Adsorption capacity of the corrosion products of nanoscale zerovalent iron for emerging contaminants†
Abstract
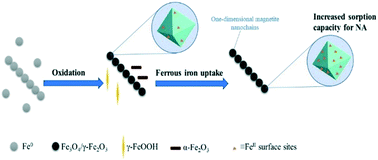
Despite the extensive use of nanoscale zerovalent iron (NZVI) in water and soil remediation, no data exist on the reactivity of secondary iron minerals formed upon the NZVI corrosion. Herein, we investigated the oxidation kinetics of NZVI by monitoring the variations of pH, oxidation–reduction potential (ORP) and dissolved Fe(II) concentration, and then examined the reactivity of resulting oxidized particles for the adsorption of an emerging contaminant (nalidixic acid (NA)). NA adsorption was found the greatest on oxidized particles and negligible on the fresh NZVI. Interestingly, the formed secondary mineral phases exhibited an unusual pH adsorption curve with an unexpected great adsorption at alkaline pH values. X-ray photoelectron spectroscopy and high resolution-transmission electron microscopy revealed a gradual increase in the Fe(II) content at the surface of the magnetite phase over the reaction time. Additional experiments and surface complexation modeling showed that the enhanced adsorption of NA onto the secondary magnetite is due to the formation of surface bound Fe(II). Fe(II) is released into the solution because, for instance, of the presence of organic buffer molecules, decreased surface Fe(II) and NA adsorption at alkaline pH values. This work sheds light on an overseen aspect of the reactivity of secondary iron minerals resulting from NZVI passivation, which can bind co-existing emerging contaminants and then affect their fate in the environment.
- This article is part of the themed collection: Environmental Remediation


 Please wait while we load your content...
Please wait while we load your content...