Doing nano-enabled water treatment right: sustainability considerations from design and research through development and implementation
M. M.
Falinski
 ab,
R. S.
Turley
ac,
J.
Kidd
ad,
A. W.
Lounsbury
ab,
M.
Lanzarini-Lopes
ad,
A.
Backhaus
ab,
H. E.
Rudel
ab,
M. K. M.
Lane
ab,
R. S.
Turley
ac,
J.
Kidd
ad,
A. W.
Lounsbury
ab,
M.
Lanzarini-Lopes
ad,
A.
Backhaus
ab,
H. E.
Rudel
ab,
M. K. M.
Lane
 ab,
C. L.
Fausey
ab,
C. L.
Fausey
 ab,
A. C.
Barrios
ad,
J. E.
Loyo-Rosales
ae,
F.
Perreault
ab,
A. C.
Barrios
ad,
J. E.
Loyo-Rosales
ae,
F.
Perreault
 ad,
W. S.
Walker
ad,
W. S.
Walker
 af,
L. B.
Stadler
ae,
M.
Elimelech
af,
L. B.
Stadler
ae,
M.
Elimelech
 ab,
J. L.
Gardea-Torresdey
ab,
J. L.
Gardea-Torresdey
 ac,
P.
Westerhoff
ac,
P.
Westerhoff
 ad and
J. B.
Zimmerman
ad and
J. B.
Zimmerman
 *abg
*abg
aNanosystems Engineering Research Center for Nanotechnology Enabled-Water Treatment, USA
bDepartment of Chemical and Environmental Engineering, Yale University, New Haven, CT 06511, USA. E-mail: julie.zimmerman@yale.edu
cDepartment of Chemistry and Biochemistry, University of Texas at El Paso, El Paso, TX 79968, USA
dSchool of Sustainable Engineering and the Built Environment, Arizona State University, Tempe, AZ 85008, USA
eDepartment of Civil and Environmental Engineering, Rice University, Houston, TX 77005, USA
fDepartment of Civil Engineering, Center for Inland Desalination Systems, University of Texas at El Paso, El Paso, TX 79968, USA
gSchool of the Environment, Yale University, New Haven, CT 06511, USA
First published on 28th September 2020
Abstract
Currently, over a billion people around the world lack access to clean drinking water, industrial wastewater treatment and reuse is limited, and conventional water treatment systems cannot adequately treat all contaminants of concern. Nanotechnology-enabled water treatment (NWT) has begun to emerge as a viable option to address many of the problems facing the water treatment status quo, either through cost reducing performance enhancements or filling unmet niches. Advancements in fundamental nanoscience allow unprecedented use of catalysis and energy from across the broad electromagnetic spectrum, as well as unique physicochemical properties, to purify drinking water, treat industrial wastewater, and access unconventional water supplies. However, before fully adopting NWT, it is imperative that the devices are both safe and sustainable, enhancing acceptance from consumers, government, non-government organizations, and industry. We suggest that we are in a unique window of time to “do nano right” by making key sustainability considerations very early in nano-water technology development. To this end, we have developed a framework based on three guiding research questions aimed at understanding the breadth of sustainability considerations for NWT at each of the four major life cycle stages – extraction, production, use, and end-of-life. In following this framework, researchers and product developers can design nano-enabled water treatment devices that perform well and are both safe and sustainable. By presenting the current state of sustainable NWT and specifying gaps in the literature, the present review aims to further develop NWT to be the best alternative to conventional water treatment across a variety of sectors.
Environmental significanceAs nanotechnology-enabled water treatment becomes more ubiquitous, potential impacts from metal extractions, production and related energy demands, material demands, worker exposures, release during the use phase, and materials entering landfills and incinerators may all increase. This review outlines the work that has been done and highlights the areas where more work is needed, with the overall goal of maintaining the functional performance of nanotechnology-enabled water treatment devices, while minimizing negative impacts on the environment or human health. |
1 Introduction
The National Academies of Engineering Grand Challenges for the 21st century report included the meaningful goal of “[providing]… clean water globally”5 to the over 1 billion people who do not currently have adequate access to safe drinking water, a significant contributor to over 5000 diarrhea-related deaths per day.5 Additionally, significant barriers are growing for industrial wastewater treatment and reuse within urban communities and for applications that are not well connected to centralized water treatment systems (e.g., agriculture, produced oil and gas, mining). For example, with the growing global population and shifting preferences for higher-value foods, especially in developing countries, agricultural production is expected to increase by roughly 70% by 2050.6 This will exacerbate current availability of suitable quality water supplies to use on arable lands, while increasing potential groundwater or surface water contamination (nitrate, pesticides, herbicides, bulk salts, etc.). The pursuit of clean water for drinking, and a myriad of other uses, faces many challenges encompassing quantity and quality issues. The issues range from securing an adequate, accessible supply to aging infrastructure and increasing costs resulting from the effects of climate change on long-term supply.11A major barrier to these challenges in both developed and developing countries is technology suitability.12 In the developing world, especially in rural areas, many of the common water treatment techniques fail to adequately treat known inorganic, organic, and biological contaminants due to mismatches between the device and the community as a result of relatively high cost and technological complexity.13 In both settings, excess stress on water supplies from contaminants of emerging concern including chemicals (e.g., per- and polyfluoroalkyl substances, pharmaceuticals14) or microbial agents (e.g., emerging viruses, antibiotic resistant genes), stricter water quality standards to protect human and ecosystem health, and the rise of nontraditional water sources (e.g., wastewater, seawater, brackish water, stormwater) challenge the capabilities of traditional water treatment technologies.15 Conventional water treatment processes generally rely upon large and chemically intensive Victorian-age technologies. While capable of meeting current regulatory limits, these processes present environmental, economic, and sustainability concerns. Some conventional processes are energy intensive, contributing to high economic costs and carbon emissions.16,17 Other processes generate an abundance of waste chemicals, produce carcinogenic byproducts during disinfection processes, or are non-selective, leading to material inefficiencies and, subsequently, waste generation.18–20 There is a need for improved water treatment technologies, where the definition of performance is expanded to meet current regulatory requirements while addressing emerging contaminants and simultaneously considering sustainability.
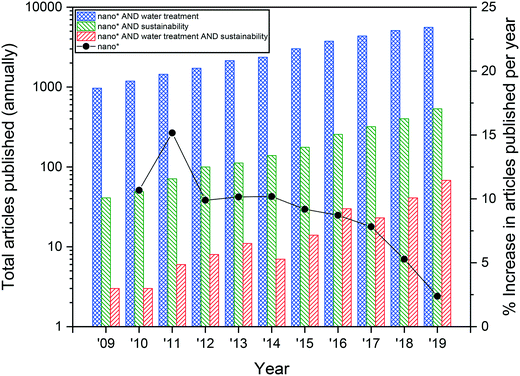
Nanotechnology-based solutions have emerged as a promising strategy to meet water quantity and quality challenges in the developed and developing world.15 This is reflected in an increasing focus on nanotechnology-enabled water treatment (NWT) research, where the total number of articles being published per year, according to data from Web of Science, has seen a nearly 6-fold increase from 2009 to 2019 (Fig. 1). The year-over-year percent increase in articles about nanotechnology and water treatment (“nano* water treatment”) outpaces the increase of all nanotechnology-related (“nano*”) articles, with average increases of 19.3% and 8.9%, respectively, suggesting that interest in NWT is becoming more significant in the field relative to other nano-enabled applications.
However, if NWT is going to emerge as a viable advancement over conventional water treatment processes, there is a need to ensure that the benefits of nanotechnology outweigh its impacts.24 Understanding the impacts of a material or technology relative to its benefits can inform design choices towards maximizing functional performance while minimizing or eliminating environmental, economic, and social impacts.25 Like any technology, nanotechnology exerts an environmental footprint that must consider embodied energy, human toxicity, and environmental health concerns, which can occur throughout the life cycle from material extraction, nanomaterial production, nano-enabled device production, device use, or at the end-of-life. Materials at the nanoscale can have very different properties than their bulk counterparts and alterations to their physicochemical structure and properties can alter their performance and hazard profiles.28 As a result, there has been an increased interest in the field of sustainable nanotechnology. While the total number of articles published in the last ten years about nanotechnology and sustainability (“nano* AND sustainability”) are far fewer than “nano* AND water treatment”, the percent increase in total number of articles per year generally outpaces that for “nano*” and “nano* AND water treatment”, with an average annual growth rate of 29.6% (Fig. 1). However, there is a clear gap in research on sustainable nanotechnology specifically for water treatment. In fact, a search for “nano* AND water treatment AND sustainability” shows that although there is increasing interest in the topic, no more than 70 articles that contain all three of those search terms have ever been published in one year. There is a need for a focused discussion about sustainable nanotechnology for future NWT devices.
NWT devices that are safe, sustainable, and functional are also essential to guarantee acceptance by stakeholders, including from governments, non-governmental organizations, industry, and consumers, lest the technology suffer rejection, similar to that faced by genetically modified organisms in Europe.29 As of right now, the literature on perception and acceptance of nanotechnology and water is quite sparse, and global surveys have found that public awareness of nanotechnology is quite low compared to other innovative technologies.30,31 This provides an opportunity to optimize NWT technologies, so as awareness increases, the chances of stakeholder rejection are minimized.
Here, we discuss considerations related to performance, safety, and sustainability across the entire life cycle of NWT. We advocate weighing these factors early in the development process (i.e., before or during technology readiness level one34), during ideation stages of technology development, because key decisions made then have been shown to impact overall sustainability of solutions as technologies come to the marketplace. Specifically, we explore the following three questions that should be asked before a nanotechnology-enabled device is employed for water treatment:
1) When and where does nanotechnology offer advantages in addressing global water challenges over existing technologies?
2) How are nanomaterial/nano-enabled devices made, and what safety and sustainability concerns are there?
3) What happens to the nanomaterials during and after their use in water treatment devices?
The first question will be addressed in section 2 by covering current strategies to maximize NWT performance through informed design using structure–property–function relationships.24 This will be followed by a review of advantages of NWT over traditional water treatment, including the introduction of potential assessment tools to ensure that nano-enabling a water treatment technology is ideal and appropriate for a given community. The other two questions are focused on the safety and sustainability of nanomaterials and NWTs. Section 3, which responds to the second question, focuses on current and future implications during the extraction of precursor materials and assessments that can be employed during nanomaterial and NWT manufacturing to limit negative impacts. These include the use and replacement of rare and critical metals in devices, the promising green chemistry assessments that can help make synthesis more sustainable, evaluations of the current implications of nanotechnology development, and concerns around worker exposure to nanoparticles. Finally, the third question is answered by section 4, which addresses the use and end-of-life safety and sustainability of NWT. In this section, risk reduction strategies through reduced exposure and reduced nanotechnology hazard are discussed, as is the current and future state of nanotechnology at the end-of-life. In seeking answers to these three questions, researchers and product developers can ensure that nano-enabled devices designed for water treatment perform well and are both safe and sustainable.
2 The promise of nanotechnology in water treatment
Before employing any technology, including NWT technologies, it is important that functional specifications and use-scenarios are well-defined. Typical factors of interest include efficacy, efficiency, cost, product lifetime, and, increasingly, environmental and social impacts. These factors are common across industries and end-users, and understanding each of these factors can inform both the selection and the design of nanomaterials to maximize structure–property–function relationships. However, it is important that new technologies are more beneficial than current technologies, while also being appropriate for the needs of a community. For example, in developing communities, there are often additional considerations that need to be identified through stakeholder engagement and an analysis of the community's capacity and needs.39 If current technologies are insufficient and/or inappropriate for a given community, new technologies, including nanotechnology, can offer advantages in efficiency, effectiveness, and appropriateness. As a result, the promise of nanotechnology in water treatment systems will be reliant on optimizing NWT technologies through nanomaterial structure and property design, strategically deciding when to use NWT-based systems instead of more traditional technologies, and determining the appropriateness of emerging technologies to satisfy community needs.2.1 Manipulating structure–property–function relationships of nanomaterials for enhanced water treatment
Nanotechnology offers a unique array of physical, chemical, and optical properties that can enhance current and future water treatment technologies.40–42 The surface area to volume ratio of engineered nanomaterials (ENMs) can enhance surface activity and efficiency of catalytic (photo-, electro-, and chemical) and sorptive water treatment processes.40–45 Nanocatalysts can degrade or transform both organic and inorganic environmental contaminants at a higher rate than bulk materials,46,47 while light absorbing properties of ENMs enhance photothermal water treatment applications such as solar desalination,48 nano-photonics enabled solar membrane distillation,50 and disinfection processes.51 The magnetic properties of some specific nanomaterials have shown potential in water treatment for particle recovery, regeneration, and ultimately reuse, eliminating the issue of waste chemicals commonly found in centralized conventional water treatment.19,52 For point-of-use applications, tunable ion release provides pathogen disinfection without the need for traditional storage, transport, and dosing of chemical oxidants.39 Similarly, non-ionic, ionic, and graphitic nanomaterials have shown promise as antimicrobial agents for direct water treatment or for preventing fouling material surfaces in the water treatment process.53–55Differences in nanomaterial performance in water treatment devices or applications can be a function of the nanomaterial composition,1,15,41,57,58 meaning that informed selection of nanomaterial classes and composites can yield a higher performance, similar to the selection of bulk materials and chemicals.59–61 However, unique to nanomaterials is the influence of altered physicochemical properties at the nanoscale. From a design and selection perspective for nano-enabling water treatment, it is critical to highlight that while two nanomaterials can have similar elemental compositions, their efficiency and effectiveness can vary greatly with structural changes (e.g., size, shape, surface functionality).24,28 Simply, a decrease in the diameter of the nanoparticle can lead to higher surface area, which can contribute to increased efficiency of adsorption, catalysis, and superparamagnetism.40–42 Changes in nanoparticle size also leads to changes in plasmonic properties, light scattering and absorption, and peak resonance.62 Further, carbon nanomaterials can have variable antimicrobial activity based on dimensionality, size, and aspect ratios with a lower aspect ratio63,64 and decreased sheet size65 resulting in more efficacious 1D and 2D materials, respectively. Shape, and, subsequently, related exposed crystal facet, in addition to size of ENMs has emerged as a critical nanomaterial attribute impacting the efficiency and effectiveness of mechanisms relevant to water treatment including sorbate selectivity,68 enhanced photodegradation of contaminants,69–71 and microbial inhibition.69,72–74 Nanoparticle crystallinity and crystal structure, which can be controlled during synthesis or post-synthesis processing of nanomaterials, has also been linked to differences in photocatalytic activity and the related degradation of contaminants,75,76 the adsorption capacity of organic and inorganic pollutants,78,79 and magnetic behavior of nanomaterials,80 among other functional benefits.
Surface functionalization of nanoparticles can serve as a method to increase not only stability and dispersibility while integrating ENMs into macroscale treatment processes (e.g., dispersion in polymers used for membranes), but also their overall performance (i.e. selective binding of target pollutants on reactive surfaces42). Surface functionalization and modifications can be added through physisorption or covalent links, via techniques like oxidation or ligand exchange,81–83 while a core/shell structure can be designed to take advantage of the properties of multiple materials. Surface functionalization can improve nanomaterial dispersion in liquid and composite matrices, which leads to improvements in desirable properties like catalytic and oxidative reactivity, photocatalytic activity, paramagnetism, sorption capacity, and antimicrobial activity,84–87 while ligand surface functionalization also contributes to enhanced selectivity for specific target contaminants88 and photocatalytic activity.89 The core/shell structure offers further opportunities to enhance nanomaterial performance in water treatment by providing multifunctionality, such as stability, tunability, reactivity, and recovery of the nanoparticle.92 Understanding and employing various sizes, shapes, surface functionalities, and core/shell structures can lead to higher-performing NWT devices, allowing for these novel technologies to compete with conventional treatment technologies.
2.2 When to nano-enable water treatment technology
Several NWT technologies have been shown to out-compete current treatment techniques for some contaminants on a price-per-unit of contaminant remediated basis.57,93 However, supplanting current technologies at-scale can be difficult even when the alternative is price-competitive due to the risk-averse nature of cities, regulators, and utilities as well as the large up-front costs of implementing a new technology.94 In these cases, there is an opportunity to account for costs beyond capital and operating expenditures, where there is an external cost to the environment or public health that is not currently borne by the manufacturer or operator. For example, municipal wastewater treatment can produce large quantities of waste sludge that can offer soil enhancement properties for land applications, but come at a potential microbiological or legacy chemical toxicity risk, depending on the disposal technique.19 Conventional water treatment processes rely on large quantities of chemicals, such as aluminum- or iron- based coagulants (which are disposed to landfills and rarely regenerated and reused20) and disinfectants (which can yield carcinogenic disinfection byproducts (DBPs)18,95,96), while nanomaterials used for water treatment have been shown to be regeneratable and limit the production of harmful DPBs.41,97,98 Beyond direct cost and sustainability considerations, alternative technologies are potentially more viable than traditional technologies if they are more effective, more efficient, or occupy an unfulfilled but critical niche. This is the case with many difficult to destroy legacy pollutants (e.g., nitrate, perchlorate) and emerging contaminants (e.g., per- and polyfluoroalkyl substances – PFAS) which are neither effectively nor efficiently remediated by conventional water treatment systems. For example, removal of naturally occurring oxyanions of human or ecosystem health concern (e.g., arsenite, selenate, and chromate) by conventional adsorbents is inhibited by competing anions (e.g., carbonate, sulfate, phosphate, etc.).100 By exploiting nanoscale phenomena and crystal facet engineering, NWT technologies have demonstrated selectively toward target contaminants over competitors, increasing overall efficiency.42 Further, while the cleaving of the strong C–F bond in PFAS cannot be accomplished by most conventional water treatment technologies, the properties that are unique to the nanoscale, such as enhanced photocatalysis and adsorption, has been demonstrated to help cleave the C–F bond.101 Centralized conventional water treatment also faces significant cost- and resource-barriers for many off-grid industrial applications, developing communities, and rural areas, leading to a need for smaller-scale systems that are promising for the implementation of nanotechnology.102–104A variety of technological assessments should be employed to understand if nanotechnology can act as a suitable replacement for current technologies, and how to best optimize nanomaterials for use. There have been many tools designed to assess the sustainability of (nano)materials and (nano)technology, some of which can be found in Table 1. While some tools, such as life cycle assessment (LCA) and risk assessment (RA), have become more established in the literature for nanotechnology assessment, many other tools are either in their infancy (i.e., they are just recently being “nanotized”) or have not been adapted for NWT.
| Assessment tool | Description | Opportunities for NWT | Unique limitations for NWT |
|---|---|---|---|
| Life cycle assessment105–108 | A systematic technique for determining the environmental impacts of products, processes, or services across their entire life cycle | Considers each part of the lifecycle, and can inform better synthesis and manufacturing techniques for NWT devices | Defining a consistent functional unit is difficult for NWT, and releases and impacts during use and at end-of-life are not well understood |
| Risk assessment109–112 | An approach to estimate the probability of adverse human or environmental health impacts caused from the exposure to materials or processes | Many commonly used NWT materials, such as TiO2, have undergone multiple risk assessments in the literature | Nanoparticle releases and transformations during water treatment and subsequent human and environmental exposure are still not well known |
| GUIDEnano tool113 | A web-based tool to guide risk mitigation and derive safety limit values based on existing studies | Decisions are based on published literature, allowing for model adjustments based on published data on physicochemical property impacts | Highly reliant on existing (eco)toxicity literature, which may not exist for emerging NWT materials, or materials with poorly studied physicochemical alteration |
| LICARA nanoSCAN114 | A tool that simultaneously evaluates risks and benefits of new or existing nanoproducts and compares them against reference products | Minimal amounts of data are required to get initial results, which can further guide design in a relatively new industry | Results are only semi-quantitative and do not consider how variability in physicochemical properties affect benefits |
| Quantitative structure–activity relationships115 | A method used to predict toxic hazards of materials in silico based on molecular structures and physicochemical properties | If enough data is collected, predictive models can be built to assess NWT materials to optimize hazard and functional performance through physicochemical design | Currently, reported data is generally not comparable across studies or aggregated in a way that can be used to build predictive models |
| Techno-economic assessment116 | A framework used to simultaneously evaluate the technical and economic performance of a product, usually compared to a reference | Very little needs to change for TEA to be used for NWT devices, since TEA considers the product as a whole | Accuracy can be improved by considering the impact of variable properties on performance and by improving predictions from bench-scale to commercial-scale production |
| Ashby-based nanomaterial selection117 | Simple, facile, and fast assessment tool allowing users to simultaneously consider benefits and risks related to nanomaterials and compare them against conventional alternatives | Easily integrated into current material selection process, and can consider impacts of varied physicochemical processes | Designed to evaluate nanomaterials, not nano-enabled devices, limiting the assessment scope |
While more standardized nanomaterial assessments are seemingly on the rise, the same cannot be said for assessments of NWT in particular. This is even the case for more established nano-assessment tools like LCA and RA. For example, while LCA is likely the most ubiquitous assessment tool used in nanotechnology, generally, a limited number of LCAs of NWTs have been published that directly consider both the functional unit of water treatment, and the environmental impact of the device (Table 2). Collectively, these LCAs indicate that in certain scenarios, the functionality provided by nanotechnology for water treatment applications show promise for overcoming the impacts of nano-enabling as well as reducing the environmental footprint of water treatment technologies. However, at present, no study fully considers the impacts of various synthetic methods, and none can accurately predict end-of-life or release impacts.
| ENM type/device | Application in water treatment | Life cycle phase | ENM synthetic route | Functional unit | Major findings and environmental impacts | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| E | M | U | EoL | ||||||
| TiO2-anion exchange resin composite | Nanocomposite sorbent for treatment of chromium and arsenic | X | X | X | Heat induced hydrolysis at 4 and 24 hours of heating | 20 million gallons water treated from 20 ppb Cr and 20 ppb As to 8 ppb Cr and 8 ppb As | Materials manufactured under lower energy conditions performed similarly to those that required higher energy during synthesis, yielding small net benefits in environmental impact | Gifford et al., 2016 (ref. 118) | |
| Fe(OH)-anion exchange resin composite | Nanocomposite sorbent for treatment of chromium and arsenic | X | X | X | Chemically-induced metal precipitation with variable rinsing | 20 million gallons water treated from 20 ppb Cr and 20 ppb As to 8 ppb Cr and 8 ppb As | Appropriate post-synthesis processing was able to increase performance by three-fold, leading to net reduction in climate change potential and human toxicity for the higher performing ENM | Gifford et al., 2016 (ref. 118) | |
| TiO2-activated membrane | Membrane-based water treatment with fouling mitigation | X | X | Altair chloride process, followed by electrodeposition onto PES membrane surface | 1 m2 of PES membrane activated with TiO2 ENM needed to treat 1 m3 feed water per hour | Production of the PES membrane had significantly higher impacts than the production and deposition of TiO2 ENMs in every impact category | Zuin et al., 2013 (ref. 119) | ||
| Iron oxide nanoparticles incorporated into polymeric beads | Core materials for recoverable sorbents | X | X | X | NaBH4 reduction of iron acetylacetonate, followed by incorporation into polymeric beads and functionalization by surfactants | 1000 L of wastewater from industrial processes | Impact of magnetic nanoparticles on climate change was highly sensitive to reclamation and reuse, where a 100% recovery had 835 times fewer climate change impacts than one with 0% recovery | Baresel et al., 2019 (ref. 120) | |
| Ginkgo biloba wood membrane decorated with Pd nanoparticles | Methylene blue (MB) removal from industrial wastewaters | X | Wood was dip-coated in a heated PdCl2 and HCl solution to obtain Pd-coated wood membranes | Filtration membrane of size 30 mm × 30 mm × 5 mm, with an operation volume of 1 L and MB concentration of 50 mg L−1 | Due to improved treatment of MB by Pd/wood membranes over non-functionalized wood membranes, use-phase impacts (human toxicity, acidification, eutrophication) decreased | Niaz et al., 2020 (ref. 121) | |||
To comprehensively assess NWT, each existing and emerging assessment tool will need to be further modified and improved. Currently, most tools operate under the assumption that all ENMs with the same composition possess the same level of both risk and performance; this limits the reliability of the results of each assessment, since risk and performance of an ENM are related to its physicochemical properties, but those relationships are hard to predict without experimental data.24 Furthermore, a lack of standardization of ENM characterizations, techniques, and terminologies make it difficult to model and predict outcomes based on different studies. Fortunately, there are standardization initiatives such as eNanoMapper,122 NanoMILE,123 and the US-EU nanoEHS platform (www.us-eu.org) designed to fill the gaps in the literature. eNanoMapper aims to standardize terminology and ontologies used in nanoEHS assessments by improving the quantity and quality of data nanomaterial researchers and modelers have at their disposal. Additionally, NanoMILE works to establish nanomaterial reference libraries and create new tools for high throughput screening and systems biology approaches, thus allowing researchers to accurately predict the impacts of certain physicochemical properties of nanomaterials on biological entities. The US-EU nanoEHS platform has funded numerous large-scale projects to allow for easier comparison and aggregation of data across studies that are not standardized. These initiatives to collect more robust, standardized, and accurate data on nanomaterials over a wide variety of physicochemical properties can enhance the likelihood that these models and predictions are specific and accurate, leading to the development of the most functional and most sustainable NWT technologies.
2.3 Using capacity factor analysis to guide appropriate technology
Before technology assessment occurs, researchers make important decisions regarding what problem to tackle, where the problem exists, and how able a community is to accept new technology. Even when NWT offers apparent advantages in terms of functionality, there is no one-size-fits all solution for each contaminant of concern for each community. This is especially acute in developing communities, where water and sanitation systems are regularly deployed at high costs (e.g., $9.1b by the World Bank in 2017 (ref. 124)), but suffer high failure rates, typically in the range of 30–60%.125 These failures are often the result of poor stakeholder management and organization, highly complex solutions, and failure to consider the local capacity for the project.124 Not only are these communities not receiving vital and life-saving water and sanitation services, these failures also represent a significant waste of time, energy, and resources that were invested in designing, building, and implementing these systems. As we pursue nano-enabling water treatment technologies, it is imperative that lessons are taken from previous failures and that new technologies are co-developed in participatory systems with the intended end users. One strategy that can be employed is capacity factor analysis (CFA). CFA is a process that provides a framework for evaluating a community's readiness to select, operate, and maintain drinking water and sanitation services (Table 3).126,127| Capacity factor | Definition (used to determine CCL) | Requirement (used to determine TRqL) |
|---|---|---|
| Service | Supply, delivery, growth | Production capability or capacity (liters per day per capita) |
| Institutional | Laws, regulations, administration, processes | Scope or scale of installation |
| Human resource | Professional, skilled labor, unskilled labor: literate, illiterate | Technology human input |
| Technical | Supply chain: spare parts, supplies, services | Failure rate (%) & required maintenance level |
| Economical & financial | Markets, mechanisms, taxes, fees, financial options | Service cost (USD$ per capacity per year) |
| Energy | Sources, access, utilization, opportunity cost | Energy demand of the technology |
| Environmental | Carrying capacity of media; stock of resources: land, water, soil type, precipitation | Technological footprint (ft2) |
| Social & cultural | Housing type transience rate, caste/class equity, female participation, community organization | Technology complexity |
Successful implementation of technologies in a community requires a thorough understanding of the capacity of a community to benefit from a technology. The community's capacity level (CCL) is measured across eight different capacity factors (CFs), as is the technology requirement level (TRqL). An in-depth guide to scoring each CCL and TRqL has been provided by Bouabid and Louis.126 Briefly, a CCL score is given by scoring weighted criteria that make up each CF and the technology assessment is scored based on how well the technology achieves certain benchmarks for each CF. While there is some clear inter-connectivity between CFs, they are each treated and scored individually. A low CCL score generally indicates that the community has low levels of formal service for a CF, while a low TRqL is indicative of a technology that is fairly simple and does not require a highly capable community to operate. Technologies that are successfully deployed in a community tend to have a TRqL less than or equal to the CCL for each CF, since this ensures that the community is equipped to handle the incoming technology.127 For NWT devices to also be successfully implemented in water treatment projects, the same must be true. Many emerging NWT technologies have sufficiently low TRqLs to make them applicable in a wide range of developing communities. For example, when considering the “Economical & financial” impacts of NWT, several technologies are less expensive than more traditional water treatment technologies on a price-per-unit remediated basis,57 even though the manufacturing costs of some ENMs are still high.102 Nanomaterials have been incorporated into sand filtration systems to treat metal contamination,129 and since this is a technique with low technological complexity, it would receive a low “Social & Cultural” TRqL score. Further, the anti-fouling properties of nanomaterials in membrane applications130 can lower the burden of maintenance for communities that employ membrane-based water treatment technology, lowering the “Technical” TRqL of their device.
Of course, there are capacity factors for which the TRqLs of NWT are high or unknown, requiring further research to ensure a match between NWT and a community. For example, some NWT devices can require pumps or other high energy equipment. For communities off the electrical grid, this can be an issue, so the “Energy” TRqL must be lowered before that technology would be appropriate in that context. Additionally, few NWTs have been scaled up to the community level, so scaled-up NWT must be proven, or point-of-use systems reliant on nanotechnology must have a high enough treatment capacity to be viable in terms of the “Service” TRqL.
However, while technological factors play a large role in the success of a project, five non-technological factors have been identified and linked to project failure or success: monitoring, coordination, design, training, and institutional environment.124 All of these factors are largely influenced by the stakeholders involved, which can include (but are not limited to) the community in need, non-governmental organizations (NGOs) and local governments, donors, and external service providers.131
3 Sustainable nanomaterials from cradle-to-gate
Once nanotechnology is deemed as a viable solution for water treatment based on its performance and functional benefits to each community of interest, researchers and product developers must ask questions of the life cycle benefits and impacts of nano-enabling the technology. To begin, considering the raw material criticality, or the technical/economic dependency and possibility of supply disruptions of the feedstock, for the nanomaterial or nanotechnology is imperative. Ideally, materials are selected to increase the performance-to-criticality ratio of any engineered nanomaterial for NWT.132 Additionally, during production, synthetic routes and the effects of post-synthesis processing, purification, and separation on the purity and structure of nanomaterials and on the environment and human health should be considered. Finally, it is important to understand and account for worker exposure and environmental releases during product assembly, especially for materials deemed hazardous or persistent. These considerations are not all intuitive, but they are vital to the safety and sustainability of a nano-enabled water treatment technology.3.1 Material extraction and transitioning to earth-abundant materials
NWT technologies have employed a wide suite of elements, crystallinities, and morphologies for their variable functions. Carbon-based ENMs (e.g., graphenes, nanotubes, fullerenes, carbon black, aerogels, etc.), zero-valent metals (e.g., Ag, Au, Cu, Fe, Pd, Pt, Zn, etc.), and metal oxides (e.g., TiO2, iron oxides, ZnO, indium oxides, etc.) each have unique properties that are relevant to a variety of water treatment processes, including adsorption, membrane processes, photocatalysis, electrocatalysis, and anti-microbial activity.15,133–135 Interestingly, multiple classes of nanomaterials based on a variety of feedstocks can demonstrate effectiveness in each of the aforementioned water treatment steps, depending on their structures and properties. As a result, material designers should increasingly consider the impacts of the extraction of the precursors used in material synthesis when choosing an ENM for a given application.136The social, economic, and environmental implications of material extraction can be systematically evaluated by the material criticality assessment,137 which considers three dimensions: “Supply Risk”, “Environmental Implications”, and “Vulnerability to Supply Restrictions”.138 “Supply Risk” is quantified over both the medium- and long-term and addresses the change in availability over time. “Environmental Implications” aims to quantify toxicity concerns, use of water and energy, and environmental emissions from extraction until the manufacturing front gate.138 “Vulnerability to Supply Restrictions” evaluates the importance of a material at the corporate, national, and global levels, its substitutability, and the ability to innovate away from that material at the corporate level.139
For metallic nanomaterials, metal criticality studies can be used as a proxy to understand the criticality of the ENM precursors, and therefore, the criticality of the finalized nanomaterial on a scale of 0–100 (with 100 being the most critical) (Table 4). Further, the synthetic efficiencies of metal and metalloid nanomaterials is quite high (often greater than 90%), meaning that the criticality of the precursors acts as a good estimate of the criticality of the produced nanomaterial.136 With an understanding of the criticality of that material against its performance, the most sustainable precursors can be chosen. For example, there are multiple metallic/metal oxide nanostructures (e.g., Pd, Pt, In, Cu, Al, Rh, and Au-based composites140–142) that have variable criticality and variable performance in the catalytic reduction of nitrate (Fig. 2) where the criticality of each nanomaterial was based up the ratio of each precursor metal present, and the criticality score of each metal precursor came from the work of Graedel et al.138,139 Of note, a more critical material is not necessarily a higher-performing material, and substituting more abundant, less expensive, and more environmentally-friendly precursors that have similar or higher performance-to-criticality ratios should be a priority for NWT device designers.
| Z | Element | Example NWT application | Supply risk (long term)138,139 | Environmental implications138,139 | Vulnerability to supply restrictions (global)138,139 | Overall criticality (global)138,139 |
|---|---|---|---|---|---|---|
| 6 | C | Anti-biofouling | — | — | — | — |
| 7 | N | Functionalization | — | — | — | — |
| 8 | O | Functionalization | — | — | — | — |
| 13 | Al | Adsorption | 0.0 | 3.1 | 57.5 | 34.4 |
| 22 | Ti | Photocatalysis | 0.0 | 2.7 | 37.1 | 26.0 |
| 26 | Fe | Adsorption | 0.0 | 0.8 | 51.7 | 32.5 |
| 27 | Co | Photocatalysis | 42.5 | 4.3 | 50.9 | 39.5 |
| 28 | Ni | Antimicrobial | 1.0 | 10.5 | 47.2 | 35.2 |
| 29 | Cu | Antimicrobial | 22.4 | 17.1 | 60.2 | 43.8 |
| 30 | Zn | Antimicrobial | 46.3 | 2.8 | 53.2 | 38.3 |
| 45 | Rh | Catalysis | 36.7 | 80.7 | 66.8 | 61.4 |
| 46 | Pd | Catalysis | 39.8 | 68.5 | 47.7 | 52.0 |
| 47 | Ag | Anti-biofouling | 77.4 | 43.5 | 56.6 | 59.2 |
| 49 | In | Catalysis | 98.0 | 21.9 | 43.6 | 54.5 |
| 50 | Sn | Catalysis | 41.3 | 10.6 | 56.0 | 36.0 |
| 78 | Pt | Catalysis | 8.6 | 72.7 | 58.1 | 46.5 |
| 79 | Au | Sensing | 6.9 | 76.3 | 61.9 | 48.4 |
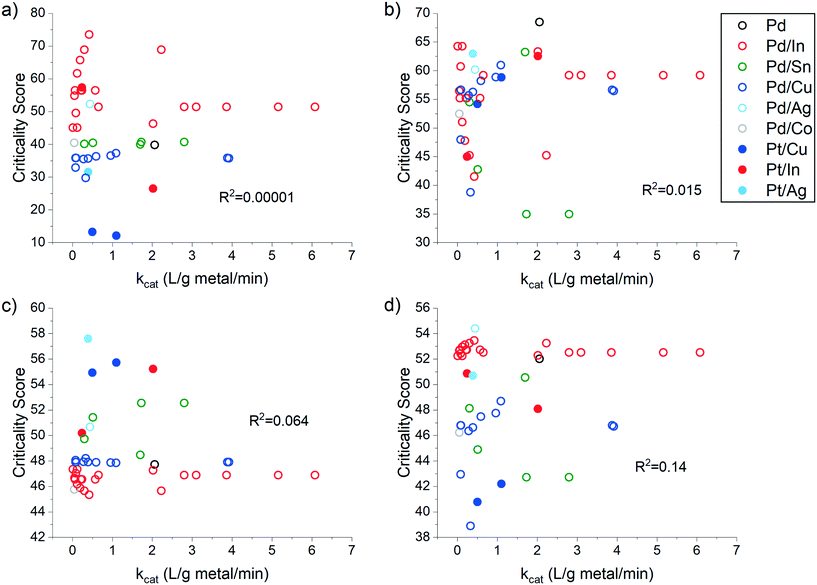 | ||
| Fig. 2 Criticality scores of Pd- and Pt-based multi-metal catalysts for nitrate reduction relative to the specific catalytic rate constant. Criticality scores represent a) Supply Risk, b) Environmental Implications, c) Vulnerability to Supply Restrictions, and d) the Overall Catalyst Criticality score. Pd-Based multi-metal catalysts are represented by empty circles, while Pt-based multi-metal catalysts are represented by filled circles. Data adapted from Graedel et al. (2015)139 and Yin et al. (2018).140 | ||
A comparison of the performance of metallic nanostructures in the catalytic reduction of nitrate140 and the overall criticality of each metal139 reveals that there is little to no correlation between the overall relative criticality of each nanocomposite and its catalytic rate constant (Fig. 2d). Further, the use of critical materials like indium can lead to nanoparticles with a high supply risk, while platinum-based ENMs have higher vulnerability to supply restrictions without a large increase in catalytic activity. This all suggests that future water treatment technologies can aim to lower the overall material criticality in pursuit of more sustainable extraction without negatively impacting performance, and that higher performing technologies do not necessarily need to rely on more critical materials. Further, while Janković and Plata found that the extraction and production of many ENMs, including those found in NWTs, only account for 0.000002–2% of all global anthropogenic material flows,136 material requirements for NWT are expected to increase as the technology becomes more ubiquitous. Nanomaterial scientists and engineers should therefore be transitioning to more earth-abundant materials to achieve long-term sustainability goals. For example, to further minimize Pd and Pt criticality concerns, mixed metal catalysts containing less critical metals like Cu and Sn are being explored for catalytic nitrate reduction to lower the overall mass loading of rare, critical metals.142 Others aim to use only non-critical materials, such as a Ni–Fe0@Fe3O4 nanocomposites,143 featuring two metals with comparatively low criticality scores.
3.2 Nanomaterial production
Given the variety of bottom-up and top-down production routes and environmental/health considerations (Table 5), no single assessment methodology is sufficient to capture the full life cycle impact. To date, atom economy, LCA, E-factor, EQ-factor, and F-factor analysis have emerged as quantitative assessments to evaluate ENM production processes from cradle-to-gate and to support ENM selection.
| ENM class | Carbonaceous | Metal oxides | Metals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Select ENMs found in water treatment | Graphenes | Carbon nanotubes | Titanium dioxide | Iron oxides | Zinc oxides | Copper oxides | Silver | Zero valent iron | Gold | Copper | Palladium | Platinum | ||
| Applications in water treatment1 | Biofouling mitigation, adsorbents, disinfection | Adsorption, photocatalysis, paramagnetism, disinfection | Biofouling mitigation, nitrate reduction, catalysis, disinfection, sensing and monitoring | |||||||||||
| Synthetic method | Synthesis advantages | Sustainability or production challenges | ||||||||||||
| Arc discharge | Minimal imperfection and structural defects2,3 | High electrical energy consumption and temperatures required,4 low throughput/yield3 | ✓3 | ✓4 | — | |||||||||
| Biosynthetic | Biological samples are abundantly available and scaling up is relatively easy; generally considered a “green” synthesis method7 | Widely unexplored field and low mechanistic understanding; safety risks7–10 | — | ✓8 | ✓7 | |||||||||
| Coprecipitation | Potential to synthesize industrial quantities of nanoparticles2 | Synthesis route dependent (solvents, solvent recycling);9 less controlled size distribution2 | — | ✓10 | — | |||||||||
| Flame pyrolysis | Most effective method for less volatile raw materials; relatively simple precursor processing7 | Energy usage (flame gas)21 | — | ✓21,22 | — | |||||||||
| Hydrothermal | Highly crystalline nanoparticle formation; good size and shape control7 | Can require high temperature and pressure;23 limited reproducibility and reliability7 | — | ✓26 | — | |||||||||
| Laser ablation | Surfactant-free formation; some property control is possible; relatively large production capacity7 | Size distribution control27 | — | ✓27 | ✓27 | |||||||||
| Mechano-chemical | Relatively simple and efficient7 | Fragmentation and defects; difficulty in size control; atmospheric contamination7,32,33 | ✓32 | ✓35 | ✓36 | |||||||||
| Microwave | Increased reaction rate compared to conventional heating7 | Controlled synthesis methods still nascent; difficult to penetrate liquid in large reactor volumes37 | — | ✓38 | ✓38 | |||||||||
| Sol–gel | Considered to be one of the simplest synthesis methods7 | High temperatures;49 post processing usually required;49 difficulty in morphology control;9 large quantities of alcohols required for purification56 | — | ✓49 | — | |||||||||
| Solvothermal | Production of highly crystallized monodisperse particles7 | Large quantities of organic solvents; energy usage from elevated temperatures for several hours or days10 | ✓66 | ✓67 | ✓77 | |||||||||
| Supercritical fluid synthesis | Fast reaction times (seconds to minutes), in situ crystallization reducing post-processing requirements, scalable, size and shape control | Energy use due to high temperature and pressure operation, solvent use when using supercritical alcohols45 | ✓45 | ✓90 | ||||||||||
| Sonochemical | Relatively ecofriendly, green, and simple7 | Controlled synthesis methods still nascent; energy for ultrasonic irradiation91 | ✓91 | ✓91 | ✓91 | |||||||||
| Template synthesis | Good control of size and morphology7 | Significant post-processing required99 | ✓99 | ✓99 | — | |||||||||
Atom economy is defined as the ratio of product atoms to reactant atoms in a synthetic route (eqn (1)),144 where the ideal atom economy equals 1 (or 100%). Efforts have been made to produce ENMs commonly found in water treatment (Cu, Ag, Au) using the atom economy approach,145 while Freund et al. have proposed integrating the atom economy approach into a framework that also considers the total number of functions that a nanomaterial can achieve.144
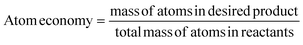 | (1) |
Complimentary to atom economy, E-factor quantifies the ratio of the mass of waste to the mass of desired product (eqn (2)).146
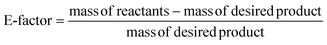 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400.56 The F-factor, in contrast, assigns one or more numeric values to the functional performance of a product, while accounting for the total number of materials or chemicals needed to achieve that function. This allows for the comparison of different products based on the material efficiency of their performance,147 although little work has been done to apply this to ENM synthesis directly. While useful as an efficiency measures, E- and F-factors do not provide any indication of the inherent nature of the waste, including its potential for bioaccumulation, persistence, and/or toxicity.
400.56 The F-factor, in contrast, assigns one or more numeric values to the functional performance of a product, while accounting for the total number of materials or chemicals needed to achieve that function. This allows for the comparison of different products based on the material efficiency of their performance,147 although little work has been done to apply this to ENM synthesis directly. While useful as an efficiency measures, E- and F-factors do not provide any indication of the inherent nature of the waste, including its potential for bioaccumulation, persistence, and/or toxicity.
LCA, as discussed earlier, is the only one of the aforementioned concepts that incorporates energy and water consumption, the environmental impacts of the input materials, and the impacts of the manufacturing process itself into a suite of standardized frameworks for evaluating the environmental impact of the output material.24 It is therefore one of the most ubiquitous assessment techniques used during the manufacturing stage. However, while there are a limited number of studies using LCA to evaluate NWT (Table 2), these studies lack any in-depth evaluation of variable manufacturing and synthesis techniques. Therefore, it is not possible to choose the most sustainable manufacturing techniques using only LCAs related to NWTs. Therefore, more common and robust LCAs that specifically consider a variety of synthetic routes for each type of nanomaterial should instead influence manufacturing decision-making for NWT technologies, until LCAs for NWTs improve in quality and scope.
LCA studies of nanomaterial and nano-enabled product manufacturing, specifically, are among the most well-represented nanotechnology assessments in the literature due to the high impact of energy and material inputs for this lifecycle stage for a variety of nano-enabled applications, including NWT.108 It has been shown that nanomaterial production can be costly, not just economically, but also in terms of energy, water use, and environmental impacts, and these impacts can vary depending on the chosen synthetic method. For example, multi-walled carbon nanotube (MWCNT) synthesis ranges from a total life cycle energy use of 483 MJ kg−1 (floating catalyst chemical vapor deposition) to 9635 MJ kg−1 (laser ablation).148 LCA can help NWT producers make more informed decisions regarding the sustainability of a material during the manufacturing stage by providing insight into ideal production methodologies. For example, the traditional sulfate TiO2 production method has greater CO2 emissions and energy use than the newer alkaline roasting of titanium slag technique that also requires a reduced amount of pure precursor material, an insight that would not be possible without a robust LCA.149
Other ENM synthetic choices that can affect life cycle impacts includes catalyst choice,150,151 feedstock, and reaction conditions152 for carbon nanomaterials, and temperature, pressure, surface stabilizers, and post-synthesis treatment for nano-metals and nano-metal oxides.9 In the case of ENM synthesis, these synthetic choices are often dictated by the goal to yield homogeneous products in terms of size, shape, and crystallinity. Emerging synthesis techniques, such as supercritical fluid synthesis, use non-toxic solvents (H2O, CO2, ethanol, etc.) for the production of controlled nano-metals and nano-metal oxides commonly found in water treatment.45 Using this, and other, emerging techniques may limit hazardous waste products while still allowing for reasonable control over particle size and shape, all while decreasing cumulative energy demand, greenhouse gas emissions, and eutrophication.153
Additionally, it may be beneficial for an application to separate nanomaterials by type (such as size or shape for nanoparticles or chirality for single-walled carbon nanotubes (SWCNTs))158–160 as several studies have shown that the size and nature of the surface of ENMs affect functional performance in water treatment applications.161 For example, separating iron oxide nanoparticles by shape and size can influence selectivity toward contaminants and magnetic properties for facile removal from the water post-contaminant adsorption.72 Size separation of nanoparticles is often accomplished through filtration, centrifugation, or size exclusion chromatography while shape separation is possible for some samples using mass or density gradient centrifugation.159 Each of these techniques have variable energy and material demands due to multi-step separation processes that depend on the nanomaterial and the desired size or shape distribution. Therefore, it is necessary for nanomaterial producers to determine the functional benefit of particle separation and compare it to the added energy and material demands.
Post-synthesis heat treatments, including annealing or calcination, can change the composition of as-synthesized nanomaterials, improving their functional performance in water treatment. For example, TiO2, a metal oxide nanoparticle commonly used in water treatment as a photocatalyst, can be converted from commonly yielded anatase or rutile to more photocatalytic crystalline morphologies through post-synthesis calcination with increased calcination temperature yielding greater photocatalytic activity.76 This means that improved activity was accompanied by increased energy demand, establishing the need to quantify the relationship between functional benefits and negative impacts. Another example considers CNTs that can be used in membranes for water purification and desalination with intrinsic anti-fouling properties.162 A post-processing step of annealing MWCNTs and graphenes alters the type and amount of functional group on the surface of the carbon nanomaterials, and can result in other drastic physical changes, which in turn affects the reactivity and antimicrobial activity.156,163,164 This post-synthesis annealing treatment also allows the MWCNT to perform as well or better than the far more expensive and energy-intensive SWCNTs.54,148
Further, base nanomaterials can be enhanced through post-synthesis processing steps including surface functionalization or ligand exchange. In one case, a ligand exchange for iron oxide nanoparticles allowed them to become multifunctional water treatment adsorbents, with the meso-2,3-dimercaptosuccinic acid (DMSA) ligand sorbing toxic soft metals like mercury, silver, lead, cadmium, and thallium while the iron oxide sorbed arsenic.165 Surface functionalization of carbonaceous nanomaterials, such as graphenes, CNTs, and fullerenes, has been shown to enhance their antimicrobial activity and relative pollutant sorption.164,166–168 Ligand exchanges can require high temperature reactions, followed by additional washing and separation steps, while surface functionalization of carbonaceous nanomaterials usually requires hazardous oxidants like nitric acid or sulfuric acid, and require further washing and separation. As with the other post-processing steps, the application of functionalization and ligand exchange relies on an optimized risk and benefit relationship.
Post-synthesis processing is intended to improve the functional performance of an ENM, but it also can be energy- and resource-intensive. As a result, it is important to use the principles of green chemistry and engineering169,170 as guidelines to limit environmental impacts of post-synthesis processing, including using benign reagents for washing/purification, maximizing atom economy while minimizing E-factor, reducing unnecessary derivatives when functionalizing nanomaterial surfaces, and aiming to improve energy efficiency in drying and calcination. The optimal route would be to weigh the specific benefits of optional post-synthesis processing with the costs of added energy, chemical, emissions, and economic burden through both a life-cycle assessment based on a unit of water treatment functionality and an economic analysis as defined by the impact-benefit ratio.171
4 Nanotechnology-enabled water treatment use and end-of-life
In addition to safety and sustainability concerns during the extraction and production phases, development of nanomaterials for water treatment devices must be accompanied by risk reduction during the use phase and mitigated impacts at the end-of-life of the NWT device. While likely relatively low, a potential risk exists for nanomaterials to be released from NWTs into treated water and wastewater during the use phase. This risk could be mitigated by either 1) designing inherently safer nanomaterials (limiting inherent hazard), or 2) preventing nanoparticle release and/or capturing released nanoparticles (limiting exposure). Additionally, after the useful life of the device, potential environmental impacts at the end-of-life (e.g., during recycling, regeneration, incineration, landfill disposal) should be understood and addressed to decrease cradle-to-grave risks for nano-enabled water treatment devices.4.1 Reducing risk during NWT use
Ideally, any NWT technology will aim to minimize risk during the water and wastewater treatment phase by limiting both factors in the standard risk equation (eqn (3)),25 where:Risk = f(hazard,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exposure) exposure) | (3) |
| Property | Physicochemical relationship | Eco- and human health relationship | NWT-relevant material example |
|---|---|---|---|
| ENM composition | Metal and metal oxide dissolution is a function of composition | Increased dissolution and ionic species release can lead to ionic interaction with cells185–188 | Limiting nAg, nCuO, and nZnO dissolution makes particles safer to mammalian cells187 |
| Size | Nanoparticle dissolution rate is a function of particle size | Decreased ionic species release and resultant toxicity due to larger size/decreased surface area186,189 | nZnO show fewer negative health outcomes toward Daphnia magna with increasing size189 |
| Uptake, biodistribution, and toxicity of non-dissolving nanoparticles is size dependent | Cellular uptake is generally optimized at 50 nm (ref. 190) | Nanoparticles of 30–50 nm interacted most efficiently with cell membrane receptors190 | |
| Some organism-nanomaterial combinations have a positive correlation between size and uptake191,192 | Smaller nano-carbon black was not readily uptaken by mouse macrophage cells191 | ||
| Some organism-nanomaterial combinations have a negative correlation between size and uptake193,194 | Larger nAu caused less DNA damage in model cell lines195 | ||
| Surface charge | ENM surface charge dictates electrostatic interaction between particles, organisms, and the surrounding environment | In most cases, negatively charged particles tend to be safer to organisms due to electrostatic repulsion mechanisms196,197 | Mice were able to tolerate a higher dose of negatively-charged, hydroxylated nano-silica196 |
| In select cases, positively charged particles can be safer117,198 | MWCNT safety toward embryonic zebrafish increased with increased surface charge198 | ||
| Shape | Dissolution rates in solution are a function of ENM shape | Lower energy, rounder edges dissolve at decreased rates with lower cell uptake199 | Spherical silver nanomaterials released ions at a significantly lower rate than trigonal prisms199 |
| Exposed crystal facets, and therefore reactivity, are a function of particle shape | Exposing less reactive crystal facets can lower reactive oxygen species (ROS) production200–202 | Enlarging the Pd {001} crystal facet limited hazardous ROS production201 | |
| “Sharpness” of a particle dictates its physical interaction with the surrounding environment | More spherical particles203,204and aggregates117 are less likely to penetrate cells/organisms | MWCNT aggregates with higher fractal dimensions showed less toxicity toward zebrafish embryos117 | |
| Surface chemistry/surface coatings | Added surface coatings alter surface properties and mechanisms of ENMs | Surface coatings can limit dissolution-based mechanisms, but could instead lead to other toxicity-based mechanisms205 | Polymer-coated CuO nanoparticles were less soluble, but enhanced the production of ROS205 |
| Surface functional groups can change the reactivity of a particle | Limiting surface reactivity through limited reactive functional groups directly correlated to limited toxic outcomes54,65,206 | Non-oxygen functionalized graphenes showed less bacterial toxicity206 |
Due to the nature of functional performance requirements (e.g., catalytic activity) that overlap with potentially hazardous nanomaterial properties (e.g., reactivity), it will be necessary to also address the exposure side of the risk equation. As such, pursuing strategies to reduce or eliminate nanomaterial release and subsequent exposure to end users or the environment during NWT use is critical. Of course, preventing release not only reduces the potential for exposure but is also vital to maintaining the performance of water treatment systems.183 Even minute losses of material over an extended period of time can have a deleterious effect on the performance of the system, even if the levels discharged into process streams are well below those which would cause toxicity concern.207
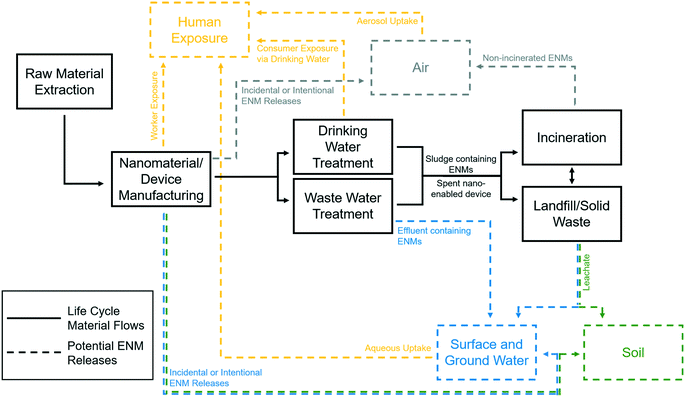
It has been shown that only 0.1–2% of all nanomaterial release occurs during the production phase,208 meaning 98–99.9% of releases happen during the use and end-of-life phases to air, water, and soil (Fig. 3). While nanomaterial release scenarios during production and at the end-of-life are generally similar regardless of nano-application, the release of nanomaterials during the NWT use phase (drinking water treatment and waste water treatment) is unique and could result from a variety of pathways and, once freed, can undergo a variety of transformations in environmental conditions (Table 7). Release during the use phase can occur by loss of unattached or weakly attached nanomaterials or dissolution of the materials themselves. In studying the release of nanomaterials from water treatment devices, results can vary based upon conditions to which the devices are subjected. For example, a study of nano-silver impregnated membranes showed differences in release based on varied flow conditions.209 While nano-silver releases were high in some cases, the device remained efficacious and much of the released silver did not enter the finished (drinking) water. However, high releases from an NWT device can markedly cut down on its useful lifespan, calling for improved designs of nanomaterial impregnation into substrates.
| Nanomaterial release scenarios | Nanomaterial immobilization strategies | Nanomaterial detection and quantification | Free nanomaterial recapture strategies |
|---|---|---|---|
| Particle dissolution209,210 | Electrospinning211 | ICP-MS techniques212 | Magnetic recovery213 |
| Physical abrasion210 | Bead enmeshment44,68 | Thermogravimetric analysis214 | Ultrafiltration215,216 |
| Free-particle release217 | In situ particle formation218 | Spectrophotometry219 | Coagulation and recovery215,216 |
| Material aging and transformations104,217 | Nanomaterial-coated sand57,129,210 | Fluorescent/colorimetric techniques219,220 | |
| Membrane integration221,222 |
Nanomaterials present in membrane reject flows, catalyst cleaning streams, packed bed backwash water, or other waste streams can enter sewers or even surface or groundwaters. NWT products may release ENMs in landfills, which could enter leachate collection systems or groundwaters. ENMs in these waste streams, or those in treated water from NWT devices, do not necessarily retain their specific shape and size, physicochemical properties, or composition and can undergo transformation depending on the local environment and matrix. Upon release, the nanomaterials may aggregate and transform with other compounds or molecules within the treated water, which may change their chemical or physical properties.223 However, some environments with varied magnitudes of ionic strength and natural organic matter, such as sea water instead of fresh water, may increase aggregation processes, which will decrease the bioavailability of nanoparticles.224,225 While ENMs are likely to accumulate in soils receiving sludges and sediments in surface waters, there remains debate on the potential of ENMs to bioaccumulate in biota.226,227 Since ENM transformations and accumulation are complex and relatively difficult to predict, reducing or eliminating release potential is more ideal.
Release prevention may be easily accomplished simply through immobilization of nanomaterials onto support media such as filters, sand, carbon block, or fibers to prevent free particle release or dissolution or by recapturing free particles after use.228,229 Immobilization may present trade-offs by adversely impacting process efficiency while extending the useful design life and enabling recovery and regeneration of the nano-enabled devices.94 To alleviate the need for additional filtration of produced or finished water containing free nanoparticles, superparamagnetic nanomaterials, usually containing iron, can be employed for post-treatment capture, and can also be regenerated for reuse, although further study is needed to ensure that free particle removal is high enough to stay below secondary maximum contaminant level values,230 such as the 0.3 mg L−1 limit set for iron.231 These materials could treat water alone or form core–shell structures where the core provides for magnetic separation and the shell is a reactive compound designed for a specific functionality such as adsorption, photocatalysis, or antimicrobial activity.
All drinking water chemicals found in conventional water treatment (e.g., aluminum, iron, cationic polymer) have small fractions of detectable residuals in finished drinking water. Thus, even with preventative measures in place, some amount of release should be anticipated and monitored via sensing and quantification of nanomaterials in the finished water.232 Ideally, sensing and quantification of released nanoparticles would produce highly accurate results in real time and in-line with the treatment system, in water matrices of variable complexity. Some techniques have been developed and show promise for this application, even if there are current limitations to their full-scale implementation. For example, one of the most robust tools for sensing and quantifying nanomaterials in water is single particle inductively coupled plasma-mass spectrometry (spICP-MS), which can detect and calculate particle size for nanomaterials as small as 10 nm for most elements.212 However, while this technique has high accuracy and can be employed to quantify nanoparticles at low concentrations and within environmentally-relevant matrices, it has not been demonstrated for real-time or in-line analyses. Other emerging methods rely on reactive fluorescent dyes that respond to redox or photocatalytic nanomaterials.233 This method has the potential to be performed in real-time and in-line and is minimally impacted by complex environmental matrices. Whether during development, piloting, or full-scale deployment of NWTs, there is a need for easy-to-use detection methods that can be applied in the field with sensitivities reflective of regulatory limits and potentially reasonable factors of safety (e.g., 10×) that could account for uncertainty if materials are present as nanomaterials.
4.2 End-of-life
Across all technologies, roughly 60% of ENMs and their transformation products are expected to end up in landfills,208 and at the end of their useful life, it is expected that many nano-enabled water treatment devices will incur the same fate. In the United States, there are currently no domestic nanotechnology-specific rules for disposal, as the US Environmental Protection Agency's general approach is that nanoparticles should be treated in the same manner as all other chemical substances.234 In the European Union (EU), although nanomaterials are defined and regulated by Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), nanomaterial waste is not regulated by REACH or any other group, and instead it is assumed that most nanomaterials will enter the waste stream with the nano-enabled product, typically ending up in landfills or incinerators.235Recycling spent nanoparticles for use in other applications has not garnered much research interest, due to the requirement that such a strategy must be simple, inexpensive, fast, and energy efficient.236 Further, a study of recycling rates of four nanomaterials commonly found in water treatment devices (TiO2, ZnO, CNTs, Ag) found that only 0.75–8% of nanoparticles entering recycling systems from representative nano-enabled products were then incorporated into the manufacture of more products, with a majority of materials either being incinerated, sent to a landfill, or exported after the recycling process was complete.237 However, while there is a gap in the literature on this topic, there has been increasing interest in ensuring materials in water treatment devices can be recovered and regenerated after use, such that nanomaterials do not need to enter the waste stream after each use.238,239
Of course, energy and material use must be considered when deciding if nanomaterials should be regenerated and reused. Some regeneration techniques, such as contaminant desorption, rely on highly acidic or highly basic chemicals to desorb contaminants, while others employ energy-intensive thermal desorption, all to varying degrees of success.240 As a result, there may be high material and/or energy cost for regeneration to divert nanomaterials from the waste stream, but with potentially greater environmental impact. Therefore, it is important to evaluate the tradeoffs between the waste produced and energy used with the nanomaterial recovery percentage and the remaining efficacy of the nanomaterials after regeneration. To our knowledge there has been no published research on the behavior of nanomaterials within water treatment modules (e.g., membranes, activated carbon, etc.) during incineration or landfill disposal, and this research is critically needed to help inform these decisions.
5 Doing nano right into the future
There are many examples in the literature and in industry of NWTs using the questions outlined above to advance the goal of “doing nano right” by continuing to improve materials and processes such that they are safe, sustainable, and effective. While efforts have been made towards nanomaterial safety and sustainability, these have not been systematically and comprehensively pursued by the nanotechnology community due to limitations of available data, of robust and nano-specific assessment tools, and of reliable and generalizable property–structure–function–hazard relationships.5.1 Nanotechnology-enabled water treatment advantages
Successfully replacing traditional water treatment technologies with NWT technologies necessitates answering the question: “When and where does nanotechnology offer advantages in addressing global water challenges over existing technologies?” Preliminary research and emerging applications indicate that the costs of NWT are lower than that of traditional water treatment by exploiting nano-properties and structures to enhance the performance of NWTs. Focusing future NWT development on the most promising applications requires improved NWT assessments. While chemical and material assessments are prevalent in the literature and in industry, few have been fully adapted as assessment techniques as appropriate and specific for nanomaterials, nanotechnologies, and NWT technologies.Outperforming traditional technologies based on factors like efficacy, efficiency, and cost may be necessary but not sufficient for the replacement of traditional water treatment technologies. By employing techniques like capacity factor analysis, a community's needs and ability to deploy a technology can be understood at a deeper level, inspiring product designers to create the best NWT technology for a community rather than the best-performing NWT technology.
5.2 Nanotechnology-enabled water treatment extraction and production
Traditional water treatment processes aren't without their own sustainability concerns. Waste production, inefficient chemical use, the production of disinfection by-products, and high emissions contribute to the environmental impact of more traditional technologies, and as a result, there is a clear opportunity for NWT to emerge as the more sustainable option. For this promise to be realized, there must be an evaluation of NWT technologies across the entire life cycle. This should include increased research activity featuring comparative LCAs between NWT devices and existing technologies to inform water treatment technology decision making. However, as some of the environmental benefits of NWT technologies compared to traditional technologies are realized, there is still a need to continuously decrease any negative impacts related to nanomaterial use, while still maintaining the improved functionality.Much of the oft-forgotten implications of nanotechnology come from the extraction of precursors and the manufacture of nanomaterials and, eventually, nano-enabled devices. As a result, designers must be able to answer the question: “How are nanomaterial/nano-enabled devices sourced and made, and what safety and sustainability concerns are there?” Unfortunately, many of the materials, particularly metals, being used for NWT applications are rare and critical. Their supply risk, environmental implications from the extraction process, and their global vulnerability to supply restrictions have the potential to be quite high, making the continued and long-term use of these metals untenable, even if the total material use for NWT technologies is less than that of more traditional techniques. However, in many cases, the increased criticality is not inherently linked to an increase in performance. In fact, less critical metals are being explored to achieve some of the same functional goals. By shifting focus toward abundant metals for NWT devices, designers can reduce the impacts of the extraction phase.
The production phase must also see improvements to ensure that more sustainable NWT technology is developed. Understanding the impacts of various nanomaterial synthesis methods and post-synthesis techniques can encourage the pursuit of more effective and efficient processes. However, there is a need for more robust analyses that incorporate considerations beyond composition, such that the impacts of imparting different structures and properties to nanomaterials can be better understood. Beyond that, as risk shifts from those consuming contaminated water to the workers producing the NWT devices, manufacturers must ensure that occupation exposure concerns are understood and mitigated.
5.3 Use and disposal of nanotechnology-enabled water treatment devices
Until scientists and engineers can systematically and comprehensively address “What happens to the nanomaterials during and after their use in water treatment devices?”, securing trust in NWT technologies by stakeholders will be an extremely difficult task. By pursing risk mitigation strategies through hazard considerations (e.g., nanomaterial selection and design) and exposure pathways (e.g., encapsulation, detection, and capture of free nanoparticles), NWT technologies can become safer and more easily accepted. While there has been a significant amount of research on safe nanomaterial compositions, there is a need to further understand the structure–property–hazard relationships of nanomaterials, as changes in nanomaterial structure and properties can impact toxicity and exposure routes. There is also an opportunity to improve detection and quantification techniques to be in-line and in real time to prevent releases to the environment or into drinking water.5.4 Future implications
Widespread use of NWT will be a relatively complex endeavor, but successful implementation can ultimately provide more accessible water and an improved quality of life, with a lower environmental impact than current technologies. As noted throughout this review, literature and emerging products indicate that sustainable design can guide the selection and design of nanomaterials, including many commonly found in NWT. However, there is a need to improve the sustainable outcomes at every stage of the life cycle, particularly in case of long-term infrastructure, such as water treatment.In some current NWT technologies, the extraction of precursor materials, especially critical metals, can be environmentally costly and damaging. Employing earth abundant metals such as iron, copper, and nickel, and exploiting nanoscale phenomena through crystal facet engineering can help realize the same catalytic efficiencies of platinum group elements or noble metals, creating a greener alternative with a lower overall energy footprint for water treatment.142,143,241 Further, improved evaluation and understanding of the environmental impacts of synthesis and post-synthesis processes of nanomaterials for NWT early on in the design process will allow for the production of materials to achieve their functional water treatment goals with limited deleterious outcomes.
The scientific community, especially in the field of nanotechnology, has become more adept at holistic material characterizations, but most of these characterizations tend to only evaluate the material before its use. As a result, many researchers have directed their efforts towards improved novel materials, without understanding how aging or fouling behavior may detrimentally impact performance or lead to unique release and hazard scenarios.242,243 Improved research in this space can inform appropriate cleaning and nanomaterial regeneration or replacement methods, allowing for a higher recycling rate and lower long-term nanomaterial requirements.
While some work has been done to characterize and quantify the release of nanomaterials into the environment from the general nanotechnology field,208 there is a lack of knowledge on the release of nanomaterials from NWT into drinking water or treated wastewater during the use phase, and there is little information on the release of nanomaterials from NWT devices, specifically, at the end-of-life. Until these releases can be quantified, characterized, and fully understood, robust risk assessments to determine acceptable levels of release are not possible. Regardless, NWT designers should aim for nanomaterial release from products to be minimized where appropriate or eliminated where possible.
Finally, stakeholder acceptance of a technology is not guaranteed, even when a technology is effective and safe. This is further complicated by the somewhat disparate concerns, needs, and desires for each stakeholder group. Stakeholder rejection of effective and safe technologies, such as GMOs in much of Europe, can limit industry growth due to perception of risk with the technology.29 As a result, better public–private partnerships to support and enhance acceptance by governmental and non-governmental organizations, consumers, and industries are needed. These partnerships should aim to establish guidelines to address regulatory uncertainty and raise public awareness to avoid misconceived risk perceptions. These groups can collaborate to ensure proper characterization and use of emerging nanoparticles, based not only on particle size, but also on ENM properties. To see successful implementation, all groups must ensure that confidence in new NWT technology is high.
Nanotechnology shows great promise for the future of global water and wastewater treatment. As NWT evolves to be more apt for developing communities and applications that are off-grid from conventional water treatment systems, it is becoming increasingly imperative to ensure that the technology is effective, safe, and sustainable so the promise of NWT can be fully realized.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Science Foundation Nanosystems Engineering Research Center on Nanotechnology-Enabled Water Treatment (ERC-1449500). The authors would like to thank Dr. Barbara Reck and Dr. Thomas Graedel for sharing raw data from their previous work, which led to the development of a figure in this manuscript.References
- X. Qu, J. Brame, Q. Li and P. J. Alvarez, Acc. Chem. Res., 2013, 46, 834–843 CrossRef CAS.
- J. Verma, S. Lal and C. J. F. Van Noorden, Int. J. Nanomed., 2014, 9, 2863–2877 Search PubMed.
- N. Arora and N. N. Sharma, Diamond Relat. Mater., 2014, 50, 135–150 CrossRef CAS.
- M. Slotte, G. Metha and R. Zevenhoven, Int. J. Energy Environ. Eng., 2015, 6, 233–243 CrossRef CAS.
- NAE Grand Challenges for Engineering, http://www.engineeringchallenges.org/, Accessed 12/1/ 2019 Search PubMed.
- J. Bruinsma, Food and Agriculture Organization of the United Nations: How to Feed the World in 2050, 2009 Search PubMed.
- P. G. Jamkhande, N. W. Ghule, A. H. Bamer and M. G. Kalaskar, J. Drug Delivery Sci. Technol., 2019, 53, 101174 CrossRef CAS.
- B. Malik, T. B. Pirzadah, M. Kumar and R. U. Rehman, in Nanotechnology: Food and Environmental Paradigm, ed. R. Prasad, V. Kumar and M. Kumar, Springer Singapore, Singapore, 2017, pp. 235–252, DOI:10.1007/978-981-10-4678-0_13.
- H. Şengül, T. L. Theis and S. Ghosh, J. Ind. Ecol., 2008, 12, 329–359 CrossRef.
- A. V. Rane, K. Kanny, V. K. Abitha and S. Thomas, in Synthesis of Inorganic Nanomaterials, ed. S. Mohan Bhagyaraj, O. S. Oluwafemi, N. Kalarikkal and S. Thomas, Woodhead Publishing, 2018, pp. 121–139, DOI:10.1016/B978-0-08-101975-7.00005-1.
- D. Flancher, J. – Am. Water Works Assoc., 2019, 111, 70–77 CrossRef.
- S. D. Alexandratos, N. Barak, D. Bauer, F. T. Davidson, B. R. Gibney, S. S. Hubbard, H. L. Taft and P. Westerhoff, ACS Sustainable Chem. Eng., 2019, 7, 2879–2888 CrossRef CAS.
- A. B. Pandit and J. K. Kumar, Annu. Rev. Chem. Biomol. Eng., 2015, 6, 217–246 CrossRef CAS.
- R. Benson, O. D. Conerly, W. Sander, A. L. Batt, J. S. Boone, E. T. Furlong, S. T. Glassmeyer, D. W. Kolpin, H. E. Mash, K. M. Schenck and J. E. Simmons, Sci. Total Environ., 2017, 579, 1643–1648 CrossRef CAS.
- X. Qu, P. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS.
- C. Ray and R. Jain, Drinking water treatment: focusing on appropriate technology and sustainability, Springer Science & Business Media, 2011 Search PubMed.
- P. J. Roeleveld, A. Klapwijk, P. G. Eggels, W. H. Rulkens and W. van Starkenburg, Water Sci. Technol., 1997, 35, 221–228 CrossRef.
- X.-F. Li and W. A. Mitch, Environ. Sci. Technol., 2018, 52, 1681–1689 CrossRef CAS.
- T. Ahmad, K. Ahmad and M. Alam, J. Cleaner Prod., 2016, 124, 1–13 CrossRef.
- O. Sahu and P. Chaudhari, J. Appl. Sci. Environ. Manage., 2013, 17, 241–257 CAS.
- F. Wu, Z. Zhou and A. L. Hicks, Environ. Sci. Technol., 2019, 53, 4078–4087 CrossRef CAS.
- W. Y. Teoh, R. Amal and L. Mädler, Nanoscale, 2010, 2, 1324–1347 RSC.
- H. Hayashi and Y. Hakuta, Materials, 2010, 3, 3794–3817 CrossRef CAS.
- L. M. Gilbertson, J. B. Zimmerman, D. L. Plata, J. E. Hutchison and P. T. Anastas, Chem. Soc. Rev., 2015, 44, 5758–5777 RSC.
- N. R. Council, Risk Assessment in the Federal Government: Managing the Process Working Papers, National Academies Press, 1983 Search PubMed.
- P. W. Dunne, A. S. Munn, C. L. Starkey, T. A. Huddle and E. H. Lester, Philos. Trans. R. Soc., A, 2015, 373, 20150015 CrossRef.
- H. Zeng, X.-W. Du, S. C. Singh, S. A. Kulinich, S. Yang, J. He and W. Cai, Adv. Funct. Mater., 2012, 22, 1333–1353 CrossRef CAS.
- M. M. Falinski, D. L. Plata, S. S. Chopra, T. L. Theis, L. M. Gilbertson and J. B. Zimmerman, Nat. Nanotechnol., 2018, 13, 708–714 CrossRef CAS.
- J. M. Lucht, Viruses, 2015, 7, 4254–4281 CrossRef.
- A. M. Waldron, D. Spencer and C. A. Batt, J. Nanopart. Res., 2006, 8, 569–575 CrossRef.
- D. A. Scheufele and B. V. Lewenstein, J. Nanopart. Res., 2005, 7, 659–667 CrossRef.
- G. Bharath, R. Madhu, S.-M. Chen, V. Veeramani, D. Mangalaraj and N. Ponpandian, J. Mater. Chem. A, 2015, 3, 15529–15539 RSC.
- C. Xu, S. De, A. M. Balu, M. Ojeda and R. Luque, Chem. Commun., 2015, 51, 6698–6713 RSC.
- S. I. Hallstedt and D. C. Pigosso, ICED17: 21st International Conference on Engineering Design, 2017, pp. 229–238 Search PubMed.
- M. Seyedi, S. Haratian and J. V. Khaki, Procedia Mater. Sci., 2015, 11, 309–313 CrossRef CAS.
- M. J. Rak, N. K. Saadé, T. Friščić and A. Moores, Green Chem., 2014, 16, 86–89 RSC.
- H. Duan, D. Wang and Y. Li, Chem. Soc. Rev., 2015, 44, 5778–5792 RSC.
- A. Mirzaei and G. Neri, Sens. Actuators, B, 2016, 237, 749–775 CrossRef CAS.
- V. A. Oyanedel-Craver and J. A. Smith, Environ. Sci. Technol., 2008, 42, 927–933 CrossRef CAS.
- I. Ali, Chem. Rev., 2012, 112, 5073–5091 CrossRef CAS.
- P. Kumari, M. Alam and W. A. Siddiqi, Sustainable Mater. Technol., 2019, 22, e00128 CrossRef CAS.
- L. N. Pincus, F. Melnikov, J. S. Yamani and J. B. Zimmerman, J. Hazard. Mater., 2018, 358, 145–154 CrossRef CAS.
- D. Sushma and S. Richa, Int. Res. J. Environ. Sci., 2015, 4, 103–106 Search PubMed.
- L. N. Pincus, A. W. Lounsbury and J. B. Zimmerman, Acc. Chem. Res., 2019, 52, 1206–1214 CAS.
- M. K. M. Lane and J. B. Zimmerman, Green Chem., 2019, 21, 3769–3781 RSC.
- S. Guo, K. Heck, S. Kasiraju, H. Qian, Z. Zhao, L. C. Grabow, J. T. Miller and M. S. Wong, ACS Catal., 2017, 8, 503–515 CrossRef.
- X. Zhao, L. Lv, B. Pan, W. Zhang, S. Zhang and Q. Zhang, Chem. Eng. J., 2011, 170, 381–394 CrossRef CAS.
- T. Arunkumar, Y. Ao, Z. Luo, L. Zhang, J. Li, D. Denkenberger and J. Wang, Renewable Sustainable Energy Rev., 2019, 115, 109409 CrossRef CAS.
- A. E. Danks, S. R. Hall and Z. Schnepp, Mater. Horiz., 2016, 3, 91–112 RSC.
- P. D. Dongare, A. Alabastri, S. Pedersen, K. R. Zodrow, N. J. Hogan, O. Neumann, J. Wu, T. Wang, A. Deshmukh, M. Elimelech, Q. Li, P. Nordlander and N. J. Halas, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6936 CrossRef CAS.
- S. Loeb, C. Li and J.-H. Kim, Environ. Sci. Technol., 2017, 52, 205–213 CrossRef.
- J. Kudr, Y. Haddad, L. Richtera, Z. Heger, M. Cernak, V. Adam and O. Zitka, Nanomaterials, 2017, 7, 243 CrossRef.
- H.-L. Yang, J. C.-T. Lin and C. Huang, Water Res., 2009, 43, 3777–3786 CrossRef CAS.
- L. M. Pasquini, R. C. Sekol, A. D. Taylor, L. D. Pfefferle and J. B. Zimmerman, Environ. Sci. Technol., 2013, 47, 8775–8783 CAS.
- S. Liu, T. H. Zeng, M. Hofmann, E. Burcombe, J. Wei, R. Jiang, J. Kong and Y. Chen, ACS Nano, 2011, 5, 6971–6980 CrossRef CAS.
- M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316–328 CrossRef CAS.
- A. S. Adeleye, J. R. Conway, K. Garner, Y. Huang, Y. Su and A. A. Keller, Chem. Eng. J., 2016, 286, 640–662 CrossRef CAS.
- V. S. Sousa and M. Ribau Teixeira, Sci. Total Environ., 2020, 707, 136077 CrossRef CAS.
- M. F. Ashby, Materials selection in mechanical design, Pergamon Press, 1992 Search PubMed.
- M. F. Ashby, Materials and the Environment: Eco-informed Material Choice, Elsevier Science, 2009 Search PubMed.
- M. F. Ashby, Y. J. M. Bréchet, D. Cebon and L. Salvo, Mater. Des., 2004, 25, 51–67 CrossRef.
- E. Townsend and G. W. Bryant, Nano Lett., 2012, 12, 429–434 CrossRef CAS.
- A. Al-Jumaili, S. Alancherry, K. Bazaka and M. V. Jacob, Materials, 2017, 10, 1066 CrossRef.
- S. Maleki Dizaj, A. Mennati, S. Jafari, K. Khezri and K. Adibkia, Adv. Pharm. Bull., 2015, 5, 19–23 Search PubMed.
- F. Perreault, A. F. de Faria, S. Nejati and M. Elimelech, ACS Nano, 2015, 9, 7226–7236 CrossRef CAS.
- M. Choucair, P. Thordarson and J. A. Stride, Nat. Nanotechnol., 2009, 4, 30–33 CrossRef CAS.
- E. Beach, S. Brown, K. Shqau, M. Mottern, Z. Warchol and P. Morris, Mater. Lett., 2008, 62, 1957–1960 CrossRef CAS.
- L. N. Pincus, H. E. Rudel, P. V. Petrović, S. Gupta, P. Westerhoff, C. L. Muhich and J. B. Zimmerman, Environ. Sci. Technol., 2020, 54, 9769–9790 CrossRef CAS.
- D. Majumder, I. Chakraborty, K. Mandal and S. Roy, ACS Omega, 2019, 4, 4243–4251 CrossRef CAS.
- W.-C. Huang, L.-M. Lyu, Y.-C. Yang and M. H. Huang, J. Am. Chem. Soc., 2012, 134, 1261–1267 CrossRef CAS.
- J. Y. T. Chan, S. Y. Ang, E. Y. Ye, M. Sullivan, J. Zhang and M. Lin, Phys. Chem. Chem. Phys., 2015, 17, 25333–25341 RSC.
- A. W. Lounsbury, R. Wang, D. L. Plata, N. Billmyer, C. Muhich, K. Kanie, T. Sugimoto, D. Peak and J. B. Zimmerman, J. Colloid Interface Sci., 2019, 537, 465–474 CrossRef CAS.
- X. Huang, X. Hou, F. Song, J. Zhao and L. Zhang, Environ. Sci. Technol., 2016, 50, 1964–1972 CrossRef CAS.
- S. Pal, Y. K. Tak and J. M. Song, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef CAS.
- T. Luttrell, S. Halpegamage, J. Tao, A. Kramer, E. Sutter and M. Batzill, Sci. Rep., 2014, 4, 4043 CrossRef.
- N. S. Allen, N. Mahdjoub, V. Vishnyakov, P. J. Kelly and R. J. Kriek, Polym. Degrad. Stab., 2018, 150, 31–36 CrossRef CAS.
- J. Lai, W. Niu, R. Luque and G. Xu, Nano Today, 2015, 10, 240–267 CrossRef CAS.
- J. Dhal, B. Mishra and G. Hota, Int. J. Environ. Sci. Technol., 2015, 12, 1845–1856 CrossRef CAS.
- P. Z. Ray and H. J. Shipley, RSC Adv., 2015, 5, 29885–29907 RSC.
- Y. C. Zhang, J. Y. Tang and X. Y. Hu, J. Alloys Compd., 2008, 462, 24–28 CrossRef CAS.
- S. Mallakpour and M. Madani, Prog. Org. Coat., 2015, 86, 194–207 CrossRef CAS.
- R. P. Rocha, O. Soares, J. L. Figueiredo and M. F. R. Pereira, C, 2016, 2, 18 Search PubMed.
- U. N. Maiti, W. J. Lee, J. M. Lee, Y. Oh, J. Y. Kim, J. E. Kim, J. Shim, T. H. Han and S. O. Kim, Adv. Mater., 2014, 26, 40–67 CrossRef CAS.
- T. Tosco, M. Petrangeli Papini, C. Cruz Viggi and R. Sethi, J. Cleaner Prod., 2014, 77, 10–21 CrossRef CAS.
- J.-M. Jian, C. Zhang, F. Wang, X. Lu, F. Wang and E. Y. Zeng, Environ. Pollut., 2019, 251, 425–433 CrossRef CAS.
- S. Zhang, T. Shao, S. S. K. Bekaroglu and T. Karanfil, Environ. Sci. Technol., 2009, 43, 5719–5725 CrossRef CAS.
- T. Cordero, J.-M. Chovelon, C. Duchamp, C. Ferronato and J. Matos, Appl. Catal., B, 2007, 73, 227–235 CrossRef CAS.
- A. Jawed, V. Saxena and L. M. Pandey, J. Water Process. Eng., 2020, 33, 101009 CrossRef.
- I. Jang, J. H. Park, K. Song, L. Kim, Y. Lee and S. G. Oh, Mater. Chem. Phys., 2014, 147, 691–700 CrossRef CAS.
- Y. Xu, V. Musumeci and C. Aymonier, React. Chem. Eng., 2019, 4, 2030–2054 RSC.
- H. Xu, B. W. Zeiger and K. S. Suslick, Chem. Soc. Rev., 2013, 42, 2555–2567 RSC.
- A. Vanderkooy, Y. Chen, F. Gonzaga and M. A. Brook, ACS Appl. Mater. Interfaces, 2011, 3, 3942–3947 CrossRef CAS.
- R. Stirling, W. S. Walker, P. Westerhoff and S. Garcia-Segura, Electrochim. Acta, 2020, 338, 135874 CrossRef CAS.
- P. Westerhoff, P. Alvarez, Q. Li, J. Gardea-Torresdey and J. Zimmerman, Environ. Sci.: Nano, 2016, 3, 1241–1253 RSC.
- G. A. Boorman, Environ. Health Perspect., 1999, 107, 207–217 CAS.
- N. Chaukura, S. S. Marais, W. Moyo, N. Mbali, L. C. Thakalekoala, T. Ingwani, B. B. Mamba, P. Jarvis and T. T. I. Nkambule, J. Environ. Chem. Eng., 2020, 8, 103659 CrossRef CAS.
- L. Al-Issai, W. Elshorbagy, M. A. Maraqa, M. Hamouda and A. M. Soliman, Water, 2019, 11, 559 CrossRef CAS.
- M. Zhang, X. Wang, T. T. Du, H. H. Wang, H. Z. Hao, Y. Y. Wang, Y. Li and T. W. Hao, Water Res., 2019, 162, 1–10 CrossRef CAS.
- Y. Liu, J. Goebl and Y. Yin, Chem. Soc. Rev., 2013, 42, 2610–2653 RSC.
- S. Sarkar, J. E. Greenleaf, A. Gupta, D. Uy and A. K. SenGupta, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 497–517 CrossRef CAS.
- N. B. Saleh, A. Khalid, Y. Tian, C. Ayres, I. V. Sabaraya, J. Pietari, D. Hanigan, I. Chowdhury and O. G. Apul, Environ. Sci.: Water Res. Technol., 2019, 5, 198–208 RSC.
- J. Brame, Q. Li and P. J. J. Alvarez, Trends Food Sci. Technol., 2011, 22, 618–624 CrossRef CAS.
- P. J. J. Alvarez, C. K. Chan, M. Elimelech, N. J. Halas and D. Villagrán, Nat. Nanotechnol., 2018, 13, 634–641 CrossRef CAS.
- M. S. Mauter, I. Zucker, F. Perreault, J. R. Werber, J.-H. Kim and M. Elimelech, Nat. Sustain., 2018, 1, 166–175 CrossRef.
- S. Gavankar, S. Suh and A. F. Keller, Int. J. Life Cycle Assess., 2012, 17, 295–303 CrossRef.
- R. Hischier and T. Walser, Sci. Total Environ., 2012, 425, 271–282 CrossRef CAS.
- H. C. Kim and V. Fthenakis, J. Ind. Ecol., 2013, 17, 528–541 CrossRef CAS.
- B. Salieri, D. A. Turner, B. Nowack and R. Hischier, Nanoimpact, 2018, 10, 108–120 CrossRef.
- D. B. Warheit, F1000Research, 2018, 7, 376 Search PubMed.
- S. Erbis, Z. Ok, J. A. Isaacs, J. C. Benneyan and S. Kamarthi, Risk Anal., 2016, 36, 1644–1665 CrossRef.
- D. R. Hristozov, S. Gottardo, A. Critto and A. Marcomini, Nanotoxicology, 2012, 6, 880–898 CrossRef CAS.
- S. N. Sørensen, A. Baun, M. Burkard, M. Dal Maso, S. Foss Hansen, S. Harrison, R. Hjorth, S. Lofts, M. Matzke, B. Nowack, W. Peijnenburg, M. Poikkimäki, J. T. K. Quik, K. Schirmer, A. Verschoor, H. Wigger and D. J. Spurgeon, Environ. Sci.: Nano, 2019, 6, 505–518 RSC.
- M. L. Fernández-Cruz, D. Hernandez-Moreno, J. Catalán, R. Cross, H. Stockmann-Juvala, J. Cabellos, V. R. Lopes, M. Matzke, N. Ferraz and J. J. Izquierdo, Environ. Sci.: Nano, 2018, 5, 381–397 RSC.
- T. van Harmelen, E. K. Zondervan-van den Beuken, D. H. Brouwer, E. Kuijpers, W. Fransman, H. B. Buist, T. N. Ligthart, I. Hincapié, R. Hischier, I. Linkov, B. Nowack, J. Studer, L. Hilty and C. Som, Environ. Int., 2016, 91, 150–160 CrossRef.
- L. Lamon, D. Asturiol, A. Vilchez, R. Ruperez-Illescas, J. Cabellos, A. Richarz and A. Worth, Comput. Toxicol., 2019, 9, 143–151 CrossRef CAS.
- M. Siegert, J. M. Sonawane, C. I. Ezugwu and R. Prasad, in Advanced Research in Nanosciences for Water Technology, ed. R. Prasad and T. Karchiyappan, Springer International Publishing, Cham, 2019, pp. 1–23, DOI:10.1007/978-3-030-02381-2_1.
- M. M. Falinski, M. A. Garland, S. M. Hashmi, R. L. Tanguay and J. B. Zimmerman, Carbon, 2019, 155, 587–600 CrossRef CAS.
- M. Gifford, M. Chester, K. Hristovski and P. Westerhoff, Environ. Sci.: Nano, 2016, 3, 1351–1360 RSC.
- S. Zuin, P. Scanferla, A. Brunelli, A. Marcomini, J. E. Wong, W. Wennekes and I. Genné, Ind. Eng. Chem. Res., 2013, 52, 13979–13990 CrossRef CAS.
- C. Baresel, V. Schaller, C. Jonasson, C. Johansson, R. Bordes, V. Chauhan, A. Sugunan, J. Sommertune and S. Welling, Heliyon, 2019, 5, e02325 CrossRef.
- F. Niaz, Q. Khan, M. Ali and W. Shen, ACS Omega, 2020, 5, 4900–4906 CrossRef CAS.
- B. Fadeel, L. Farcal, B. Hardy, S. Vázquez-Campos, D. Hristozov, A. Marcomini, I. Lynch, E. Valsami-Jones, H. Alenius and K. Savolainen, Nat. Nanotechnol., 2018, 13, 537–543 CrossRef CAS.
- I. Lynch, C. Weiss and E. Valsami-Jones, Nano Today, 2014, 9, 266–270 CrossRef CAS.
- L. A. Ika, A. Diallo and D. Thuillier, Int. J. Proj. Manag., 2012, 30, 105–116 CrossRef.
- M. Palaniappan, P. H. Gleick and E. Change, A review of decision-making support tools in the water, sanitation, and hygiene sector, Pacific Institute, Oakland California, 2008 Search PubMed.
- A. Bouabid and G. E. Louis, J. Environ. Manage., 2015, 161, 335–343 CrossRef.
- J. J. Henriques and G. E. Louis, J. Environ. Manage., 2011, 92, 214–222 CrossRef CAS.
- G. E. Louis and A. Bouabid, Capacity Assessment for Sustainable Sanitation Services in Lower-Income Communities, International Council of Systems Engineering Mid-Atlantic Regional Conference Proceedings, 2004 Search PubMed.
- S.-M. Lee, C. Laldawngliana and D. Tiwari, Chem. Eng. J., 2012, 195–196, 103–111 CrossRef CAS.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. J. Alvarez, Water Res., 2008, 42, 4591–4602 CrossRef CAS.
- A. A. Chisholm, C. L. Genda, S. Pailla, J. L. Martin, A. M. Thrash, K. J. Sheehy and G. E. Louis, Proceedings of the 2013 IEEE Systems and Information Engineering Design Symposium, 2013, pp. 201–206 Search PubMed.
- S. I. Hallstedt and O. Isaksson, J. Cleaner Prod., 2017, 161, 40–52 CrossRef.
- I. Gehrke, A. Geiser and A. Somborn-Schulz, Nanotechnol., Sci. Appl., 2015, 8, 1–17 CAS.
- R. K. Thines, N. M. Mubarak, S. Nizamuddin, J. N. Sahu, E. C. Abdullah and P. Ganesan, J. Taiwan Inst. Chem. Eng., 2017, 72, 116–133 CrossRef CAS.
- P. Xu, G. M. Zeng, D. L. Huang, C. L. Feng, S. Hu, M. H. Zhao, C. Lai, Z. Wei, C. Huang, G. X. Xie and Z. F. Liu, Sci. Total Environ., 2012, 424, 1–10 CrossRef CAS.
- N. Z. Janković and D. L. Plata, Environ. Sci.: Nano, 2019, 6, 2697–2711 RSC.
- N. R. Council, Minerals, Critical Minerals, and the U.S. Economy, The National Academies Press, Washington, DC, 2008 Search PubMed.
- T. E. Graedel, R. Barr, C. Chandler, T. Chase, J. Choi, L. Christoffersen, E. Friedlander, C. Henly, C. Jun, N. T. Nassar, D. Schechner, S. Warren, M.-y. Yang and C. Zhu, Environ. Sci. Technol., 2012, 46, 1063–1070 CrossRef CAS.
- T. E. Graedel, E. M. Harper, N. T. Nassar, P. Nuss and B. K. Reck, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4257 CrossRef CAS.
- Y. B. Yin, S. Guo, K. N. Heck, C. A. Clark, C. L. Coonrod and M. S. Wong, ACS Sustainable Chem. Eng., 2018, 6, 11160–11175 CrossRef CAS.
- C. A. Clark, C. P. Reddy, H. Xu, K. N. Heck, G. Luo, T. P. Senftle and M. S. Wong, ACS Catal., 2020, 10, 494–509 CrossRef CAS.
- K. N. Heck, S. Garcia-Segura, P. Westerhoff and M. S. Wong, Acc. Chem. Res., 2019, 52, 906–915 CrossRef CAS.
- Z. A. Jonoush, A. Rezaee and A. Ghaffarinejad, J. Cleaner Prod., 2020, 242, 118569 CrossRef CAS.
- R. Freund, U. Lächelt, T. Gruber, B. Rühle and S. Wuttke, ACS Nano, 2018, 12, 2094–2105 CrossRef CAS.
- S. B. Kalidindi, U. Sanyal and B. R. Jagirdar, Inorg. Chem., 2010, 49, 3965–3967 CrossRef CAS.
- R. A. Sheldon, J. Chem. Technol. Biotechnol., 1997, 68, 381–388 CrossRef CAS.
- J. Clark, R. Sheldon, C. Raston, M. Poliakoff and W. Leitner, Green Chem., 2014, 16, 18–23 RSC.
- D. Kushnir and B. A. Sandén, J. Ind. Ecol., 2008, 12, 360–375 CrossRef CAS.
- S. Middlemas, Z. Z. Fang and P. Fan, J. Cleaner Prod., 2015, 89, 137–147 CrossRef CAS.
- A. Szabó, C. Perri, A. Csató, G. Giordano, D. Vuono and J. B. Nagy, Materials, 2010, 3, 3092–3140 CrossRef.
- C. J. Lee, J. Park and J. A. Yu, Chem. Phys. Lett., 2002, 360, 250–255 CrossRef CAS.
- W. Shi, K. Xue, E. R. Meshot and D. L. Plata, Green Chem., 2017, 19, 3787–3800 RSC.
- M. P. Tsang, G. Philippot, C. Aymonier and G. Sonnemann, ACS Sustainable Chem. Eng., 2018, 6, 5142–5151 CrossRef CAS.
- M. He, X. F. Liu, B. Liu and J. H. Yang, J. Colloid Interface Sci., 2019, 537, 414–421 CrossRef CAS.
- S. M. Ansar, F. S. Mohammed, G. von White, M. Budi, K. C. Powell, O. T. Mefford and C. L. Kitchens, J. Phys. Chem. C, 2016, 120, 6842–6850 CrossRef CAS.
- L. M. Gilbertson, D. G. Goodwin, A. D. Taylor, L. Pfefferle and J. B. Zimmerman, Environ. Sci. Technol., 2014, 48, 5938–5945 CrossRef CAS.
- W. Abdelwahed, G. Degobert, S. Stainmesse and H. Fessi, Adv. Drug Delivery Rev., 2006, 58, 1688–1713 CrossRef CAS.
- Y. Mori, Kona Powder Part. J., 2015, 102–114 CrossRef CAS.
- J. D. Robertson, L. Rizzello, M. Avila-Olias, J. Gaitzsch, C. Contini, M. S. Magoń, S. A. Renshaw and G. Battaglia, Sci. Rep., 2016, 6, 27494 CrossRef CAS.
- Y. Yomogida, T. Tanaka, M. Zhang, M. Yudasaka, X. Wei and H. Kataura, Nat. Commun., 2016, 7, 12056 CrossRef CAS.
- W. Li, K. R. Yang, X. Yao, Y. He, Q. Dong, G. W. Brudvig, V. S. Batista and D. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 5616–5622 CrossRef CAS.
- R. Das, M. E. Ali, S. B. A. Hamid, S. Ramakrishna and Z. Z. Chowdhury, Desalination, 2014, 336, 97–109 CrossRef CAS.
- M. M. M. Alyobi, C. J. Barnett and R. J. Cobley, Crystals, 2017, 7, 11 CrossRef.
- A. C. Barrios, Y. Wang, L. M. Gilbertson and F. Perreault, Environ. Sci. Technol., 2019, 53, 14679–14687 CrossRef CAS.
- W. Yantasee, C. L. Warner, T. Sangvanich, R. S. Addleman, T. G. Carter, R. J. Wiacek, G. E. Fryxell, C. Timchalk and M. G. Warner, Environ. Sci. Technol., 2007, 41, 5114–5119 CrossRef CAS.
- Y. Wang and L. M. Gilbertson, Green Chem., 2017, 19, 2826–2838 RSC.
- H. H. Cho, K. Wepasnick, B. A. Smith, F. K. Bangash, D. H. Fairbrother and W. P. Ball, Langmuir, 2010, 26, 967–981 CrossRef CAS.
- M. Rajabi, K. Mahanpoor and O. Moradi, RSC Adv., 2017, 7, 47083–47090 RSC.
- P. T. Anastas and J. B. Zimmerman, Environ. Sci. Technol., 2003, 37, 94A–101A CrossRef.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- L. M. Gilbertson, A. A. Busnaina, J. A. Isaacs, J. B. Zimmerman and M. J. Eckelman, Environ. Sci. Technol., 2014, 48, 11360–11368 CrossRef CAS.
- M. Gifford, M. Chester, K. Hristovski and P. Westerhoff, Water Res., 2018, 128, 246–254 CrossRef CAS.
- R. A. Yokel and R. C. MacPhail, J. Occup. Med. Toxicol., 2011, 6, 7 CrossRef CAS.
- C. Geraci, D. Heidel, C. Sayes, L. Hodson, P. Schulte, A. Eastlake and S. Brenner, J. Nanopart. Res., 2015, 17, 366 CrossRef.
- M. N. Shepard and S. Brenner, Ann. Occup. Hyg., 2013, 58, 251–265 Search PubMed.
- L. V. Stebounova, H. Morgan, V. H. Grassian and S. Brenner, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2012, 4, 310–321 CrossRef CAS.
- P. C. Raynor, in Assessing Nanoparticle Risks to Human Health, ed. G. Ramachandran, William Andrew Publishing, Oxford, 2011, pp. 167–193, DOI:10.1016/B978-1-4377-7863-2.00007-8.
- L. Hodson, C. Geraci and P. Schulte, Continuing to Protect the Nanotechnology Workforce: NIOSH Nanotechnology Research Plan for 2018–2025, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH), Cincinnati, OH, 2019, DHHS (NIOSH) Publication 2019-116 Search PubMed.
- K. R. Babik, M. M. Dahm, K. H. Dunn, K. L. Dunn and M. K. Schubauer-Berigan, J. Occup. Environ. Hyg., 2018, 15, 44–56 CrossRef CAS.
- Y. Ding, T. A. J. Kuhlbusch, M. Van Tongeren, A. S. Jiménez, I. Tuinman, R. Chen, I. L. Alvarez, U. Mikolajczyk, C. Nickel, J. Meyer, H. Kaminski, W. Wohlleben, B. Stahlmecke, S. Clavaguera and M. Riediker, J. Hazard. Mater., 2017, 322, 17–28 CrossRef CAS.
- C. Oksel, V. Subramanian, E. Semenzin, C. Y. Ma, D. Hristozov, X. Z. Wang, N. Hunt, A. Costa, W. Fransman, A. Marcomini and T. Wilkins, Environ. Sci.: Nano, 2016, 3, 869–882 RSC.
- W. A. Heitbrink, L.-M. Lo and K. H. Dunn, J. Occup. Environ. Hyg., 2015, 12, 16–28 CrossRef CAS.
- P. Westerhoff, A. Atkinson, J. Fortner, M. S. Wong, J. Zimmerman, J. Gardea-Torresdey, J. Ranville and P. Herckes, Nat. Nanotechnol., 2018, 13, 661–669 CrossRef CAS.
- B. Fubini, M. Ghiazza and I. Fenoglio, Nanotoxicology, 2010, 4, 347–363 CrossRef CAS.
- Z.-m. Xiu, Q.-b. Zhang, H. L. Puppala, V. L. Colvin and P. J. Alvarez, Nano Lett., 2012, 12, 4271–4275 CrossRef CAS.
- D. A. Notter, D. M. Mitrano and B. Nowack, Environ. Toxicol. Chem., 2014, 33, 2733–2739 CrossRef CAS.
- O. Bondarenko, K. Juganson, A. Ivask, K. Kasemets, M. Mortimer and A. Kahru, Arch. Toxicol., 2013, 87, 1181–1200 CrossRef CAS.
- N. V. Hudson-Smith, P. L. Clement, R. P. Brown, M. O. P. Krause, J. A. Pedersen and C. L. Haynes, Environ. Sci.: Nano, 2016, 3, 1236–1240 RSC.
- S. Lopes, F. Ribeiro, J. Wojnarowicz, W. Łojkowski, K. Jurkschat, A. Crossley, A. M. Soares and S. Loureiro, Environ. Toxicol. Chem., 2014, 33, 190–198 CrossRef CAS.
- P. Foroozandeh and A. A. Aziz, Nanoscale Res. Lett., 2018, 13, 339 CrossRef.
- H. T. Kong, Y. Zhang, Y. J. Li, Z. F. Cui, K. Xia, Y. H. Sun, Q. F. Zhao and Y. Zhu, Int. J. Mol. Sci., 2013, 14, 22529–22543 CrossRef.
- M. Zhang, M. Yang, T. Morimoto, N. Tajima, K. Ichiraku, K. Fujita, S. Iijima, M. Yudasaka and T. Okazaki, Carbon, 2018, 127, 93–101 CrossRef CAS.
- Q. Feng, Y. Liu, J. Huang, K. Chen, J. Huang and K. Xiao, Sci. Rep., 2018, 8, 2082 CrossRef.
- C. P. Adams, K. A. Walker, S. O. Obare and K. M. Docherty, PLoS One, 2014, 9, e85981 CrossRef.
- C. Lopez-Chaves, J. Soto-Alvaredo, M. Montes-Bayon, J. Bettmer, J. Llopis and C. Sanchez-Gonzalez, Nanomed.: Nanotechnol., Biol. Med., 2018, 14, 1–12 CrossRef CAS.
- K. Greish, G. Thiagarajan, H. Herd, R. Price, H. Bauer, D. Hubbard, A. Burckle, S. Sadekar, T. Yu, A. Anwar, A. Ray and H. Ghandehari, Nanotoxicology, 2012, 6, 713–723 CrossRef CAS.
- A. M. El Badawy, R. G. Silva, B. Morris, K. G. Scheckel, M. T. Suidan and T. M. Tolaymat, Environ. Sci. Technol., 2011, 45, 283–287 CrossRef CAS.
- L. M. Gilbertson, F. Melnikov, L. C. Wehmas, P. T. Anastas, R. L. Tanguay and J. B. Zimmerman, Nanotoxicology, 2015, 1–10 CrossRef.
- C. Graf, D. Nordmeyer, C. Sengstock, S. Ahlberg, J. Diendorf, J. Raabe, M. Epple, M. Koller, J. Lademann, A. Vogt, F. Rancan and E. Ruhl, Langmuir, 2018, 34, 1506–1519 CrossRef CAS.
- M. Ramani, S. Ponnusamy, C. Muthamizhchelvan and E. Marsili, Colloids Surf., B, 2014, 117, 233–239 CrossRef CAS.
- Y. Chang, K. Li, Y. Feng, Y. Cheng, M. Zhang, Z. Wang, Z. Wu and H. Zhang, Nanotoxicology, 2017, 11, 907–922 CrossRef CAS.
- Y. Feng, Y. Chang, X. Sun, N. Liu, Y. Cheng, Y. Feng, H. Zhang and X. Li, Toxicol. Sci., 2017, 156, 480–491 CAS.
- Z. Chu, S. Zhang, B. Zhang, C. Zhang, C.-Y. Fang, I. Rehor, P. Cigler, H.-C. Chang, G. Lin, R. Liu and Q. Li, Sci. Rep., 2014, 4, 4495 CrossRef.
- X. Shi, A. von dem Bussche, R. H. Hurt, A. B. Kane and H. Gao, Nat. Nanotechnol., 2011, 6, 714–719 CrossRef CAS.
- F. Perreault, R. Popovic and D. Dewez, Environ. Pollut., 2014, 185, 219 CrossRef CAS.
- A. F. Faria, F. Perreault and M. Elimelech, ACS Appl. Nano Mater., 2018, 1, 1164–1174 CrossRef CAS.
- J. Olabarrieta, S. Zorita, I. Peña, N. Rioja, O. Monzón, P. Benguria and L. Scifo, Appl. Catal., B, 2012, 123–124, 182–192 CrossRef CAS.
- A. A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- Y. Bi, B. Han, S. Zimmerman, F. Perreault, S. Sinha and P. Westerhoff, Water Res., 2018, 143, 77–86 CrossRef CAS.
- C. M. Park, K. H. Chu, N. Her, M. Jang, M. Baalousha, J. Heo and Y. Yoon, Sep. Purif. Rev., 2017, 46, 255–272 CrossRef CAS.
- Y. Liao, C.-H. Loh, M. Tian, R. Wang and A. G. Fane, Prog. Polym. Sci., 2018, 77, 69–94 CrossRef CAS.
- K. Flores, R. S. Turley, C. Valdes, Y. Ye, J. Cantu, J. A. Hernandez-Viezcas, J. G. Parsons and J. L. Gardea-Torresdey, Appl. Spectrosc. Rev., 2019, 1–26, DOI:10.1080/05704928.2019.1694937.
- C. D. Powell, A. J. Atkinson, Y. Ma, M. Marcos-Hernandez, D. Villagran, P. Westerhoff and M. S. Wong, J. Nanopart. Res., 2020, 22, 48 CrossRef CAS.
- D. G. Goodwin, A. S. Adeleye, L. Sung, K. T. Ho, R. M. Burgess and E. J. Petersen, Environ. Sci. Technol., 2018, 52, 4491–4513 CrossRef CAS.
- L. Reijnders, in Health and Environmental Safety of Nanomaterials, ed. J. Njuguna, K. Pielichowski and H. Zhu, Woodhead Publishing, 2014, pp. 222–250, DOI:10.1533/9780857096678.3.222.
- L. Reijnders, J. Cleaner Prod., 2006, 14, 124–133 CrossRef.
- A. Nagar and T. Pradeep, ACS Nano, 2020, 14, 6420–6435 CrossRef CAS.
- T. Zhang, G. V. Lowry, N. L. Capiro, J. Chen, W. Chen, Y. Chen, D. D. Dionysiou, D. W. Elliott, S. Ghoshal, T. Hofmann, H. Hsu-Kim, J. Hughes, C. Jiang, G. Jiang, C. Jing, M. Kavanaugh, Q. Li, S. Liu, J. Ma, B. Pan, T. Phenrat, X. Qu, X. Quan, N. Saleh, P. J. Vikesland, Q. Wang, P. Westerhoff, M. S. Wong, T. Xia, B. Xing, B. Yan, L. Zhang, D. Zhou and P. J. J. Alvarez, Environ. Sci.: Nano, 2019, 6, 1283–1302 RSC.
- M. D. Montaño, G. V. Lowry, F. von der Kammer, J. Blue and J. F. Ranville, Environ. Chem., 2014, 11, 351–366 CrossRef.
- Y. Bi and P. Westerhoff, Chemosphere, 2019, 223, 275–284 CrossRef CAS.
- C. Ursino, R. Castro-Muñoz, E. Drioli, L. Gzara, M. H. Albeirutty and A. Figoli, Membranes, 2018, 8, 18 CrossRef.
- Y. Wen, J. Yuan, X. Ma, S. Wang and Y. Liu, Environ. Chem. Lett., 2019, 17, 1539–1551 CrossRef CAS.
- M. Amde, J.-f. Liu, Z.-Q. Tan and D. Bekana, Environ. Pollut., 2017, 230, 250–267 CrossRef CAS.
- A. C. Mensch, R. T. Hernandez, J. E. Kuether, M. D. Torelli, Z. V. Feng, R. J. Hamers and J. A. Pedersen, Environ. Sci. Technol., 2017, 51, 11075–11084 CrossRef CAS.
- N. Gong, K. Shao, C. Che and Y. Sun, Mar. Pollut. Bull., 2019, 149, 110532 CrossRef CAS.
- H. Pérez-Hernández, F. Fernández-Luqueño, E. Huerta-Lwanga, J. Mendoza-Vega and D. Álvarez-Solís José, Land Degrad. Dev., 2020, 1–18 Search PubMed.
- W.-C. Hou, P. Westerhoff and J. D. Posner, Environ. Sci.: Processes Impacts, 2013, 15, 103–122 RSC.
- H. O'Neal Tugaoen, S. Garcia-Segura, K. Hristovski and P. Westerhoff, Sci. Total Environ., 2018, 613-614, 1331–1338 CrossRef.
- Y. Zhang, B. Wu, H. Xu, H. Liu, M. Wang, Y. He and B. Pan, Nanoimpact, 2016, 3–4, 22–39 CrossRef.
- K. D. Good, L. E. Bergman, S. S. Klara, M. E. Leitch and J. M. VanBriesen, J. – Am. Water Works Assoc., 2016, 108, E1–E17 CrossRef.
- W. Tang, Y. Su, Q. Li, S. Gao and J. K. Shang, Water Res., 2013, 47, 3624–3634 CrossRef CAS.
- G. Odling and N. Robertson, Catal. Sci. Technol., 2019, 9, 533–545 RSC.
- X. Bi, H. Ma and P. Westerhoff, Environ. Sci. Technol., 2018, 52, 13289–13297 CrossRef CAS.
- U. E. P. Agency, 2008.
- S. F. Hansen and A. Baun, Dose–Response, 2011, 10, 364–383 Search PubMed.
- O. Myakonkaya, Z. Hu, M. F. Nazar and J. Eastoe, Chem. – Eur. J., 2010, 16, 11784–11790 CrossRef CAS.
- A. Caballero-Guzman, T. Sun and B. Nowack, Waste Manage., 2015, 36, 33–43 CrossRef CAS.
- R. K. Gautam and M. C. Chattopadhyaya, in Nanomaterials for Wastewater Remediation, ed. R. K. Gautam and M. C. Chattopadhyaya, Butterworth-Heinemann, Boston, 2016, pp. 297–309, DOI:10.1016/B978-0-12-804609-8.00012-1.
- S. Lata and S. R. Samadder, J. Environ. Manage., 2016, 166, 387–406 CrossRef CAS.
- S. Lata, P. K. Singh and S. R. Samadder, Int. J. Environ. Sci. Technol., 2015, 12, 1461–1478 CrossRef CAS.
- A. S. Fajardo, P. Westerhoff, C. M. Sanchez-Sanchez and S. Garcia-Segura, Appl. Catal., B, 2020, 119465, DOI:10.1016/j.apcatb.2020.119465.
- F. Schüth, M. D. Ward and J. M. Buriak, Chem. Mater., 2018, 30(11), 3599–3600 CrossRef.
- S. L. Scott, ACS Catal., 2018, 8, 8597–8599 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |