 Open Access Article
Open Access ArticleEnvironmental and health risks of nanorobots: an early review
Rickard
Arvidsson
 *a and
Steffen Foss
Hansen
*a and
Steffen Foss
Hansen
 b
b
aEnvironmental Systems Analysis, Chalmers University of Technology, Vera Sandbergs Allé 8, SE 412 96 Gothenburg, Sweden. E-mail: rickard.arvidsson@chalmers.se
bDepartment of Environmental Engineering, Technical University of Denmark, DTU Building 115, 2800 Kgs. Lyngby, Denmark
First published on 27th August 2020
Abstract
Nanorobots for biomedical applications have experienced extensive research and rapid development during the last decade, up to a point where they can now deliver cargos to designated sites in organisms under laboratory conditions. Despite this development, research into nanorobot risks and discussions about potential regulation of nanorobots have so far been limited. This early review of risks related to nanorobots first provides a brief overview of the current state of the technology. The overview outlines three main types of nanorobots: helices, nanorods and DNA nanorobots. Several different designs exist for each of these categories. Second, early indications of potential hazards are reviewed and discussed. Two potential hazards are highlighted: (i) the use of hazardous materials and UV light in nanorobots, and (ii) the loss of propulsion/targeting control. Third, how current regulations are adapted to nanorobots is discussed. Current regulations for medical devices are clearly not adapted to nanorobots and it is even unclear which specific regulations might be applicable. In order to make the most of the use of nanorobots, we recommend they should be subject to broad, risk-related studies as well as dialogues with stakeholders and the public about the definition, purpose and controllability of nanorobot applications. A list of ten priority questions to be addressed in future risk-related studies of nanorobots is provided.
Environmental significanceSo far, discussions and research about environmental and health risks related to nanomaterials have focused on so-called passive nanomaterials, such as metal-oxide nanoparticles and carbon nanotubes. Much less attention has been given to so-called active nanomaterials. This includes nanorobots, which are individual nano-sized devices able to perform designated tasks, so far given limited attention regarding their potential future risks. This paper provides a review of early indications of potential environmental and health hazards related to nanorobots, investigates the applicability of existing regulatory frameworks, and provides recommendations on engaging expert stakeholders and the public. |
1. Introduction
In a foundational paper, Roco1 distinguished between a number of generations of nanotechnology, the two earliest being passive and active nanostructures. Tour2 used a similar categorization and defined passive nanotechnology as when “the nano part does nothing particularly elaborate”. Active nanotechnology was defined as when “the nano entity does something elaborate such as absorbing a photon and releasing an electron, thereby driving a device, or moving in a specific and definable fashion across a surface”. Although the exact distinction between the passive and active nanotechnology can be tricky, conventional nanoparticles and nanotubes currently used in existing nanoproducts3,4 generally belong to the category of passive nanomaterials. This is where most efforts in terms of risk-related research have occurred during the 2000s, in particular for a limited set of nanomaterials, including silver nanoparticles, titanium dioxide nanoparticles, silica nanoparticles, cerium dioxide nanoparticles, zinc oxide nanoparticles, iron nanoparticles, quantum dots, fullerenes, carbon nanotubes and graphene.5–9 Much less attention has been given to active nanomaterials, probably because of their limited production and use in society. However, one type of active nanomaterial is clearly on the march. Often referred to as science fiction, nanorobots are currently being extensively researched and developed, especially for medical applications where there is an effort to merge nanotechnology with pharmaceuticals.10,11 The most frequent application mentioned is drug delivery, in particular for site-specific cancer treatment through the delivery of tumor-killing drugs.12 Other envisioned areas of applications beside medicine include environmental monitoring and water remediation.13Despite this development, research on risks related to nanorobots have so far been limited. History shows several examples of how the introduction of new technology that offered great benefits into society later turned out to also cause notable environmental and health impacts.14,15 Regulation was generally imposed only at a late stage, long after the first signs of negative side effects appeared. A telling example from medicine is ionizing radiation, discovered around 1900, which brought important diagnostic and therapeutic benefits through X-rays and radioisotopes. Already at that time, there were early reports that exposure to radiation could cause severe damage, but these were largely overlooked. X-rays were even used for fitting shoes and removing unwanted hair in beauty shops in the 1930s and 1940s. Such misuses were possible because of the lack of regulation ensuring the safety of ionizing radiation. Only very slowly have such regulations evolved as the knowledge about radiation risks grew bigger, eventually enabling the beneficial use of radiation while keeping risks reasonably low.16 Another important medical discovery – perhaps the most important of all times – is antimicrobials, which have saved millions of lives by killing malign bacteria. In parallel with such predominantly beneficial uses, antimicrobials have also been used as growth promotor in industrialized animal husbandry. Animals fed with sub-therapeutic amounts of antimicrobials for long time periods showed enhanced properties, such as increased growth rate, food conversion, egg production and milk yield. Much a consequence of such extensive use, antimicrobial resistance began to be reported as early as the 1940s, threatening the use of antimicrobials in medicine. Bans of antimicrobials as growth promotors did not appear until the mid 1980s.17
Considering the examples of late lessons from early warnings for a number of previous medical technologies, it is important to address risks of such emerging technologies at an early stage of development, which is where we find nanorobots today. In this paper, we present an early review of environmental and health risks related to nanorobots. First, we provide a brief overview of the progress in nanorobot developments, including which types of nanorobots are under development and in which applications they are likely to first emerge. Second, we review existing knowledge about nanorobots in the context of environmental and health risks, identifying a set of potential hazards to be further studied and scrutinized. Third, we discuss how nanorobots, if proven risky to the environment or human health, might become regulated. Finally, recommendations for the further enquiry into potential risks of nanorobots are provided.
A precise definition of the term ‘nanorobot’ is currently lacking. It is here tentatively defined as an individual nano-sized device able to perform a designated task. The nanometer size (referring approximately to the 1–100 nm size range) follows naturally from the term nanorobot, while the ability to perform tasks is central to any (also a macro-sized) robot and sets nanorobots apart from conventional, passive nanomaterials. This definition is similar to the one provided by Gao and Wang13 for ‘nanomachine’: a nanoscale device that performs a task. The main difference is that our definition specifies individual devices themselves as being nanorobots, rather than devices consisting of entire swarms of nano-sized robots. It should be noted that this definition excludes a range of micro-sized robots. A specific example is MagnetoSperm, aimed at imitating a sperm cell in movement.18 This device has a thickness, length and width of about 5.2, 320 and 42 μm, respectively, which is here considered too large for a nanorobot. Another excluded example is the artificial bacterial flagellum described by Qiu et al.,19 which is about 1.2 μm wide and 16 μm long. Also excluded are metal–organic-framework-based biomedical microrobots, called MOFBOTS, which are at least a few micrometers in diameter.20 Naturally, the delimitation to the nanoscale also excludes larger micro-sized robots, such as the 400 × 800 μm tumbling micromotor.21 The exclusion of such micro-sized devises in this review is done to enable a focus on nano-sized devises, which might potentially exhibit unique hazardous properties due to their small size.22 However, we have been rather inclusive in our selection, including for example robots of some hundred nanometers in at least one dimension, even though most contemporary definitions of nanomaterials have an upper size limit of 100 nm.23
2. Technological development of nanorobots
The technological development of both micro- and nano-sized robots stretches from Richard Feynman's famous talk “There's Plenty of Room at the Bottom” in 1959 until now, intertwined with the development of other technologies, such as magnetic control and nanostructure synthesis.24 The first nano-sized robots were reported in 2005, including the nanocar made mainly by fullerene molecules.25 During the 2010s, three main designs of nanorobots have emerged: helices (also called nanoswimmers), nanorods (also called nanoswimmers, nanomotors or, if longer, nanowires) and DNA nanorobots. These will be described in more detailed in Sections 2.1–2.3 and a non-exhaustive list of nanorobot examples is presented in Table 1. These three categories generally fulfill the tentative definition of nanorobot in section 1 by being both nano-sized and able to perform some tasks. However, sometimes the task to be performed has not yet been entirely realized for specific nanorobot designs, but there is generally an ambition to execute successful drug delivery or some similar task, like biological imaging. In general, it seems that the nanorods and DNA nanorobots have reached the furthest towards actually accomplishing drug delivery and similar tasks.| Nanorobot | Materials | Nanometer size | Propulsion/navigation | Reported speed (μm s−1) | Reference |
|---|---|---|---|---|---|
| a Note that since DNA nanorobots are well-defined molecules, their size in terms of nm is not always provided. Also, their speed is not reported to the same extent as for helices and nanorods. | |||||
| Helices | |||||
| Helical propeller | Glass, cobalt | 200–300 nm wide | Magnetism | 40 | 26 |
| Artificial bacterial flagellum | Indium, gallium, arsenic, chromium, nickel, gold | 200 nm wide | Magnetism | 1.2 (average) | 27 |
| Repolymerized flagella-bound nanoparticles | Magnetic materials, bacterial flagella | Nanoparticles: 40–400 nm | Magnetism | 2.5 (max) | 29 |
| Flagella: 20 nm wide | |||||
| Nanorods | |||||
| Drug-delivering nanorod | Gold, nickel, polymer | 250 nm wide | Ultrasound/magnetism | 50 (max) | 31 |
| Antibacterial nanorod | Gold, lysozyme | <300 nm wide | Ultrasound/bioreceptors | n.a. | 32 |
| Coated nanowire | Gold, graphene oxide, DNA | 200 nm wide | Ultrasound/bioreceptors | n.a. | 33 |
| Enzyme-bound nanowire | Gold, nickel, polymer, asparaginase | ca. 500 nm wide | Ultrasound/magnetism | 32 (average) | 34 |
| Light-driven nanorod | Gold, iron oxide | 300 nm wide | Visible light/magnetism | 33 | 37 |
| Light-driven nanowire | Silicon, platinum | 500–1000 wide | Visible or near-infrared light/morphology | 5–35 | 38 |
| Match-like nanorod | Silica, silver, silver chloride | <210 nm wide | UV light, silver chloride decomposition | 4–14 | 39 |
| Nanorod with flagellum tail | Gold, nickel, polymer | 300–600 nm wide | Acoustic waves/magnetism | 60 (max) | 46 |
| V-Shaped nanorod | Platinum | 700 nm wide | Hydrogen peroxide decomposition | 2–7 | 41 |
| Nanofish | Gold, nickel, silver | 200 nm wide | Magnetism | 31 (max) | 42 |
| Two-armed nanoswimmer | Gold, nickel, silver | 200 nm wide | Magnetism | 39 (max) | 43 |
| Machine-learning optimized nanoparticles | Polystyrene, platinum | 398 nm | Hydrogen peroxide decomposition | n.a. | 44 |
| 2D nanoswimmer | Barium ferrite, platinum | 4.6 nm wide | Hydrogen peroxide decomposition/magnetism | n.a. | 45 |
| DNA nanorobots | |||||
| DNA walker | DNA | n.a. | Bioreceptors | n.a. | 49 |
| Molecular spiders | Protein, DNA | n.a. | Bioreceptors | n.a. | 50 |
| I-switch | DNA | n.a. | Diffusion/bioreceptors | n.a. | 51 |
| Hexagonal barrel cage | DNA | 35 × 35 × 45 nm | Diffusion/bioreceptors | n.a. | 52 |
| Tetrahedron DNA nanoparticle | DNA | 8×10 nm | Diffusion/bioreceptors | n.a. | 53 |
| Icosahedral DNA nanocapsules | DNA | n.a. | Diffusion/bioreceptors | n.a. | 55 |
| Nanosheet/tubular nanorobot | DNA | 90 × 50 × 2 nm | Diffusion/bioreceptors | n.a. | 56 |
2.1 Helices
A number of robots with screw-like helix tails for movement have been developed, often resembling bacterial flagella or other biological entities. Most of them are rather to be categorized as micro-sized robots, including the above-mentioned MagnetoSperm and the MOFBOTS. However, there exist examples of helix-like robots that are approaching the nanometer size range. One example is the helical cobalt-covered glass propeller developed by Ghosh and Fischer,26 which is 200–300 nm wide and 1–2 μm long (Fig. 1a). The propeller is meant to mimic a bacterial flagellum in terms of swimming behavior. Thanks to the magnetic cobalt layer, the propeller can be moved and navigated through magnetic fields (both backward and forward), reaching speeds of about 40 μm s−1. The navigation control in a water-based solution is exemplified by ‘writing’ micrometer-scale letters and symbols, such as ‘R’, ‘@’ and ‘H’, using the propeller's trajectory. Several propellers can be controlled simultaneously in this way.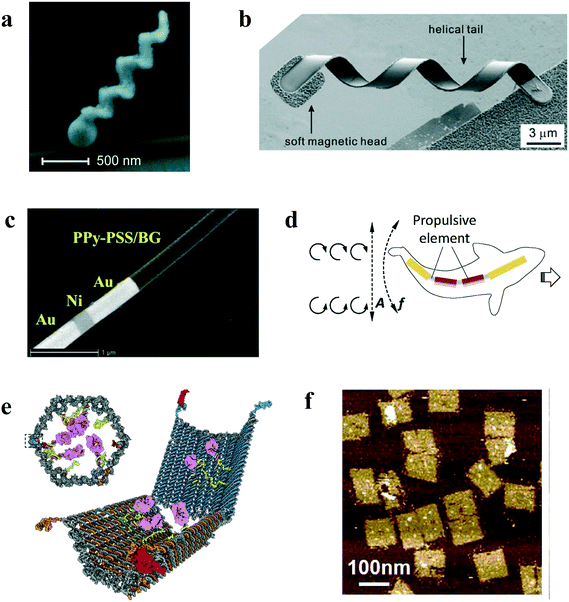 | ||
| Fig. 1 Examples of nanorobots. (a) 200–300 nm glass propeller. Reprinted (adapted) with permission from Ghosh and Fischer.26 Copyright (2009) American Chemical Society. (b) 200 nm thick artificial bacterial flagellum made by a chromium–nickel–gold head and an indium gallium arsenide-gallium arsenide-chromium tail. Reprinted (adapted) with permission from Zhang et al.57 Copyright (2009) American Chemical Society. (c) Gold (Au)–nickel (Ni)–gold (Au)–polymer (PPyPSS) nanorod loaded with an antiseptic drug (brilliant green, BG). Reprinted (adapted) with permission from Garcia-Gradilla et al.31 Copyright (2013) American Chemical Society. (d) Fish-like 200 nm wide nanorod consisting of gold–nickel–nickel–gold segments with three flexible silver hinges linking the segments. Reprinted (adapted) with permission from Li et al.42 Copyright (2016) Wiley-VCH Verlag GmbH & Co. (e) Hexagonal cage-like DNA robot able to transport payloads (in pink). Reprinted (adapted) with permission from Douglas et al.52 Copyright (2012) Science. (f) DNA nanorobots consisting of 90 nm × 50 nm × 2 nm sheets able to fold into tubular drug carriers. Reprinted (adapted) with permission from Li et al.56 Copyright (2018) Nature Publishing Group. | ||
Another helix design that might be considered a nanorobot is the artificial bacterial flagellum by Zhang et al.,27 which can be 200 nm thick, 2.5 μm wide and 2.5 μm long (Fig. 1b). It consists of a magnetic chromium–nickel–gold head attached to an indium gallium arsenide–gallium arsenide–chromium tail. It is the tail that resembles a bacterial flagellum (both in size and shape) and the head enables magnetic propulsion and control. The artificial bacterial flagellum can swim at an average speed of 1.2 μm s−1 under magnetic fields thanks to its magnetic head. Similar to the helical glass propeller, the artificial bacterial flagellum can move both forward, backward and turn in water-based media depending on the shape of the helix and the direction of the magnetic field. It was furthermore shown that the artificial bacterial flagellum can be controlled into pushing and rotating polystyrene microparticles.
In addition to these two examples of nanorobot helices, several similar helix-like nanorobots have been developed, where most are micro-sized but some dwell somewhere in the borderland between the nano- and micro-size ranges.28 There are also attempts to use actual bacterial flagella by de-polymerizing them into flagellin proteins using heating, then repolymerizing them back into flagella and attaching them to magnetic particles 40–400 nm in size.29 Multiple flagella can be attached to the same nanoparticle, but a functional group that can bind to the nanoparticles must be introduced during the repolymerization. The repolymerized flagella have outer diameters of about 20 nm, lengths of 5–10 μm and can obtain different shapes (normal, curly and coiled) depending on if ethylene glycol or dimethyl sulfoxide is added. The idea is that reconfiguring its geometry might be beneficial when navigating through heterogenous biological environments (such as the human body) compared to movement in pure water. By applying magnetic fields, the nanoparticle–flagella clusters can swim at velocities up to 2.5 μm s−1. An advantage of this helical nanorobot design is that the specific nanoparticles can be changed for different purposes while maintaining the repolymerized flagella for movement. However, for both propulsion and navigation purposes, the nanoparticles must be magnetic.
2.2 Nanorods
The nanorods typically consist of cylindrical rods with different metal segments,30 although different shapes are also used for the same purpose. A particularly notable example from a medical point of view is the 250 nm wide and 1800 nm long rod with gold-nickel-gold segments developed by Garcia-Gradilla et al.31 These nanorods move due to ultrasound waves and can move in serum at about 50 μm s−1 and, albeit at lower speed, in saliva (about 10 μm s−1). Thanks to the magnetic properties of nickel, such nanorods can be steered along predetermined trajectories. For example, the developers made it ‘write’ the letters ‘U’, ‘C’, ‘S’ and ‘D’ with its trajectory. The nanorod can be functionalized, making it carry drug cargoes. An important potential application of the gold–nickel–gold nanorod was shown by functionalizing it with a polypyrrole–polystyrene sulfonate segment. This organic segment can bind to the antiseptic drug brilliant green (Fig. 1c) and deliver this drug to designated destinations. The drug can then become released due to changes in pH.Another potential medical use of nanorods was demonstrated by Kiristi et al.32 They used ultrasound-powered porous gold nanorods less than 300 nm wide and functionalized them with the bactericidal substance lysozyme, which can kill both Gram-negative and Gram-positive bacteria. They showed that the lysozyme-functionalized gold nanorods could kill up to about 80% of the Gram-positive bacteria M. lysodeikticus in a sample within a few minutes. The number of nanorods influenced the death rate, with about 5000 being required for a rate of 80%. Glycosidic bonds in the cell walls of bacteria act as bioreceptors for the lysozyme. Although there was no navigation control for this nanorobot, the movement in itself greatly increased the lysozyme-bacteria interactions and thus also the bacterial killing capacity versus pure lysozyme only.
Several nanorod designs aim at cancer detection and treatment. MicroRNAs (miRNA) are small RNA strands, some of which can be associated with diseases such as cancer and diabetes. In the work by Esteban-Fernández de Ávila et al.,33 graphene oxide-covered gold nanowires 200 nm wide were used to detect miRNA. The ultrasound-propelled nanowires can penetrate cancer cells where DNA strands bound to the graphene oxide surface detach and bind to the miRNA. This causes the graphene oxide–gold nanowires to send out a fluorescence signal, thereby detecting the presence of a specific miRNA and thus possibly also a cancer cell.
Moving beyond detecting cancer and towards treating it, Uygun et al.34 used gold–nickel–gold–polymer nanowires as an effective anti-cancer agent. These nanowires were propelled by ultrasound at an average speed of 32 μm s−1 in human serum and magnetically guided thanks to the nickel. They furthermore had asparaginase enzymes bound to the polymer segment, which can deplete cancer cells of the essential amino acid asparagine and thereby inhibit their growth. A 92% inhibition of lymphoma cancer cells was observed, compared to only 17% inhibition for free, non-nanowire-bound asparagine.
While the chemical (e.g. hydrogen decomposition), magnetic, acoustic (e.g. ultrasound) and biological (e.g. attaching bacterial flagella) have conventionally been the main mechanisms of propulsion and navigation for nanorods,35 recent studies have also reported using light for nanorod propulsion.36 An example is 300 nm wide gold-iron oxide nanorods, which can be powered by visible light in diluted hydrogen peroxide, reaching speeds of 33 μm s−1 due to hydrogen peroxide decomposition at the iron oxide end.37 Due to the superparamagnetic iron, magnets can be used to steer the rods in designated trajectories. Similarly, platinum nanoparticle-coated silicon nanowires in a quinone solution can achieve propulsion if irradiated with light at the visible or near-infrared spectrum.38 Speeds of about 5–35 μm s−1 could then be achieved depending on the power intensity of the light. Changing the end-surface morphology of the nanowire can result in different propulsion patterns, from linear to circular. A final example of light-propelled nanorods is the <210 nm wide match-like silver–silica nanorod with a silver chloride tail.39 The nanorods were dispersed in water and irradiated with UV light, inducing a photocatalytic decomposition of the silver chloride tail. Depending on the length of the match-like nanorod, speeds of 4–14 μm s−1 can be achieved.
