W. Zidek, J. Jankowski, J. Dzubiella and M. Ballauff, Interaction of human serum albumin with short polyelectrolytes: a study by calorimetry and computer simulations, Soft Matter, 2015, 11, 4630–4639 RSC.
- L. Chiappisi, M. Simon and M. Gradzielski, Towards bioderived intelligent nanocarriers for controlled pollutant recovery and pH-sensitive binding, ACS Appl. Mater. Interfaces, 2015, 7, 6139–6145 CrossRef CAS PubMed.
- H. Wu, Z. Liu and H. Yang, Evaluation of starch-based flocculants for the flocculation of dissolved organic matter from textile dyeing secondary wastewater, Chemosphere, 2017, 174, 200–207 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |