The adsorption and oxidation of SO2 on MgO surface: experimental and DFT calculation studies†
Abstract
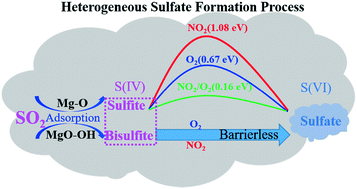
The heterogeneous oxidation of sulfur dioxide (SO2) to sulfate on the surface of MgO particles was investigated by in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and density functional theory (DFT). The in situ DRIFTS spectra show that the major products of SO2 adsorption on MgO particles are sulfite, bisulfite and sulfate, while the coexisting NO2 and O2 promote the conversion of sulfite/bisulfite into sulfate. DFT calculations show that the main adsorption products of SO2 on the perfect MgO (001) surface, the hydroxylated MgO (001) surface and the step sites of the MgO (001) surface are sulfite (SO2,gas + Olattice → SO3,ads), bisulfite and sulfate, respectively. The oxidation of sulfite to sulfate was hardly promoted by the presence of O2 at room temperature, because the process needs to overcome an energy barrier of 0.67 eV. The existence of NO2 couldn't promote the formation of sulfate, because the direct oxidation of sulfite into sulfate by NO2 is difficult (ΔEa = 1.08 eV). However, coexisting NO2 with O2 can facilitate the oxidation of SO2, which illustrates the micro-mechanism of the synergistic effect between SO2 and NO2 on MgO. In contrast to sulfite, surface bisulfite formed through the adsorption of SO2 on surface OH could be oxidized by O2 or NO2via barrierless processes. This mechanism highlights the synergistic effects of O2/NO2 as well as surface hydroxyl on the heterogeneous oxidation of SO2 on the MgO surface. Atmospheric implications of the heterogeneous oxidation of SO2 on MgO under ambient atmosphere are discussed.
- This article is part of the themed collection: Nanomaterials in air


 Please wait while we load your content...
Please wait while we load your content...