In addition to these examples of nanorods, often with intended or even realized medical applications, several other nanorod designs exist, often being about 200–300 nm wide and including the elements nickel, gold and/or platinum as segments.40 However, nanorobots with shapes other than purely cylindrical but otherwise similar to nanorods have also been developed. One example is a 700 nm wide and 4000–4500 nm long V-shaped platinum nanorod.41 It can move in a hydrogen peroxide medium through the decomposition of hydrogen peroxide occurring predominantly at the non-pointy end of the V shape:
| H2O2 → H2 + O2 |
Adopting a structured approach to nanorobot design, Zeng et al.44 used machine learning to design optimal micro-/nanorobots for the purpose of catalytic water cleaning, assuming hydrogen peroxide decomposition propulsion. Different aspect ratios, shapes, catalytic materials and other parameters were tested. The results suggested a spherical 398 nm platinum-coated polystyrene nanoparticle, which was then synthesized and successfully used for decomposing methyl blue dyes. In addition, two-dimensional (2D) nanoswimmers were designed by the same group, consisting of 4.6 nm thick platinum-coated barium ferrite platelets.45 Again, hydrogen peroxide decomposition was the propulsion mechanism, and steering was enabled by magnetic control of the iron in the barium ferrite. The 2D nanoswimmers showed excellent performance in catalytically removing stains on cloth. These two non-cylindrical nanorobots are thus clearly intended for remediation and cleaning rather than for medicine.
Finally, constituting some sort of a hybrid between nanorods and helices is a nanorobot consisting of a 300–600 nm wide rod with nickel–gold segments and an attached polypyrrole tail.46 When exposed to acoustic waves, the polypyrrole tail begins to oscillate and propels the nanorod forward at speeds up to 60 μm s−1. Although this nanorobot does not consist of a helical structure, and is thus classified as a nanorod, the polypyrrole tail acts much like an artificial flagellum in a similar way as the tails of some helical nanorobots (see section 2.1).
2.3 DNA nanorobots
DNA nanorobots consist of deoxyribonucleic acid molecules, thus using DNA as construction material for nano-sized devices.11,47 Sometimes, they are based on DNA origami, where DNA molecules are folded to create patterns and shapes.48 An example of such a nanorobot is the DNA walker developed by Gu et al.,49 which consists of a trigonal arrangement of double helices, resembling a symmetrical three-legged wheel with ‘feet’ that act like ligands. These DNA ‘feet’ can bind to a larger DNA origami sheet ‘landscape’, across which the DNA walker can ‘walk’ by rotating 120° and binding to a new bioreceptor in each step. It can deliver cargo across the DNA sheet landscape to a designated site, illustrated by the DNA walker delivering several 5 nm gold nanoparticles in the study. Other structures able to walk across a DNA origami ‘landscape’ are the molecular spiders, which consist of protein bodies with three DNA ‘legs’ and a fourth capture ‘leg’, specifically made by so-called DNA enzymes.50 Due to the detailed design of the DNA origami ‘landscape’, the molecular spiders can ‘walk’ across the landscape as the legs dissociate from one site and reattach to a new site. Since the spiders have three legs, complete dissociation is hindered as dissociated legs are held in place due to the binding of the two other legs and quickly reattach. The spiders can be made to follow pre-designed one-dimensional tracks in the ‘landscape’ and even execute commands like ‘turn’ and ‘stop’. The capture leg is used to capture the spiders from solution and place them at the starting position. Although the DNA walkers and molecular spiders have impressive programmability, it should be noted that their controlled movement and navigation seems to be limited to pre-designed DNA ‘landscapes’.Moving away from such landscapes, DNA nanorobots have also been used in the in vivo environments of living organism. An example of such a device is a DNA nanorobot called the I-switch, which consists of three DNA strands.51 The I-switch can change shape depending on pH and the two shapes emit light of different wavelength when the nanorobot is tagged with a fluorescent molecule. This property can be used for tempo-spatial mapping of pH changes in living organisms, as shown for both wild type and mutant nematodes (C. elegans). Florescence-tagged I-switches were translocated to certain nematode cells and taken up through receptor-mediated endocytosis so that pH changes in the cells could be tempo-spatially mapped. Since many phenomena in cells, including neurodegeneration and spermatogenesis, are modulated based on changes in pH in the range where the I-switch is sensitive to changing shape, there might be several mapping applications for this DNA nanorobot.
An example of a DNA nanorobot with potential medical implications is an origami-based hexagonal barrel-shaped cage-like robot with dimensions of 35 nm × 35 nm × 45 nm (Fig. 1e).52 The cage can be loaded with various materials, such as gold nanoparticles and in particular biologically active payloads, such as antibody fragments. The cage has ‘locks’ that can be ‘unlocked’ by binding to protein receptor ‘keys’. This causes the DNA nanorobot to undergo a drastic reconfiguration that releases the payloads. The release of payload was shown in human cells, such as leukaemia cells and lymphocytes. Similar delivery of therapeutics in vivo was achieved by tetrahedral DNA nanoparticles.53 The tetrahedral is made by six self-assembling DNA strands, to which six strands of a particular type of RNA, called siRNA, were bound. The siRNA can be used to silence target genes in tumors. The application was demonstrated by delivering the tetrahedral-bound siRNA to target tumors in nude mice through injection in the tail vein. Another similar DNA nanorobot is the cage-like icosahedral DNA nanocapsule which can be used to encapsulate biomacromolecules.54 The nanocapsule can target specific cells and deliver the molecules to the cytosol.55 The release of the cargo is controlled by photoirridiation. The application was illustrated in vivo by delivering a neurosteroid, which can promote neurogenesis and neuron survival, in the nematode C. elegans. A final example of a DNA nanorobot is a 90 nm × 50 nm × 2 nm DNA sheet (Fig. 1f) that can fold into a tubular nanorobot.56 The sheets can bind certain cargos while open and then and fold into a tube, thus encapsulating the cargo. Furthermore, the nanorobot can be functionalized with DNA ligands on the outside of the tube, which can bind to a receptor protein specifically expressed in tumor cells on the inside of blood vessels and unfold. This way, the tubular nanorobot was intravenously injected and able to transport thrombin to target blood vessel tumors in mice, where the nanorobot unfolded to release its cargo. Since thrombin is a molecule able to kill cancer cells in blood vessels, this leads to tumor cell necrosis and tumor growth inhibition.
