Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Environmental context determines the impact of titanium oxide and silver nanoparticles on the functioning of intertidal microalgal biofilms†
Claire
Passarelli
 *a,
Xianjin
Cui
*a,
Xianjin
Cui
 b,
Eugenia
Valsami-Jones
b,
Eugenia
Valsami-Jones
 b and
Graham J. C.
Underwood
b and
Graham J. C.
Underwood
 *a
*a
aUniversity of Essex, School of Life Sciences, Wivenhoe Park, Colchester CO43SQ, UK. E-mail: claire.passarelli@gmail.com; gjcu@essex.ac.uk
bFacility for Environmental Nanoscience Analysis and Characterisation (FENAC), School of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
First published on 14th September 2020
Abstract
Coastal environments are receiving habitats for most nanoparticle (NP) waste. Coastal sediments, into which NPs accumulate, support microalgal biofilms that provide important ecosystem processes: primary production, enhanced sediment stabilisation, and nutrient recycling. We assessed the impact of realistic concentrations of titanium oxide (TiO2) and silver (Ag) NPs on marine microalgal biofilms and associated ecosystem processes in simulated natural conditions, by exposing natural biofilms to TiO2 and Ag-NPs for one-month periods in outdoor tidal mesocosms under three contrasted environmental contexts (seasons). Ag-NPs had no significant effects on microalgal biomass, sediment biostabilisation potential and sediment–water oxygen and nutrient fluxes, even at concentrations (25 μg l−1) higher than current estimated levels (25 ng l−1). TiO2-NPs had no significant effect at current expected concentrations (25 μg l−1), but higher concentrations (25 mg l−1) resulted in decreased microalgal biomass; decreased ability of biofilms to biostabilise sediment, therefore limiting their coastal protection potential; reduced primary production and modified nutrient recycling. TiO2-NPs impacts were dependent on the environmental context: most effect was seen in winter, while no toxicity on biofilms was demonstrated in early spring. Our findings demonstrate that while Ag-NPs, being liable to dissolution into Ag+ ions under the conditions tested, are not expected to have an environmental impact if current predictions of environmental loading prevail, TiO2-NPs may have ecological consequences in coastal environments in addition to direct impacts on microbial biomass.
Environmental significanceAg and TiO2 nanoparticles are emerging contaminants of coastal waters. Their toxicology has been well studied in laboratory conditions, but their real impact on coastal ecosystems is poorly characterised. We demonstrated that under environmentally relevant conditions TiO2-NPs can have a negative effect on multiple coastal processes, such as coastal protection potential, oxygen fluxes and nutrient recycling. These effects were dependent on environmental context (season of year) and measurements of biomass alone are not sufficient to evaluate impacts on ecosystem functioning. Our research therefore both provides a first step towards the comprehension of NP impact in coastal environments, and guides future research by demonstrating the importance of assessing impacts in a variety of contexts, and directly on ecosystem processes. |
Introduction
The production of engineered nanomaterials has increased since the early 1990s. Titanium oxide (TiO2) nanoparticles (NPs) are produced in great quantities,1,2 possess photocatalytic and anti-fouling properties and are widely used in paints, sunscreens and other cosmetics, and food packaging.3–5 Silver NPs (Ag-NPs) are used in many products;6 for example, in textiles, medical materials and household appliances because of their anti-microbial properties.6,7A consequence of the development and use of products containing NPs is the production of “nano-waste” and the release of NPs to the environment.4,7–9 The efficiency of sewage treatment works (STW) in removing NPs from their waste water streams can be high, reaching 50–90%.10,11 For instance, sulfidation of Ag-NPs in STW will limit, but not suppress, the release of Ag-NPs into surface waters.10–12 TiO2-NPs do not dissolve or transform easily and can be released in the environment through STW discharge.13 Through these STW discharges, rivers and runoff waters, coastal habitats are the recipient of large amounts of NPs.4,15 In addition to these inputs, TiO2-NPs can be released directly in coastal waters as they are an important component of sunscreens, which are currently the dominant source of TiO2-NPs in the marine environment.16,17
The high ionic strength of seawater may promote NP aggregation, as NPs become less stabilised by electrical repulsive force in seawater.5,18,19 Aggregation can then lead to increased deposition into sediments.20 Ag-NPs fate in seawater will depend on their size, concentrations, and concentrations of other ions, such as Cl− and S2−.6 Up to 90% of Ag-NPs can deposit from seawater to sediment in less than 24 h.20 TiO2-NPs can also be incorporated into natural organic matter aggregates (marine snow), increasing their deposition onto sediments.18,19 These processes mean that sediment-inhabiting (benthic) organisms may have the highest exposure to NPs arriving in coastal waters.4,21
In coastal environments, photosynthetic microbial biofilms dominated by microalgae (predominantly benthic diatoms) are the main primary producers of unvegetated mudflats.23–25 These microphytobenthic biofilms are of key importance for the functioning of coastal systems, where they influence the recycling of nutrients,26 form the basis of food webs27,28 and become a potential entry point of NPs in marine trophic networks.21,29 Biofilms stabilise sediments, thus promoting coastal protection by limiting sediment erosion.30,31 In culture, microalgal growth can be reduced at Ag-NP concentrations from 200 μg l−1,6 and at TiO2-NP concentrations from 5 mg l−1.32 In periphytic biofilms, concentrations between 1 to 5 mg l−1 of TiO2-NPs reduced microalgal development and enhanced the biofilm metabolism,33 through an alteration of the composition of the microbial community.33,34 Impacts of Ag and TiO2-NPs on coastal microphytobenthic biofilms have the potential to substantially influence the functioning of coastal systems, and decrease the ecosystem services they provide to human populations.31,35
The toxicity of TiO2-NPs and Ag-NPs towards aquatic and marine microorganisms (both microalgae and bacteria) is clearly demonstrated in cultures under laboratory conditions. However, experiments conducted in more complex environments to determine if current levels of exposure to NPs are endangering microorganisms or microbial processes are more equivocal. Ag-NPs appear to have limited toxicity on the abundance of, and processes driven by, microorganisms in freshwater or estuarine biofilms,15,36–39 except at concentrations (1000 μg l−1) far higher than modelled environmental concentrations (1–100 ng l−1).40–42 Shifts in species composition of bacterial communities have been recorded at Ag-NP concentrations of 200 μg l−1.36,39,43,44 The link between alteration of community composition by NPs and modification of associated processes is not straightforward; for instance, bacterial activity to degrade hydrocarbons can be maintained in presence of Ag-NPs, despite significant modification of the composition of the bacterial communities.36 Toxicity of NPs to microorganisms has also been shown to be taxon specific,37 and influenced by environmental parameters, such as presence of UV light for TiO2-NPs2 and Ag-NPs,45 and temperature for Ag-NPs.46
In this study, we used environmentally relevant experimental conditions47 to determine if TiO2-NPs and Ag-NPs affect coastal biofilms and their associated ecosystem processes. TiO2 were chosen as the most produced NP,1,2 and Ag-NPs as the NP used in the highest diversity of products.6 Both have also been identified as NPs “of concern” regarding environmental degradation,48 and are likely to behave differently in the environment, given that Ag-NPs are soluble and redox sensitive, whereas TiO2-NPs are relatively inert and biopersistent.5 We performed experiments on natural intertidal biofilms, maintained in outdoor tidal mesocosms subject to natural weather conditions during three contrasted periods of the year. We simulated the constant inputs of NPs into coastal waters by dosing the mesocosms with close to expected natural concentrations of NPs (based on published modelled and measured concentrations) at regular intervals over a 28 day-period. In addition, we used a 1000 times higher concentration of TiO2 and Ag-NPs, to allow for comparison with other laboratory and mesocosms studies, as well as a combined TiO2 + Ag-NP treatment to look for potential synergistic or antagonistic effects.49 We analysed the effects of NPs on microphytobenthic biomass, and also on biofilm-associated ecosystem processes: the ability to biostabilise sediment (as assessed by biofilm extracellular carbohydrate matrix content); primary production, organic carbon fluxes; and the cycling of inorganic nutrients across the sediment–water interface.
We hypothesised (1) that at current concentrations NPs will have little impact on biofilm biomass or ecosystem processes, while higher concentrations would have acute and chronic impact on both biomass and processes. (2) That changes in ecosystem processes will be dependent on changes in microalgal biomass. Finally, as microphytobenthic biofilms undergo a seasonal cycle of species-changes50,51 and altered ecophysiology throughout the year,52 we hypothesised (3) that the impact of NPs will depend on the physiological condition of the biofilm throughout the seasonal cycle, especially as such dynamics are associated with seasonal variations in light and temperature. Based on the above hypothesis, we proposed that TiO2-NPs would be more toxic to biofilms in summer, when light and UV levels are highest.2
Materials & methods
Experimental set-up
Multiple cores (6.8 cm in diameter, 10 cm deep) of surface sediment supporting microphytobenthic biofilms were sampled from an intertidal mudflat on the Essex coast (51°46′45.7′′N 1°02′49.0′′E, UK) and placed in experimental tanks with natural seawater (ESI† Fig. S1). Microphytobenthic biofilms from this site have been characterised,53,54 and are dominated by diatoms, with a minimal contribution from cyanobacteria. Such biofilms are characteristic of diatom-dominated intertidal biofilms found widely across NW European coastal habitats.24,25For each NP treatment (see below), 4 independent tidal mesocosms were established. Mesocosm are particularly well suited to understand the impact of NPs on complex systems such as microbial biofilms.55 Each mesocosm consisted of an experimental tank containing 4 sediment cores, and a reservoir tank. Seawater was pumped in and out of the experimental tanks twice per 24 h, and advancing 1 h per day to match the natural rhythm of tides on the Essex coast. During low tide, the top of cores was exposed to air, and cores were covered with a few centimetres of water at high tide (ESI† Fig. S1).
Three 28 day long experiments were implemented, to capture different environmental contexts (Tables 1 and 2): in November to December 2016 (TiO2-NPs only – termed winter); one in February to March 2017 (early spring) and in June to July 2017 (summer).
| Name | Early spring | Summer | Winter |
|---|---|---|---|
| a Temperatures over 40 °C reached around noon for a couples of hours, on 11 occasions. | |||
| Month | Feb./Mar. 2017 | Jun./Jul. 2017 | Nov./Dec. 2016 |
| Temperature (°C) | 8.0 ± 0.1 | 22.3 ± 0.1 | 7.5 ± 0.1 |
| Temperature variations (°C) | 0.1 to 25.8 | 9.6 to 48.8a | −4.2 to 17.0 |
| Light intensity (μmol s−1 m−2) | 168.3 ± 5.4 | 541 ± 13 | 61.0 ± 3.0 |
| Photoperiod (h d−1) | 11.2 ± 0.7 | 17.7 ± 0.4 | 8.8 ± 0.0 |
| O2 concentration (μmol l−1) | 228 ± 1 | 138 ± 1 | 209 ± 4 |
| TOC (mg l−1) | 14.5 ± 2.4 | 10.1 ± 0.5 | 26.3 ± 1.6 |
| NO2− concentration (μmol l−1) | 0.42 ± 0.02 | 0.43 ± 0.02 | 0.47 ± 0.05 |
| NO3− concentration (μmol l−1) | 19.9 ± 0.4 | 12.4 ± 0.5 | 17.3 ± 1.0 |
| NH4+ concentration (μmol l−1) | 1.81 ± 0.18 | 2.6 ± 0.15 | 1.89 ± 0.42 |
| SiO44− concentration (μmol l−1) | 6 ± 0.1 | 2.1 ± 0.0 | 11.4 ± 0.3 |
| PO43− concentration (μmol l−1) | 0.83 ± 0.04 | 0.78 ± 0.03 | 1.62 ± 0.22 |
| Variable | Early spring | Summer | Winter |
|---|---|---|---|
| a A positive flux is a net flux from sediment into the overlaying water. | |||
| Month | Feb./Mar. 2017 | Jun./Jul. 2017 | Nov./Dec. 2016 |
| Chl a concentration (μg g−1 sed dw) | 86 ± 4 | 24 ± 1 | 88 ± 8 |
| Chl a/phaeophytin ratio | 1.56 ± 0.04 | 1.22 ± 0.07 | 1.63 ± 0.06 |
| Colloidal carbohydrate concentration (μg g−1 sed dw) | 54.7 ± 2.0 | 69.8 ± 2.4 | 175 ± 147.5 |
| Carbohydrate/Chl a ratio | 0.71 ± 0.04 | 3.07 ± 0.13 | 2.34 ± 0.27 |
| Photosynthetic potential Fv/Fm | 0.45 ± 0.00 | 0.47 ± 0.00 | 0.41 ± 0.01 |
| Benthic respiration (mmol O2 m−2 h−1)a | −0.99 ± 0.10 | −2.34 ± 0.23 | −1.59 ± 0.32 |
| Net primary production (mmol O2 m−2 h−1)a | 0.08 ± 0.21 | 0.90 ± 0.24 | 0.65 ± 0.25 |
| Gross primary production (mmol O2 m−2 h−1)a | 1.21 ± 0.23 | 3.30 ± 0.36 | 2.16 ± 0.39 |
| Production/respiration ratio | 1.05 ± 0.17 | 1.42 ± 0.12 | 1.45 ± 0.24 |
The mesocosms were located outside in an unshaded area, exposed to natural light and temperature conditions (recorded every 10 minutes throughout the experiment with a HOBO logger set at the same height as the top of a core). Salinity of system water was monitored daily and kept constant by addition of seawater (after heavy rain events) or distilled water (to compensate evaporation). To prevent depletion of nutrients, all seawater was replaced with fresh natural seawater in experimental and reservoir tanks at weekly intervals.
NP preparation and addition
We established 6 experimental treatments:(i) no added NP (control treatment);
(ii) and (iii) two TiO2-NP treatments: target concentrations of 25 μg l−1 and 25 mg l−1; 25 μg l−1 was chosen to approximate the estimated current concentration of TiO2-NP in wastewater treatment plant effluents;3,16,42
(iv) and (v) two Ag-NP treatments: target concentrations of 25 ng l−1 and 25 μg l−1; 25 ng l−1 was chosen to approximate the estimated current concentration of Ag-NP in wastewater treatment plant effluents;11,14,41
(vi) one treatment with TiO2 and Ag-NP together (target concentrations of 25 μg l−1 of TiO2-NP and 25 ng l−1 of Ag-NP).
Uncoated TiO2-NPs (anatase![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rutile (80
rutile (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 vol%), sold as 20 nm diameter) were purchased from American Elements (Los Angeles, USA). The mixture of both forms was chosen to mimic natural conditions where NPs will originate from diverse sources.3,56,57 There are indeed numerous applications that do not require a specific phase of TiO2 (e.g. painting, photocatalytic applications…).56,58,59 PVP-coated silver nanospheres (sold as 25 nm in diameter) were bought from NanoComposix (San Diego, USA). PVP-coated NPs, which disperse easily in water, are likely to be found on coasts where most NPs will arrive from rivers and run-off water. The size of NPs in water was assessed by TEM and DLS and found different from the manufacturer's specifications for both NPs (ESI† Fig. S2a and b): TiO2-NPs were larger than expected (most individual particles had length and width between 50 and 200 nm), while Ag-NPs exhibited two peaks in the size distribution, one around 15 nm and another around 40 nm (ESI† Fig. S2 for more details on the size and shape of NPs).
20 vol%), sold as 20 nm diameter) were purchased from American Elements (Los Angeles, USA). The mixture of both forms was chosen to mimic natural conditions where NPs will originate from diverse sources.3,56,57 There are indeed numerous applications that do not require a specific phase of TiO2 (e.g. painting, photocatalytic applications…).56,58,59 PVP-coated silver nanospheres (sold as 25 nm in diameter) were bought from NanoComposix (San Diego, USA). PVP-coated NPs, which disperse easily in water, are likely to be found on coasts where most NPs will arrive from rivers and run-off water. The size of NPs in water was assessed by TEM and DLS and found different from the manufacturer's specifications for both NPs (ESI† Fig. S2a and b): TiO2-NPs were larger than expected (most individual particles had length and width between 50 and 200 nm), while Ag-NPs exhibited two peaks in the size distribution, one around 15 nm and another around 40 nm (ESI† Fig. S2 for more details on the size and shape of NPs).
NP target concentrations (see treatments above) were obtained by diluting NP stocks into the experimental mesocosms. For each TiO2-NP or Ag-NP treatment, a stock solution was prepared at the beginning of the experiment in ultra-high purity water. Immediately before each addition into the mesocosms, the stocks were sonicated for 30 min and then 9 ml of sonicated stock solution was added in each 9 l mesocosm under that treatment. The same stock solution was used for the whole experiment. The release of Ag+ ions from Ag-NP meant that the stock solution contained a mixture of Ag-NP and Ag ions (see ESI† S2 for more details on Ag-NP dissolution).
Most NPs settle in the first 24 h when in contact with seawater,60,61 so that without regular addition of NPs, the concentrations of NPs in the water of the mesocosms would have declined. The mesocosm systems were also subjected to weekly water changes with fresh seawater, to prevent nutrient limitation and simulate estuarine flushing processes. To accommodate these factors, NPs were added into mesocosms after every water change, then every two days until the next water change in order to maintain the concentration of NPs in water close to the target concentration. NPs could precipitate on the top of the sediment core (i.e. on the biofilm), but also on the bottom of the tank and in the reservoir.
Measurements
Sampling took place before any NP addition (T0), then after 4 and 28 days; the sampling design is summarized in Fig. S3.† Briefly, each independent replicate mesocosm (4 replicate mesocosm per treatment) contained 4 cores: one core was used for sediment–water flux measurement throughout the experiment (repeated measures design), while the 3 remaining cores were used for mini-core sampling and lens-tissue extractions of biofilms throughout the experiment. Mini-cores were never taken in the same spot twice during an experiment.Sediment mini-cores (1.4 cm diameter, 2 mm deep) were kept frozen at −20 °C until analysis for chlorophyll a, phaeophytin and carbohydrate concentrations. Chlorophyll a and phaeophytin concentration were determined spectrophotometrically,62 and colloidal carbohydrate concentration was measured with colorimetry.54,63,64 Colloidal carbohydrate concentration was measured as this is directly related to biostabilisation potential through the production of mucilaginous matrices containing colloidal EPS.30,54,64
Lens tissue extractions were used to isolate epipelic diatoms from the sediment.65 Lens-tissues were placed on the surface of the sediment after emersion of the cores (falling tide), and left for 1 h under illumination (light used for flux measurements as described below). Lens-tissue extracts were then resuspended in filtered natural seawater by gentle mixing and dark-adapted 30 min at a seasonally relevant temperature. Photosynthetic potential (Fv/Fm, the maximum PSII photochemical efficiency) of microalgae was then measured using a FRR fluorimeter.66,67
On each sampling day, one core per tank was used for oxygen, carbon and nutrient sediment–water exchange measurements as described in Thornton et al.50 Each sediment core was enclosed within a long core tube with seawater from the site, and maintained at in situ temperature during two sequential 3 hour incubations: one incubation in the dark (during the in situ immersion period) followed by one in the light (during the in situ emersion period). Overlaying water was sampled before and after each incubation to measure oxygen, total organic carbon (TOC, i.e. particulate organic carbon + dissolved organic carbon) and nutrient (nitrate, nitrite, ammonium, silicate and phosphate ions) concentrations. Oxygen concentrations were measured using Winkler titration;68 TOC concentrations with a Skalar FormacsHT TOC analyser, and nutrient concentrations using SEAL Analytical AACE auto-analyser. Fluxes between sediment and water were determined for each core from the concentration changes, and normalised (based on surface core area) to flux per square meter per hour. A technical problem during the winter experiment meant that flux measures were made on day 7 instead of day 4.
All cores used for flux measurements were sieved (1 mm mesh size) at the end of the experiment, and macrofauna preserved in 100% ethanol. All individual animals were counted and identified and width or length measurements taken. Biovolume of individuals were calculated using geometric shapes and published morphometric relationships for Hydrobia, Macoma (mollusca) and Hediste polychaete annelid worms.69–71
Statistical analyses
Differences in the measured variables between experimental treatments (control and NP treatments) were tested at two time points: after 4 days (short term, acute toxicity) and 28 days (longer term, chronic toxicity), using multiple comparison Kruskal-Wallis tests (also called non-parametric ANOVA; R statistical framework72). When significant differences between treatments were found, we performed pairwise post hoc Mann Whitney tests with Bonferroni correction. This correction for multiple comparison meant that our statistical power was too low determine significant differences between two individual treatments.To compare inorganic nutrient fluxes, non-metric multi-dimensional scaling analyses (nMDS, 500 iterations; Primer 5 software73) were performed for each experiment, with similarity matrix constructed with normalised Euclidian distances using the dark and light flux values for all nutrients. ANOSIM was then used to test the difference in nutrient fluxes between treatments. When relevant, SIMPER analyses were used to determine which fluxes were responsible for the most difference between NP treatments (control included).
Non-metric multi-dimensional scaling (nMDS, Bray Curtis similarity with square root transformation and Wisconsin double standardisation; R version 3.6.1 with the package vegan72,74) was used to visualise differences in macrofauna community structure. nMDS was based on total biovolume of each taxa to provide a better reflection of the potential impact of the taxa on the overall assemblage functioning.
Results
Nanoparticles and environmental context
TiO2-NPs ranged in size between 50 and 200 nm (ESI† Fig. S2c), and with a zeta potential at −17.5 ± 0.9 mV, aggregated in seawater and were deposited on the biofilm surface (ESI† Fig. S4a). Ag-NPs showed no sign of aggregation and deposition in seawater, despite a zeta potential of −9 mV, due to their PVP coating (ESI† Fig. S2d). Ag-NPs were more consistent in size, with a median size of 12 nm (ESI† Fig. S2a and d). Analysis of dissolution of Ag-NPs in seawater revealed greater dissolution of Ag-NPs into Ag+ ions in summer seawater than in early spring seawater (ESI† Fig. S2e).A number of point measurements of Ag concentrations in the surface sediments were taken at the end of our early spring and summer experiments with ICP-MS (1 measurement per treatment per experiment; sediment pooled from the 4 cores of each treatment). Concentrations were around 0.02 μg Ag g−1 sediment (dry weight) in control cores and between 0.02 and 0.17 μg g−1 in cores treated with silver. These low numbers confirm a low deposition of Ag-NPs onto the sediment cores. There was a trend for sediment Ag concentrations to be lower in summer compared to early spring.
The environmental conditions in the three seasonal periods (contexts) were well contrasted (Tables 1 and 2), with the experiments in November to December 2016, February to March 2017 and June to July 2017 representing the physical, chemical and biological conditions in the Colne estuary in winter, early spring and summer, respectively.25 To mirror the natural annual cycle of microphytobenthic biofilms which start growing in early spring, then decline in winter,52 results from the three contexts are presented in the sequence, early spring, summer, winter.
Microscopic identification of microalgae in lens-tissue extracts confirmed the presence of living benthic diatoms in our sediment cores. The sediment cores supported healthy microphytobenthic biofilms, with chlorophyll a/phaeophytin ratio above 1 and Fv/Fm measurements above 0.4 on all lens-tissue extracts of the epipelic diatoms (Table 1). Fv/Fm measurements did not show any sign of decrease throughout the experiments in control tanks, showing that the mesocosm conditions were appropriate to support the healthy development of microalgal biofilms.
Macrofaunal communities were significantly different between seasons (ESI† Fig. S4b), with Macoma balthica and Hediste diversicolor being more abundant in summer compared to other seasons (ESI† Table S1). Dolicopodid larvae were absent from cores in the summer experiment. In early spring, H. diversicolor were significantly less abundant in treatments with TiO2-NPs (KW test, p < 0.05), but otherwise there were no significant effects of experimental treatments on the densities of macrofauna.
Experimental treatments
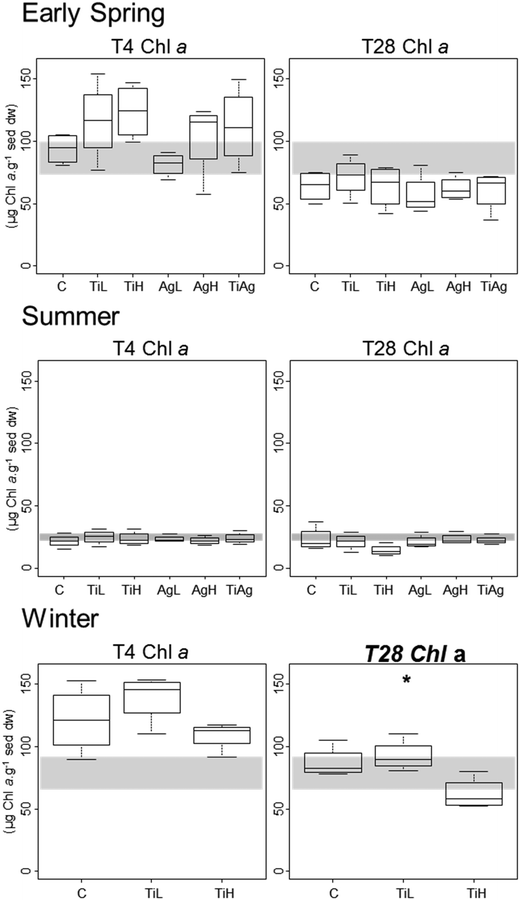
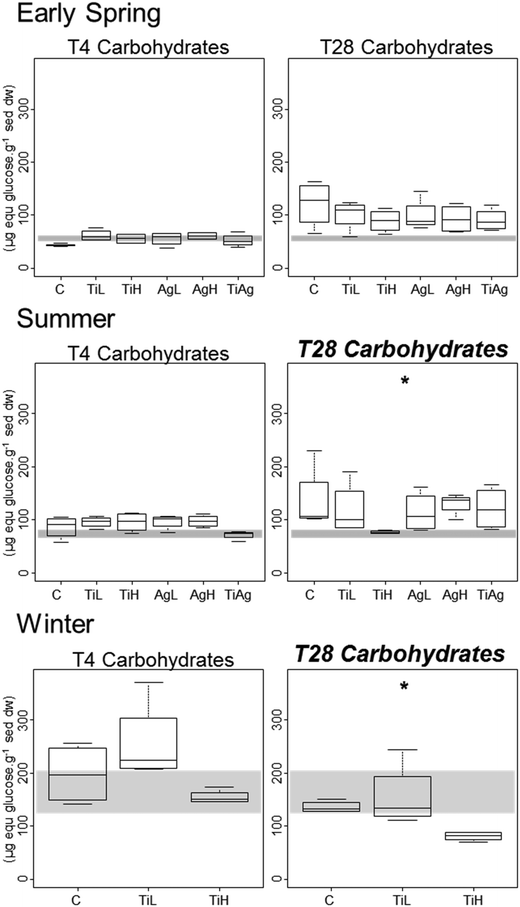
Microphytobenthic biomass on our sediment cores was measured using the proxy of chlorophyll a content in the first 2 mm of the sediment, after 4 and 28 days of experiment (Fig. 1). No significant differences between treatments were found after 4 days regardless of the season (Kruskal-Wallis, p > 0.05). After 28 days in winter, significant differences were observed (Kruskal-Wallis, p = 0.026), with chl a content 28% lower in the presence of high concentrations of TiO2-NPs compared to control treatment.Colloidal carbohydrate content of the surface sediment (first 2 mm), a proxy for sediment biostabilisation potential, was measured after 4 and 28 days of experiment (Fig. 2). No significant effects of the NP treatments were found after 4 days (Kruskal-Wallis, p > 0.05). However, after 28 days in both summer and winter, TiO2-NPs significantly impacted biofilm development (Kruskal-Wallis, p = 0.023 in summer, p = 0.024 in winter). At high concentrations TiO2-NPs caused a 44% reduction of colloidal carbohydrate content in sediment in summer, and 40% reduction in winter (compared with the control treatment). A similar trend, although not statistically significant, was seen in early spring (p > 0.1, 28% reduction). These indicates a potential for TiO2-NPs to decrease the capacity of microalgal biofilms to stabilise sediments.
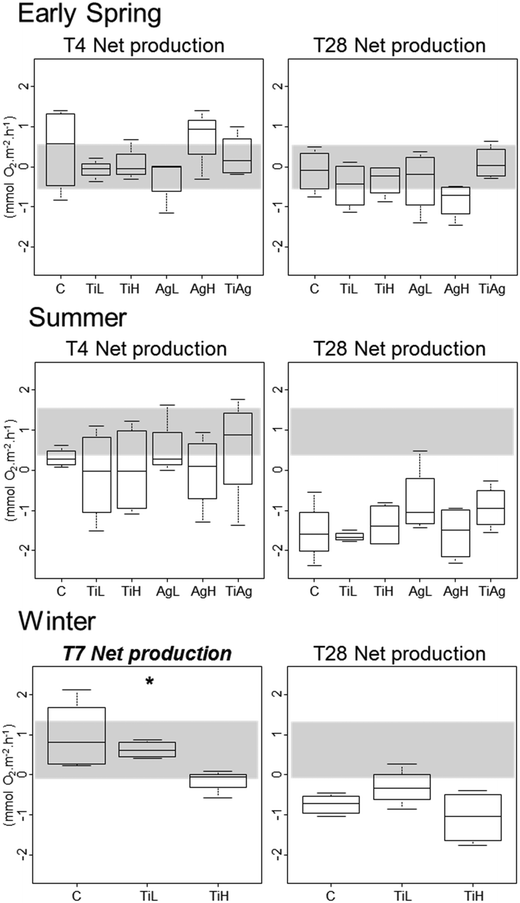
Neither TiO2-NPs nor Ag-NPs showed any influence on biofilm photosynthetic potential of diatoms in the biofilms, regardless of the experimental conditions, with no significant differences between treatments for either Fv/Fm, or gross primary production (calculated from oxygen fluxes between sediment and water in the dark and in the light; Kruskal-Wallis, p > 0.05; data not shown). In winter, net primary production (NPP, i.e. oxygen fluxes from sediment to water in the light; Fig. 3) was significantly different between treatments after 7 days of experiment (Kruskal-Wallis, p < 0.05): the sediment switched from a net oxygen production to a net oxygen consumption in presence of high concentrations of TiO2-NPs. However, no significant influence of NPs on NPP was observed at the end of the winter experiment, or in other seasons (Kruskal-Wallis, p < 0.05).
Other carbon-related sediment–water fluxes, i.e. benthic respiration and TOC fluxes, did not show any differences between treatments in any of the experiments (data not shown; mean TOC fluxes per season given in Table S2†).
The influence of NPs on inorganic nutrient sediment–water fluxes was analysed considering all nutrient fluxes together: nitrate, nitrite, ammonium, phosphate and silicate fluxes in both dark and light conditions (mean fluxes per season, Table S2†). Nutrients fluxes were not significantly different between NP treatments and controls in any season after 4 days of treatment. At the end of the experiments, nutrient fluxes were different between treatments in winter only (Fig. 4 for winter, ANOSIM, R = 0.22, p = 0.043; Fig. S4,†p > 0.05 for other seasons). SIMPER analysis in winter showed that alterations of fluxes of ammonium, nitrate and silicate in the dark, and flux of ammonium in the light, explained most of the difference between treatments in winter (Fig. 4).
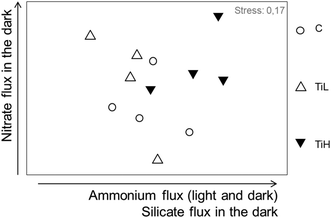 | ||
| Fig. 4 nMDS plot based on nutrient fluxes from sediment to water after 28 days of experiment in winter. C: control, no NP. TiL: target concentration 25 μg l−1 of TiO2-NP. TiH: target concentration 25 mg l−1 of TiO2-NP. The arrows indicate the fluxes responsible for most difference between treatments (SIMPER analysis). For early spring and summer data, see Fig. S5.† | ||
Discussion
Lack of observed toxicity of silver nanoparticles under environmentally relevant conditions
The PVP-coated Ag-NPs used showed no sign of aggregation or sinking even at high concentrations, but some of them dissolved in seawater (ESI† Fig. S2), and greater dilution could be expected at the low concentrations used in our experimental mesocosms.75 Ag+ ions originating from the NP could form complexes with Cl− or S2− ions, or link with microbial biofilms at the surface of the sediment.21 AgCl complexes can then act as Ag-NP precursor, but such formation remains low in seawater with high Cl−,76 especially at low Ag concentrations.77 Ag-NPs have been found to accumulate in biofilms,55 in particular in simulated marine environments,7 but our point measurements of Ag content in sediment suggest such accumulation was limited in the experimental set up deployed.Using environmentally relevant concentrations (25 ng l−1) or higher concentrations (25 μg l−1), our experiments showed no significant impact of silver (Ag) NPs on marine biofilms or associated ecosystem functions, regardless of the environmental context. This result did not support our original hypothesis 1, which was based on the evidence that Ag-NPs are used mainly for their anti-microbial properties (they were registered as biocide in the USA in 1954) and on the large body of literature investigating the toxicity of Ag-NPs.6 Ecological risk assessments indicate that Ag-NPs are the NPs of most concern at current estimated concentrations (higher risk characterisation ratio, i.e. concentrations in environment vs. estimated safe concentration).78 Several reasons might explain our findings.
Seawater can increase agglomeration and sedimentation of Ag-NPs with a charged surface,20 therefore decreasing their toxicity, though this process was not apparent in our experimental tanks (Fig. S2†). The dissolution of PVP-coated Ag-NPs into ionic form, including Ag+, AgCl2− and AgCl32− has been shown to increase in seawater compared to ultra-pure water.79 Our data (Fig. S2e†) does suggest a higher dissolution of Ag-NPs under summer conditions than in early spring conditions, indicating a temperature dependence of dissolution.46 In freshwater, PVP-coated AgNPs don't exhibit such seasonal differences in dissolution.80 The precipitation of Ag+ with Cl− or S2− in seawater and sediments lowers the bioavailability of silver ions, which are partly responsible for Ag-NP toxicity.4,81–84 The toxicity of Ag-NPs can however be greater than Ag+ ion toxicity at equivalent concentrations:38,85,86 a combination of dissolution followed by precipitation would overall lessen the potential toxic impact. PVP coated Ag-NPs, as used in our study, may have limited toxicity compared to uncoated NPs87–89 but coatings can also increase NP toxicity37,90 so that the overall effect of coatings on the environmental effect of Ag-NP is difficult to forecast.
Most studies demonstrating Ag-NP toxicity towards microorganisms use concentrations that are higher than those used in our study (25 ng l−1 as environmentally relevant, and 25 μg l−1 as 1000 times higher). Growth limitation of marine biofilms was found at concentrations from 200 μg l−1,43 in estuarine plankton from 500 μg l−1,91 in freshwater biofilms at 10 mg l−1,87 and marine benthic bacteria at 50 mg l−1;37 though no effect on freshwater microorganisms was found at 500 μg l−1 (ref. 92) or on estuarine bacteria at 1000 μg l−1.15 In wetland mesocosms, Ag-NPs at 2.5 mg l−1 showed a very significant impact on aquatic plants and carbon chemistry in the water.93 Other studies showed short-term toxic effects (for one or a few days) at lower or similar concentrations (tens of μg l−1 to mg l−1), such as increased stress for freshwater bacteria at 10 μg l−1,94 but microbial assemblages can then recover.20,82,86
Our study demonstrated that a regular exposure to low but environmentally realistic concentrations of Ag-NPs for one month had little effect on microphytobenthic biofilms, in terms of biomass, photosynthesis, and nutrient exchange processes. This is likely due to a combination of biological protection mechanisms and resistance, and chemical transformations of Ag-NPs in the environment. Microorganisms in coastal biofilms are embedded in a matrix of extracellular polymeric substances (EPS),95 which can provide protection from toxicants.96 Microbial EPS can trap and accumulate Ag-NPs and Ag+ ions efficiently,29,38,85 limiting their access to the cells.85 As a result, cells protected with EPS are less susceptible to Ag-NP toxicity than cells without EPS.85,97 Longer-term exposure to Ag-NPs could however potentially alter coastal biofilms and associated processes through modification of the microbial community.44
Toxicity of titanium oxide nanoparticles on coastal biofilms
In our experiments TiO2-NPs did not remain permanently in suspension in seawater and many were deposited on the sediment cores or the bottom of the tanks within a day of NP addition (visual observation, see Fig. S4a†). TEM images and zeta potential measurements showed that our the TiO2-NPs did aggregate rapidly in seawater (Fig. S2c†), behaviour which is consistent with other studies.19 TiO2-NPs are also known to associate with “marine snow” which also leads to sedimentation in natural seawater.18 Based on the doses of TiO2-NPs added, the surface area of our tanks and cores, and an estimated 90% of NPs sinking after each addition, we can estimate a final sediment load at 56 mg TiO2 m−2 for TiL and 56 g TiO2 m−2 for TiH treatments at the end of 28 days in our experiments.TiO2-NPs displayed toxic effects on microphytobenthic biofilms at high concentrations (25 mg l−1), with decreased microphytobenthic biomass and affected nutrient recycling in winter. Unlike the behaviour of Ag-NPs, TiO2-NPs are chemically stable under environmental conditions; and thus their toxicity is not associated with their dissolution into Ti ions, but with the NPs themselves.98 No such effect was observed at low concentrations (i.e. current expected concentration in STW effluents; 25 μg l−1).3,16
TiO2-NPs limited the biostabilisation potential of biofilms in both summer and winter, with concentrations of colloidal carbohydrate being 40–44% lower in presence of TiO2-NPs at high concentrations compared to controls. This result was surprising as colloidal carbohydrates, and EPS in general, can act as a protection mechanism for cells,34 and EPS production increases under a variety of stresses.99,100 Other studies showed that diatoms in periphytic biofilms exposed to TiO2-NPs and CuO-NPs showed an increased production of EPS33,87 protecting cells by mitigating ROS-associated stress.34 Different classes of EPS do not display the same response to NP exposure:33 Miao et al.87 showed a higher content of protein than carbohydrates in the EPS produced following such stress. In our experiment, TiO2-NPs might have affected cells by direct shading effect, therefore limiting light-dependent colloidal carbohydrate and EPS production.64,101 Also, NPs may disrupt EPS biochemical production pathways, which have been shown to alter under temperature and salinity stresses,102 and could show similar responses in presence of NPs.
TiO2-NPs occur commonly as two different crystalline structures, anatase and rutile; a mix of both phases of titanium was chosen in this experiment to take into account the diversity of TiO2-NPs arriving on coasts from different sources,3,56,57 for instance: coatings, paints, sunscreens. Toxicity of TiO2-NPs is twofold: a direct physical effect on cells, associating with and shading them, interrupting energy transduction along the membrane and limiting cells access to light.103 TiO2-NPs also lead to the production of reactive oxygen species (ROS) when in contact with cells,2,5,103 especially ˙OH that damage membranes and proteins. Anatase has a higher photocatalytic activity103,104 than rutile: it produces more ROS in presence of light. It is suggested that a mixture of both forms is more toxic that exposure to each form separately.103
The concentrations at which we observed toxic effects (25 mg l−1) were consistent with literature date on the toxicity of TiO2-NPs: significant toxicity was observed from one or a few mg l−1 in cultures1,2,32 and on river periphyton,98 to 50 mg l−1 in freshwater biofilms.103 Current expected concentrations of TiO2-NPs in the water (around 25 μg l−1) did not impact coastal biofilm growth or ecosystem functioning in our experiments. However, these findings must be nuanced by two facts: Binh et al.104 showed toxic effects of TiO2-NPs on freshwater biofilms at similarly low concentrations (30 μg l−1), but only after 22 weeks of experiment. Similar long-term chronic impacts may occur for coastal biofilms, which our 4 week experiments would not have detected. This view is consistent with high concentrations of TiO2-NPs showing toxicity mainly at the end of the experiments, when exposure time was longest. There is also uncertainty concerning the actual levels of environmental exposure; currently available measures of several types of NP concentrations in sediments are on average 1000 times higher than those predicted by modelling.40 Therefore our “high concentration” treatment might not be so far from current concentrations of TiO2-NPs in coastal sediments.
Mixtures of Ag-NPs and TiO2-NPs at low concentrations did not impact significantly biofilm biomass or associated biogeochemical processes. No “cocktail effect” was therefore demonstrated; different results may however be obtained at higher concentrations. TiO2-NPs can reduce Ag-NP toxicity towards bacteria in dark conditions, but increase the toxicity in the light.105 The interaction also seems to be dependent on light quality and cycle,49 but also on salinity, as chloride (Cl−) concentration in seawater limits the availability of Ag+ ions.21 NPs also interact with other types of pollutants: the disruption of membranes by TiO2-NPs can facilitate the entry of other pollutants, such as heavy metals, in the cells, therefore increasing their toxicity.106 Approaches to determine the active concentrations of mixtures of NPs, other toxicants, and physical and chemical cofounding variables at the site of biological impact at scales relevant to microbial processes in situ are needed to better understand environmental impacts of NPs in combination with other stressors.
Influence of environmental context on the toxicity of nanoparticles
The toxicity of TiO2-NPs was dependent on environmental context: no effects were seen in early spring, with most effects demonstrated in winter, thus supporting the first part of our third hypothesis. Differences in water chemistry (ionic or organic carbon content for instance) will influence the interactions between NPs and their abiotic environment, the fate of potential ions they release and therefore both NP bioavailability and toxicity.5,6 Higher temperatures increased the dissolution of Ag-NPs, increasing the release of potentially toxic ions (see Fig. S2e†);46 however, no toxicity was observed for Ag-NPs in our experiments, regardless of the temperature. Environmental conditions will influence how NPs aggregate and sink onto the sediment.5,6,18,94,105 In sediments as well as in the water, chemical parameters such as sulphide content will affect toxicity.88 Furthermore, TiO2-NPs are photoactive compounds, which means that the levels and wavelength range of light will influence their toxicity.2,103,107 For instance, TiO2-NPs are toxic to phytoplankton only in the presence of UV radiation.2In our experiments, we expected the greatest toxicity of TiO2-NPs to be in summer, where light and UV levels were higher.2 Some significant effects were observed in summer, but greatest toxicity to biofilms was found in winter when light and temperature were the lowest. In the summer and winter experiments where significant toxicity was observed, biofilms were characterised by high production to respiration ratios, and high amounts of colloidal carbohydrate per unit Chl a (Table 2). High production to respiration ratios, and high colloidal carbohydrates to Chl a ratios, indicate physiologically-active populations of microalgae,52 with different biogeochemical processes active compared with other seasons.52 We can hypothesise that these differences explain the observed differences in toxicity. For instance, degradation processes are preponderant in winter,52 and may be enhanced in the presence of ROS produced when NPs are present; this could explain the increased toxicity seen on this season. It is also interesting to note that chronic exposure to silver NPs in a wetland mesocosm also showed a higher sensitivity of prokaryotes in winter,44 while winter communities of freshwater diatoms were more resistant to herbicides.108 Our results highlight the necessity to repeat experiments under different environmental contexts encompassing different seasons and physiological states of the biological community under investigation, if we are to understand NP toxicity on the environment.
Environmental impact of titanium oxide nanoparticles
Our experiments investigated the effect of NPs on coastal microphytobenthic biofilms, and on the ecosystem processes that they influence. Mesocosm approaches allow for controlled experimental manipulation of intact intertidal sediment communities while maintaining as close as possible natural environmental conditions.54,55,109,110 The impact of grazing fish111 and wave action on biofilm resuspension112 were not simulated, but our approach simulated important environmental drivers of sediment-biofilm ecology: tidal cover, natural daylight quality and periodicities, rainfall, temperature, and nutrient resupply. Microphytobenthic activity (Chl a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phaeophytin ratio, Fv/Fm) indicated healthy biofilm functioning was sustained throughout the experimental periods, permitting conclusions about the environmental impacts of addition of NPs on biofilm related ecosystem functions to be drawn.
phaeophytin ratio, Fv/Fm) indicated healthy biofilm functioning was sustained throughout the experimental periods, permitting conclusions about the environmental impacts of addition of NPs on biofilm related ecosystem functions to be drawn.
Sediment biostabilisation potential, as measured by colloidal carbohydrate content in the sediment,30,31 was significantly reduced in both summer and winter at high concentrations of TiO2-NPs. Further research may help determine the threshold at which such toxicity is observed, its longer-term effects and whether shifts of microbial assemblages occur. Similar reductions of sediment biostabilisation potential have been demonstrated at high concentration of Ag-NPs for freshwater biofilms, where the structure of the biofilm was affected and sediment adhesiveness was largely reduced.113 Experiments in stream mesocosms with Ag-NPs showed reduced mechanical stability of freshwater biofilms despite unaffected microbial viability and biofilm architecture being unaffected at concentrations of 600 μg l−1.114 By reducing the colloidal carbohydrate content of microphytobenthic biofilms, TiO2-NPs have a potentially significant environmental effect in reducing biostabilisation of sediment, leading to increased resuspension of biofilms and sediment in sheltered estuaries by wind driven waves.112 Coastal sediment protection through biostabilisation is an important ecosystem service provided by microbial biofilm,31 as coastal erosion has been estimated to cost about 500 million dollars per year in the US only.115
In our experiments, NPs had no effect on biofilm photosynthetic maximum potential (Fv/Fm), or respiration, but a short-term negative influence of TiO2-NPs on net primary production was observed in winter. Some studies in cultures or mesocosms have shown deleterious effect of Ag-NPs81,87,91,97 and TiO2-NPs103 on the photosynthetic activity of microalgae, but usually at high concentrations compared to current expected environmental concentrations; other studies have shown limited impact.38 One long-term study on the effect of TiO2-NPs on freshwater biofilms at environmentally relevant concentration showed a decrease in photosynthetic activity after 4 to 6 months.104
We found that TiO2-NPs influenced nutrient recycling, especially increasing ammonium fluxes from sediment into the overlaying water. This is to our knowledge the first demonstration of the effects of TiO2-NPs on nutrient recycling in coastal systems. Ag-NPs at high concentrations limit the growth of nitrifying bacteria in culture, limiting nitrification in estuarine sediments,37,90 and limit the growth of ammonia-oxidising bacteria.37 Reduced ammonia-oxidation and nitrification will increase sediment ammonium concentrations, therefore increasing efflux of ammonium from sediment;116 this cascading effect is consistent with what we observed in our experiment with TiO2-NPs treatments. Reductions in microphytobenthic biomass will also reduce microphytobenthic demand for ammonium in surface sediments,116 increasing ammonium fluxes from sediment to water. The next step will be to assess if these effects are the result of the modification of the algal, bacterial or archaeal assemblages, or result from direct influence of TiO2-NPs on physiological processes. NPs can modify the composition of microbial assemblages,21,22,36,46,98 and therefore the ability of the community as a whole to drive ecosystem processes, noting that redundancy between microbial groups means that some processes, such as hydrocarbon degradation, may stay unaffected despite shifts in microbial assemblages.36
The link between reduction of biomass by NPs and modification of ecosystem processes is far from straightforward. We found that the biostabilisation potential of biofilms and primary production was affected by NPs while photosynthetic biomass was not (in summer and after 4 days in winter, respectively), and that a decrease of microphytobenthic biomass following TiO2-NP addition (at the end of the experiment, in winter) did not result in any alteration of oxygen fluxes. These observations do not support our hypothesis 2; and therefore, evaluating the environmental impact of NPs therefore requires not only the undertaking of experiments in environmentally relevant contexts (this study),47 but also a measure of the impact of NPs on both communities and ecosystem processes.
Conclusion
This study has shown that TiO2-NPs can limit the growth of coastal microphytobenthic biofilms in simulated natural conditions, while PVP coated Ag-NPs do not appear to be toxic in these conditions. TiO2-NPs have the potential for ecological impacts on the functioning of coastal systems, as they limit the coastal protection potential of biofilms, temporarily limit the primary production and alter nutrient recycling. Effects on biogeochemical processes were not directly dependent on changes in microalgal biomass, and all toxic effects varied between environmental contexts such as seasons. Our research therefore both provides a first step towards the comprehension of NP impact in coastal environments, and guides future research by demonstrating the importance of assessing impacts in a variety of contexts, and directly on ecosystem processes.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors wish to thank J. Green and R. Smart for their technical support in running the experiments. This research received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 702217, and from NERC SSB programme, module Blue Carbon, grant NE/K001914/1; this support is gratefully acknowledged. The authors acknowledge access to the NERC funded FENAC facility.References
- S. Manzo, S. Buono, G. Rametta, M. Miglietta, S. Schiavo and G. Di Francia, The diverse toxic effect of SiO2 and TiO2 nanoparticles toward the marine microalgae Dunaliella tertiolecta, Environ. Sci. Pollut. Res., 2015, 22, 15941–15951 CrossRef CAS.
- R. J. Miller, S. Bennett, A. A. Keller, S. Pease and H. S. Lenihan, TiO2 Nanoparticles Are Phototoxic to Marine Phytoplankton, PLoS One, 2012, 7, e30321 CrossRef CAS.
- A. B. A. Boxall, Q. Chaudhry, C. Sinclair, A. Jones, R. Aitken, B. Jefferson and C. Watts, Current and Future Predicted Environmental Exposure to Engineered Nanoparticles, Central Science Laboratory (UK), York, 2007 Search PubMed.
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Nanomaterials in the environment: behavior, fate, bioavailability, and effects, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS.
- M. Amde, J.-F. Liu, Z.-Q. Tan and D. Bekana, Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review, Environ. Pollut., 2017, 230, 250–267 CrossRef CAS.
- E. McGillicuddy, I. Murray, S. Kavanagh, L. Morrison, A. Fogarty, M. Cormican, P. Dockery, M. Prendergast, N. Rowan and D. Morris, Silver nanoparticles in the environment: Sources, detection and ecotoxicology, Sci. Total Environ., 2017, 575, 231–246 CrossRef CAS.
- D. Cleveland, S. E. Long, P. L. Pennington, E. Cooper, M. H. Fulton, G. I. Scott, T. Brewer, J. Davis, E. J. Petersen and L. Wood, Pilot estuarine mesocosm study on the environmental fate of Silver nanomaterials leached from consumer products, Sci. Total Environ., 2012, 421–422, 267–272 CrossRef CAS.
- S. Bartke, N. Hagemann, N. Harries, J. Hauck and P. Bardos, Market potential of nanoremediation in Europe - Market drivers and interventions identified in a deliberative scenario approach, Sci. Total Environ., 2018, 619–620, 1040–1048 CrossRef CAS.
- F. Gottschalk, T. Y. Sun and B. Nowack, Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies, Environ. Pollut., 2013, 181, 287–300 CrossRef CAS.
- C. M. Whiteley, M. Dalla Valle, K. C. Jones and A. J. Sweetman, Challenges in assessing release, exposure and fate of silver nanoparticles within the UK environment, Environ. Sci.: Processes Impacts, 2013, 15, 2050–2058 RSC.
- A. Johnson, I. Cisowska, M. Jürgens, V. Keller, A. Lawlor and R. Williams, Exposure assessment for engineered silver nanoparticles throughout the rivers of England and Wales, Centre for Ecology & Hydrology, 2011 Search PubMed.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Fate and transformation of silver nanoparticles in urban wastewater systems, Water Res., 2013, 47, 3866–3877 CrossRef CAS.
- T. Tong, A. N. Hill, M. A. Alsina, J. Wu, K. Y. Shang, J. J. Kelly, K. A. Gray and J.-F. Gaillard, Spectroscopic Characterization of TiO2 Polymorphs in Wastewater Treatment and Sediment Samples, Environ. Sci. Technol. Lett., 2015, 2, 12–18 CrossRef CAS.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS.
- A. Bradford, R. D. Handy, J. W. Readman, A. Atfield and M. Mühling, Impact of Silver Nanoparticle Contamination on the Genetic Diversity of Natural Bacterial Assemblages in Estuarine Sediments, Environ. Sci. Technol., 2009, 43, 4530–4536 CrossRef CAS.
- A. P. Gondikas, F. von der Kammer, R. B. Reed, S. Wagner, J. F. Ranville and T. Hofmann, Release of TiO2 Nanoparticles from Sunscreens into Surface Waters: A One-Year Survey at the Old Danube Recreational Lake, Environ. Sci. Technol., 2014, 48, 5415–5422 CrossRef CAS.
- R. Arvidsson, S. Molander and B. A. Sandén, Particle Flow Analysis. Exploring Potential Use Phase Emissions of Titanium Dioxide Nanoparticles from Sunscreen, Paint, and Cement, J. Ind. Ecol., 2012, 15, 844–854 CrossRef.
- J. J. Doyle, V. Palumbo, B. D. Huey and J. E. Ward, Behavior of Titanium Dioxide Nanoparticles in Three Aqueous Media Samples: Agglomeration and Implications for Benthic Deposition, Water, Air, Soil Pollut., 2014, 225, 2106 CrossRef.
- I. Corsi, E. Bergami and G. Grassi, Behavior and Bio-Interactions of Anthropogenic Particles in Marine Environment for a More Realistic Ecological Risk Assessment, Front. Environ. Sci., 2020, 8, 60 CrossRef.
- V. Echavarri-Bravo, L. Paterson, T. J. Aspray, J. S. Porter, M. K. Winson, B. Thornton and M. G. J. Hartl, Shifts in the metabolic function of a benthic estuarine microbial community following a single pulse exposure to silver nanoparticles, Environ. Pollut., 2015, 201, 91–99 CrossRef CAS.
- T. J. Baker, C. R. Tyler and T. S. Galloway, Impacts of metal and metal oxyde nanoparticles on marine organisms, Environ. Pollut., 2014, 186, 257–271 CrossRef CAS.
- S. Jomini, H. Clivot, P. Bauda and C. Pagnout, Impact of manufactured TiO2 nanoparticles on planktonic and sessile bacterial communities, Environ. Pollut., 2015, 202, 196–204 CrossRef CAS.
- V. Méléder, Y. Rincé, L. Barillé, P. Gaudin and P. Rosa, Spatiotemporal changes in microphytobenthos assemblages in a macrotidal flat (Bourgneuf bay, France), J. Phycol., 2007, 43, 1177–1190 CrossRef.
- G. J. C. Underwood and J. Kromkamp, Primary Production by Phytoplankton and Microphytobenthos in Estuaries, Adv. Ecol. Res., 1999, 29, 93–163 CAS.
- D. B. Nedwell, G. J. C. Underwood, T. J. McGenety, C. Whitby and A. J. Dumbrell, The Colne Estuary: A Long-Term Microbial Ecology Observatory, Adv. Ecol. Res., 2016, 55, 227–281 Search PubMed.
- D. C. O. Thornton, L. F. Dong, G. J. C. Underwood and D. B. Nedwell, Sediment-water inorganic nutrient exchange and nitrogen budgets in the Colne Estuary, UK, Mar. Ecol.: Prog. Ser., 2007, 337, 63–77 CrossRef CAS.
- A. Carpentier, S. Como, C. Dupuy, C. Lefrançois and E. Feunteun, Feeding ecology of Liza spp. in a tidal flat: Evidence of the importance of primary production (biofilm) and associated meiofauna, J. Sea Res., 2013, 92, 86–91 CrossRef.
- J. Widdows and M. Brinsley, Impact of biotic and abiotic processes on sediment dynamics and the consequences to the structure and functioning of the intertidal zone, J. Sea Res., 2002, 48, 143–156 CrossRef.
- C.-S. Chen, J. M. Anaya, S. Zhang, J. Spurgin, C.-Y. Chuang, C. Xu, A.-J. Miao, E. Y.-T. Chen, K. A. Schwehr, Y. Jiang, A. Quigg, P. H. Santschi and W.-C. Chin, Effects of Engineered Nanoparticles on the Assembly of Exopolymeric Substances from Phytoplankton, PLoS One, 2011, 6, e21865 CrossRef CAS.
- K. S. Black, T. J. Tolhurst, D. M. Paterson and S. E. Hagerthey, Working with natural cohesive sediments, Journal of hydraulic engineering., 2002, 128, 2–8 CrossRef.
- C. Passarelli, F. Olivier, D. M. Paterson, T. Meziane and C. Hubas, Organisms as cooperative ecosystem engineers in intertidal flats, J. Sea Res., 2014, 92, 92–101 CrossRef.
- B. Xia, B. Chen, X. Sun, K. Qu, F. Ma and M. Du, Interaction of TiO2 nanoparticles with the marine microalga Nitzchia closterium: Growth inhibition, oxidative stress and internalization, Sci. Total Environ., 2015, 508, 525–533 CrossRef CAS.
- J. Hou, T. Li, L. Miao, G. You, Y. Xu and S. Liu, Effects of titanium dioxide nanoparticles on algal and bacterial communities in periphytic biofilms, Environ. Pollut., 2019, 251, 407–414 CrossRef CAS.
- N. Zhu, S. Wang, C. Tang, P. Duan, L. Yao, J. Tang, P. K. Wong, T. An, D. D. Dionysiou and Y. Wu, Protection Mechanisms of Periphytic Biofilm to Photocatalytic Nanoparticle Exposure, Environ. Sci. Technol., 2019, 53, 1585–1594 CrossRef CAS.
- C. Passarelli, C. Hubas and D. M. Paterson, in Mudflat Ecology, ed. P. G. Beninger, Springer Nature, Switzerland AG, 2018 Search PubMed.
- J. Beddow, B. Stölpe, P. A. Cole, J. R. Lead, M. Sapp, B. P. Lyons, B. McKew, M. Steinke, F. Benyahia, I. Colbeck and C. Whitby, Estuarine sediment hydrocarbon-degrading microbial communities demonstrate resilience to nanosilver, Int. Biodeterior. Biodegrad., 2014, 96, 206–215 CrossRef.
- J. Beddow, B. Stolpe, P. A. Cole, J. R. Lead, M. Sapp, B. P. Lyons, I. Colbeck and C. Whitby, Nanosilver inhibits nitrification and reduces ammonia-oxidising bacterial but not archeal amoA gene abundance in estuarine sediment, Environ. Microbiol., 2017, 19, 500–510 CrossRef CAS.
- A. G. González, S. Mombo, J. Leflaive, A. Lamy, O. S. Pokrovsky and J.-L. Rols, Siver nanoparticles impact phototrophix biofilm communities to a considerably higher degree than ionic silver, Environ. Sci. Pollut. Res., 2015, 22, 8412–8424 CrossRef.
- A. Y. Grün, C. B. App, A. Breidenbach, J. Meier, G. Metreveli, G. E. Schaumann and W. Manz, Effects of low dose silver nanoparticle treatment on the structure and community composition of bacterial freshwater biofilms, PLoS One, 2018, 13, e0199132 CrossRef.
- T. Y. Sun, F. Gottschalk, K. Hungerbühler and B. Nowack, Comprehensive probalistic modelling of environmental emissions of engineered nanomaterials, Environ. Pollut., 2014, 185, 69–76 CrossRef CAS.
- B. Giese, F. Klaessig, B. Park, R. Kaegi, M. Steinfeldt, H. Wigger, A. von Gleich and F. Gottschalk, Risks, Release and Concentrations of Engineered Nanomaterial in the Environment, Sci. Rep., 2018, 8, 1565 CrossRef.
- T. Y. Sun, N. A. Bornhöft, K. Hungerbühler and B. Nowack, Dynamic Probabilistic Modelling of Environmental Emissions of Engineered Nanomaterials, Environ. Sci. Technol., 2016, 50, 4701–4711 CrossRef CAS.
- J. Fabrega, R. Zhang, J. C. Renshaw, W.-T. Liu and J. R. Lead, Impact of silver nanoparticles on natural marine biofilm bacteria, Chemosphere, 2011, 85, 961–966 CrossRef CAS.
- C. S. Ward, J.-F. Pan, B. P. Colman, Z. Wang, C. A. Gwin, T. C. Williams, A. Ardis, C. K. Gunsch and D. E. Hunt, Conserved Microbial Toxicity Responses for Acute and Chronic Silver Nanoparticle Treatments in Wetland Mesocosms, Environ. Sci. Technol., 2019, 53, 3268–3276 CrossRef CAS.
- X. Zhao, T. Toyooka and Y. Ibukin, Synergistic bactericidal effect by combined exposure to Ag nanoparticles and UVA, Sci. Total Environ., 2013, 458–460, 54–62 CrossRef CAS.
- D. Batista, C. Pascoal and F. Cássio, Temperature modulates AgNP impacts on microbial decomposer activity, Sci. Total Environ., 2017, 601–602, 1324–1332 CrossRef CAS.
- P. A. Holden, J. L. Gardea-Torresdey, F. Klaessig, R. F. Turco, M. Mortimer, K. Hund-Rinke, E. A. Cohen Hubal, D. Avery, D. Barceló, R. Behra, Y. Cohen, L. Deydier-Stephan, P. L. Ferguson, T. F. Fernandes, B. Herr Harthorn, W. M. Henderson, R. A. Hoke, D. Hristozov, J. M. Johnston, A. B. Kane, L. Kapustka, A. A. Keller, H. S. Lenihan, W. Lovell, C. J. Murphy, R. M. Nisbet, E. J. Petersen, E. R. Salinas, M. Scheringer, M. Sharma, D. E. Speed, Y. Sultan, P. Westerhoff, J. C. White, M. R. Wiesner, E. M. Wong, B. Xing, M. Steele Horan, H. A. Godwin and A. E. Nel, Considerations of Environmentally Relevant Test Conditions for Improved Evaluation of Ecological Hazards of Engineered Nanomaterials, Environ. Sci. Technol., 2016, 50, 6124–6145 CrossRef CAS.
- R. Arvidsson, A. Baun, A. Furberg, S. F. Hansen and S. Molander, Proxy Measures for Simplified Environmental Assessment of Manufactured Nanomaterials, Environ. Sci. Technol., 2018, 52, 13670–13680 CrossRef CAS.
- X. Zou, J. Shi and H. Zhang, Coexistence of silver and titanium dioxide nanoparticles: Enhancing or reducing environmental risks?, Aquat. Toxicol., 2014, 154, 168–175 CrossRef CAS.
- D. C. O. Thornton, L. F. Dong, G. J. C. Underwood and D. B. Nedwell, Factors affecting microphytobenthic biomass, species composition and production in the Colne Estuary (UK), Aquat. Microb. Ecol., 2002, 27, 285–300 CrossRef.
- G. J. C. Underwood, Seasonal and spatial variation in epipelic diatom assemblages in the Severn estuary, Diatom Res., 1994, 9, 451–472 CrossRef.
- C. Passarelli, T. Meziane, N. Thiney, D. Boeuf, B. Jesus, M. Ruivo, C. Jeanthon and C. Hubas, Seasonal variations of the composition of microbial biofilms in sandy tidal flats: Focus on fatty acids, pigments and exopolymers, Estuarine, Coastal Shelf Sci., 2015, 153, 29–37 CrossRef CAS.
- G. J. C. Underwood, J. Phillips and K. Saunders, Distribution of estuarine benthic diatom species along salinity and nutrient gradients, Eur. J. Phycol., 1998, 33, 173–183 CrossRef.
- B. A. McKew, J. D. Taylor, T. J. McGenety and G. J. C. Underwood, Resistance and resilience of benthic biofilm communities from a temperate saltmarsh to dessication and rewetting, ISME J., 2011, 5, 30–41 CrossRef.
- M. Desmau, A. Carboni, M. Le Bars, E. Doelsch, M. F. Benedetti, M. Auffan, C. Levard and A. Gelabert, How Microbial Biofilms Control the Environmental Fate of Engineered Nanoparticles?, Front. Environ. Sci., 2020, 8, 82 CrossRef.
- H. Shi, R. Magaye, V. Castranova and J. Zhao, Titanium dioxide nanoparticles: a review of current toxicological data, Part. Fibre Toxicol., 2013, 10, 15 CrossRef CAS.
- A. Azimzada, J. M. Farner, I. Jreije, M. Hadioui, C. Liu-Kang, N. Tufenkji, P. Shaw and K. J. Wilkinson, Single- and Multi-Element Quantification and Characterization of TiO2 Nanoparticles Released From Outdoor Stains and Paints, Front. Environ. Sci., 2020, 8, 91 CrossRef.
- X. Chen and S. S. Mao, Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS.
- L. Sang, Y. Zhao and C. Burda, TiO2 Nanoparticles as Functional Building Blocks, Chem. Rev., 2014, 114, 9283–9318 CrossRef CAS.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. Ji, Stability and Agregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS.
- F. Seitz, R. R. Rosenfeldt, S. Schneider, R. Schulz and M. Bundschuh, Size-, surface- and crystalline structure composition-related effects of titanium dioxide nanoparticles during their aquatic life cycle, Sci. Total Environ., 2014, 493, 891–897 CrossRef CAS.
- A. Jensen, in Handbook of Phycological Methods. Physiological and Biochemical Methods, ed. J. A. Hellebust and J. S. Craigie, Cambridge University Press, Cambridge, 1978, pp. 59–74 Search PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- B. J. Bellinger, G. J. C. Underwood, S. E. Ziegler and M. R. Gretz, Significance of diatom-derived polymers in carbon flow dynamics within estuarine biofilms determined through isotopic enrichment, Aquat. Microb. Ecol., 2009, 55, 169–187 CrossRef.
- J. W. Eaton and B. Moss, Estimation of numbers and pigment content in epipelic algal populations, Limnol. Oceanogr., 1966, 11, 584–595 CrossRef.
- Z. S. Kolber, O. Prášil and P. G. Falkowski, Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols, Biochim. Biophys. Acta, 1998, 1367, 88–106 CrossRef CAS.
- D. J. Suggett, C. M. Moore, A. E. Hickman and R. J. Geider, Interpretation of fast repetition rate (FRR) fluorescence: signatures of phytoplankton community structure versus physiological state, Mar. Ecol.: Prog. Ser., 2009, 376, 1–19 CrossRef.
- J. D. H. Strickland and D. R. Parsons, A practical handbook of seawater analysis, Fisheries Research Board of Canada, Ottawa, 1972 Search PubMed.
- C. Casagranda and C. F. Boudouresque, A sieving method for rapid determination of size-frequency distribution of small-gasteropods. Example of the mud snail Hydrobia ventrosa (Gastropoda: Prosobranchia), Hydrobiologia, 2002, 485, 143–152 CrossRef.
- G. Ejdung, E. Flach, L. Byrén and H. Hummel, Predation by crustaceans on native and non-native Baltic clams, Aquat. Biol., 2009, 6, 15–24 CrossRef.
- P. Esselink and L. Zwarts, Seasonal trend in burrow depth and tidal variation in feeding activity of Nereis diversicolor, Mar. Ecol.: Prog. Ser., 1989, 56, 243–254 CrossRef.
- R Core Team, R: A language and environment for statistical computing, 2018 Search PubMed.
- PRIMER-E Limited, Primer 5, 2002 Search PubMed.
- J. Oksanen, G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O'Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs and H. Wagner, vegan: Community Ecology Package, R package version 2.4-3, 2017 Search PubMed.
- C. Toncelli, K. Mylona, I. Kalantzi, A. Tsiola, P. Pitta, M. Tsapakis and S. Pergantis, Silver nanoparticles in seawater: A dynamic mass balance at part per trillion silver concentrations, Sci. Total Environ., 2017, 601–602, 15–21 CrossRef CAS.
- A. Sing, W.-C. Hou, T.-F. Lin and R. Zepp, Roles of Silver Chloride Complexations in Sunlight-Driven Formation of Silver Nanoparticles, Environ. Sci. Technol., 2019, 53, 11162–11169 CrossRef.
- H. Liu, X. Gu, C. Wei, H. Fu, P. J. J. Alvarez, Q. Li, S. Zheng, X. Qu and D. Zhu, A Threshold Concentration of Silver Ions Exists for Sunlight-Induced Formation of Silver Nanoparticles in the Presence of Natural Organic Matter, Environ. Sci. Technol., 2018, 52, 4040–4050 CrossRef CAS.
- R. Arvidsson, Risk Assessments Show Engineered Nanomaterials To Be of Low Environmental Concern, Environ. Sci. Technol., 2018, 52, 2436–2437 CrossRef CAS.
- K. A. Huynh and K. L. Chen, Aggregation Kinetics of Citrate and Polyvinylpyrrolidone Coated Silver Nanoparticles in Monovalent and Divalent Electrolyte Solutions, Environ. Sci. Technol., 2011, 45, 5567–5571 CrossRef.
- L.-J. A. Ellis, M. Baalousha, E. Valsami-Jones and J. R. Lead, Seasonal variability of natural water chemistry affects the fate and behaviour of silver nanoparticles, Chemosphere, 2018, 191, 616–625 CrossRef CAS.
- E. Navarro, F. Piccapietra, B. Wagner, F. Marconi, R. Kaegi, N. Odzak, L. Sigg and R. Behra, Toxicity of Silver Nanoparticles to Chlamydomonas reinhardtii, Environ. Sci. Technol., 2008, 42, 8959–8964 CrossRef CAS.
- K. Doiron, E. Pelletier and K. Lemarchand, Impact of polymer-coated silver nanoparticles on marine microbial communities: A microcosm study, Aquat. Toxicol., 2012, 124–12, 22–27 CrossRef.
- C. Levard, E. M. Hotze, B. P. Colman, A. L. Dale, L. Truong, X. Y. Yang, A. J. Bone, G. E. J. Brown, R. L. Tanguay, R. T. Di Giulio, E. S. Bernhardt, J. N. Meyer, M. R. Wiesner and G. V. Lowry, Sulfidation of Silver Nanoparticles: Natural Antidote to Their Toxicity, Environ. Sci. Technol., 2013, 47, 13440–13448 CrossRef CAS.
- C. Levard, S. Mitra, T. Yang, A. D. Jew, A. Raju Badireddy, G. V. Lowry and G. E. J. Brown, Effect of Chloride on the Dissolution Rate of Silver Nanoparticles and Toxicity to E. coli, Environ. Sci. Technol., 2013, 47, 5738–5745 CrossRef CAS.
- G. Pletikapić, V. Žutić, I. Vinković Vrček and V. Svetličić, Atomic force microscopy characterization of silver nanoparticles interactions with marine diatom celles and extracellular polymeric substance, J. Mol. Recognit., 2012, 25, 309–317 CrossRef.
- P. Das, M. A. Xenopoulos, C. J. Williams, M. Ehsanul Hoque and C. D. Metcalfe, Effects of silver nanoparticles on bacterial activity in natural waters, Environ. Toxicol. Chem., 2012, 31, 122–130 CrossRef CAS.
- L. Miao, C. Wang, J. Hou, P. Wang, Y. Ao, Y. Li, Y. Yao, B. Lv, Y. Yang, G. You and Y. Xu, Influence of silver nanoparticles on benthic oxygen consumption of microbial communities in freshwater sediments determined by microelectrodes, Environ. Pollut., 2017, 224, 771–778 CrossRef CAS.
- S. Bao, H. Wang, W. Zhang, Z. Xie and T. Fang, An investigation into the effects of silver nanoparticles on natural microbial communities in two freshwater sediments, Environ. Pollut., 2016, 219, 696–704 CrossRef CAS.
- B. P. Colman, B. P. Espinasse, C. J. Richardson, C. W. Matson, G. V. Lowry, D. E. Hunt, M. R. Wiesner and E. S. Bernhardt, Emerging Contaminant or an Old Toxin in Disguise? Silver Nanoparticle Impacts on Ecosystems, Environ. Sci. Technol., 2014, 48, 5229–5236 CrossRef CAS.
- J. Beddow, B. Stolpe, P. Cole, J. R. Lead, M. Sapp, B. P. Lyons, I. Colbeck and C. Whitby, Effects of engineered silver nanoparticles on the growth and activity of ecologically important microbes, Environ. Microbiol. Rep., 2014, 6, 448–458 CrossRef CAS.
- M. S. Baptista, R. J. Miller, E. R. Halewood, S. K. Hanna, C. M. R. Almeida, V. M. Vasconcelos, A. A. Keller and H. S. Lenihan, Impacts of Silver Nanoparticles on a Natural Estuarine Plankton Community, Environ. Sci. Technol., 2015, 49, 12968–12974 CrossRef CAS.
- H. S. Jiang, L. Yin, N. N. Ren, L. Xian, S. Zhao, W. Li and B. Gontero, The effect of chronic silver nanoparticles on aquatic systems in microcosms, Environ. Pollut., 2017, 223, 395–402 CrossRef CAS.
- B. P. Colman, L. F. Baker, R. S. King, C. W. Matson, J. M. Unrine, S. M. Marinakos, D. E. Gorka and E. S. Bernhardt, Dosing, Not the Dose: Comparing Chronic and Pulsed Silver Nanoparticle Exposures, Environ. Sci. Technol., 2018, 52, 10048–10056 CrossRef CAS.
- C. M. Wilke, T. Tong, J.-F. Gaillard and K. A. Gray, Attenuation of Microbial Stress Due to Nano-Ag and Nano-TiO2 Interactions under Dark Conditions, Environ. Sci. Technol., 2016, 50, 11302–11310 CrossRef CAS.
- G. J. C. Underwood and D. M. Paterson, The Importance of Extracellular Carbohydrate Production by Marine Epipelic Diatoms, Adv. Bot. Res., 2003, 40, 183–239 CAS.
- A. W. Decho, in Oceanography and Marine Biology: an Annual review, ed. M. Barnes, Aberdeen University Press, 1990, vol. 28, pp. 73–153 Search PubMed.
- A.-J. Miao, K. A. Schwehr, C. Xu, S.-J. Zhang, L. Zhiping, A. Quigg and P. H. Santschi, The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances, Environ. Pollut., 2009, 157, 3034–3041 CrossRef CAS.
- M. V. Wright, C. W. Matson, L. F. Baker, B. T. Castellon, P. S. Watkins and R. S. King, Titanium dioxide nanoparticle exposure reduces algal biomass and alters algal assemblage composition in wastewater effluent-dominated stream mesocosms, Sci. Total Environ., 2018, 626, 357–365 CrossRef CAS.
- D. J. Steele, D. J. Franklin and G. J. C. Underwood, Protection of cells from salinity stress by extracellular polymeric substances in diatom biofilms, Biofouling, 2014, 30, 987–998 CrossRef CAS.
- R. S. Wotton, in Oceanography and Marine Biology: an Annual Review, ed. R. N. Gibson, R. J. A. Atkinson and J. D. M. Gordon, Aberdeen University Press, 2004, vol. 42, pp. 57–94 Search PubMed.
- R. G. Perkins, G. J. C. Underwood, V. Brotas, G. C. Snow, B. Jesus and L. Ribeiro, Responses of microphytobenthos to light: primary production and carbohydrate allocation over an emersion period, Mar. Ecol.: Prog. Ser., 2001, 223, 101–112 CrossRef.
- S. N. Aslam, J. Strauss, D. N. Thomas, T. Mock and G. J. C. Underwood, Identifying metabolic pathways for production of extracellular polymeric substances by the diatom Fragilariopsis cylindrus inhabiting sea ice, ISME J., 2018, 12, 1237–1251 CrossRef CAS.
- K. Li, J. Qian, P. Wang, C. Wang, J. Liu, X. Tian, B. Lu and M. Shen, Crystalline phase-dependent eco-toxicity of titania nanoparticles to freshwater biofilms, Environ. Pollut., 2017, 231, 1433–1441 CrossRef CAS.
- C. T. T. Binh, E. Adams, E. Vigen, T. Tong, M. A. Alsina, J.-F. Gaillard, K. A. Gray, C. G. Peterson and J. J. Kelly, Chronic addition of a common engineered nanomaterial alters biomass, activity and composition of stream biofilm communities, Environ. Sci.: Nano, 2016, 3, 619–630 RSC.
- C. M. Wilke, B. Wunderlich, J.-F. Gaillard and K. A. Gray, Synergistic Bacterial Stress Results from Exposure to Nano-Ag and Nano-TiO2 Mixtures under Light in Environmental Media, Environ. Sci. Technol., 2018, 52, 3185–3194 CrossRef CAS.
- L. Canesi, G. Frenzilli, T. Balbi, M. Bernardeschi, C. Ciacci, S. Corsolini, C. Della Torre, R. Fabbri, C. Faleri, S. Focardi, P. Guidi, A. Kočan, A. Marcomini, M. Mariottini, M. Nigro, K. Pozo-Gallardo, L. Rocco, V. Scarcelli, A. Smerilli and I. Corsi, Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis, Aquat. Toxicol., 2014, 153, 53–65 CrossRef CAS.
- D. Sánchez-Qiles and A. Tovar-Sánchez, Are sunscreens a new environmental risk associated with coastal tourism?, Environ. Int., 2015, 83, 158–170 CrossRef.
- F. Larras, B. Montuelle, F. Rimet, N. Chèvre and A. Bouchez, Seasonal shift in the sensitivity of a natural benthic microalgal community to a herbicide mixture: impact on the protective level of thresholds derived from species sensitivity distributions, Ecotoxicology, 2014, 23, 1109–1123 CrossRef CAS.
- M. Ubertini, S. Lefebvre, C. Rakotomalala and F. Orvain, Impact of sediment grain-size and biofilm age on epipelic microphytobenthos resuspension, J. Exp. Mar. Biol. Ecol., 2015, 467, 52–64 CrossRef.
- C. Lavergne, M. Hugoni, C. Hubas, D. Debroas, C. Dupuy and H. Agogué, Diel rhythm does not shape the vertical distribution of bacterial and archaeal 16S rRNA transcript diversity in intertidal sediments: a mesocosm study, Microb. Ecol., 2018, 75, 364–374 CrossRef CAS.
- A. Carpentier, S. Como, C. Dupuy, C. Lefrançois and E. Feunteun, Feeding ecology of Liza spp. in a tidal flat: Evidence of the importance of primary production (biofilm) and associated meiofauna, J. Sea Res., 2014, 92, 86–91 CrossRef.
- N. S. Redzuan and G. J. C. Underwood, Movement of microphytobenthis and sediment betwee, mudflats and salt marsh during spring tides, Front Mar Sci, 2020, 7, 496 CrossRef.
- H. Schmidt, M. Thom, M. Madzgalla, S. U. Gerbersdorf, G. Metreveli and W. Manz, Exposure to Silver Nanoparticles Affects Biofilm Structure and Adhesiveness, Journal of Aquatic Pollution and Toxicology, 2017, 1, 2–9 Search PubMed.
- A. Y. Grün, J. Meier, G. Metreveli, G. E. Schaumann and W. Manz, Sublethal concentrations of silver nanoparticles affect the mechanical stability of biofilms, Environ. Sci. Pollut. Res., 2016, 23, 24277–24288 CrossRef.
- Heinz Centre for Science Economics and the Environment, Evaluation of erosion hazards, Washington, DC, 2000 Search PubMed.
- D. C. O. Thornton, G. J. C. Underwood and D. B. Nedwell, Effect of illumination and emersion period on the exchange of ammonium across the estuarine sediment-water interface, Mar. Ecol.: Prog. Ser., 1999, 184, 11–20 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en00440e |
| This journal is © The Royal Society of Chemistry 2020 |