Open Access Article
Open Access ArticleProbing the immune responses to nanoparticles across environmental species. A perspective of the EU Horizon 2020 project PANDORA
Annalisa
Pinsino
 *a,
Neus G.
Bastús
b,
Martí
Busquets-Fité
*a,
Neus G.
Bastús
b,
Martí
Busquets-Fité
 c,
Laura
Canesi
d,
Paola
Cesaroni
e,
Damjana
Drobne
f,
Albert
Duschl
c,
Laura
Canesi
d,
Paola
Cesaroni
e,
Damjana
Drobne
f,
Albert
Duschl
 g,
Marie-Ann
Ewart
h,
Ignasi
Gispert
c,
Jutta
Horejs-Hoeck
g,
Paola
Italiani
ij,
Birgit
Kemmerling
k,
Peter
Kille
l,
Petra
Procházková
g,
Marie-Ann
Ewart
h,
Ignasi
Gispert
c,
Jutta
Horejs-Hoeck
g,
Paola
Italiani
ij,
Birgit
Kemmerling
k,
Peter
Kille
l,
Petra
Procházková
 m,
Victor F.
Puntes
bno,
David J.
Spurgeon
m,
Victor F.
Puntes
bno,
David J.
Spurgeon
 p,
Claus
Svendsen
p,
Colin J.
Wilde
p,
Claus
Svendsen
p,
Colin J.
Wilde
 h and
Diana
Boraschi
h and
Diana
Boraschi
 *ij
*ij
aInstitute for Biomedical Research and Innovation, National Research Council, Palermo 90146, Italy. E-mail: annalisa.pinsino@irib.cnr.it
bCatalan Institute of Nanoscience and Nanotechnology (ICN2), CSIC and BIST, Campus UAB, Bellaterra, Barcelona, Spain
cApplied Nanoparticles S.L., Barcelona, Spain
dDepartment of Earth Environment and Life Sciences, University of Genoa, Genoa 16126, Italy
eALTA S.r.l.u, Siena, Italy
fDepartment of Biology, Biotechnical Faculty, University of Ljubljana, Ljubljana 1000, Slovenia
gDepartment of Biosciences, Paris-Lodron University Salzburg, Salzburg 5020, Austria
hAvantiCell Science Ltd, Ayr KA6 5HW, UK
iInstitute of Biochemistry and Cell Biology, National Research Council, Napoli 80131, Italy. E-mail: diana.boraschi@ibbc.cnr.it
jStazione Zoologica Anton Dohrn, Napoli 80121, Italy
kCenter for Plant Molecular Biology – ZMBP, Eberhard-Karls University Tübingen, Tübingen 72076, Germany
lSchool of Biosciences, Cardiff University, Cardiff CF10 3AX, UK
mInstitute of Microbiology of the Czech Academy of Sciences, Prague 142 20, Czech Republic
nInstitució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona 08010, Spain
oVall d Hebron Institut de Recerca (VHIR), Barcelona 08035, Spain
pUK Centre for Ecology and Hydrology, Wallingford OX10 8BB, UK
First published on 14th October 2020
Abstract
Understanding how engineered nanomaterials affect immune responses of living organisms requires a strong collaborative effort between immunologists, toxicologists, ecologists, physiologists, inorganic chemists, nanomaterial scientists and experts in law and risk management. This perspective aims to provide a new viewpoint on the interaction between engineered nanomaterials and the immune defensive systems across living species, gained within the EU Horizon 2020 project PANDORA. We consider the effects of nanoparticle exposure on immune functions in plants, marine and terrestrial invertebrates and their relation to the current state of knowledge for vertebrates (in particular humans). These studies can shed light on the broader perspective of defensive and homeostatic mechanisms (immunity, inflammation, stress responses, microbiota, stem cell differentiation) suggesting ways to: i) perform a comparative analysis of the nanoparticle impact on immunity across model organisms; ii) inspire best practices in experimental methodologies for nanosafety/nanotoxicity studies; iii) regroup and harmonise fragmented research activities; iv) improve knowledge transfer strategies and nano-security; v) propose innovative tools and realistic solutions, thereby helping in identifying future research needs and tackling their challenges.
Environmental significanceThis perspective is based on the experience gained within a collaborative work that compared the immune reaction to NPs in plants, invertebrates and vertebrates. Each species uses a shared number of core defensive mechanisms upon exposure to NPs (recognition, uptake, gene regulation, production of defensive molecules), and then adapts them and adds others to its own specific requirements. In general, an immune reaction to NPs is not detrimental for the organism and resolves rapidly with NP elimination, while in few cases it can produce damage or have beneficial effects. Each species has its own peculiarities, but the commonality of their basic defensive mechanisms becomes a valuable tool for NP-related environmental health risk assessment and management. |
1. Nanoparticles: health, immunity and environment
Assessing the safety of nanoparticles (NPs) has been identified as a priority, because of the potential challenges that this kind of agent could pose to the environmental and human health. Over the past decades, there has been a significant increase in the number and production volumes of nano-enabled products coming to market. As the diversity of NP production and use has increased, the need to identify, measure and manage the associated hazards and risk has been recognised. Despite significant progress, many gaps still remain in our knowledge of how NPs interact with organisms. Gaps in understanding include details of the mechanistic pathways of NP toxicity at the cellular level (e.g., interactions with biological macromolecules, effects of signal transduction mechanisms, cellular stress and cell function), at the level of tissues/organs (e.g., blood vessels, immune cells), individual organisms and population/community (e.g., resource allocation species sensitivity, ecosystem process impacts). Such information would significantly improve the basis for NP risk assessment and management.One of the major shortcomings in our understanding of the effects of NPs relates to the impact of NP exposure on the immune system. The conserved nature of many immune mechanisms suggests that comparative studies on the immune responses of organisms covering different phyla and hierarchical levels of organization can together provide a knowledge base for understanding the mechanisms and consequences of NP exposure on human and environmental health. Key topics for comparative nano-immunosafety studies include: i) the identification of species differences and/or species similarities in the immunological mechanisms triggered by NPs; ii) the extrapolation of predictive markers of risk vs. safety by a cross-species comparison of innate immune defence capacity; iii) the design of predictive in vitro assays, in the practical and vigilant application of the 3R principle, to measure the nano-immunological risk to the environment and human health, for industrial and environmental nanosafety testing application.
The full functioning of innate immunity is of central importance for the survival and fitness of organisms, because of the critical role of immunity in preserving tissue and cellular homeostasis. The particulate nature of NPs dictates a preferential interaction with those cells in the immune system that are deputed to recognition and elimination of foreign particulate matters.1 NPs can interact with the immune system in a number of ways, which in the majority of cases end in a defensive reaction that resolves with the elimination of the particles and the re-establishment of homeostasis.2,3 In some instances, however, reaction to NPs may trigger an anomalous immune response. This can be evident as a quantitatively exaggerated or insufficient response (immune hyperreactivity or immunodeficient response) and also as a response sustained over extended timescales, beyond those taking place under normal physiological conditions, again implying hyperreactivity.4 While such reactions may be at the basis of inflammatory and other pathologies, on the other hand the NP capability to activate, regulate or modulate immune responses is of great interest, because of potential developments into treatment strategies for numerous disorders, such as autoimmune and inflammatory diseases, and cancer.5 Thus, assessing the impact of NPs on the immune system not only is an issue of common immuno-nanosafety (reactive mechanisms shared among all organisms), but it can also provide an improved understanding of the impact of NP exposure on the nano-immune interaction that can be exploited for targeted immunomodulation, disease management and crop protection.
By studying and comparing humans, plants and invertebrates, we can learn much about the general and specific mechanisms of the NP effects on immunity. The interactions of NPs with cells and molecules of the innate immune system represents the best ground for understanding the common mechanisms of immune defence. Innate immunity includes natural barriers at the organism–environment interface that limit internal exposure to pathogens, and an array of different cellular, molecular and chemical effector and regulatory players participating to the defensive responses. The full operational capacity of innate immunity is a major determinant in the survival and fitness of all organisms. Therefore, the immunosafety of engineered NPs is a key element of environmental nanosafety. The fact that living species have been consistently exposed to potential pathogens and other threats through their extended evolutionary history has directed the evolution of innate immune protective signalling pathways and mechanisms towards diversification (mainly dependent on species and environmental characteristics) of specialized immune-related molecules, which mediate cellular responses.6 Ecological immunology attempts to explain variability in immune defence, and, in turn, shows that the optimal immune response changes with environmental changes, establishing an evolutionary ecology framework around immunity.7 Since the appropriate functioning of the immune system is important for maintaining individual health, an agent that compromises its function can have implications for survival, reproduction and, hence, population fitness. Here we discuss the potential of NPs to act as agents affecting the immune status in different environmentally relevant species, with a focus on the emerging experimental models launched by the EU Commission H2020 project PANDORA (probing safety of nano-objects by defining immune responses of environmental organisms).8
2. Nanosafety and nanotoxicity: considerations from an immunological perspective
Living organisms have developed highly efficient defence biological barriers to block foreign species/substances and prevent damage to the organism's tissues. In the context of understanding the biological effects of NPs, an important consideration is the extent by which particles may enter the organism, distribute across tissues, pass into cells and interact with organelles, DNA and other macromolecules. The external body surfaces (e.g., skin, lungs and intestine) are where NPs first interact with a living organism. Their active defensive barrier functions determine the extent of NP–tissue interaction and internal distribution. Plants and invertebrates possess additional types of physical/biological barriers (e.g., exoskeleton, peritrophic membrane) that can further prevent the NP entry.9 These barriers largely rely on cells working as a basic functional unit (e.g., epithelium), which prevent NPs to go deeper into tissues.10 If poor tissue permeability is a significant barrier to particle entry, NPs may still enter tissues in a number of ways.11 The physico-chemical properties of NPs (e.g., size, surface charge and shape), their bio-modification during translocation in the body, and the physiological and pathological conditions of the animal, all influence the NP capability of interact with the barriers. On the other hand, there is no available evidence that the effects on barriers depend on the NP concentration. As an example of the complexity of NP–barrier interaction, NP translocation through the intestinal barrier is a multistep process that involves NP diffusion through the mucus layer, contact with enterocytes and/or M cells, uptake via endocytosis, and translocation to the subepithelial tissue via transcytosis.12 Some invertebrates (e.g., mussels, earthworms, arthropods) have fine food sorting systems, and food particles are taken and digested intracellularly by cells in the gut lumen.13,14 NPs may follow the same route as the food particles. In some coelomates (e.g., mussels, echinoderms) free circulating immune cells are present in all coelomic spaces, including the perivisceral coelomic cavities and the open water-vascular system, and in the major organs and tissues. In the open circulatory system, the NPs are likely to be entrapped by phagocytic cells and to be moved quickly through the body fluids. Thus, some types of barriers may likely fail to prevent the NP entry. Although the anatomical, physiological and biochemical barriers vary widely from one species to another, all organisms are organized into similar levels of anatomical structures (sub-cellular entity, cell, tissue, organ, apparatus, organism). In the Fig. 1 are shown the anatomical maps of some invertebrates, in which the location of immune cells is shown, relative to the main barriers.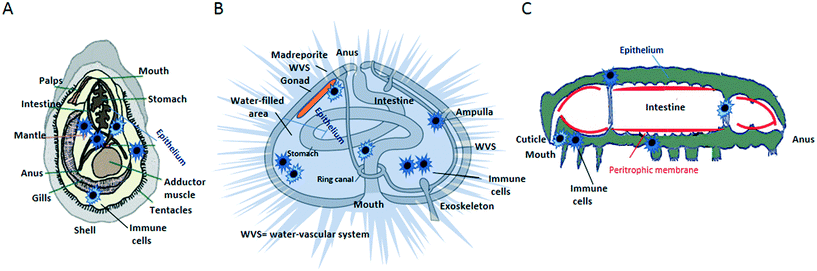 | ||
| Fig. 1 Anatomical maps of some invertebrates, showing the location of immune cells relative to organs and barriers. A) Mussel; B) echinoderm; C) arthropod. | ||
Changes in cell/tissue/organ functions following NP exposure may indirectly affect immune functions. A relevant example is the effect of biocidal NPs on the structure and/or function of the gut microbiota.15 The resident microbiota plays a central role in the stimulation, training, and function of the host immune system. In healthy physiological conditions, the immune system and microbiota cooperate in inducing protective responses to foreign substances and in maintaining regulatory pathways.16 All organisms initiate immune reactions to maintain metabolic homeostasis and self-integrity after suffering from stress, injury or invaders. They require mechanisms to recognize damage, to discriminate between the molecular signatures of invading pathogens (“non-self” molecules) and cellular constituents that generally pose no health risk (“self” molecules), and to discriminate “self” from “damaged self”.17 An interesting question is whether it could be possible to predict innate defence responses against NPs based on how the immune system manages pathogens. NPs may (or may not) set the immune system in motion, communicate with cells, receptors and proteins, trigger signalling cascades, and generate unpredictable immune responses (activation or suppression) as well as other harmful outcomes,18 depending on their physico-chemical characteristics, especially their surface properties.19 In the case of nanomedical applications, it may be attractive to design NPs able to engage with the immune system in a specific manner, e.g., immunostimulation or immunosuppression, depending on the intended use. This has encouraged the development of NP and cell-based hybrid systems that cannot be detected/eliminated by the immune system, making them highly biocompatible and physiologically integrable within tissues in humans.11 For example, nanoporous silicon NPs coated with cellular membranes purified from leukocytes are able to elude the immune system, avoiding opsonization and phagocytosis, and to transmigrate across the endothelial barrier to enter a tissue.20
Notably, some NPs may selectively accumulate in organs with high adsorptive, filtering and detoxification capacity (e.g., spleen, liver, kidney, intestine and lung), engulfed by the mononuclear phagocytic system (MPS) that includes both resident macrophages and circulating blood monocytes.21 Phagocytic cells are responsible of several important processes, including clearance (engulfment/elimination of putative threats), biodistribution (selective localisation in organs and tissues) and balance between tolerance and toxicity (e.g., between production of reactive oxygen species –ROS– and antioxidant response). Each organ can count on the availability of an exclusive equipment of cells that coordinate and perform local innate immune reactions. The cells responsible of innate immune activities include both “professional” immune cells (such as mononuclear and polymorphonuclear phagocytes, mast cells and innate lymphoid cells) and non-professional defence cells, such as endothelial cells, epithelial cells, and fibroblasts.22
Experimental methodologies for nanosafety/nanotoxicity studies must allow us to capture the relevant details of a complex nano-immune interaction. While in vitro models facilitate the analysis of particle effects on isolated single cells and tissues, in vivo models allow for a clearer evaluation of the NP effects in whole organs or in the entire organism. While exposure time, route, and NP concentration help in determining the modes of interaction between NPs and the immune system (or any other system they come in contact with), there is a parameter that is of major importance in any kind of nano-bio interaction, i.e., the changes particles experience when coming in contact with biological fluids. Mimicking such interactions in the laboratory is always challenging because of the complexity and multiplicity of interactions taking place in real life environments. For instance, the presence of natural organic matter (including toxins) may play a role in the NP partition, which is difficult to control but that should be considered, as they are part of a natural environment. This is a critical point in the context of ecotoxicological assays and how and to which purpose we want to use them. Thus, given the advantages of the in vivo and vitro/ex vivo approaches in providing nanosafety information, future studies should aim to improving the exposure conditions of the ecotoxicological assays in order to better mimicking complex environments and provide realistic and valid nanosafety data.
Aggregation/agglomeration influences the fate of the NPs in the environment, particularly in aqueous environments. NP colloidal stability is influenced by the solution ionic strength, organic matter composition, pH and solvent composition. In some conditions NPs can agglomerate, and return in colloidal solution when the conditions change, while in some cases NPs can aggregate, forming irreversible larger clusters. Heteroaggregation/agglomeration, in which NPs are admixed with other environmental molecules/particles, is likely the most common form in which NPs are present in the environment and come in contact with living organisms. Such complexes can also associate with other environmental biological and non-biological contaminants, such as bacterial toxins (e.g. Gram-negative endotoxin) and chemicals.23 Since these complexes are the real-life forms in which NPs come in contact with living organisms, the future ecotoxicological assays should consider using complex environmental matrices to adequately reflect the exposure conditions in real life. Although nude particles can rapidly aggregate/agglomerate in a fluid, coated particles acquire chemical functions of the coat that modify the particle behaviour, for example by reducing the formation of aggregates/agglomerates. Within biological fluids (blood, haemolymph, coelomic fluid, mucus, etc.), which are rich in biomacromolecules (e.g., lipids, sugars, nucleic acids, and particularly proteins), NPs readily adsorb on their surface various organic molecules that form a complex biomolecular corona and lead to an increase in the NP hydrodynamic range.24 Large aggregates/agglomerates are transported in vesicles via different mechanisms of endocytosis/exocytosis (based on the size, shape and surface characteristics, and the cell internalization machinery).25 For example, NPs of <200 nm can be internalized via clathrin-coated pits by non-phagocytic cells, whereas at increasing size a shift towards caveolae-mediated internalization become predominant.26 NPs can also enter cells by a passive uptake, which can or not disturb the membrane depending the size of the NPs. Small NPs have a higher probability to be internalized by passive uptake than large ones.25
The bio-corona composition may vary among different NPs and upon contact with different biological fluids that contain different mixtures of biomolecules. The bio-corona evolves with time, with a progressive loss of molecules of lower avidity (soft corona) and the establishment of a hard corona of strongly interacting biomolecules. The structure of this macromolecular coating is most likely a key factor in making the NPs “visible” to cells for uptake, contributing to clearance from circulation and possibly reducing their interactions with other cells and tissues.27 On the other hand, functionalizing particles with some capping agents (e.g., polyethylene glycol) reduces accumulation of proteins on the NP surface, making them functionally “invisible” to the immune system in humans.28,29 The majority of the bio-corona studies have been performed in vitro, and have contributed to unravel the role of the bio-corona in the immune recognition of NPs. Conversely, the in vivo approach is important for a better understanding of the mechanisms of particle clearance vs. toxicity, which would be greatly improved by examining the differences in clearance kinetics and type of toxic effect across living species.
Vertebrates and invertebrates display common immune mechanisms but they have also developed divergent immune tools for attaining challenge-specific protection. As an example, whereas vertebrates use somatic rearrangement of immune receptors and immunoglobulins, invertebrates privilege alternative splicing of pattern-recognition genes.30 Whereas vertebrates have developed an array of different cell types as effectors of innate immune responses (e.g., monocytes, macrophages, mast cells, neutrophils, basophils, eosinophils, NK cells, ILC, epithelial cells, fibroblasts), invertebrates use a limited range of multifunctional cells. Plants do not have specialised immune cells, with all cells being able of innate immune reactivity and with systemically spreading signals emanating from injured sites.31
The innate immune system also exhibits adaptive traits, a feature that has been termed “innate immune memory” (or “trained immunity” in vertebrates).32 The innate immune memory is the ability of the immune system to record and recall information of previously met pathogens to activate a faster and more efficient protective response upon re-exposure to the same or different pathogens/stressful agents.33 The phenomenon is well known in plants and invertebrates, which also show some degree of specificity,34,35 and has been also observed in vertebrates (in particular mouse and man), in which however the memory is as non-specific as innate immunity itself.32 How NPs may induce/alter innate memory is an open issue that requires special attention, as alteration of immune memory can modify the capability of an organism to adapt to its environment. NPs can modulate stimulus-induced memory in human monocytes, mostly by shifting the memory response towards tolerance.36 Innate memory in plants is known as systemic acquired resistance (SAR), which leads to the activation of a better protective response (e.g., increase in the levels of pattern-recognition receptors, accumulation of dormant signalling enzymes, alterations in chromatin state) not only in the tissue re-exposed to the same or similar insult but also in an unexposed tissue.37 If found able to induce an epigenetic and metabolic reprogramming of immune responses, as they are able to promote root growth (B. Kemmerling, unpublished results), NPs would support the design of new agriculture strategies able to reduce yield losses, thereby allowing for reduction of conventional pesticides. Invertebrates show a high degree of memory in their immune responses, including recall immunity to infections, natural transplantation immunity, and individual/trans-generational immunological priming.34 How NPs can affect the establishment of innate memory in invertebrates is however still largely unknown. The only available results describing nanoplastics-induced innate memory and some underlying molecular and cellular mechanisms come from mussels.38 Upon a primary exposure to amino-modified nanopolystyrene, Mytilus haemocytes respond with changes in some immunological parameters (e.g., lysozyme release in the haemolymph, lysosomal acidification, membrane destabilization), whereas a second exposure induces an immunological memory by shifting the immune parameters towards a tolerant condition (recovery).