3. Potential nanorobot hazards
Several of the nanorobot designs described in section 2 offer the promise of significant health-related benefits, such as improved cancer therapy. However, considering what can been learned from previous late lessons with promising technologies offering great societal benefits, such as the X-rays and antimicrobials discussed in section 1, risks can outweigh the benefits for some applications of a technology. So far, only a few references to environmental and health risks can be found in the literature about nanorobots. Kostarelos58 wrote briefly about the safety of nanorobots, commenting that nanorobots “will need to be toxicologically inert, degradable or expelled from the body”. One might note that this mainly refers to human toxicity and not subsequent environmental effects that might occur after the nanorobots have become expelled from bodies. Gao and Wang13 wrote about the use of nanorobots (mainly nanorods) for environmental sensing, monitoring and remediation. They comment that “the potential toxicity of micro/nano-scale motors needs to be evaluated to prevent potential adverse environmental impacts”. However, they do not provide any specific recommendations on how that could be accomplished, despite envisioning wide-spread use of nanorobots in the environment. Surana et al.59 did a study on DNA nanorobots and their compatibility with the immune system of higher organisms. They comment that foreign, ‘non-self’ DNA from other organisms can be harmful and therefore immunogenic, since they trigger the immune system: “Even though DNA is a natural biopolymer, when present at the wrong place at the wrong time it can elicit a strong inflammatory reaction”. Therefore, they asserted that it is important to consider the various cellular and systemic responses that such DNA architectures might elicit, which are likely to be specie-specific. Such considerations have a dual purpose: it is both to keep the organism in questions safe from the DNA nanorobot but also to ensure the proper medical function of the DNA nanorobot in cells. Again, the focus is on human toxicological responses rather than on environmental toxicity.Some consideration of safety can be found in studies describing DNA nanorobots. The developers of the I-switch noted that the nematodes injected where “viable and healthy”, indicating that the I-switch is non-toxic to nematodes given the applied concentrations.51 In the study by Li et al.56 about the DNA nanosheet/tubular nanorobot, an assessment of the safety of the nanorobot was conducted. It was noted that the nanorobots did not elicit any thrombi or increased blood coagulation in non-tumor-bearing mice at relevant concentrations. In addition, no immunological or cytotoxic responses were shown. They also did not cause any thrombi or blood coagulation in Bama miniature pigs, which is an animal similar to humans in anatomy and physiology. Although these results provide an early indication that such nanorobots might be safe, they are also limited to human toxicity impacts.
To these considerations of nanorobot risks in the previous literature, we might add a number of potential hazards. That foreign DNA can elicit immunologic and inflammatory responses has been noted above. Other materials used in contemporary nanorobot designs (see e.g.Table 1) might also potentially have hazardous properties that warrant further investigations. For example, the nickel used in order to magnetically control the propulsion of several helices and nanorods is known to be allergenic, carcinogenic (though not in pure metallic form), toxic at high doses and in certain forms, as well as teratogenic at high doses.60 Allergenic reactions have already been seen for people working with nano-sized nickel powder.61 The silver sometimes used for making hinges in designs with several connected nanorods is also known to be toxic to organisms in the environment – both in nano-form and when dissolved into silver ions.62,63 However, silver is not toxic to humans. High silver intake results in discoloration of the skin and internal organs (argyria and argyrosis, respectively), both which do not seem to bring any negative health effects.62 In addition, the UV light used for propulsion in some nanorod designs is known to be able to cause skin damage and, in the worst case, skin cancer.
Foreign DNA, nickel, silver and UV light are all established hazards. Whether their use in specific nanorobot applications constitute risks remains to be investigated. Novel hazards associated with nanorobots might be related to the control of nanorobot propulsion and navigation – whether by chemical propulsion, magnetic fields, sound waves, bioreceptor binding and/or light – potentially making the nanorobots travel to places in the human body and elsewhere where they are not supposed to. Should loss of propulsion control or targeting of an erroneous site occur, hazardous drugs might be delivered to healthy cells. An erroneous targeting might cause high concentrations locally, so that a small number of nanorobots potentially causes much harm.
Besides potential hazards, an additional aspect of risk is whether organisms will become exposed to the potential hazard. Whereas nanoparticles have typically been perceived as extrasomatic risks, released to the environment and subsequently taken up by organisms,64 the mainly medical applications envisioned for nanorobots mean that exposure and uptake to humans might be inherent in the use of nanorobots rather than unintentional. Environmental exposure might then potentially occur subsequent to excretion or discarding of the nanorobots. In addition, the use of nanorobots for environmental remediation also seems to imply a direct exposure to organisms in the environment,13 in that sense being similar to pesticides applied to agricultural land. The probability of nanorobot exposure to relevant organisms might thus be high for these two promising applications.
4. Regulating nanorobots
As in the early development of X-rays and antimicrobials, there is currently no regulation targeting the use of nanorobots specifically. Meeting the current approval requirements for medical products and devices is arguably one of most lengthy, thorough and expensive regulatory processes around, with various phases of clinical testing, safety and benefit assessment. However, regulations in the EU and elsewhere have still been criticized for being insufficient when it comes to more complex drugs.65 It even remains unclear whether nanorobots are to be consider a medical device or a medicinal product, for which different sets of regulations apply in the EU – the Regulation of Medical Devices and Medicinal Products Directive, respectively. Currently, the ‘mechanism of action’ is key to decide whether a product should be regulated as one or the other. These can be pharmacological, immunological or metabolic means, which is why the categorization of nanorobots would depend on if they use complex mechanisms of action combining mechanical, chemical, pharmacological and immunological properties, as well as if they have both diagnostic and therapeutic functions.66One of few regulations that includes specific considerations for nanomaterials is the EU Regulation on Medical Devices (Regulation (EU) 2017/745),67 where nanomaterials are defined according to the European Commission's recommendation: “a natural, incidental or manufactured material containing particles in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1–100 nm”.68 This definition, currently under revision, was clearly developed with nanoparticles in mind (considering the reference to particle size distribution) and not with a focus on active nanomaterials. An annex of the Medical Devises Regulation calls for “special attention” to be given to nanomaterials without further specification, and again focuses on nanoparticles: “devices shall be designed and manufactured in such a way as to reduce as far as possible the risks linked to the size and the properties of particles which are or can be released into the patient's or user's body, unless they come into contact with intact skin only.” If there is a high or medium potential for internal exposure, devices incorporating or consisting of nanomaterial should also be classified as ‘Class III’. This constitutes the highest risk classification in the EU and is reserved for the most critical devices, for which explicit prior authorization with regard to conformity with current standards and rules is required for them to be placed on the market. However, these rules for classification of medical devices and conformity assessment procedures were adopted in 1993 – a decade before the more widespread use of nanotechnology. Current regulations thus seldom include nanomaterials, and when they do, the focus is on nanoparticles. Already more than ten years ago, when nanorobots were hardly developed, the European Medicines Agency69 argued that appropriate expertise will need to be mobilized for the evaluation of the quality, safety, efficacy and risk management of novel applications of nanotechnology, such as nanostructures allowing transport across biological barriers, remote control of nanoprobes, and multifunctional chemical structures for drug delivery and targeting of disease. Back then, such novel applications of nanotechnology did not exist, but current developments of nanorobots are increasingly making such applications possible.
5. Recommendations
The main applications envisioned for nanorobots are such that they might potentially become administrated directly to the human body or the environment (section 3). Such applications with potential for exposure, akin to those of pharmaceutical and pesticide applications, warrant consideration into the risks related to nanorobots. We identified two main potential hazards related to nanorobots at this early stage: (i) the use of conventional hazards, such as hazardous materials and UV light, as well as (ii) the loss of propulsion and navigation control (section 3). Furthermore, we note a lack of nano-specific regulation, making it uncertain whether current regulation will be able to identify and regulate nanorobot hazards at an early stage of development (section 4). In order to address this situation, we provide three recommendations for future research and action. The recommendations are based on three lessons learned from failing to respond to early warnings in the past,14 which seem particularly relevant to the discussion about nanorobot risks: (i) acknowledge and respond to ignorance, uncertainty and risk in technology appraisal, (ii) ensure use of ‘lay’ knowledge, as well as specialist expertise, and (iii) systematically scrutinize claimed benefits and risks. Following the three recommendations would allow for making the most of nanorobots while avoiding that their use later turns out to cause harm to the environment and/or human health.The first recommendation, based on lessons (i) and (iii), is to conduct studies of the environmental and human health risks of different nanorobot designs before they are in widespread use, moving away from the view that nanoparticles are the only aspects of nanotechnology for which risk assessment and regulation are needed. With nanoparticles, discussions about their risks started early in the development of nanotechnology. We have since then learned how important it is that sufficient funding of risk-related research is provided and that studies are initiated early on in order to map different risks. Although it is currently unknown whether nanorobots constitute a potential risk to human health and the environment, it is possible to start processes where this can be investigated. At the moment, risk assessments of medical devices and medicinal products are largely focused on establishing whether a drug or device is safe to use under a specific set of conditions using pre-defined test methods, which means that novel risks will probably come as surprises under the current regulatory framework. It would therefore be wise to conduct broader studies of the potential risks of nanorobots, considering different potential hazards and other risk-related aspects. Table 2 provides a non-exhaustive list of ten questions which we recommend be addressed in such risk-related studies of nanorobots, including the consideration of the potential hazards outlined in section 3. Among these, question 3 about the future production and use of nanorobots is fundamental. Currently, nanorobots have only begun to be tested at laboratory scale for applications such as medicine and environmental remediation. Their future production and use will depend on the technical performance of nanorobots in these applications. In medical applications specifically, an important prerequisite for successful use is that the nanorobots can evade the immune systems of the organisms.70 For DNA nanorobots in particular, this can be challenging since foreign DNA is immunogenic,59 although conducted tests for the tetrahedron DNA nanoparticles53 and nanosheet/tubular DNA nanorobot56 did not indicate any immunogenic response in mice. Another prerequisite for future nanorobot use that convenient, large-scale fabrication methods are achieved. Without a high-enough production and use of nanorobots, any associated risks will remain low. However, it should be noted that all technologies are sparsely used in the earliest beginning of their development, in their so-called embryonic phase.71 Therefore, an initially low production and use should not be taken as evidence of future low production and use. Detailed monitoring of production volumes, along with technology forecasting and scenario analyses, are recommended for addressing question 3.
| 1. What is the toxicity of nanorobots and their constituents to humans and other organisms? |
| 2. Are nanorobots more hazardous than previous generations of passive nanomaterials? |
| 3. How many nanorobots are expected to be produced and used in the future? |
| 4. What is the likely future exposure of nanorobots to humans and organisms in the environment? |
| 5. In which ways can the propulsion and navigation of different nanorobots be obscured? |
| 6. How can existing regulations be adapted to cover potential risks of active nanomaterials such as nanorobots? |
| 7. How can nanorobots be designed to be safe? |
| 8. How can the benefits of nanorobots be quantified and compared to the potential risks? |
| 9. What is peoples' risk perception of nanorobots? |
| 10. What are the main societal concerns related to nanorobots? |
The second recommendation, based on lesson (ii), is that policy-makers and regulators should reach out to relevant expert stakeholders and initiate dialogue about how, when and why nanorobots might be used and address some of the issues that we know from discussions about nanoparticles are likely to be contentious for the future of nanorobots. Such issues include the discussion about nanomaterial and nanoparticle definitions, which are on-going but have not yet resulted in consensus.23 Over time, stakeholder positions seem to have become increasingly entrenched with the emergence of increasing evidence indicating that nanoparticles might be associated with harmful effects on the environment and human health.72 There is no need to wait with discussions about definitions until early indications of harm emerges. Discussions about a regulatory relevant definition of nanorobots should start now when the stakes are not yet so high for stakeholders involved. The tentative definition of nanorobots provided in section 1 of this paper provides a starting point for such discussions.
The third recommendation, also based on lesson (ii), is the initiation of broader public dialogue about risks and benefits of nanorobots, as well as how regulatory measures can be implemented in order to maximize the benefits of nanorobots while minimizing potential risks. For nanotechnology in general, there was an early effort already in 2004 to explore of the general public's attitudes towards nanotechnologies.73 Many of the identified areas of concern are still highly relevant for nanotechnologies in general and nanorobots in particular. For instance, the public raised questions about the purpose and controllability of nanotechnologies, whether health and environmental considerations had been adequately addressed, whether existing regulation was up to the task, and whether lessons from the past had been learned. For nanorobots, we recommend reengaging in a dialogue with the public, listening to their concerns, and ensuring these concerns are addressed up-front.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the Swedish Research Council Formas and the Mistra Environmental Nanosafety program is gratefully acknowledged.References
- M. C. Roco, Nanoscale Science and Engineering: Unifying and Transforming Tools, AIChE J., 2004, 50(5), 890–897 CrossRef.
- J. M. Tour, Nanotechnology: The Passive, Active and Hybrid Sides - Gauging the Investment Landscape from the Technology Perspective, Nanotechnology law & Business, 2007, 4(3), 361–373 Search PubMed.
- M. E. Vance, T. Kuiken, E. P. Vejerano, S. P. McGinnis, M. F. Hochella Jr. and D. Rejeski, et al., Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory, Beilstein J. Nanotechnol., 2015, 6, 1769–1780 CrossRef.
- S. F. Hansen, L. R. Heggelund, P. Revilla Besora, A. Mackevica, A. Boldrin and A. Baun, Nanoproducts – what is actually available to European consumers?, Environ. Sci.: Nano, 2016, 3(1), 169–180 RSC.
- R. Arvidsson, Risk Assessments Show Engineered Nanomaterials To Be of Low Environmental Concern, Environ. Sci. Technol., 2018, 52(5), 2436–2437 CrossRef.
- K. L. Garner and A. A. Keller, Emerging patterns for engineered nanomaterials in the environment: a review of fate and toxicity studies, J. Nanopart. Res., 2014, 16(8), 2503 CrossRef.
- R. Arvidsson, A. Baun, A. Furberg, S. F. Hansen and S. Molander, Proxy Measures for Simplified Environmental Assessment of Manufactured Nanomaterials, Environ. Sci. Technol., 2018, 52(23), 13670–13680 CrossRef CAS.
- T. Y. Sun, N. A. Bornhöft, K. Hungerbühler and B. Nowack, Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials, Environ. Sci. Technol., 2016, 50(9), 4701–4711 CrossRef CAS.
- Y. Wang and B. Nowack, Dynamic probabilistic material flow analysis of nano-SiO2, nano iron oxides, nano-CeO2, nano-Al2O3, and quantum dots in seven European regions, Environ. Pollut., 2018, 235, 589–601 CrossRef CAS.
- C. Toumey, Nanobots today, Nat. Nanotechnol., 2013, 8, 475 CrossRef CAS.
- L. M. Smith, Molecular robots on the move, Nature, 2010, 465, 167 CrossRef CAS.
- R. Boffard, Tiny bots to embark on fantastic voyage, Engineering & Technology, 2015, 10(5), 34–36 Search PubMed.
- W. Gao and J. Wang, The Environmental Impact of Micro/Nanomachines: A Review, ACS Nano, 2014, 8(4), 3170–3180 CrossRef.
- P. Harremoës, D. Gee, M. MacGarvin, A. Stirling, J. Keys and B. Wynne, et al.Late lessons from early warnings: the precautionary principle 1896–2000, European Environment Agency, Copenhagen, 2001 Search PubMed.
- D. Gee, P. Grandjean, S. Foss Hansen, S. van den Hove, M. MacGarvin and J. Martin, et al.Late lessons from early warnings: science, precaution, innovation, European Environment Agency, Copenhagen, 2013 Search PubMed.
- B. Lambert, Radiation: early warnings; late effects, ed. P. Harremoës, D. Gee, M. MacGarvin, A. Stirling, J. Keys and B. Wynne, et al., Late lessons from early warnings: the precautionary principle 1896–2000, European Environment Agency, Copenhagen, 2001 Search PubMed.
- L.-E. Edqvist and K. B. Pedersen, Antimicrobials as growth promoters: resistance to common sense, ed. P. Harremoës, D. Gee, M. MacGarvin, A. Stirling, J. Keys and B. Wynne, et al., Late lessons from early warnings: the precautionary principle 1896–2000, European Environment Agency, Copenhagen, 2001 Search PubMed.
- I. S. M. Khalil, H. C. Dijkslag, L. Abelmann and S. Misra, MagnetoSperm: A microrobot that navigates using weak magnetic fields, Appl. Phys. Lett., 2014, 104(22), 223701 CrossRef.
- F. Qiu, R. Mhanna, L. Zhang, Y. Ding, S. Fujita and B. J. Nelson, Artificial bacterial flagella functionalized with temperature-sensitive liposomes for controlled release, Sens. Actuators, B, 2014, 196, 676–681 CrossRef.
- X. Wang, X.-Z. Chen, C. C. J. Alcântara, S. Sevim, M. Hoop and A. Terzopoulou, et al., MOFBOTS: Metal–Organic-Framework-Based Biomedical Microrobots, Adv. Mater., 2019, 31(27), 1901592 CrossRef.
- C. Bi, M. Guix, B. V. Johnson, W. Jing and D. J. Cappelleri, Design of Microscale Magnetic Tumbling Robots for Locomotion in Multiple Environments and Complex Terrains, Micromachines, 2018, 9(2), 68 CrossRef.
- A. Nel, T. Xia, L. Madler and N. Li, Toxic Potential of Materials at the Nanolevel, Science, 2006, 311(5761), 622–627 CrossRef CAS.
- M. Boholm and R. Arvidsson, A Definition Framework for the Terms Nanomaterial and Nanoparticle, Nanoethics, 2016, 10(1), 25–40 CrossRef.
- S. Martel, Swimming microorganisms acting as nanorobots versus artificial nanorobotic agents: A perspective view from an historical retrospective on the future of medical nanorobotics in the largest known three-dimensional biomicrofluidic networks, Biomicrofluidics, 2016, 10(2), 021301 CrossRef.
- Y. Shirai, A. J. Osgood, Y. Zhao, K. F. Kelly and J. M. Tour, Directional Control in Thermally Driven Single-Molecule Nanocars, Nano Lett., 2005, 5(11), 2330–2334 CrossRef CAS.
- A. Ghosh and P. Fischer, Controlled Propulsion of Artificial Magnetic Nanostructured Propellers, Nano Lett., 2009, 9(6), 2243–2245 CrossRef CAS.
- L. Zhang, J. J. Abbott, L. Dong, B. E. Kratochvil, D. Bell and B. J. Nelson, Artificial bacterial flagella: Fabrication and magnetic control, Appl. Phys. Lett., 2009, 94(6), 064107 CrossRef.
- X.-Z. Chen, M. Hoop, F. Mushtaq, E. Siringil, C. Hu and B. J. Nelson, et al., Recent developments in magnetically driven micro- and nanorobots, Appl. Mater. Today, 2017, 9, 37–48 CrossRef.
- J. Ali, U. K. Cheang, J. D. Martindale, M. Jabbarzadeh, H. C. Fu and K. M. Jun, Bacteria-inspired nanorobots with flagellar polymorphic transformations and bundling, Sci. Rep., 2017, 7(1), 14098 CrossRef.
- T. Mirkovic, N. S. Zacharia, G. D. Scholes and G. A. Ozin, Fuel for Thought: Chemically Powered Nanomotors Out-Swim Nature's Flagellated Bacteria, ACS Nano, 2010, 4(4), 1782–1789 CrossRef CAS.
- V. Garcia-Gradilla, J. Orozco, S. Sattayasamitsathit, F. Soto, F. Kuralay and A. Pourazary, et al., Functionalized Ultrasound-Propelled Magnetically Guided Nanomotors: Toward Practical Biomedical Applications, ACS Nano, 2013, 7(10), 9232–9240 CrossRef CAS.
- M. Kiristi, V. V. Singh, B. Esteban-Fernández de Ávila, M. Uygun, F. Soto and D. Aktaş Uygun, et al., Lysozyme-Based Antibacterial Nanomotors, ACS Nano, 2015, 9(9), 9252–9259 CrossRef CAS.
- B. Esteban-Fernández de Ávila, A. Martín, F. Soto, M. A. Lopez-Ramirez, S. Campuzano and G. M. Vásquez-Machado, et al., Single Cell Real-Time miRNAs Sensing Based on Nanomotors, ACS Nano, 2015, 9(7), 6756–6764 CrossRef.
- M. Uygun, B. Jurado-Sánchez, D. A. Uygun, V. V. Singh, L. Zhang and J. Wang, Ultrasound-propelled nanowire motors enhance asparaginase enzymatic activity against cancer cells, Nanoscale, 2017, 9(46), 18423–18429 RSC.
- J. Li, B. Esteban-Fernández de Ávila, W. Gao, L. Zhang and J. Wang, Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification, Sci. Robot., 2017, 2(4), eaam6431 CrossRef.
- Z. Zhan, F. Wei, J. Zheng, W. Yang, J. Luo and L. Yao, Recent advances of light-driven micro/nanomotors: toward powerful thrust and precise control, Nanotechnol. Rev., 2018, 7(6), 555 CAS.
- D. Zhou, L. Ren, Y. C. Li, P. Xu, Y. Gao and G. Zhang, et al., Visible light-driven, magnetically steerable gold/iron oxide nanomotors, Chem. Commun., 2017, 53(83), 11465–11468 RSC.
- J. Wang, Z. Xiong, X. Zhan, B. Dai, J. Zheng and J. Liu, et al., A Silicon Nanowire as a Spectrally Tunable Light-Driven Nanomotor, Adv. Mater., 2017, 29(30), 1701451 CrossRef.
- Y. Wang, C. Zhou, W. Wang, D. Xu, F. Zeng and C. Zhan, et al., Photocatalytically Powered Matchlike Nanomotor for Light-Guided Active SERS Sensing, Angew. Chem., Int. Ed., 2018, 57(40), 13110–13113 CrossRef CAS.
- J. G. S. Moo, C. C. Mayorga-Martinez, H. Wang, B. Khezri, W. Z. Teo and M. Pumera, Nano/Microrobots Meet Electrochemistry, Adv. Funct. Mater., 2017, 27(12), 1604759 CrossRef.
- J. Bao, Z. Yang, M. Nakajima, Y. Shen, M. Takeuchi and Q. Huang, et al., Self-Actuating Asymmetric Platinum Catalytic Mobile Nanorobot, IEEE Trans. Robot., 2014, 30(1), 33–39 Search PubMed.
- T. Li, J. Li, H. Zhang, X. Chang, W. Song and Y. Hu, et al., Magnetically Propelled Fish-Like Nanoswimmers, Small, 2016, 12(44), 6098–6105 CrossRef CAS.
- T. Li, J. Li, K. I. Morozov, Z. Wu, T. Xu and I. Rozen, et al., Highly Efficient Freestyle Magnetic Nanoswimmer, Nano Lett., 2017, 17(8), 5092–5098 CrossRef CAS.
- M. Zeng, S. Yuan, D. Huang and Z. Cheng, Accelerated Design of Catalytic Water-Cleaning Nanomotors via Machine Learning, ACS Appl. Mater. Interfaces, 2019, 11(43), 40099–40106 CrossRef CAS.
- M. Zeng, D. Huang, P. Wang, D. King, B. Peng and J. Luo, et al., Autonomous Catalytic Nanomotors Based on 2D Magnetic Nanoplates, ACS Appl. Nano Mater., 2019, 2(3), 1267–1273 CrossRef CAS.
- D. Ahmed, T. Baasch, B. Jang, S. Pane, J. Dual and B. J. Nelson, Artificial Swimmers Propelled by Acoustically Activated Flagella, Nano Lett., 2016, 16(8), 4968–4974 CrossRef CAS.
- Y. Krishnan, DNA's new avatar as nanoscale construction material, Resonance, 2008, 13(2), 195–197 CrossRef CAS.
- P. W. K. Rothemund, Folding DNA to create nanoscale shapes and patterns, Nature, 2006, 440, 297 CrossRef CAS.
- H. Gu, J. Chao, S.-J. Xiao and N. C. Seeman, A proximity-based programmable DNA nanoscale assembly line, Nature, 2010, 465, 202 CrossRef CAS.
- K. Lund, A. J. Manzo, N. Dabby, N. Michelotti, A. Johnson-Buck and J. Nangreave, et al., Molecular robots guided by prescriptive landscapes, Nature, 2010, 465(7295), 206–210 CrossRef CAS.
- S. Surana, J. M. Bhat, S. P. Koushika and Y. Krishnan, An autonomous DNA nanomachine maps spatiotemporal pH changes in a multicellular living organism, Nat. Commun., 2011, 2(1), 340 CrossRef.
- S. M. Douglas, I. Bachelet and G. M. Church, A Logic-Gated Nanorobot for Targeted Transport of Molecular Payloads, Science, 2012, 335(6070), 831 CrossRef CAS.
- H. Lee, A. K. R. Lytton-Jean, Y. Chen, K. T. Love, A. I. Park and E. D. Karagiannis, et al., Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery, Nat. Nanotechnol., 2012, 7(6), 389–393 CrossRef CAS.
- D. Bhatia, S. Surana, S. Chakraborty, S. P. Koushika and Y. Krishnan, A synthetic icosahedral DNA-based host–cargo complex for functional in vivo imaging, Nat. Commun., 2011, 2(1), 339 CrossRef.
- A. T. Veetil, K. Chakraborty, K. Xiao, M. R. Minter, S. S. Sisodia and Y. Krishnan, Cell-targetable DNA nanocapsules for spatiotemporal release of caged bioactive small molecules, Nat. Nanotechnol., 2017, 12(12), 1183–1189 CrossRef CAS.
- S. Li, Q. Jiang, S. Liu, Y. Zhang, Y. Tian and C. Song, et al., A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo, Nat. Biotechnol., 2018, 36, 258 CrossRef CAS.
- L. Zhang, J. J. Abbott, L. Dong, K. E. Peyer, B. E. Kratochvil and H. Zhang, et al., Characterizing the Swimming Properties of Artificial Bacterial Flagella, Nano Lett., 2009, 9(10), 3663–3667 CrossRef CAS.
- K. Kostarelos, Nanorobots for medicine: how close are we?, Nanomedicine, 2010, 5(3), 341–342 CrossRef.
- S. Surana, A. R. Shenoy and Y. Krishnan, Designing DNA nanodevices for compatibility with the immune system of higher organisms, Nat. Nanotechnol., 2015, 10, 741 CrossRef CAS.
- E. Denkhaus and K. Salnikow, Nickel essentiality, toxicity, and carcinogenicity, Crit. Rev. Oncol. Hematol., 2002, 42(1), 35–56 CrossRef CAS.
- S. F. Hansen, The emergence of nano risk-immunity, Frontiers in Nanoscience and Nanotechnology, 2016, 2(3), 131–134 Search PubMed.
- S. N. Luoma, Silver nanotechnologies and the environment: Old problems or new challanges?, Washington DC, Woodrow Wilson International Center for Scholars, Project on Emerging Nanotechnologies and The PEW Charitable Trusts, 2008 Search PubMed.
- N. Lubick, Nanosilver toxicity: ions, nanoparticles—or both?, Environ. Sci. Technol., 2008, 42(23), 8617 CrossRef CAS.
- M. R. Wiesner, G. V. Lowry, K. L. Jones, J. M. F. Hochella, R. T. Di Giulio and E. Casman, et al., Decreasing Uncertainties in Assessing Environmental Exposure, Risk, and Ecological Implications of Nanomaterials, Environ. Sci. Technol., 2009, 43(17), 6458–6462 CrossRef CAS.
- Editorial, Regulating nanomedicine, Nat. Mater., 2007, 6, 249 CrossRef.
- S. F. Hansen and A. Baun, European Regulation Affecting Nanomaterials - Review of Limitations and Future Recommendations, Dose-Response, 2012, 10(3), 364–383 CrossRef CAS.
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC.
- European Commission, Commission recommendation of 18 October 2011 on the definition of nanomaterial, Brussels, 2011 Search PubMed.
- European Medicines Agency, Reflection paper on nanotechnology-based medicinal products for human use (EMEA/CHMP/70769/2006), London, 2006.
- G. M. Patel, G. C. Patel, R. B. Patel, J. K. Patel and M. Patel, Nanorobot: A versatile tool in nanomedicine, J. Drug Targeting, 2006, 14(2), 63–67 CrossRef CAS.
- A. Grübler, Technology and Global Change, Cambridge University Press, Cambridge, 1998 Search PubMed.
- S. F. Hansen and A. Baun, DPSIR and Stakeholder Analysis of the Use of Nanosilver, Nanoethics, 2015, 9(3), 297–319 CrossRef.
- Royal Society and Royal Academy of Engineering, Nanoscience and nanotechnologies: opportunities and uncertainties, London, 2004 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |
