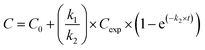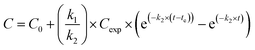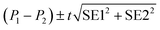Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparative biokinetics of pristine and sulfidized Ag nanoparticles in two arthropod species exposed to different field soils†
Iva
Talaber
 a,
Cornelis A. M.
Van Gestel
a,
Cornelis A. M.
Van Gestel
 b,
Anita
Jemec Kokalj
b,
Anita
Jemec Kokalj
 *a,
Gregor
Marolt
c,
Sara
Novak
a,
Primož
Zidar
a and
Damjana
Drobne
a
*a,
Gregor
Marolt
c,
Sara
Novak
a,
Primož
Zidar
a and
Damjana
Drobne
a
aBiotechnical Faculty, University of Ljubljana, Jamnikarjeva 101, Ljubljana, Slovenia. E-mail: anita.jemec@bf.uni-lj.si
bDepartment of Ecological Science, Faculty of Science, Vrije Universiteit, De Boelelaan 1085, 1081 HV Amsterdam, The Netherlands
cFaculty of Chemistry and Chemical technology, University of Ljubljana, Večna pot 113, Ljubljana, Slovenia
First published on 13th August 2020
Abstract
Silver nanoparticles (Ag NPs), incidentally or intentionally released to soil ecosystems, have the potential to accumulate in organisms. Due to the great variety of natural (field) soil and its interactions with Ag NPs, the few available biokinetic studies performed on standard soils are not sufficient for an environmentally relevant assessment of Ag NP bioaccumulation. In this study, we analyzed the biokinetics of pristine and sulfidized Ag NPs (Ag2S NPs) in two arthropod species with different routes of exposure to soil contaminants (isopod Porcellio scaber and springtail Folsomia candida) exposed to 4 different soils, widely differing in chemical and physical properties. Using the biokinetic constant of uptake as proxy for bioavailability, we found soil pH and soil texture to be the strongest predictors of Ag bioavailability to isopods and springtails, respectively. Both organisms accumulated Ag when exposed to pristine Ag NPs, which suggests a risk for food-chain accumulation of Ag. In contrast, no Ag bioaccumulation was detected in the case of the poorly soluble Ag2S NPs, which is the more environmentally relevant form of Ag NPs. Our study corroborates the dominant role of dissolution in Ag NP bioavailability and draws attention to the biological differences between different soil dwelling organisms modulating their Ag NP exposure.
Environmental significanceThe majority of silver nanoparticle (Ag NPs) biokinetic studies have tested environmentally less relevant pristine nanoparticles in standard soils, which does not reflect the situation in the field. In line with this, our key finding is that Ag NP bioavailability to isopods and springtails depends on the soil properties, mainly soil pH and texture, respectively, and is negligible for the environmentally relevant sulfidized Ag form. These findings are applicable to other soil dwelling organisms with similar routes of exposure and physiology. Our study provides the much-needed empirical data and a set of biokinetic constants for the validation of Ag NP bioavailability models based on specific soil properties and the parameterization of Ag NP uptake models. |
Introduction
Bioaccumulation of silver nanoparticles (Ag NPs) is of significant interest when studying their environmental fate. Bioaccumulation of NPs, i.e. their accumulation in or on organisms, depends on their bioavailability, defined as the ability to interact with an organism.1 The bioavailable fraction of Ag NPs is lower than their total concentration in most natural environments, particularly in soils,2 where it is affected by a complex interplay of soil properties and interactions with other ligands present in the soil solution. Ag NPs that reach the soil are mostly transformed by the environment and lose their pristine characteristics. The majority of released engineered Ag NPs pass through the anoxic environment of wastewater treatment plants where they can become sulfidized to poorly soluble Ag2S.3–5 Potential application of sewage sludge to agricultural soils results in the direct exposure of soil organisms to several different silver species; Ag2S NP, soluble pristine Ag NPs, and Ag ions.6 This means that the bioavailability of all Ag forms (species) has to be evaluated in order to understand and assess the bioaccumulation potential in soil dwelling organisms. Moreover, there are many indications that the bioavailability of pristine Ag NPs correlates with the amount of Ag ions released,2,7 indicating that the bioaccumulation of Ag in organisms exposed to Ag NPs is predominantly due to the bioavailability of Ag ions. The latter is dependent on the rate of dissolution of Ag NPs and the mobility of released Ag ions in the soil.Metal bioaccumulation is assessed with biokinetic studies of uptake and elimination that relate metal body concentrations to time of exposure to a certain metal NP concentration. To derive kinetic rate constants, biokinetic models of varying complexity can be applied, where different transformation processes and NP species can be included in the model descriptions.7,8 Due to analytical challenges in discriminating between ionic and particulate metal forms associated with NP exposure in soil and organisms, models based on total metal concentrations are still frequently applied. The simplest model considers the organism as a single compartment, but more compartments can be assumed, depending on the physiological traits of the test organism.7 It has been argued that the data obtained for soil invertebrates are frequently not precise enough to discriminate more than one compartment9 and that parameterization of simple models should be pursued.8
Studies of Ag NP uptake and elimination kinetics in soil organisms are scarce, and only a few of these considered the effect of soil properties.10,11 It is clear that differences in chemical and physical properties of natural soils have an effect on Ag NP bioavailability, especially their organic matter content, soil texture, ionic composition and pH (ref. 6) and that Ag NPs show a different fate in different environments.12
We performed biokinetic experiments on four different soils spiked with Ag NPs, in two model terrestrial arthropods susceptible to soil contamination: the springtail Folsomia candida (Collembola, Entognatha) and the woodlouse Porcellio scaber (Isopoda, Crustacea), inhabiting soils throughout the world. These animals were chosen to address differences in organism physiology and feeding behavior manifesting in different routes of exposure to Ag NPs in soil. Isopods are mainly exposed to soil contaminants through oral uptake of soil particles,13 and accumulate metals in proportion to their concentration in the soil.14,15 Springtails, however, are mainly exposed to contaminants in the pore water through the ventral tube,16 an organ on the first abdominal segment, with an important role in fluid and electrolyte balance capable of direct uptake of water.17 In some studies, metal uptake in springtails indeed was best explained with soil porewater concentrations,18,19 but other studies did not confirm this, suggesting that ingestion of soil particles and/or other food items (fungi, yeast, litter) may also play a role.20
The aim of this study was to investigate (i) bioaccumulation of pristine and sulfidized Ag NPs in two arthropod species, and (ii) differences in Ag NP bioavailability between different soils. We discuss the role of the ionic Ag form in the bioaccumulation of Ag NPs as well as how differences in feeding strategies and organism physiology influence their biokinetics.
Methods
Nanoparticles
All silver nanoparticles were obtained within the framework of the EC H2020 NanoFASE project and their characteristics have been described previously.21 Ag2S NPs (5.5 mM polyvinylpyrrolidone; 20.3 ± 9.8 nm; hereafter referred to as 20 nm Ag2S NPs) and Ag NPs (5.5 mM sodium citrate, 25 mM tannic acid, 47.3 ± 5.3 nm; hereafter referred to as 50 nm Ag NPs) were synthesized and supplied by Applied Nanoparticles SL (Barcelona, Spain). TEM images of stock Ag2S NPs and 50 nm Ag NPs and solutions are provided by Baccaro et al.21 Ag NPs (PVP-stabilized) of nominal size 3–8 nm were provided by Amepox Microelectronics (Poland). The characterization, including TEM images of these Ag2S NPs has been published previously.19 Different batches of 50 nm Ag NPs and Ag2S NPs were used for isopods and springtail tests while the 3–8 nm Ag NPs were only tested with isopods. Stock solutions of the different Ag forms were prepared in water by vortexing and added to the amount of soil needed to reach the desired nominal test concentration.The concentration of free Ag+ in working solutions of Ag NPs and Ag2S NPs (1 g L−1) was analyzed according to previously published protocol.22 Briefly, test dispersions were ultra-centrifuged in duplicate at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min at 20 °C (Beckman Coulter L8-70M class H preparative ultracentrifuge with Type 70.1 Ti rotor and 10 mL thick wall polyallomer tubes). The supernatants were acidified with 65% HNO3 (analytical grade purity; Fischer Scientific, Leicester, UK) and the total Ag content was quantified by flame AAS (AAS, Perkin Elmer Analyst 100, Waltham, Massachusetts, USA). Negligible concentrations of free Ag+ were measured, representing 0.008, 0.066 and 0.120% of the total Ag working concentration (1 g L−1) in case of 3–8 nm Ag NPs, 50 nm Ag NPs and Ag2S NPs, respectively. We thus consider that none of the working Ag solutions had evident excess of Ag+ that might originate from the synthesis.
000g for 30 min at 20 °C (Beckman Coulter L8-70M class H preparative ultracentrifuge with Type 70.1 Ti rotor and 10 mL thick wall polyallomer tubes). The supernatants were acidified with 65% HNO3 (analytical grade purity; Fischer Scientific, Leicester, UK) and the total Ag content was quantified by flame AAS (AAS, Perkin Elmer Analyst 100, Waltham, Massachusetts, USA). Negligible concentrations of free Ag+ were measured, representing 0.008, 0.066 and 0.120% of the total Ag working concentration (1 g L−1) in case of 3–8 nm Ag NPs, 50 nm Ag NPs and Ag2S NPs, respectively. We thus consider that none of the working Ag solutions had evident excess of Ag+ that might originate from the synthesis.
Test organisms
The terrestrial crustaceans Porcellio scaber (Crustacea: Isopoda), referred to as isopods, originated from a laboratory culture at the Department of Biology, University of Ljubljana (Večna pot 111, Ljubljana), originally derived from individuals collected from a compost heap in a non-polluted garden in Ljubljana, Slovenia. Prior to the tests, animals were acclimatized to the specific soil type for 14 d in a climate chamber at 22 ± 1 °C with a 16/8 h light/dark period of 120 and 16 lux, respectively (LI-1000 Data Logger, LI – COR, Nebraska, USA), while being fed with dry hazel leaves (Corylus avellana). Only adult animals (30–60 mg fresh body mass) of both sexes with intact antennae were used. Molting individuals and gravid females were excluded.Springtails of the species Folsomia candida (Entognatha: Isotomidae) have been cultured at the Department of Ecological Science of the Vrije Universiteit Amsterdam for many years. Cultures were kept in a climate room at 16 ± 1 °C, 75% relative humidity (RH) and a 16/8 h light/dark period. To obtain age-synchronized animals, adult springtails were placed in boxes with a moistened layer of plaster of Paris for 2 days. After removal of the adults and hatching of the eggs produced, the juveniles were allowed to grow until an age of 20–22 d before use in the tests. Age synchronizations were performed at 20 ± 1 °C, 75% RH and a 16/8 h light/dark period. The animals were fed dry baker's yeast. Unfortunately, age synchronizations of F. candida were unsuccessful in some cases and adults taken directly from the culture were used for tests on all three Ag forms in North Wales soil, on Ag2S NPs in Lufa 2.2 soil and on the 50 nm Ag NPs in Woburn soil.
Test soils
Four different soil types were used: standard Lufa 2.2 (LUFA, Speyer, Germany) and three field soils (hereafter referred to as North Wales, Woburn and Dorset) obtained within the framework of the EC H2020 NanoFASE project. The latter three soils were collected from different sites in the UK. Together, these soils represent a variety of soils properties (Table 1). All soils were sieved through 2 mm mesh and air-dried prior to use. The <2 mm fraction was used for the analysis of physical and chemical properties. Soil texture (as percentage of silt, sand and clay) was determined with Stokes setting,23 cation exchange capacity (CEC) with ammonium acetate extraction,24 Water holding capacity (WHC) according to OECD (2004)25 and organic matter content as loss of ignition at 500 °C in an ashing oven.26 pH-CaCl2 was measured at the beginning of the biokinetic experiment with a method described in Topuz and Gestel.11| Soil type | pH-CaCl2 | WHC (%) | Organic matter content (%) | Clay (%) | Silt (%) | Sand (%) | CEC (cmolc kg−1) |
|---|---|---|---|---|---|---|---|
| North Wales | 5.5 | 76.5–80.0 | 16.7 | 12.6 | 29.7 | 57.7 | 33.3 |
| Woburn | 6.9 | 31.9–32.1 | 1.8 | 12.6 | 11.8 | 75.6 | 13.3 |
| Lufa 2.2 | 6.0 | 43.3–45.0 | 4.2 | 15.3 | 17.4 | 67.3 | 9.7 |
| Dorset | 4.2 | 34.5–38.0 | 8.4 | 3.5 | 4.7 | 91.8 | 7.8 |
Among the four soils tested, Dorset soil was most acidic, followed by North Wales, Lufa 2.2 and Woburn. North Wales soil had a much higher organic matter content compared to the other soils, with Woburn having the lowest. North Wales had highest content of clay and Woburn had a highest proportion of bigger grains (sand) (Table 1).
Experimental set-up
Working Ag solutions were freshly prepared prior to each application into soil. Soil was spiked with 50 nm Ag NPs, 3–8 nm Ag NPs, 20 nm Ag2S NPs and ionic Ag to reach a nominal concentration of 10 μg Ag per g dry soil for all treatments. Each of the four batches of soil was further distributed to the test vials. This nominal concentration was chosen as being close to environmentally relevant.21 For the ionic Ag treatment, AgNO3 (99.5% pure, Sigma Aldrich, Steinheim, Germany) was used. In the test on springtails with AgNO3 in Dorset soil, due to a calculation error a nominal concentration of only 5.95 μg Ag per g was spiked into the soil. Soil, water and added Ag were mixed with a spatula for 10 minutes to assure a homogeneous mixture and sampled in duplicate for Ag concentration analysis. Water was later added to reach moisture contents of 40% or 50% of the WHC for the isopod and springtail tests, respectively. Soil samples, weighing 100 ± 5 mg each, were dried for 3 hours at 105 °C to reach a constant weight and stored in the desiccator until Ag concentration analyses.Biokinetic experiments with P. scaber were carried out for 6 weeks. For the first 3 weeks animals were exposed to spiked soil and afterwards transferred to clean soil for another 3 weeks, representing the uptake and elimination phase, respectively. For each soil type a control without NPs was included, and sampled in the same way as the other exposure groups. Each experimental unit consisted of a glass jar with a lid, containing 30 g moist soil from the same batch of spiked soil, a piece of dried hazel leaf (∼100 mg) and 5 randomly selected P. scaber specimens. For each exposure and the control, 8 experimental units were prepared for 8 sampling days. Samples were collected at days 1, 7, 14, 21 (uptake phase) and 22, 29, 35 and 42 (elimination phase) of experiment. An additional experimental unit was prepared for the control, and sampled at day 0. Collectively, we prepared 148 experimental units and used 740 isopods. On each sampling day all surviving animals from one jar were sampled and weighted. The gut was removed and the rest of the specimens (samples) were individually frozen at −20 °C for further analysis. During the experiment, additional soil samples were collected in duplicate (100 ± 5 mg) at days 7, 14, and 21, and processed in the same way as other soil samples.
Biokinetic experiment with F. candida followed OECD guideline 317 (OECD, 2010)27 and lasted 28 days, including a 14 day uptake phase in spiked soil followed by a 14 day elimination phase in clean soil. For each sampling time, three replicate test jars were prepared. Each test jar contained approximately 25 g dry-weight equivalent of moist soil, which moisture level was maintained by replenishing water loss on a weekly basis. Eight F. candida adult specimens (20–22 d old) were introduced into each test jar, and a few grains of dry baker's yeast were added for food. If necessary, additional yeast grains for food were added on a weekly basis. Test jars were kept in a climate room at 20 °C and a 12 h light/12 h dark cycle. During both the uptake and elimination phase, animals were sampled at 24 and 48 hours as well as 3 (or 4), 7, 10 and 14 days. Approximately 100 ml of tap water was added to a test jar causing the animals to be released from soil and float atop the water surface. All surviving animals from three replicate jars were transferred to plastic boxes containing a thin layer of plaster of Paris and allowed to empty their gut for one hour prior to storage. At each sampling time point, three animals were sampled from each of the three replicate jars, giving a total of nine animals, frozen at −20 °C, freeze dried for 24 hours and then weighed using a micro balance (Mettler Toledo GmbH).
Total Ag analysis
All samples were lyophilized and weighed before digestion.The extraction of total Ag from isopods and soil was performed by microwave assisted acid digestion in aqua regia (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 = 65% HNO3
4 = 65% HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36% HCl; analytical grade purity, Fisher Scientific, Loughborough, UK). Digestion was conducted in quartz inserts at 200 °C and 800 W power, with step 1 (heating) lasting 25 min, step 2 (constant temperature) lasting 15 min, and 45 min cooling to 60 °C (Milestone Ethos E microwave lab station; Milestone, Bergamo, Italy). Springtails were digested in a block heater using a 7
36% HCl; analytical grade purity, Fisher Scientific, Loughborough, UK). Digestion was conducted in quartz inserts at 200 °C and 800 W power, with step 1 (heating) lasting 25 min, step 2 (constant temperature) lasting 15 min, and 45 min cooling to 60 °C (Milestone Ethos E microwave lab station; Milestone, Bergamo, Italy). Springtails were digested in a block heater using a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of concentrated HNO3 (65%; Mallbaker Ultrex Ultra-Pure) and HClO4 (70%; Mallbaker Ultrex Ultra-Pure). The temperature was raised step-wise up to 180 °C and maintained until complete digestion and evaporation of all acid. If digestion was not complete, the procedure was repeated. Resulting sample residues were dissolved in 300 μL of 1 M HCl. Before analysis, the content of each tube was thoroughly mixed using a vortex mixer.
1 mixture of concentrated HNO3 (65%; Mallbaker Ultrex Ultra-Pure) and HClO4 (70%; Mallbaker Ultrex Ultra-Pure). The temperature was raised step-wise up to 180 °C and maintained until complete digestion and evaporation of all acid. If digestion was not complete, the procedure was repeated. Resulting sample residues were dissolved in 300 μL of 1 M HCl. Before analysis, the content of each tube was thoroughly mixed using a vortex mixer.
The total Ag concentration in soil and P. scaber samples was determined by flame atomic absorption spectroscopy (AAS) (Perkin-Elmer AAnalyst100, Massachusetts, USA). Springtail samples were analyzed by graphite furnace AAS (PinAAcle 900Z, Perkin Elmer, Germany) calibrated using Ag standards prior to analysis as well as after every 15 samples to ensure the accuracy of measurements. The LOD was 1.62 μg Ag per g animal dry weight.
Calculation of uptake and elimination kinetic rate constants
Silver uptake and elimination kinetics were described with the first-order one-compartment biokinetic model, which consists of two equations describing uptake and elimination of Ag:Uptake (t < te)
where C = silver concentration in the animals at time t (μg Ag per g dry body weight), C0 = Ag concentration in animals at time 0 (μg Ag per g dry body weight), Cexp = silver exposure concentration (μg Ag per g dry soil), k1 = uptake rate constant (g dry soil per g dry body weight per day), k2 = elimination rate constant (day−1), t = time (day), and te = time at which animals were transferred to clean soil (day). For C0 we used the average Ag concentration in control animals, that was 6.6 ± 2.3 and 0.03 ± 0.01 μg Ag per g dry body weight for isopods and springtails, respectively.
Both equations were fitted to the data of the uptake and elimination phases simultaneously, using the user-defined non-linear fitting OriginC type function in the OriginPro 8.5 software, which estimated the parameters k1 and k2 and corresponding standard errors with the iteration procedure.
For each parameter estimated by the model, the respective 95% confidence interval (95% CI) was calculated. Often, the lower limit of the 95% CI was negative. This means, that there was insufficient evidence to conclude that a parameter is significantly different from zero at the 95% level of confidence (p < 0.05). In these cases, it was assumed that the parameter value was zero.
95% confidence intervals for parameters were calculated as:
| P ± t(0.05, n − df) × SE |
Statistically significant difference between parameters (P1, P2) was evaluated with the standard method:28
When the estimate of k2 (elimination rate constants) was not significantly different from zero, we calculated k1 again, because k1 and k2 are dependent. Hence, to derive a better estimate of the uptake rate constant (k1), we applied the model again by fixing k2 close to zero (assigning a value of 0.00001). We chose this approach to be able to apply the same model to all datasets.
Uptake rate constants (k1) were related to soil properties by Pearson correlation and multidimensional scaling (MDS) analysis, performed with the XLSTAT software in Microsoft Excel.
Results
Total Ag concentrations in the test soils
Measured total Ag concentrations in the test soils are presented in Table S1.† We report the total Ag concentrations at the start of the experiment, which were used as Cexp in the biokinetic model. In the isopod tests we sampled the soil each consecutive week during the uptake phase. Total Ag concentrations in the test soils did not vary more than 10% (data not shown).Uptake and elimination kinetics of Ag in different exposures
P. scaber Ag body concentrations from exposures to ionic Ag and pristine Ag NPs showed an increase during exposure to spiked soil and mostly no evident decrease after transfer to clean soil (Fig. 1). No Ag bioaccumulation was detected in P. scaber exposed to Ag2S NPs, as Ag body concentrations showed no clear temporal increase (Fig. 1), were not significantly different from control values (Fig. S1†) and the estimated parameters k1 and k2 were not significantly different from zero (Table 2).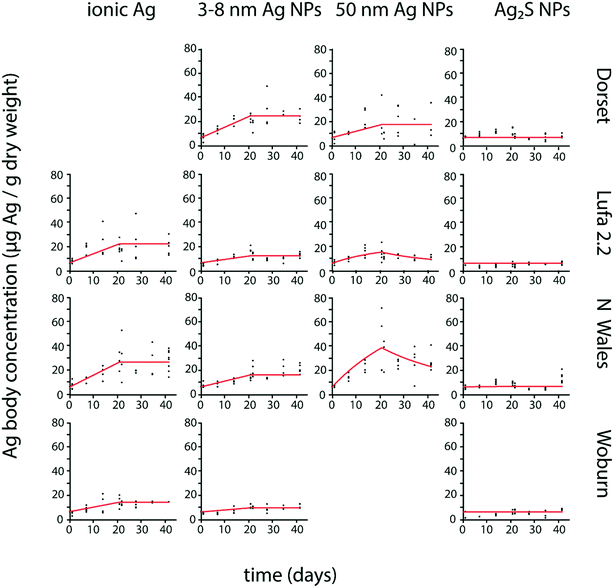 | ||
| Fig. 1 Uptake and elimination kinetics of Ag in Porcellio scaber exposed to different forms of Ag (ionic Ag, 3–8 nm Ag NPs, 50 nm Ag NPs and 20 nm Ag2S NPs) in four different test soils (Dorset, Lufa 2.2, North Wales, Woburn). Uptake and elimination period lasted 21 days each. Estimated kinetic parameters are shown in Table 2. | ||
| Soil type | Ionic Ag | 50 nm Ag NPs | 3–8 nm Ag NPs | 20 nm Ag2S NPs | |||||
|---|---|---|---|---|---|---|---|---|---|
| k 1 | k 2 | k 1 | k 2 | BAF | k 1 | k 2 | k 1 | k 2 | |
| Lufa 2.2 | 0.057 (0.043–0.071) | 0 | 0.051 (0.031–0.071) | 0.049 (0.017–0.081) | 1.1 | 0.021 (0.015–0.027) | 0 | 0 | 0 |
| Woburn | 0.028 (0.022–0.031) | 0 | — | 0.017 (0.011–0.023) | 0 | 0 | 0 | ||
| North Wales | 0.064 (0.056–0.076) | 0 | 0.141 (0.089–0.193) | 0.031 (0.009–0.053) | 4.5 | 0.036 (0.030–0.042) | 0 | 0 | 0 |
| Dorset | — | 0.051 (0.032–0.070) | 0 | — | 0.086 (0.075–0.097) | 0 | 0 | 0 | |
Except for a few cases (Table 3), k1 and k2 values were significantly different from zero for F. candida exposed to all tested Ag forms, and Ag body concentrations showed an increase, mostly followed by a decrease after transfer to clean soil (Fig. 2).
| Soil type | Ionic Ag | 50 nm Ag NPs | 20 nm Ag2S NPs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k 1 | k 2 | BAF | k 1 | k 2 | BAF | k 1 | k 2 | BAF | |
| Lufa 2.2 | 0.063 (0.042–0.085) | 0.100 (0.050–0.150) | 0.63 | 0 | 0 | 0 | 0.016 (0.004–0.026) | 0.676 (0.163–1.189) | 0.02 |
| Woburn | 0.152 (0.111–0.193) | 0.047 (0.023–0.071) | 3.23 | 0.203 (0.065–0.341) | 0.263 (0.066–0.460) | 0.77 | 0.016 (0.009–0.023) | 0.148 (0.077–0.218) | 0.11 |
| North Wales | 0 | 0 | — | 0.010 (0.007–0.013) | 0 | — | 0 | 0 | — |
| Dorset | 0.276 (0.182–0.370) | 0.097 (0.051–0.143) | 2.85 | 0.381 (0.264–0.499) | 0.033 (0.005–0.061) | 11 | 0.164 (0.018–0.309) | 1.514 ± (0.065–2.963) | 0.11 |
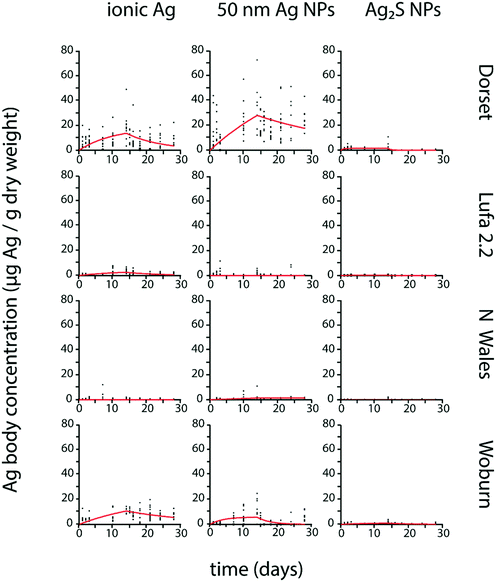 | ||
| Fig. 2 Uptake and elimination kinetics of Ag in Folsomia candida exposed to different forms of Ag (ionic Ag, 50 nm Ag NPs, and 20 nm Ag2S NPs) in four different test soils (Dorset, Lufa 2.2, North Wales, Woburn). Uptake and elimination period lasted 14 days each. Estimated kinetic parameters are shown in Table 3. | ||
Differences in biokinetic parameters between soil types
The highest k1 for ionic Ag in P. scaber was found in North Wales and Lufa 2.2 followed by Woburn. The Dorset soil was not tested. In F. candida, the highest k1 for ionic Ag was estimated in Dorset, followed by Woburn and Lufa 2.2. No significant Ag uptake was detected in North Wales (Table 3).
The highest k1 for 3–8 nm Ag NPs in P. scaber was estimated in Dorset, followed by North Wales and lastly Lufa 2.2 and Woburn (Table 2). The uptake of 3–8 nm AgNPs in F. candida was not tested.
The highest k1 for 50 nm Ag NP uptake in P. scaber was found in North Wales, followed by Dorset and Lufa 2.2. Woburn soil was not tested (Table 2). The highest k1 for 50 nm Ag NPs in F. candida was estimated in Dorset, followed by Woburn and lastly North Wales. No uptake was detected in Lufa 2.2 (Table 3).
In P. scaber, no uptake was detected in case of Ag2S NP exposure in any of the soils, as k1 was 0 in all soils (Table 2). In F. candida, the highest k1 for Ag2S NP uptake was estimated in Dorset, much lower k1 was estimated in Woburn and Lufa 2.2. No uptake was detected in North Wales (Table 3).
In F. candida, k2 for ionic Ag was highest in Dorset and Lufa 2.2, followed by Woburn. No elimination was detected in North Wales (Table 3). The highest k2 for 50 nm Ag NPs from F. candida was estimated in Woburn, and much lower in Dorset. No elimination was detected in Lufa 2.2 and North Wales (Table 3).
In F. candida, the highest k2 for Ag2S NP elimination was found in Dorset and Lufa 2.2, while a much lower k2 was estimated for Woburn. No elimination was detected in North Wales (Table 3).
Relationship between uptake rate constant and soil properties
The relationship of k1 and soil properties was analyzed with Pearson correlation (Table 4) and visualized with the MDS plot (Fig. 3). Only a few correlations were statistically significant. Scatter plots of k1 with chosen variables are shown in Fig. S3.† In isopods, the k1 for 3–8 nm Ag NPs was negatively correlated with pH and clay content of the test soils. The same trend was found for ionic Ag, although only three soils were tested (Fig. 3 and S3†). In springtails, k1 for all Ag forms was negatively correlated with clay content and positively with sand content of the test soils. In either organism, k1 did not significantly correlate with organic matter content or CEC (Table 4 and Fig. S3†).| P.s ionic Ag | P.s.50nm Ag NPs | P.s.3–8nm Ag NP | F.c. ionic Ag | F.c.50nm Ag NPs | F.c. Ag2S NPs | pH | OM | CEC | Clay | Silt | Sand | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P.s. ionic Ag | 1.00 | |||||||||||
| P.s.50nm Ag NPs | 0.06 | 1.00 | ||||||||||
| P.s.3–8nm Ag NPs | 0.97 | 0.00 | 1.00 | |||||||||
| F.c. ionic Ag | 0.58 | −0.66 | 0.70 | 1.00 | ||||||||
| F.c.50nm Ag NPs | 0.58 | −0.51 | 0.74 | 0.97 | 1.00 | |||||||
| F.c. Ag2S NPs | 0.88 | −0.35 | 0.94 | 0.89 | 0.88 | 1.00 | ||||||
| pH | −0.99 | −0.21 | −0.95 | −0.46 | −0.49 | −0.81 | 1.00 | |||||
| OM | 0.36 | 0.95 | 0.32 | −0.41 | −0.25 | −0.03 | −0.50 | 1.00 | ||||
| CEC | −0.23 | 0.94 | −0.24 | −0.73 | −0.55 | −0.55 | 0.09 | 0.81 | 1.00 | |||
| Clay | −0.85 | 0.16 | −0.96 | −0.84 | −0.90 | −0.95 | 0.81 | −0.15 | 0.32 | 1.00 | ||
| Silt | −0.40 | 0.85 | −0.50 | −0.95 | −0.86 | −0.77 | 0.27 | 0.65 | 0.89 | 0.65 | 1.00 | |
| Sand | 0.60 | −0.68 | 0.71 | −0.99 | 0.95 | 0.90 | −0.49 | −0.42 | −0.77 | −0.83 | −0.96 | 1.00 |
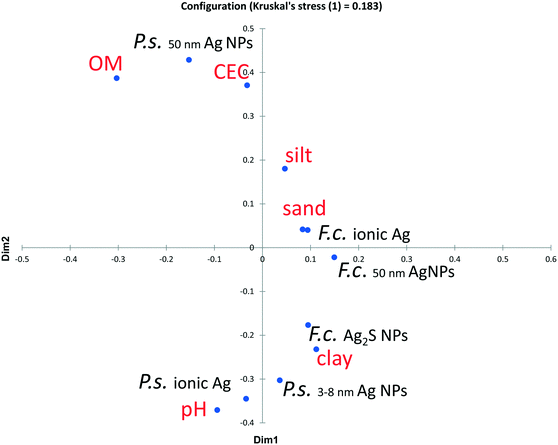 | ||
| Fig. 3 Multidimensional scaling analysis (MDS plot) of the Pearson correlation matrix presented in Table 4. The proximity of elements in the plot indicates the strength of Pearson correlation, being either positive or negative. P.s. – Porcellio scaber, F.c. – Folsomia candida, OM – organic matter content and CEC – cation exchange capacity. | ||
Discussion
This study showed clear differences in Ag biokinetics depending on the Ag form, soil type and test organism. Ag uptake (temporal increase in Ag body concentration upon exposure to spiked soil) was recorded in both organisms following exposure to pristine Ag NPs and ionic Ag, while in the case of the sulfidized Ag2S NPs no uptake of Ag in the isopod P. scaber and only slight uptake of Ag in the springtail F. candida was recorded. A different Ag biokinetic response was observed for the two species; generally, the Ag taken up by F. candida was eliminated to some extent after transfer to clean soil, whereas in P. scaber in most cases no significant elimination was detected. Consequently, bioaccumulation of Ag in P. scaber was higher compared to F. candida in all cases, except for the sulfidized Ag2S NPs. It has to be emphasized that although the spiked soil was most probably ingested by the isopods in all exposures including the exposure to Ag2S NPs, the material that merely passes the gut does not contribute to body Ag concentration as the gut was removed before analysis. Hence in our study, uptake and elimination are not synonymous with consumption and defecation, respectively, but uptake is nevertheless related to the ingestion of the soil.Uptake rate constants varied both between the Ag forms and the soil types for each test species. Each of these results will be discussed below. Significant variation in metal uptake rate constants is frequently reported especially for soil invertebrates,9 because total metal concentrations in the bulk soil or soil solution do not accurately reflect the bioavailable metal fraction. In our study, total Ag concentration in soil was used as the measure of exposure in the biokinetic model, which means that different estimates of the uptake rate constant reflect differences in Ag bioavailability between different test soils.29 Bioavailable Ag in case of exposure to Ag NPs can include both Ag ions and NPs, where NPs can also be bioavailable in terms of attaching to body surfaces of the test organisms.
Biokinetics of Ag depends largely on the Ag form
Despite the variation in biokinetic parameters across different soil types observed in both test species, some general patterns did appear, reflecting the influence of Ag form on the Ag biokinetics. The highest uptake in both organisms was found following exposures to ionic Ag and pristine Ag NPs. While in isopods, Ag uptake rate constants were significantly higher for ionic Ag than for pristine Ag NPs (3–8 nm), in springtails they mostly did not significantly differ between ionic Ag and Ag NPs (50 nm). Both for ionic and pristine Ag NPs, it is assumed that uptake of Ag is predominantly due to the bioavailability of free Ag ions.2,7 This interpretation is consistent with the results of this study. Firstly, uptake rate constants for ionic Ag and pristine Ag NPs across different soils were significantly correlated in both isopods and springtails. Secondly, in the most acidic Dorset soil, where the highest dissolution of pristine Ag NPs is expected,30 isopod uptake rate constant for the 3–8 nm Ag NPs was higher compared to 50 nm Ag NPs, which could be due to a higher dissolution rate expected for smaller NPs having a higher surface-to-volume ratio.31 However, further analysis of Ag in pore water would be needed to support this suggestion.A marked distinction between ionic Ag and pristine Ag NPs, as opposed to sulfidized Ag2S NPs was observed. Upon exposure to Ag2S NPs, the Ag uptake (i.e. a temporal increase in Ag body concentration) was much lower in springtails and undetectable in isopods. The absence of Ag uptake upon exposure to the presumably insoluble Ag2S NPs was expected for the isopod, since there is considerable evidence that metal uptake in P. scaber exposed to metal NPs is due to ions released.32,33 However, Kampe et al.34 recently reported Ag2S in sewage sludge was taken up by P. scaber in their study. Nevertheless, they proposed that the Ag2S in their experimental set-up may have been sparingly soluble and implied the role of the ionic form in Ag uptake. Analogously, the same could be argued for the detected Ag uptake in springtails upon exposure to Ag2S NPs, considering that it was highest in the acidic Dorset soil, where a higher dissolution is expected30 and Ag uptake was at least an order of magnitude lower compared to that of pristine Ag NPs and ionic Ag in every tested soil. Clearly, under the assumption that Ag2S NPs are sparingly soluble, the dissolved Ag ions would also be taken up by the isopods, but the amount of assimilated Ag in the isopods' storage tissue (the hepatopancreas) in this case might be too low to be distinguished from the relatively high Ag background level in our test isopods (Fig. S1†). We acknowledge that the average Ag concentration in control isopods (6.6 ± 2.3 μg Ag per g dry body weight (n = 153)) observed in this study is higher than the only other published value (a closely related species Porcellionides pruinosus (2.3 ± 1.3 μg Ag per g dry body weight (n = 3))35), but this most probably represents the natural variability in Ag background concentration in isopods. It must be pointed out however, that if we assume that Ag2S NPs in our study were sparingly soluble, prolonging the time of exposure to Ag2S NPs might result in a detectable Ag uptake in isopods. However, no conclusions can be made without proof of Ag2S NP dissolution. With this, the uptake of particulate Ag in case of springtails cannot be excluded. Nevertheless, based on the estimated uptake rate constants in isopods (Table 2) and springtails (Table 3), it can be concluded that Ag2S NPs are less bioavailable compared to pristine Ag NPs.
Although generally Ag was not eliminated from the isopods, elimination was detected in the case of the 50 nm Ag NPs in Lufa 2.2 and North Wales soils. This suggests some particle-specific interaction with either the organism or the soil, presumably connected to hetero- or homo-aggregation of these particles. It is frequently assumed that the metal elimination rate constant is a property intrinsic to the organism and not influenced by soil properties or the form of Ag. The first was for instance shown for the earthworm Eisenia andrei,36 the second for the isopod P. pruinosus35 and the earthworm Eisenia fetida.21 Hence, the Ag elimination in isopods observed in only two cases might not reflect the elimination of Ag from the storage tissue, but rather the elimination of the material adsorbed/attached to body surface, which could be dependent on the chemical and physical properties of both the soil and the NPs tested. This however, needs further investigation, in particular as to why this was not the case for the smaller sized Ag NPs (3–8 nm). In addition to particle size, also the type of the coating material may affect the aggregation and dissolution behaviour of NPs,37 and therefore these aspects might affect its biokinetics.
Biokinetics of Ag depends on test species physiology
We observed different Ag biokinetic responses in isopods and springtails. The most evident difference was that isopods generally accumulated Ag, while springtails generally eliminated the Ag taken up to a great extent, corresponding to high and low bioaccumulation factors (BAF), respectively. P. scaber is known to accumulate high amounts of metal in the hepatopancreas, providing isopods with a successful sequestration mechanism allowing the storage of excessive amounts of ingested metals in metal granules located in the hepatopancreas.32 Evidently, the capacity for Ag storage was not yet exceeded at the exposure concentrations employed in this study, which are considered low32 and environmentally relevant. This is in line with results of the biokinetic study with another isopod species, P. pruinosus, exposed to a higher concentration of Ag (30 and 60 μg Ag per g dry soil), where accumulation was observed of both ionic Ag and of the same 3–8 nm pristine Ag NPs as used in our study.35 In a previous study on the biokinetics of these 3–8 Ag NPs in F. candida, Ag elimination was generally higher in case of particulate Ag compared to ionic Ag collectively resulting in a higher bioaccumulation factor (BAF) for the ionic Ag exposure.19 The same was observed for F. candida in our study, despite relatively large variation in elimination rate constants. The only exception was the lower elimination estimated for 50 nm Ag NP exposure in the acidic Dorset soil, where the BAF was much higher than for any other exposure.The total Ag body concentrations during the uptake phase generally were higher in isopods compared to springtails. This agrees with the fact that among terrestrial invertebrates, isopods are known to be among those with the highest metal-storing capacities.15 Lastly, the most evident difference between the two species is in their route of metal uptake. While isopods are exposed to Ag mainly orally through ingestion of soil particles,13 springtails are mainly exposed to metals in the pore water.16 Accordingly, less Ag is taken up by the springtail, because only a small fraction of the total Ag concentration is present in the pore water and as such bioaccessible, as opposed to the isopod, where total soil Ag is consumed. It has been argued that complexation of Ag ions with soil organic matter might increase Ag bioavailability to soil ingesting organisms, such as isopods. When organic matter is consumed and digested, the presence of surfactant molecules and specific gut lumen conditions and its microbial community might facilitate the release of Ag into solution.36 It has also been demonstrated that metals which are unavailable for chemical extraction are available and are accumulated by P. scaber, meaning that isopods are probably able to acquire metals from several soil fractions, not just the water-soluble one.38 Additionally, as bioavailability of metals from ingested soil is expected to be highly dependent on the pH in the different compartments of the gastrointestinal tract, being generally larger at low pH,39 the slightly acidic conditions in the intestinal tract of P. scaber40 could contribute to the high bioavailability of Ag to isopods in the organic soil observed in this study.
Effect of soil properties on Ag bioavailability
Fully exploring the role of soil properties on Ag bioavailability would require testing a much larger number of soils. Nevertheless, this study may be seen as a first step in this direction, using a sample of soils exhibiting a wide range in specific soil properties, with an interpretation based on the aspects of the mobility of ionic and particulate Ag in soils. Silver is generally expected to be more mobile in mineral compared to organic soils and more dissolved Ag ions are expected at low pH.30,41,42 The mobility of Ag NPs appears to be lower in soils with higher clay content,42 implying a higher mobility in sandier soils.Among the soils tested in our study, Dorset soil was most acidic, had the highest sand content and lowest content of clay. Indeed, in this soil highest Ag uptake was observed in F. candida for all Ag forms tested and in P. scaber for the 3–8 nm Ag NPs. Considering the significant positive correlation between the k1 values for ionic Ag and 3–8 nm Ag NPs, we presume that the uptake of ionic Ag would also be highest in the Dorset soil in case of P. scaber, had it been tested. Moreover, the higher Ag bioavailability in Dorset soil compared to the other soils is most probably due to a higher dissolution of Ag NPs and higher Ag mobility. Ag NPs have been reported to preferentially interact with granulometric clays,42 implying a decreased bioavailability in soils with high clay contents. Accordingly, a negative correlation of k1 values and clay content was observed for all Ag forms in F. candida and for 3–8 nm Ag NPs in P. scaber (Table 4).
The negative relationship of Ag bioavailability and pH was more pronounced in P. scaber, where uptake of ionic Ag and 3–8 nm Ag NPs was high also in the second most acidic soil, the North Wales soil. This further supports the higher mobility of Ag NPs at low pH. On the contrary, in F. candida uptake was lowest in this soil. Incidentally, this soil had the highest organic matter content. Although there was no clear relationship between k1 values and organic matter content in either organism (Table 4 and Fig. S4†), Ag was least bioavailable to F. candida in the soil with the highest organic matter content. This is presumably due to complexation of Ag with organic matter,30,43 and consequent decreased Ag concentration in the pore water. As argued above, the bioavailability of Ag to P. scaber in this situation might be higher compared to F. candida, due to larger contribution of soil constituents in the ingested soil.
In conclusion, we demonstrated that compared to pristine Ag NPs, sulfidized Ag2S NPs are considerably less bioavailable to both isopods and springtails. From an environmental perspective this is a positive finding, since most Ag NPs released to the environment become sulfidized with time and less bioavailable. Isopods and springtails have different Ag-bioaccumulation capacity, depending on their metal metabolism and feeding habits, which calls for integrating data from different test species. We clearly showed that for a single organism, Ag bioaccumulation upon exposure to pristine Ag NPs differs widely between different field soils and is higher in acidic sandy soils. Other soil properties, especially organic matter content, contribute to differences in Ag bioavailability between organisms. Adsorption of Ag NPs to body surfaces presumably contributes to the variation in Ag elimination dynamics observed in both species and should be addressed in future biokinetic studies.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Thanks are due to Silas Pape and Rudo A. Verweij for performing the springtail tests, Katja Lobe and Neja Medvešek for performing the isopod tests and Geert Cornelis for sharing the results of the analyses of physical and chemical soil properties performed at the Swedish University of Agricultural Science. This study was funded by the EU H2020 project NanoFASE (Nanomaterial Fate and Speciation in the Environment; grant no. 646002) and supported by the Slovenian Research Agency (ARRS) through research programmes P1-0153 and P1-0184.References
- E. J. Petersen, M. Mortimer, R. M. Burgess, R. Handy, S. Hanna, K. T. Ho, M. Johnson, S. Loureiro, H. Selck and J. J. Scott-Fordsmand, Strategies for robust and accurate experimental approaches to quantify nanomaterial bioaccumulation across a broad range of organisms, Environ. Sci.: Nano, 2019, 6, 1619–1656 RSC
.
- G. Cornelis, K. Hund-Rinke, T. Kuhlbusch, N. Van den Brink and C. Nickel, Fate and bioavailability of engineered nanoparticles in soils: a review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2720–2764 CrossRef CAS
.
- C. Levard, E. M. Hotze, B. P. Colman, A. L. Dale, L. Truong, X. Y. Yang, A. J. Bone, G. E. Brown Jr, R. L. Tanguay and R. T. Di Giulio, Sulfidation of silver nanoparticles: natural antidote to their toxicity, Environ. Sci. Technol., 2013, 47, 13440–13448 CrossRef CAS PubMed
.
- B. Thalmann, A. Voegelin, B. Sinnet, E. Morgenroth and R. Kaegi, Sulfidation kinetics of silver nanoparticles reacted with metal sulfides, Environ. Sci. Technol., 2014, 48, 4885–4892 CrossRef CAS PubMed
.
- P. Wang, N. W. Menzies, E. Lombi, R. Sekine, F. P. C. Blamey, M. C. Hernandez-Soriano, M. Cheng, P. Kappen, W. J. G. M. Peijnenburg and C. Tang, Silver sulfide nanoparticles (Ag2S-NPs) are taken up by plants and are phytotoxic, Nanotoxicology, 2015, 9, 1041–1049 CrossRef PubMed
.
- M. Bundschuh, J. Filser, S. Lüderwald, M. S. McKee, G. Metreveli, G. E. Schaumann, R. Schulz and S. Wagner, Nanoparticles in the environment: where do we come from, where do we go to?, Environ. Sci. Eur., 2018, 30, 1–17 CrossRef CAS PubMed
.
- N. W. Van Den Brink, A. J. Kokalj, P. V. Silva, E. Lahive, K. Norrfors, M. Baccaro, Z. Khodaparast, S. Loureiro, D. Drobne and G. Cornelis, Tools and rules for modelling uptake and bioaccumulation of nanomaterials in invertebrate organisms, Environ. Sci.: Nano, 2019, 6, 1985–2001 RSC
.
- M. Baalousha, G. Cornelis, T. A. J. Kuhlbusch, I. Lynch, C. Nickel, W. Peijnenburg and N. W. Van Den Brink, Modeling nanomaterial fate and uptake in the environment: current knowledge and future trends, Environ. Sci.: Nano, 2016, 3, 323–345 RSC
.
- M. M. Ardestani, N. M. van Straalen and C. A. M. van Gestel, Uptake and elimination kinetics of metals in soil invertebrates: a review, Environ. Pollut., 2014, 193, 277–295 CrossRef CAS PubMed
.
- W. A. Shoults-Wilson, B. C. Reinsch, O. V. Tsyusko, P. M. Bertsch, G. V. Lowry and J. M. Unrine, Role of particle size and soil type in toxicity of silver nanoparticles to earthworms, Soil Sci. Soc. Am. J., 2011, 75, 365–377 CrossRef CAS
.
- E. Topuz and C. A. M. van Gestel, The effect of soil properties on the toxicity and bioaccumulation of Ag nanoparticles and Ag ions in Enchytraeus crypticus, Ecotoxicol. Environ. Saf., 2017, 144, 330–337 CrossRef CAS PubMed
.
- L. Degenkolb, M. Kaupenjohann and S. Klitzke, The Variable Fate of Ag and TiO 2 Nanoparticles in Natural Soil Solutions—Sorption of Organic Matter and Nanoparticle Stability, Water, Air, Soil Pollut., 2019, 230, 62 CrossRef
.
- M. G. Vijver, H. T. Wolterbeek, J. P. M. Vink and C. A. M. van Gestel, Surface adsorption of metals onto the earthworm Lumbricus rubellus and the isopod Porcellio scaber is negligible compared to absorption in the body, Sci. Total Environ., 2005, 340, 271–280 CrossRef CAS PubMed
.
- A. Heikens, W. Peijnenburg and A. J. Hendriks, Bioaccumulation of heavy metals in terrestrial invertebrates, Environ. Pollut., 2001, 113, 385–393 CrossRef CAS PubMed
.
- S. P. Hopkin, D. T. Jones and D. Dietrich, The isopod Porcellio scaber as a monitor
of the bioavailability of metals in terrestrial ecosystems: towards a global ‘woodlouse watch’scheme, Sci. Total Environ., 1993, 134, 357–365 CrossRef
.
- M. T. Fountain and S. P. Hopkin, Folsomia candida (Collembola): a “standard” soil arthropod, Annu. Rev. Entomol., 2005, 50, 201–222 CrossRef CAS PubMed
.
- C.-G. Chen, T. Chen, B.-Z. Hua and T.-R. Wan, Structure and functions of the ventral tube of the clover springtail Sminthurus viridis (Collembola: Sminthuridae), Sci. Rep., 2019, 9, 1–6 CrossRef PubMed
.
- C. E. Smit and C. A. M. Van Gestel, Effects of soil type, prepercolation, and ageing on bioaccumulation and toxicity of zinc for the springtail Folsomia candida, Environ. Toxicol. Chem., 1998, 17, 1132–1141 CrossRef CAS
.
- P. L. Waalewijn-Kool, K. Klein, R. M. Forniés and C. A. M. van Gestel, Bioaccumulation and toxicity of silver nanoparticles and silver nitrate to the soil arthropod Folsomia candida, Ecotoxicology, 2014, 23, 1629–1637 CrossRef CAS PubMed
.
- M. Vijver, T. Jager, L. Posthuma and W. Peijnenburg, Impact of metal pools and soil properties on metal accumulation in Folsomia candida (Collembola), Environ. Toxicol. Chem., 2001, 20, 712–720 CrossRef CAS PubMed
.
- M. Baccaro, A. K. Undas, J. de Vriendt, J. H. J. Van Den Berg, R. J. B. Peters and N. W. van den Brink, Ageing, dissolution and biogenic formation of nanoparticles: how do these factors affect the uptake kinetics of silver nanoparticles in earthworms?, Environ. Sci.: Nano, 2018, 5, 1107–1116 RSC
.
- T. Romih, A. Jemec, M. Kos, S. B. Hocevar, S. Kralj, D. Makovec and D. Drobne, The role of PVP in the bioavailability of Ag from the PVP-stabilized Ag nanoparticle suspension, Environ. Pollut., 2016, 218, 957–964 CrossRef CAS PubMed
.
-
Methods of soil analysis, part 3: Chemical methods, ed. D. L. Sparks, A. L. Page, P. A. Helmke and R. H. Loeppert, 2020, vol. 14, John Wiley & Sons Search PubMed
.
- L. van Reeuwijk, Procedures for Soil Analysis - sixth edition, ISRIC Technical Papers, 2002, Wageningen, the Netherlands, ISRIC Search PubMed.
-
Oecd, Guideline for the testing of chemicals No. 222. Earthworm reproduction test (Eisenia fetida/Eisenia andrei), 2004 Search PubMed
.
- S. I. F. Standarder, Vattenundersökningar - Bestämning av torrsubstans och glödgningsrest i vatten, slam och sediment, Svenska Institutet för standarder, Stockholm, 1981 Search PubMed.
-
T. N. Oecd, 317: Bioaccumulation in Terrestrial Oligochaetes, Guidelines for the Testing of Chemicals, 2010 Search PubMed
.
- N. Schenker and J. F. Gentleman, On judging the significance of differences by examining the overlap between confidence intervals, Am. Stat., 2001, 55, 182–186 CrossRef
.
- K. Veltman, M. A. J. Huijbregts, M. G. Vijver, W. J. G. M. Peijnenburg, P. H. F. Hobbelen, J. E. Koolhaas, C. A. M. van Gestel, P. C. J. van Vliet and A. J. Hendriks, Metal accumulation in the earthworm Lumbricus rubellus. Model predictions compared to field data, Environ. Pollut., 2007, 146, 428–436 CrossRef CAS PubMed
.
- R. Benoit, K. J. Wilkinson and S. Sauvé, Partitioning of silver and chemical speciation of free Ag in soils amended with nanoparticles, Chem. Cent. J., 2013, 7, 75 CrossRef PubMed
.
- T. S. Peretyazhko, Q. Zhang and V. L. Colvin, Size-controlled dissolution of silver nanoparticles at neutral and acidic pH conditions: kinetics and size changes, Environ. Sci. Technol., 2014, 48, 11954–11961 CrossRef CAS PubMed
.
- Ž. P. Tkalec, D. Drobne, K. Vogel-Mikuš, P. Pongrac, M. Regvar, J. Štrus, P. Pelicon, P. Vavpetič, N. Grlj and M. Remškar, Micro-PIXE study of Ag in digestive glands of a nano-Ag fed arthropod (Porcellio scaber, Isopoda, Crustacea), Nucl. Instrum. Methods Phys. Res., Sect. B, 2011, 269, 2286–2291 CrossRef
.
- S. Novak, D. Drobne, M. Golobič, J. Zupanc, T. Romih, A. Gianoncelli, M. Kiskinova, B. Kaulich, P. Pelicon and P. Vavpetič, Cellular internalization of dissolved cobalt ions from ingested CoFe2O4 nanoparticles: in vivo experimental evidence, Environ. Sci. Technol., 2013, 47, 5400–5408 CrossRef CAS PubMed
.
- S. Kampe, R. Kaegi, K. Schlich, C. Wasmuth, H. Hollert and C. Schlechtriem, Silver nanoparticles in sewage sludge: Bioavailability of sulfidized silver to the terrestrial isopod Porcellio scaber, Environ. Toxicol. Chem., 2018, 37, 1606–1613 CrossRef CAS PubMed
.
- P. S. Tourinho, C. A. M. van Gestel, A. J. Morgan, P. Kille, C. Svendsen, K. Jurkschat, J. F. W. Mosselmans, A. M. V. M. Soares and S. Loureiro, Toxicokinetics of Ag in the terrestrial isopod Porcellionides pruinosus exposed to Ag NPs and AgNO 3 via soil and food, Ecotoxicology, 2016, 25, 267–278 CrossRef CAS PubMed
.
- M. Diez-Ortiz, E. Lahive, P. Kille, K. Powell, A. J. Morgan, K. Jurkschat, C. A. M. Van Gestel, J. F. W. Mosselmans, C. Svendsen and D. J. Spurgeon, Uptake routes and toxicokinetics of silver nanoparticles and silver ions in the earthworm Lumbricus rubellus, Environ. Toxicol. Chem., 2015, 34, 2263–2270 CrossRef CAS PubMed
.
- A. R. Whitley, C. Levard, E. Oostveen, P. M. Bertsch, C. J. Matocha, F. von der Kammer and J. M. Unrine, Behavior of Ag nanoparticles in soil: effects of particle surface coating, aging and sewage sludge amendment, Environ. Pollut., 2013, 182, 141–149 CrossRef CAS PubMed
.
- M. Udovic, D. Drobne and D. Lestan, Bioaccumulation in Porcellio scaber (Crustacea, Isopoda) as a measure of the EDTA remediation efficiency of metal-polluted soil, Environ. Pollut., 2009, 157, 2822–2829 CrossRef CAS PubMed
.
- W. Peijnenburg and T. Jager, Monitoring approaches to assess bioaccessibility and bioavailability of metals: matrix issues, Ecotoxicol. Environ. Saf., 2003, 56, 63–77 CrossRef CAS PubMed
.
- M. Zimmer and W. Topp, Homeostatic responses in the gut of Porcellio scaber (Isopoda: Oniscidea) optimize litter degradation, J. Comp. Physiol., B, 1997, 167, 582–585 CrossRef
.
- J. Liu and R. H. Hurt, Ion release kinetics and particle persistence in aqueous nano-silver colloids, Environ. Sci. Technol., 2010, 44, 2169–2175 CrossRef CAS PubMed
.
- G. Cornelis, C. DooletteMadeleine Thomas, M. J. McLaughlin, J. K. Kirby, D. G. Beak and D. Chittleborough, Retention and dissolution of engineered silver nanoparticles in natural soils, Soil Sci. Soc. Am. J., 2012, 76, 891–902 CrossRef CAS
.
- C. Coutris, E. J. Joner and D. H. Oughton, Aging and soil organic matter content affect the fate of silver nanoparticles in soil, Sci. Total Environ., 2012, 420, 327–333 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en00291g |
| This journal is © The Royal Society of Chemistry 2020 |