An important issue that should be considered when examining the interaction between NPs and immunity is the role of microbiota. This is particularly important for NPs that are ingested (as in the case of most aquatic and terrestrial/earth-dwelling invertebrates and vertebrates) but also for NPs to which organisms are exposed through the skin or inhalation, because symbiotic and commensal microbiota populate the gut and all the barrier surfaces or environmental organisms and it is well known that microbiota profoundly influence and shape immunity at the barrier level.39 However, little is currently known on how the interaction between NPs and commensal microbes can impact on immune responses, in particular in the long term (Fig. 2).
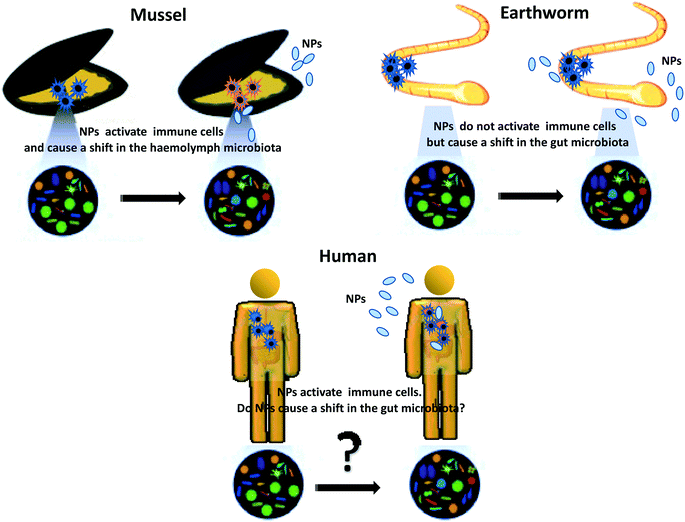 | ||
| Fig. 2 Impact of NPs on the microbiota–immune axis in vivo: differences across environmental organisms. | ||
Recent data show that TiO2NPs impact the immune system of mussels and induce a shift in the microbiota composition of the haemolymph40 upon exposure in vivo, while CuONPs cause a shift in the earthworm gut microbiota despite the absence of an evident immune reaction.41 The only available data on impact of NPs on the human microbiota–immune axis come from in vitro studies, which underlines an essential a lack of information on the realistic impact of the NPs ingested via food in humans.39 Whether chronic dietary exposure to NPs may be viewed as a risk factor that facilitates disease development remains an open issue.
In the next section, we will provide an overview of the environmentally relevant organisms and approaches used, within the framework of the EU PANDORA project, to study nano-immune interactions and identify their common vs. divergent mechanisms.
3. Nanotoxicological evaluations: in vivo and in vitro models for studying nano-immune interactions
The 3R principle of reducing, refining, and replacing animal experimentation42 has led to new approaches to in vivo experimental studies, with an increasing use of invertebrate animal models. Studying the interactions between NPs and the immune system across animal phyla allows us to identify both conserved mechanisms and species-specific defence solutions. Plants differ from animals because they are autotrophs, but nevertheless show conserved/converging defensive mechanisms that could greatly help in clarifying the mechanisms of defence and repair in animal species. Some invertebrate organisms, by virtue of being easy to handle, breed and maintain, are becoming popular experimental models, in particular in ecotoxicological research and in comparative immunology. However, the different demands of the two disciplines have brought scientists to select different model species for their studies. As a consequence, our current knowledge of the immune functions of species used in ecotoxicology is faulty, and likewise we have an incomplete knowledge of the ecotoxicological aspects in species whose immune functions are well known.In this section, we report and discuss recent studies aiming at integrating nanotoxicological evaluations with assessment of immune-related effects in plant, human and invertebrate animal models, in vivo and in vitro (Fig. 3). We will examine the similarities and differences in the defensive responses of each organism upon exposure to NPs. Comparison will contribute to increasing our knowledge of the commonalities in the immune reaction to NPs, and allow us to identify pathways and biomarkers of nano-risk that are shared across species. Notably, several features must be considered in order to correctly evaluate the NP interaction with the immune defences in the selected experimental models: experimental conditions (in vivo, in vitro/ex vivo); environmental factors; basal immunological state; immunological memory.
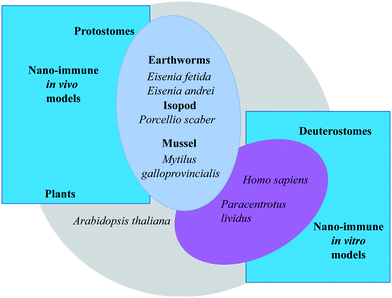 | ||
| Fig. 3 Overview of the experimental models for investigating the interactions of nanomaterials with the immune system used within the EU Commission H2020 project PANDORA: from the plants to humans. | ||
3.1 Plants
Plants are in constant contact with the rising number of NPs released into the environment. Given the importance of plants in determining ecosystem functions such as stability and resistance,43,44 studying plant-NP interactions is an important element to assess the risk of NPs for the environment.Plants have a well understood immune system able to detect and react to danger, and are easily accessible to genetic and biochemical analyses and therefore provide a valuable tool for NP-related risk assessment.45 Plant immunity is an exceptional example of sensing capability based on networks of immune receptors that act as sensors of invading pathogens.46 Receptors evolve rapidly to keep up with the pathogens' variability, and interact with each other creating systemic signals emanating from infection sites that induce an effective immune response. The task of mounting an immune reaction is not dependent on specialized immune cells, but each plant cell is able to react against invaders. The plant immune system is organised in three layers, the pathogen-associated molecular patterns (PAMP)-triggered immunity (PTI) (preliminary defence), the effector triggered immunity (ETI) (secondary defence), and the exosome-mediated cross-kingdom RNA interference (CKRI) system (additional defence).47 Resistance against a non-adapted pathogen involves the rapid production of cell wall appositions (physical barriers), and inducible-specialized metabolites at the infection sites via PTI and ETI signalling.48,49
The growth and metabolic functions in response to NPs vary among plants.50 Reports reveal contradictory effects of NPs depending on the experimental conditions (field or lab studies), medium of plant growth, duration of exposure and species.51 For example, TiO2NPs improve light absorption efficiency and promote the activity of Rubisco activase, accelerating growth in spinach;52 whereas they reduce the photosynthetic rate thereby decelerating growth in Ulmus elongata.53Arabidopsis thaliana leaf cells exposed to large particles of single-walled carbon nanotubes form endocytosis-like structures in the plasma membrane, whereas the same particles fail to penetrate the cell walls in other plants.54 Notably, the interaction of plant cells with NPs leads to the modulation of plant gene expression and related biological pathways, which in turn influence plant growth and development.55 For example, exposure of A. thaliana to gold NPs induces the expression of a few key regulatory molecules and microRNAs (miRs) thereby stimulating seed germination, growth, and antioxidant metabolic activity.56A. thaliana is a small model plant with a short life cycle, which allows for easy manipulation and study. The observations made in this species for studying NP uptake and metabolism encourage more relevant and significant research in this field, due to commonalities between Arabidopsis and edible plants. Well controlled and field studies will allow us to get an overview about the potential of plants to recognize and react to NPs, keeping in mind that NPs bear also the potential to improve plant yield and health and reduce crop loss by specific, localized and efficient application of nano-fertilizers, pesticides or immunostimulating agents.51
3.2 Protostomes
The mechanisms of interaction of NPs interact with different components of the earthworm immune system are still not well defined. There are data showing that AgNPs, both in vivo and in vitro on Eisenia fetida coelomocytes, can transiently affect the expression of a number of immune-related genes, such as those involved in oxidative stress (CAT, SOD), energy metabolism (aspartate aminotransferase, glucose-6-phosphate isomerise, phosphoglucomutase) and immune response (lysozyme, MyD88, PKC, MEKK1).64,65 The intracellular signalling pathway involved in the AgNP-induced modulation of earthworm immunity apparently involve the myeloid differentiation factor 88 (MyD88) (encoding a central adaptor protein of toll-like receptors), and the mitogen activated protein kinase (MAPK) cascades.65 This upstream signalling cascade induces activation of nuclear factor (NF)-κB, resulting in transcription of genes encoding inflammatory cytokines in humans.66 Cytokine production is necessary for defence, but it can also induce to tissue damage by inducing the generation of ROS, enzymes and other toxic defensive molecules.67 These findings underline the presence of conserved innate immune activation mechanisms and pathways between earthworm and human immunity. In agreement, the 1H NMR-based metabolomic analysis of Eisenia fetida earthworms exposed to TiO2NPs in soil showed significant changes in the metabolic profile consistent with oxidative stress as the main mechanism of immune-related inflammation/toxicity.68 Upon exposure to another type of particle, CuO2NPs, in soil, the earthworm Metaphire posthuma showed an immune metabolic impairment in term of nitric oxide generation, superoxide dismutase, phenoloxidase, catalase and alkaline phosphatase activity.69
Since earthworms ingest soil and their associate microbiota, the NP effects on the soil microbial communities70–72 will affect the composition of the microbiota in the earthworm gut. This is particularly important, in terms of effects on immune functions, because the interaction between immunity and microbiota at the gut level defines the defensive capacity of an organism (from earthworms to human beings), and any variation of microbiota can affect immune competence. Unfortunately, little is known on how NPs might interact with earthworms' bacterial communities and even less on how this might affect their immunity. Earthworms capture NPs from contaminated soil using mucus glycoproteins/glycans of gut/microbial origin.73 Exposing the oligochaete Enchytraeus crypticus to 50–100 nm plastic particles caused a significant shift in the gut microbiota, including a significant decrease in the relative abundance of the families Rhizobiaceae, Xanthobacteraceae and Isosphaeraceae,74 but how these changes may affect immunity is unknown. The earthworm Eisenia fetida can survive in soil containing high ZnONP concentrations, and accumulate zinc ions in the body. ZnONPs enhance the activity of cellulolytic bacteria (Bacillus and Pseudomonas sp.) in the gut, contributing to bioconversion (digestion) of lignocellulosic waste.75 The metagenomic analysis of Eisenia fetida earthworms exposed to ZnNPs in soil shows a decrease in biodiversity of the gut microbiota, mainly due to a significant decrease in the relative abundance of the phylum Firmicutes.76 Reduction in the abundance of these bacteria correlates with inflammatory/autoimmune diseases in mammals.77 Although there is no strong evidence that this type of correlation occurs in earthworms, we can speculate that NPs impacting the gut microbiota can consequently affect the earthworm immunity upon in vivo exposure. This is based on the consideration that amoebocytes, the most active immune cells in the earthworm, are involved in a broad range of defensive functions in the gut78 reflecting the abundance of immune cells in the gut of vertebrates. However, recent data show that in vivo exposure to CuONPs can cause significant changes in the gut microbiota and microbiome of earthworms, but it does not affect the expression of immune-related genes (e.g., coelomic cytolytic factor, lysenin/fetidin, lysozyme) and resistance/susceptibility to a bacterial infection.41 Future studies of gene expression based on a greater time resolution of immune responses may aid the understanding of whether and how NPs could impact on the dialogue between gut commensals and immune cells in the earthworms.
The interaction of NPs with the mussel biological fluids, in particular the haemolymph, allowed for the identification of specific proteins forming a stable biocorona on the NP surface, and to assess the features of the haemocyte interaction with the biocorona-coated NPs. Depending on the NPs and its bio-corona, this interaction can lead to distinct responses (recognition, internalization, activation/inhibition of immune responses), and therefore results in distinct immunomodulatory pathways.83 These findings underlie the strong similarities with the features of the nano-immune interactions in mammalian cells, suggesting that this model could be suitable for investigating the mechanistic basis and the biological outcomes of such interactions in conditions that reflect those in both mammals and environmental organisms. Notably, recent data showed that the immunomodulatory effects of different types of NPs are linked with shifts in the microbiota composition of the haemolymph, underlying a relationship between innate immunity and host microbiota in mussels, as it occurs in mammals.40
3.3 Deuterostomes
Current studies of the interaction of NPs with the immune system of the sea urchin Paracentrotus lividus show that, as in humans, a number of sea urchin extracellular proteins (glycoproteins, cytoskeletal proteins) form a biocorona on the particle surface in vitro.93 The protein-coated TiO2NPs are internalised more efficiently, transiently down-regulating the expression of genes encoding for proteins involved in immune response (NF-κB, FGFR2, JUN, p38MAPK), and increasing a few antioxidant metabolic pathways (pentose phosphate, cysteine–methionine, glycine–serine).2 Conversely, studies on sea urchin phagocytes exposed to PVP-AuNPs in vitro underline the transient involvement of the TLR4/ERK signalling pathway based on increased protein levels.94 The notion that these NPs do not cause a pathological immune response and associated irreversible damage in sea urchins may prompt us to consider them immunologically safe also for other environmental species and human beings, given the commonality of involved defensive mechanisms.
Thus, the combined use of in vivo and in vitro investigations, its proximity to mammalian reactivity as well as the similar features with invertebrate immune responses, make the sea urchin a valuable model for the study of the nano-immune interactions across evolution.
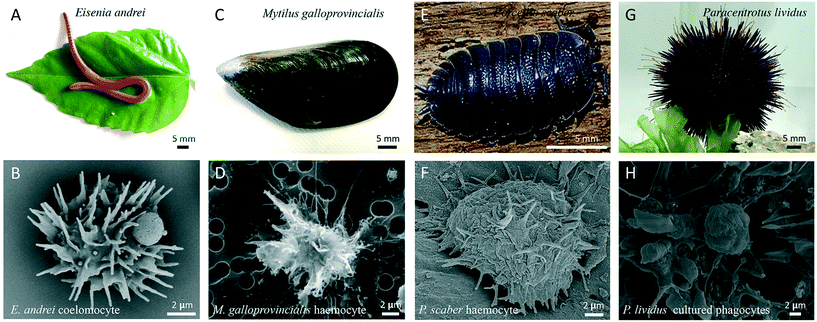
Representative images of the invertebrate models described in this section and their immune cells are shown in the Fig. 4.
An efficient and predictive method that can be applied for assessing the general impact of NPs on human blood is the whole blood assay,97,98 in which an aliquot of anti-coagulated blood is challenged with NPs. This assay encompasses NP coating with serum proteins and interaction with all types of circulating cells, without however allowing for the identification of specific cell types affected by NPs. Since phagocytes are the first defence cells that confront particulate matter, we can consider as the best models of innate immunity those based on monocytes isolated from blood of healthy donors (MAT, monocytes activation test), and on macrophages and dendritic cells differentiated in vitro from fresh monocytes.99 These models allow us to observe not only the direct activation of immune cells by NPs but also the capacity of NPs to interfere with the physiological course of an inflammatory response4 or with the induction of innate memory.36 The in vitro systems based on human primary cells present the main disadvantage of all in vitro systems, i.e., they do not reflect the complexity of an in vivo system, and, in addition, they suffer of donor-to-donor variations. Such variations are however sufficiently limited in the case of innate immune responses, with a general homogeneity of qualitative reaction and variability evident only in quantitative terms.100 Conversely, in the case of innate memory responses the variability is much higher, as also observed in adaptive immune responses, as memory depends on the individual history of exposure. This notion underlines the need of using human primary cells in immunoassays for assessing nano-risk, since they are more predictive evaluation of the NPs effect in vivo. Also, it warrants a personalised evaluation of the NP effects on immunity, as each individual subject may react differently to an NP challenge.
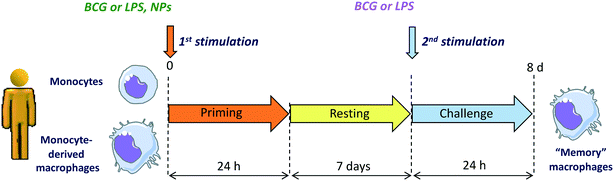
In light of the notion that an unbalanced immune response underlies many diseases including autoimmunity, inflammatory diseases and inflammation-related cancers, the potential effects of NPs on the human immune responses requires extensive attention. To obtain a realistic overview on the effects of NPs on human immune responses, it is necessary to examine both the direct effects of NPs on innate immune effector cells (capacity to induce an innate/inflammatory reaction that does not resolve and become chronic) and their interference with an ongoing defensive innate/inflammatory response (capacity to exacerbate or inhibit a defensive response). In the latter context, the effects of NPs on the development of innate memory could be of great interest. NPs could be appropriately designed for modulating innate memory to treat two opposing conditions, i.e., diseases where an innate/trained memory should be inhibited (autoimmune, inflammatory and cardiovascular diseases) and those in which memory responses should be potentiated (cancer, sepsis, immunosuppression). Recent work describes that AuNPs could interfere with the induction of BCG-induced memory by shifting the human memory monocytes towards a tolerant phenotype.36 Another recent study shows that pristine graphene can directly prime mouse macrophages towards a potentiated secondary response to TLR-activating stimuli, by increasing the production of inflammatory cytokines and decreasing the production of regulatory factors.101 The effect of NPs on innate memory is a new field with great therapeutic potential. A representative in vitro model for assessing the capacity of NPs to induce innate immune memory is shown in the Fig. 5.
4. Exploiting similarities and differences: improving models and tools for basic and translational studies on nano-immune interactions
A number of aspects determine the outcome and the possible risk to environmental and human health posed by exposure to engineered NPs, which include the inherent nature of the particle, the level of environmental exposure, the route of the exposure, and the susceptibility of the living species and of the single individuals. As our studies focus on immunity, and more specifically on innate immunity, we have examined the interaction of NPs with the innate immune system of different living species to assess similarities and differences. The first general observation is that each species uses a number of similar defensive mechanisms, adapting them to its own specific requirements (due to environmental conditions, range of microbial species within the environment, lifestyle, etc.). Whether this is indeed adaptation of common mechanisms/tools or parallel evolution is still a matter of debate, but we see that physical/mechanical barriers are common and very efficient mechanisms of defence, that toll-like receptors are present in all living species, and are used to recognition of invaders and initiators of a defensive reaction, and that animals use phagocytic cells in the gut as specialised innate immune effectors. Phagocytosis and ROS production, for direct pathogen elimination, and kinase-dependent signalling for upregulating expression of effector proteins are also common to many animal species. The number of known innate immune receptors and pathways of many species are rapidly increasing and provides a rich resource to improving our understanding of the mechanisms of innate immunity. The species-specific range of the relevant innate receptors is obvious, as their number and their ligand selectivity can differ significantly among species. The question is if the differences are a problem or, on the contrary, they can be considered precious tools for a deeper understanding of human immunity and for knowledge exploitation towards targeted immunomodulation for medical purposes. We tend to favour the latter, based on the experience gained within a collaborative work of comparative nano-immune interaction assessment across living species.17 Non-human models have a great value in unravelling the signalling pathways used in innate immune responses, in identifying conserved mechanisms/molecules of innate immunity, and in evaluating the in vivo impact of exposure to NPs. For instance, the use on invertebrate models reveals that the large majority of NPs either do not cross the body barriers, or are rapidly excreted, while only in some infrequent conditions they are retained within the body. Another important notion is that the interaction between NPs and immune cells occurs mainly in the gut (from invertebrates to human beings), that resident microbiota influences immune responses, and that phagocytic cells are the main/only immune cells involved in the first interaction with the foreign particles. Each organism has its own array of few or many phagocytic cell types, which may preferentially interact with one or another foreign agent, but the basic mechanism is common: recognition, uptake, degranulation, gene regulation with the production of defensive molecules. As discussed previously, each model has its own advantages and disadvantages, specificities and commonalities, thus each model gives us the opportunity to unravel a piece of the story. We believe that the new frontier in our understanding of nano-immune interactions also revolves around the differences. Studies on resistance and tolerance to anthropogenic challenges (including engineered NPs and nano-/microplastics) will most likely contribute to identifying not only some key species-specific or common protective molecules, but also the mechanisms at the basis of the individual innate memory profile, i.e., the pattern of epigenetic reprogramming that defines the individual capacity to optimally react to a challenge and that is shaped by the previous exposure to other challenges. While in short-lived animals this immune memory lasts for a lifetime and is usually shared by the entire community (living in the same environment and exposed to the same challenges), the innate memory profiles in human beings are expected to be highly variable and therefore warrant to a personalised approach in the nano-risk assessment.Which are the models and tools that we can use to assess nano-immune interactions, which information will they provide, and how can we use them optimally to answer to different questions?
We have focused on protostomes, including the earthworm Eisenia, the isopod P. scaber, the blue mussel Mytilus, and the plant A. thaliana for in vivo studies, while deuterostomes (including human models and the sea urchin P. lividus) have been used directly in vitro/ex vivo, as discussed previously.
In plants, uptake and response to NPs is dependent on the plant species and the capacity of NPs to cross the mechanical barriers. While in some cases NPs can induce stress responses, it is notable that in several instances the presence of NPs showed positive effects on growth and yield. This opens novel opportunities in the use of NPs in increasing the fitness of plants of agronomic interest. Whether such effect includes an increased resistance to pests, in terms of induction of immune memory, is currently under investigation.
From earthworms and isopods, we learned the major importance of gut and resident microbiota in determining the immune response to foreign particles (including NPs). A disturbed host-microbiota interaction, in particular in the gut, can impact on the immune system, increasing susceptibility to infections. NPs may have significant effects in altering the microbiota balance, indirectly affecting the host immune defensive competence. Thus, some protostomes become a practical choice to study the effect of ingested NPs on the gut microbiota interaction with the host immunity and its ability to overcome infections, a study that cannot be easily performed in human beings.
Mytilus shows, in response to exposure to high NP concentrations, a stress response that is very similar to that observed in humans, with activation of phagocytes and release of ROS. This warrants the use of Mytilus as easy in vivo model for evaluating the phagocyte response to NPs.
The comparison of the sea urchin and human genomes allows for discriminating between conserved immune genes and new human-specific genes. The possibility of exploiting long-term in vitro cultures of sea urchin immune cells makes it possible to compare in vivo effects of NPs with the evaluation of cell-specific molecular mechanisms, and to focus on conserved molecules across evolution. Table 1 reports the main features and mechanisms involved in the nano-immune interaction across the selected species.
| Parameter | Organism | |||||
|---|---|---|---|---|---|---|
| Plant | Earthworm | Mussel | Terrestrial isopod | Sea urchin | Human | |
| Exposure route | Soil | Soil | Seawater | Soil | Seawater | Skin, mucosal cells in gastrointestinal and respiratory tract |
| Contact level | Root cells | Gut and skin epithelial cells, coelomocytes, mucus, microbiota | Gills, gut epithelial cells, haemolymph, microbiota, haemocytes | Microbiota. No direct contact with gut epithelial cells and haemocytes | Coelomic fluid, free immune cells, gut epithelial cells | Gut and skin and mucosal epithelial and immune cells |
| Biocorona | ND | Lysenins | Complement component C1q, haemolymph components | ND | Depends on NP surface characteristics (several proteins) | Depends on NP surface characteristics and route of exposure (several proteins) |
| Uptake by immune cells | Endocytosis-like | Endocytosis | Endocytosis | No uptake | Endocytosis | Endocytosis |
| Immune-related effects/activation | Regulatory molecules, miRs, PRRs, MAPKs, energy metabolism, upregulation Rubisco activase | PRRs, MyD88, MAPKs, NF-κB, coelomic cytolytic factors, lysozyme, energy metabolism, antimicrobial peptides | PRRs, MAPKs, NF-κB, lysozyme, energy metabolism, antimicrobial peptides | Changes in protein, lipid, nucleic acid and carbohydrate levels. No immune response | PRRs, MAPKs, NFκB, cytokines, growth factors, energy metabolism | PRRs, MAPKs, NF-κB, energy metabolism, cytokines, chemokines, complement system, growth factors, etc. |
| Oxidative stress and apoptosis | ROS production, antioxidant metabolism, Rubisco activase, apoptotic enzymes | ROS production, antioxidant metabolism, apoptotic enzymes | ROS production, antioxidant metabolism, apoptotic enzymes | ND | Antioxidant metabolism | ROS production, antioxidant metabolism, apoptotic enzymes |
| Immune memory | ND | ND | Compensatory recall responses to maintain immune homeostasis | ND | ND | Innate immune memory |
Based on the above consideration, we can state that the realistic evaluation of nanosafety risk demands a ‘toolbox’ of cell-based models able to reproduce with fidelity the functions of barrier tissues and of the immune system. This is true for both environmental and human health risk. An important issue in this context is the accessibility of tests and assays, which would allow their use across several institutions, companies, agencies and private labs, without the need of specialised instrumentation and personnel. Within the PANDORA consortium, we have contributed to this ‘toolbox’ through adaptation of human immune cell models to be readily-available and user-friendly, whilst retaining the analytical reproducibility and predictive value obtainable only with primary cells (that retain the functions of their tissue of origin under test conditions in culture). To this end, human blood monocytes were extracted from blood transfusion by-products, distributed in multiwell plates, either used immediately after isolation or differentiated into macrophages or dendritic cells, frozen with a novel protocol in analysis-ready format and, in the case of poorly-adherent cells such as dendritic cells, anchoring them on bio printable scaffolds in NP-receptive 3D culture arrangement. Upon thawing the different types of cells were able to react to NPs in a fashion that is qualitatively and quantitatively superimposable to that of cells that did not experience freezing–thawing. The reproducibility of immune cell response, or more often the absence of response to NP challenge, translated to user-friendly cryopreserved cell models offers a robust platform against which effects of materials of uncharacterized or non-specific nano-composition can be tested with confidence.
The next-generation in vitro human models, including co-culture with multiple cell types, 3D spheroids, 3D tissue sections, microfluidic organ-on-a-chip, air–liquid interface systems, are showing great capacity in helping to bridge the gap between in vitro and in vivo exposure/effect. Notably, 3D organoids are self-organizing, multi-cellular aggregates that closely resemble function and architecture of human tissues.102 Furthermore, microfluidic technologies can model fluidic properties in organ-like tissues, making it possible to study small fluidic volumes in motion.103,104 Despite the availability of outstanding organoid models representing the lungs, gastrointestinal tract and many other organs, one of the greatest challenges for the coming years will be to further improve organoid-like tissues to include the interaction of tissues/organoids with the immune cells that in real life reside in the organ and with those that enter it during an inflammatory reaction.
5. Inclusive and sustainable research and innovation: the future perspectives of research on nano-immune interactions
In the study of nano-immune interactions, which is specifically relating to nanosafety for both environmental and human health, an aspect that should be duly considered is the so-called responsible research and innovation (RRI). RRI has gained increasing importance in EU research policy (with its inclusion in Horizon 2020) and academia. RRI considers scientific investigations in a larger context by including the environmental and societal perspective. RRI is a policy concept experiencing a dynamic development, in which sustainability, social justice/inclusion, open innovation, open science and open in the world are the overarching goals. These are the backbone of the Horizon 2020 RRI strategy and that will inform the upcoming European Green Deal. The RRI approach consolidates a new instrumental model (transdisciplinary, network-governed, externally validated, problem-solving, impact-driven).105The RRI strategy (“Science with and for Society”) is the approach that the PANDORA partners have tried to use to increase the researchers' capacity to think and act towards a more responsible, acceptable, and ethical science. The EU-funded project PANDORA encompassed five Academic Institutions, four Research Centres, and two small and medium-sized enterprises (SMEs) distributed within seven European countries (Italy, Austria, Czech Republic, Germany, Slovenia, United Kingdom, Spain), all with proven experience in higher education and training. Eleven PhD students have been involved in an overarching training programme including training-by-research, joint courses of technical, scientific and transferrable skills, completed with an intense inter-sectorial networking exchange plan. PANDORA merged scientists, NP producers and experts in law and risk management into a broader approach to environmental nanosafety. Within this complex framework, the younger scientists have been encouraged to critically reflect106 on the research process within, around and outside the laboratory, and to aim at collaboration and quality by privileging care and sustainability in their research planning and conduction.107 Such attitude/behaviour was particularly suited to the goals of their studies, dealing with environmental nanosafety, and helped them in progressing towards the fundamental dimensions of RRI: courage, rigor, safety, sustainability, inter- and trans-disciplinarity, critical creativity and elegance.
6. Conclusions: what's next?
By evaluating the impact of engineered NPs on the immune reactivity of many living organisms across evolution, from plants to human beings, we can draw some conclusions that can help us in choosing the direction of future research. First is that immune responses across evolution are not too different apart for some specialized mechanisms due to anatomical peculiarities (e.g., plant cells that do not move). As a consequence, reaction to NPs turned out to be pretty similar in different species, and mainly based on immediate elimination, no recognition, or recognition/reaction that rapidly resolves. Only in few cases a detrimental reaction could be observed, depending on the dose and chemical composition of the NPs, while in other cases it was possible to observe beneficial effects.Based on these findings, what should we do in the future? Abandoning nanosafety research, only because in general NPs do not seem to be detrimental, is not a good idea because there are too many special situations that could increase risk. Just to make an example, the immune system of elderly or chronically ill individuals is weakened, and therefore we should expect that agents that are innocuous in healthy people may cause significant damage. Another case: even when exposure to NPs or other individual agents does not cause a damaging effect, co-exposure to several agents at the same time may synergize and provoke unexpected detrimental reactions.
Thus, we may foresee that future research of nano-immune interactions may explore these directions:
• Development and refinement of in vivo/in vitro models that can realistically predict the nanorisk associated to acute and chronic exposure to NPs. Goals will be having models that discriminate between a normal immune response and a pre-pathological reaction, models that predict risk for many different environmental species and human beings, models that predict realistic risk upon exposure to combined challenges, models that predict risk in frail organisms.
• Exploiting the potential of NPs in modulating some immune functions (such as innate memory) for designing new immunotherapeutic or immunopreventive approaches for human beings and crop production in agriculture.
Building on the environmental impact of nanosafety research and its impact on society for reshaping all these studies along the principles of RRI, contributing at the same time in building a new generation of researchers that privilege rigor, cooperation and sustainability.
Authors' contributions
AP and DB conceived and wrote the manuscript. NGB, MBF, LC, PC, DD, AD, MAE, IG, JHH, PI, BK, PK, PP, VFP, DJS, CS, CJW contributed to writing, critical reviewing and editing the manuscript.Conflicts of interest
There are no conflits to declare.Acknowledgements
This work is based on the EU Commission H2020 project PANDORA (GA 671881) that has supported the studies of all authors. The authors wish to acknowledge the EM Facility of CAS (Prague, Czech Republic; supported by the project LO1509 of the Ministry of Education, Youth and Sports) for providing the electron microscopy images. A special thanks to Giuliana Donini (EU Commission, Bruxelles, Belgium), who has expertly supported this project throughout all stages of its development.References
- D. Boraschi, P. Italiani, R. Palomba, P. Decuzzi, A. Duschl, B. Fadeel and S. M. Moghimi, Nanoparticles and innate immunity: new perspectives on host defence, Semin. Immunol., 2017, 34, 33 CrossRef CAS.
- A. Alijagic, D. Gaglio, E. Napodano, R. Russo, C. Costa, O. Benada, O. Kofroňová and A. Pinsino, Titanium dioxide nanoparticles temporarily influence the sea urchin immunological state suppressing inflammatory-relate gene transcription and boosting antioxidant metabolic activity, J. Hazard. Mater., 2020, 384, 121389 CrossRef CAS.
- M. Auguste, T. Balbi, M. Montagna, R. Fabbri, M. Sendra, J. Blasco and L. Canesi, In vivo immunomodulatory and antioxidant properties of nanoceria (nCeO 2) in the marine mussel Mytilus galloprovincialis, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2019, 219, 95 CAS.
- Y. Li, P. Italiani, E. Casals, D. Valkenborg, I. Mertens, G. Baggerman, I. Nelissen, V. F. Puntes and D. Boraschi, Assessing the immunosafety of engineered nanoparticles with a novel in vitro model based on human primary monocytes, ACS Appl. Mater. Interfaces, 2016, 8, 28437 CrossRef CAS.
- G. Hu, M. Guo, J. Xu, F. Wu, J. Fan, Q. Huang, G. Yang, Z. Lv, X. Wang and Y. Jin, Nanoparticles targeting macrophages as potential clinical therapeutic agents against cancer and inflammation, Front. Immunol., 2019, 10, 1998 CrossRef CAS.
- G. W. Litman, J. P. Cannon and L. J. Dishaw, Reconstructing immune phylogeny: new perspectives, Nat. Rev. Immunol., 2005, 5, 866 CrossRef CAS.
- B. M. Sadd and P. Schmid-Hempel, Principles of ecological immunology, Evol. Appl., 2009, 2, 113 CrossRef.
- PANDORA website, https://www.pandora-h2020.eu/index.
- A. Kumar, P. Srivastava, P. Sirisena, S. K. Dubey, R. Kumar, J. Shrinet and S. Sunil, Mosquito Innate Immunity, Insects, 2018, 9, 95 CrossRef.
- H. Meng, W. Leong, K. W. Leong, C. Chen and Y. Zhao, Walking the line: the fate of nanomaterials at biological barriers, Biomaterials, 2018, 174, 41 CrossRef CAS.
- S. Barua and S. Mitragotri, Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects, Nano Today, 2014, 9, 223 CrossRef CAS.
- I. L. Bergin and F. A. Witzmann, Nanoparticle toxicity by the gastrointestinal route: evidence and knowledge gaps, Int. J. Biomed. Nanosci. Nanotechnol., 2013, 3 Search PubMed.
- M. van der Zande, A. J. Kokalj, D. J. Spurgeon, S. Loureiro, P. V. Silva, Z. Khodaparast, D. Drobne, N. J. Clark, N. W. van den Brink, M. Baccaro, C. A. M. van Gestel, H. Bouwmeesteraf and R. D. Handy, The gut barrier and the fate of engineered nanomaterials: a view from comparative physiology, Environ. Sci.: Nano, 2020, 7, 1874 RSC.
- L. C. Smith, V. Arizza, M. A. B. Hudgell, G. Barone, A. G. Bodnar, K. M. Buckley, V. Cunsolo, N. Dheilly, N. Franchi, S. D. Fugmann, R. Furukawa, J. Garcia-Arraras, J. H. Henson, T. Hibino, Z. H. Irons, C. Li, C. M. Lun, A. J. Majeske, M. Oren, P. Pagliara, A. Pinsino, D. A. Raftos, J. P. Rast, B. Samasa, D. Schillaci, C. S. Schrankel, L. Stabili, K. Stensväg and E. Sutton, Echinodermata: the complex immune system in Echinoderms, in Advances in Comparative Immunology, ed. E. L. Cooper, Springer, Gewerbestrase, Switzerland, 2018, vol. 13, pp. 409–501 Search PubMed.
- J. Li, M. Tang and Y. Xue, Review of the effects of silver nanoparticle exposure on gut bacteria, J. Appl. Toxicol., 2019, 39, 27 CrossRef CAS.
- Y. Belkaid and T. Hand, Role of the microbiota in immunity and inflammation, Cell, 2014, 157, 121 CrossRef CAS.
- I. R. Cohen and S. Efroni, The immune system computes the state of the body: crowd wisdom, machine learning, and immune cell reference repertoires help manage inflammation, Front. Immunol., 2019, 10, 10 CrossRef.
- D. Boraschi, A. Alijagic, M. Auguste, F. Barbero, E. Ferrari, S. Hernadi, C. Mayall, S. Michelini, N. I. N. Pacheco, A. Prinelli, E. Swart, B. J. Swartzwelter, N. G. Bastús, L. Canesi, D. Drobne, A. Duschl, M. A. Ewart, J. Horejs-Hoeck, P. Italiani, B. Kemmerling, P. Kille, P. Prochazkova, V. F. Puntes, D. J. Spurgeon, C. Svendsen, C. J. Wilde and A. Pinsino, Addressing nanomaterial immunosafety by evaluating innate immunity across living species, Small, 2020, 2000598 CrossRef CAS.
- D. F. Moyano, Y. Liu, D. Peer and V. M. Rotello, Modulation of immune response using engineered nanoparticle surfaces, Small, 2016, 12, 76–82 CrossRef CAS.
- A. Parodi, N. Quattrocchi, A. L. van de Ven, C. Chiappini, M. Evangelopoulos, J. O. Martinez, B. S. Brown, S. Z. Khaled, I. K. Yazdi, M. V. Enzo, L. Isenhart, M. Ferrari and E. Tasciotti, Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions, Nat. Nanotechnol., 2013, 8, 61–68 CrossRef CAS.
- H. Herd Gustafson, D. Holt-Casper, D. W. Grainger and H. Ghandehar, Nanoparticle uptake: the phagocyte problem, Nano Today, 2015, 10, 487–510 CrossRef.
- O. Takeuchi and S. Akira, Pattern recognition receptors and inflammation, Cell, 2010, 140, 805 CrossRef CAS.
- M. Bundschuh, J. Filser, S. Lüderwald, M. S. McKee, G. Metreveli, G. E. Schaumann, R. Schulz and S. Wagner, Nanoparticles in the environment: where do we come from, where do we go to?, Environ. Sci. Eur., 2018, 30, 6 CrossRef.
- F. Barbero, L. Russo, M. Vitali, J. Piella, I. Salvo, M. L. Borrajo, M. Busquets-Fite, R. Grandori, N. G. Bastús, E. Casals and V. Puntes, Formation of the protein corona: the interface between nanoparticles and the immune system, Semin. Immunol., 2017, 34, 52–60 CrossRef CAS.
- L. Shang, K. Nienhaus and G. U. Nienhaus, Engineered nanoparticles interacting with cells: size matters, J. Nanobiotechnol., 2014, 12, 5 CrossRef.
- T. Mironava, M. Hadjiargyrou, M. Simon, V. Jurukovski and M. H. Rafailovich, Gold nanoparticles cellular toxicity and recovery: effect of size, concentration and exposure time, Nanotoxicology, 2010, 4, 120–137 CrossRef CAS.
- M. Neagu, Z. Piperigkou, K. Karamanou, A. B. Engin, A. O. Docea, C. Constantin, C. Negrei, D. Nikitovic and A. Tsatsakis, Protein bio-corona: critical issue in immune nanotoxicology, Arch. Toxicol., 2017, 91, 1031 CrossRef CAS.
- Y. Li, R. Lin, L. Wang, J. Huang, H. Wu, G. Cheng, Z. Zhou, T. MacDonald, L. Yang and H. Maoa, PEG-b-AGE polymer coated magnetic nanoparticle probes with facile functionalization and anti-fouling properties for reducing non-specific uptake and improving biomarker targeting, J. Mater. Chem. B, 2015, 3, 3591–3603 RSC.
- T. J. Anchordoquy, Y. Barenholz, D. Boraschi, M. Chorny, P. Decuzzi, M. A. Dobrovolskaia, Z. S. Farhangrazi, D. Farrell, A. Gabizon, H. Ghandehari, B. Godin, N. M. La-Beck, J. Ljubimova, S. M. Moghimi, L. Pagliaro, J. H. Park, D. Peer, E. Ruoslahti, N. J. Serkova and D. Simberg, Mechanisms and barriers in cancer nanomedicine: addressing challenges, looking for solutions, ACS Nano, 2017, 11, 12–18 CrossRef CAS.
- G. W. Litman, L. J. Dishaw, J. P. Cannon, R. N. Haire and J. P. Rast, Alternative mechanisms of immune receptor diversity, Curr. Opin. Immunol., 2007, 19, 526 CrossRef CAS.
- J. D. Jones and J. L. Dangl, The plant immune system, Nature, 2006, 444, 323 CrossRef CAS.
- M. G. Netea, J. Domínguez-Andrés, L. B. Barreiro, T. Chavakis, M. Divangahi, E. Fuchs, L. A. B. Joosten, J. W. M. van der Meer, M. M. Mhlanga, W. J. M. Mulder, N. P. Riksen, A. Schlitzer, J. L. Schultze, C. Stabell Benn, J. C. Sun, R. J. Xavier and E. Latz, Defining trained immunity and its role in health and disease, Nat. Rev. Immunol., 2020, 20, 375–388 CrossRef CAS.
- J. C. Sun, S. Ugolini and E. Vivier, Immunological memory within the innate immune system, EMBO J., 2014, 33, 1295–1303 CAS.
- D. Melillo, R. Marino, P. Italiani and D. Boraschi, Innate immune memory in invertebrate metazoans: a critical appraisal, Front. Immunol., 2018, 9, 1915 CrossRef.
- J. Sharrock and J. C. Sun, Innate immunological memory: from plants to animals, Curr. Opin. Immunol., 2020, 62, 69–78 CrossRef CAS.
- B. J. Swartzwelter, F. Barbero, A. Verde, M. Mangini, M. Pirozzi, A. C. De Luca, V. F. Puntes, L. C. C. Leite, P. Italiani and D. Boraschi, Gold nanoparticles modulate BCG-induced innate immune memory in human monocytes by shifting the memory response towards tolerance, Cell, 2020, 9, 284 CrossRef CAS.
- E. M. Reimer-Michalski and U. Conrath, Innate immune memory in plants, Semin. Immunol., 2016, 28, 319 CrossRef CAS.
- M. Auguste, T. Balbi, C. Ciacci, B. Canonico, S. Papa, A. Borello, L. Vezzulli and L. Canesi, Shift in immune parameters after repeated exposure to nanoplastics in the marine bivalve Mytilus, Front. Immunol., 2020, 11, 426 CrossRef CAS.
- B. Lamas, N. Martins Breyner and E. Houdeau, Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: potential consequences for host health, Part. Fibre Toxicol., 2020, 17, 19 CrossRef.
- M. Auguste, A. Lasa, A. Pallavicini, S. Gualdi, L. Vezzulli and L. Canesi, Exposure to TiO2 nanoparticles induces shifts in the microbiota composition of Mytilus galloprovincialis hemolymph, Sci. Total Environ., 2019, 670, 129–137 CrossRef CAS.
- E. Swart, J. Dvorak, S. Hernádi, T. Goodall, P. Kille, D. Spurgeon, C. Svendsen and P. Prochazkova, The effects of in vivo exposure to copper oxide nanoparticles on the gut microbiome, host immunity, and susceptibility to a bacterial infection in earthworms, Nanomaterials, 2020, 10, 1337 CrossRef CAS.
- W. M. S. Russell and R. L. Burch, The principles of humane experimental technique, Methuen & Co. Ltd, London, 1959, vol. 238 Search PubMed.
- D. Tilman and J. A. Downing, Biodiversity and stability in grasslands, Nature, 1994, 367, 363–365 CrossRef.
- S. Naeem, J. M. K. Knops, D. Tilman, K. M. Howe, T. Kennedy and S. Gale, Plant diversity increases resistance to invasion in the absence of covarying extrinsic factors, Oikos, 2000, 91, 97–108 CrossRef.
- A. A. Gust, R. Pruitt and T. Nürnberger, Sensing danger: key to activating plant immunity, Trends Plant Sci., 2017, 22, 779 CrossRef CAS.
- C. H. Wu, L. Derevnina and S. Kamoun, Receptor networks underpin plant immunity, Science, 2018, 360, 1300–1301 CrossRef CAS.
- R. Zhang, F. Zheng, S. Wei, S. Zhang, G. Li, P. Cao and S. Zhao, Evolution of disease defense genes and their regulators in plants, Int. J. Mol. Sci., 2019, 20, 335 CrossRef.
- E. Miedes, R. Vanholme, W. Boerjan and A. Molina, The role of the secondary cell wall in plant resistance to pathogens, Front. Plant Sci., 2014, 5, 358 Search PubMed.
- L. Chae, T. Kim, R. Nilo-Poyanco and S. Y. Rhee, Genomic signatures of specialized metabolism in plants, Science, 2014, 344, 510–513 CrossRef CAS.
- V. Mishra, R. K. Mishra, A. Dikshit and A. C. Pandey, in Emerging technologies and management of crop stress tolerance, ed. P. Ahmad and S. Rasool, Elsevier Inc, Amsterdam, 2014, ch. 8, pp. 159–180 Search PubMed.
- J. Gardea-Torresdey and J. R. Peralta-Videa, Nanomaterials in plants, Curr. Opin. Environ. Sci. Health, 2018, 6, 1–84 CrossRef.
- S. Mingyu, J. Liu, S. Yin, M. Linglan and F. Hong, Effects of nano-anatase on the photosynthetic improvement of chloroplast damaged by linolenic acid, Biol. Trace Elem. Res., 2008, 124, 173–183 CrossRef.
- J. Gao, G. Xu, H. Qian, P. Liu, P. Zhao and Y. Hu, Effects of nano-TiO2 on photosynthetic characteristics of Ulmus elongata seedlings, Environ. Pollut., 2013, 176, 63–70 CrossRef CAS.
- C. X. Shen, Q. F. Zhang, J. Li, F. C. Bi and N. Yao, Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes, Am. J. Bot., 2010, 97, 18 CrossRef.
- Z. Hossain, G. Mustafa and S. Komatsu, Plant responses to nanoparticle stress, Int. J. Mol. Sci., 2015, 16, 26644–26653 CrossRef CAS.
- V. Kumar, P. Guleria, V. Kumar and S. KumarYadav, Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana, Sci. Total Environ., 2013, 461–462, 462–468 CrossRef CAS.
- M. Bilej, P. Procházková, M. Šilerová and R. Josková, Earthworm Immunity, in Invertebrate Immunity, ed. K. Söderhäll, Springer, Gewerbestrase, Switzerland, 2018, ch. 4, pp. 66–79 Search PubMed.
- A. Irizar, C. Rivas, N. Garcia-Velasco, F. Goni de Cerio, J. Etxebarria, I. Marigomez and M. Soto, Establishment of toxicity thresholds in subpopulations of coelomocytes (amoebocytes vs. eleocytes) in Eisenia fetida exposed in vitro to a variety of metals: implications for biomarker measurements, Ecotoxicology, 2015, 24, 1004–1013 CrossRef CAS.
- E. L. Cooper and P. Roch, The capacities of earthworms to heal wounds and to destroy allografts are modified by polychlorinated biphenyls (PCB), J. Invertebr. Pathol., 1992, 60, 59–63 CrossRef CAS.
- P. Prochazkova, R. Roubalova, F. Skanta, J. Dvorak, N. I. N. Pacheco, M. Kolarik and M. Bilej, Developmental and immune role of a novel multiple cysteine cluster TLR from Eisenia andrei earthworms, Front. Immunol., 2019, 10, 1277 CrossRef CAS.
- J. Dvořák, V. Mančíková, V. Pižl, D. Elhottová, M. Šilerová, R. Roubalová, F. Škanta, P. Procházková and M. Bilej, Microbial environment affects innate immunity in two closely related earthworm species Eisenia andrei and Eisenia fetida, PLoS One, 2013, 8, e79257 CrossRef.
- H. L. Drake and M. A. Horn, As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes, Annu. Rev. Microbiol., 2007, 61, 169–189 CrossRef CAS.
- E. Thursby and N. Juge, Introduction to the human gut microbiota, Biochem. J., 2017, 474, 1823–1836 CrossRef CAS.
- Y. Hayashi, L. H. Heckmann, V. Simonsen and J. J. Scott-Fordsmand, Time-course profiling of molecular stress responses to silver nanoparticles in the earthworm Eisenia fetida, Ecotoxicol. Environ. Saf., 2013, 98, 219–226 CrossRef CAS.
- Y. Hayashi, P. Engelmann, R. Foldbjerg, M. Szabó, I. Somogyi, E. Pollák, L. Molnár, H. Autrup, D. S. Sutherland and J. Scott-Fordsmand, Earthworms and humans in vitro: characterizing evolutionarily conserved stress and immune responses to silver nanoparticles, Environ. Sci. Technol., 2012, 46, 4166–4173 CrossRef CAS.
- T. Liu, L. Zhang, D. Joo and S. C. Sun, NF-κB signaling in inflammation, Signal Transduction Targeted Ther., 2017, 2, 17023 CrossRef.
- S. Sakon, X. Xue, M. Takekawa, T. Sasazuki, T. Okazaki, Y. Kojima, J. H. Piao, H. Yagita, K. Okumura, T. Doi and H. Nakano, NF-kB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death, EMBO J., 2003, 22, 3898–3909 CrossRef CAS.
- M. L. Whitfield Aslund, H. McShane, M. J. Simpson, A. J. Simpson, J. K. Whalen, W. H. Hendershot and G. I. Sunahara, Earthworm sublethal responses to titanium dioxide nanomaterial in soil detected by 1H NMR metabolomics, Environ. Sci. Technol., 2012, 46, 1111 CrossRef CAS.
- A. Gautam, A. Ray, S. Mukherjee, S. Das, K. Pal, S. Das, P. Karmakar, M. Ray and S. Ray, Immunotoxicity of copper nanoparticle and copper sulfate in a common Indian earthworm, Ecotoxicol. Environ. Saf., 2018, 148, 620–631 CrossRef CAS.
- A. D. Samarajeewa, J. R. Velicogna, J. I. Princz, R. M. Subasinghe, R. P. Scroggins and L. A. Beaudette, Effect of silver nano-particles on soil microbial growth, activity and community diversity in a sandy loam soil, Environ. Pollut., 2017, 220, 504 CrossRef CAS.
- D. S. Read, M. Matzke, H. S. Gweon, L. K. Newbold, L. Heggelund, M. Diez Ortiz, E. Lahive, D. Spurgeon and C. Svendsen, Soil pH effects on the interactions between dissolved zinc, non-nano- and nano-ZnO with soil bacterial communities, Environ. Sci. Pollut. Res., 2016, 23, 4120 CrossRef CAS.
- C. Forstner, T. G. Orton, A. Skarshewski, P. Wang, P. M. Kopittke and P. G. Dennis, Effects of graphene oxide and graphite on soil bacterial and fungal diversity, Sci. Total Environ., 2019, 671, 140 CrossRef CAS.
- Y. Shweta, Metagenomics explorations of earthworm gut micro-biome to detoxify nps in soil system, Int. J. Recent Sci. Res., 2016, 7, 10113–10116 Search PubMed.
- B. K. Zhu, Y. M. Fang, D. Zhu, P. Christie, X. Ke and Y. G. Zhu, Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus, Environ. Pollut., 2018, 239, 408–415 CrossRef CAS.
- S. Gupta, T. Kushwah and S. Yadav, Exposure to ZnO-NPs enhanced gut- associated microbial activity in Eisenia fetida, J. Toxicol. Environ. Health Sci., 2015, 7, 9–17 CrossRef CAS.
- E. Yausheva, E. Sizova, S. Lebedev, A. Skalny, S. Miroshnikov, A. Plotnikov, Y. Khlopko, N. Gogoleva and S. Cherkasov, Influence of zinc nanoparticles on survival of worms Eisenia fetida and taxonomic diversity of the gut microflora, Environ. Sci. Pollut. Res., 2016, 23, 13245–13254 CrossRef CAS.
- D. A. Hill, C. Hoffmann, M. C. Abt, Y. Du, D. Kobuley, T. J. Kirn, F. D. Bushman and D. Artis, Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis, Mucosal Immunol., 2010, 3, 148–158 CrossRef CAS.
- E. L. Cooper and E. A. Stein, Oligochaetes, in Invertebrate Blood Cells, ed. N. A. Ratcliffe and A. F. Rowley, Academic Press, London, San Francisco, 1981, pp. 75–140 Search PubMed.
- OSPAR Commission, Background documents and technical annexes for biological effects monitoring, 2013, p. 239, www.ospar.org Search PubMed.
- L. Canesi, C. Ciacci, R. Fabbri, A. Marcomini, G. Pojana and G. Gallo, Bivalve molluscs as a unique target group for nanoparticle toxicity, Mar. Environ. Res., 2012, 76, 16 CrossRef CAS.
- L. Canesi and P. Prochazkova, The invertebrate immune system as a model for investigating the environmental impact of nanoparticles, in Nanoparticles and immune system, ed. D. Boraschi and A. Duschl, Acad. Press, Oxford, 2013, vol. 7, pp. 91–112 Search PubMed.
- L. Canesi and C. Pruzzo, Specificity of innate immunity in bivalves: a lesson from bacteria, in Lessons in Immunity: From Single-cell Organisms to Mammals, ed. L. Ballarin and F. Cammarata, Elsevier, Academic Press, London, 2016, vol. 6, pp. 9–91 Search PubMed.
- L. Canesi, C. Ciacci and T. Balbi, Invertebrate models for investigating the impact of nanomaterials on innate immunity: the example of the marine mussel Mytilus spp, Curr. Bionanotechnol., 2016, 2, 77–83 CrossRef.
- D. Bouchon, M. Zimmer and J. Dittmer, The terrestrial isopod microbiome: an all-in-one toolbox for animal-microbe interactions of ecological relevance, Front. Microbiol., 2016, 7, 1472 Search PubMed.
- S. Novak, T. Romih, B. Drašler, G. Birarda, L. Vaccari, P. Ferraris, S. Sorieul, M. Zieba, V. Sebastian, M. Arruebo, S. B. Hočevar, A. J. Kokalj and D. Drobne, The in vivo effects of silver nanoparticles on terrestrial isopods, Porcellio scaber, depend on a dynamic interplay between shape, size and nanoparticle dissolution properties, Analyst, 2019, 144-2, 488–497 RSC.
- O. Malev, P. Trebše, M. Piecha, S. Novak, B. Budič, M. D. Dramićanin and D. Drobne, Effects of CeO2 nanoparticles on terrestrial isopod Porcellio scaber: comparison of CeO2 biological potential with other nanoparticles, Arch. Environ. Contam. Toxicol., 2017, 72, 303 CrossRef CAS.
- S. Novak, D. Drobne, J. Valant and P. Pelicon, Internalization of consumed TiO2 nanoparticles by a model invertebrate organism, J. Nanomater., 2012, 658752 Search PubMed.
- A. Hamdoun and D. Epel, Embryo stability and vulnerability in an always changing world, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 1745–1750 CrossRef CAS.
- T. Hibino, M. Loza-Coll, C. Messier, A. J. Majeske, A. H. Cohen, D. P. Terwilliger, K. M. Buckley, V. Brockton, S. V. Nair, K. Berney, S. D. Fugmann, M. K. Anderson, Z. Pancer, R. A. Cameron, L. C. Smith and J. P. Rast, The immune gene repertoire encoded in the purple sea urchin genome, Dev. Biol., 2006, 300, 349 CrossRef CAS.
- C. Falugi, M. G. Aluigi, M. C. Chiantore, D. Privitera, P. Ramoino, M. A. Gatti, A. Fabrizi, A. Pinsino and V. Matranga, Toxicity of metal oxide nanoparticles in immune cells of the sea urchin, Mar. Environ. Res., 2012, 76, 114 CrossRef CAS.
- A. Pinsino, R. Russo, R. Bonaventura, A. Brunelli, A. Marcomini and V. Matranga, Titanium dioxide nanoparticles stimulate sea urchin immune cell phagocytic activity involving TLR/p38 MAPK-mediated signalling pathway, Sci. Rep., 2015, 5, 14492 CrossRef CAS.
- A. Pinsino and A. Alijagic, Sea urchin Paracentrotus lividus immune cells in culture: formulation of the appropriate harvesting and culture media and maintenance conditions, Biol. Open, 2019, 8 DOI:10.1242/bio.039289.
- A. Alijagic, O. Benada, O. Kofroňová, D. Cigna and A. Pinsino, Sea urchin extracellular proteins design a complex protein corona on titanium dioxide nanoparticle surface influencing immune cell behavior, Front. Immunol., 2019, 10, 2261 CrossRef CAS.
- A. Alijagic, F. Barbero, D. Gaglio, E. Napodano, O. Benada, O. Kofroňová, V. F. Puntes, N. G. Bastús and A. Pinsino, Gold nanoparticles coated with polyvinylpyrrolidone and sea urchin extracellular molecules induce transient immune activation, J. Hazard. Mater., 2021, 402, 123793 CrossRef CAS.
- J. L. Wilding and W. F. Bodmer, Cancer cell lines for drug discovery and development, Cancer Res., 2014, 74, 2377 CrossRef CAS.
- N. Ninan, H. Albrecht and A. Blencowe, Mammalian Cell-Based Assays for Studying Bio-Nano Interactions, in Micro and Nano Technologies, Characterization of Nanomaterials, ed. S. M. Bhagyaraj, O. S. Oluwafemi, N. Kalarikkal and S. Thomas, Woodhead Publishing, Sawston, Cambridge, 2018, vol. 5, pp. 129–166 Search PubMed.
- M. Daneshian, S. von Aulock and T. Hartung, Assessment of pyrogenic contaminations with validated human whole-blood assay, Nat. Protoc., 2009, 4, 1709–1721 CrossRef CAS.
- Site of the JRC ECVAM, among the validated assays alternative to animal experimentation, https://tsar.jrc.ec.europa.eu/test-method/tm2002–05.
- S. Michelini, M. Sarajlic, A. Duschl and J. Horejs-Hoeck, IL-1β induces expression of costimulatory molecules and cytokines but not immune feedback regulators in dendritic cells, Hum. Immunol., 2018, 79, 610 CrossRef CAS.
- P. Italiani, E. M. C. Mazza, D. Lucchesi, I. Cifola, C. Gemelli, A. Grande, C. Battaglia, S. Bicciato and D. Boraschi, Transcriptomic profiling of the development of the inflammatory response in human monocytes in vitro, PLoS One, 2014, 9, e87680 CrossRef.
- F. Lebre, J. B. Boland, P. Gouveia, A. L. Gorman, A. M. L. E. Lundahl, R. I. Lynch, F. J. O'Brien, J. Coleman and E. C. Lavelle, Pristine graphene induces innate immune training, Nanoscale, 2020, 12, 11192 RSC.
- N. Iakobachvili and P. J. Peters, Humans in a Dish: The potential of organoids in modeling immunity and infectious diseases, Front. Microbiol., 2017, 8, 2402 CrossRef.
- R. Mittal, F. W. Woo, C. S. Castro, M. A. Cohen, J. Karanxha, J. Mittal, T. Chhibber and V. M. Jhaveri, Organ-on-chip models: Implications in drug discovery and clinical applications, J. Cell. Physiol., 2019, 234, 8352 CrossRef CAS.
- C. M. Sakolish, M. B. Esch, J. J. Hickman, M. L. Shuler and G. J. Mahlera, Modeling barrier tissues in vitro: methods, achievements, and challenges, EBioMedicine, 2016, 5, 30 CrossRef.
- S. Basu, J. Jongerden and G. Ruivenkamp, Sci. Public Policy, 2017, 1–15, DOI:10.1093/scipol/scx008Perspective.
- D. Schuurbiers, in Social Responsibility in Research Practice. Engaging applied scientists with the socio-ethical context of their work, ed. P. Brey, P. Kroes and A. Meijers, 2010, https://www.wtmc.eu/social-responsibility-in-research-practice-engaging-applied-scientists-wih-the-socio-ethical-context-of-their-work/ Search PubMed.
- S. Kumar, Soil, soul, society: a new trinity for our time, Leaping Hare Press, 2015 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |