Irradiation intensity dependent heterogeneous formation of sulfate and dissolution of ZnO nanoparticles†
Tao
Wang
 a,
Yangyang
Liu
a,
Yue
Deng
a,
Hanyun
Cheng
a,
Yang
Yang
a,
Kejian
Li
a,
Xiaozhong
Fang
a and
Liwu
Zhang
a,
Yangyang
Liu
a,
Yue
Deng
a,
Hanyun
Cheng
a,
Yang
Yang
a,
Kejian
Li
a,
Xiaozhong
Fang
a and
Liwu
Zhang
 *ab
*ab
aShanghai Key Laboratory of Atmospheric Particle Pollution and Prevention, Department of Environmental Science & Engineering, Fudan University, Shanghai, 200433, Peoples' Republic of China. E-mail: zhanglw@fudan.edu.cn
bShanghai Institute of Pollution Control and Ecological Security, Shanghai, 200092, Peoples' Republic of China
First published on 19th December 2019
Abstract
Atmospheric photochemistry is widely regarded as an important pathway for SO2 oxidation. The influence of irradiation intensity, especially photochemistry under weak sunlight during heavy haze events is not known. This work investigates the irradiation-intensity-dependence of the dust-related heterogeneous process using in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and ex situ flow reaction. Illumination of the particles alters the surface oxygen species, and further accelerates the significant oxidation of sulfite species to sulfate products. The sulfate formation rate grows rapidly from the dark state to faint irradiation, while it becomes insensitive to irradiation intensity variation under strong illumination. According to the photo-electrochemical tests and concentration dependence experiments, this interesting phenomenon can be interpreted by the presence of excess SO2 under weak sunlight and sufficient photoinduced active species (PAS) under strong illumination. This uneven promotion effect is beneficial for explaining the sulfate burst under faint sunlight during heavy haze events. More significantly, the uptake of SO2 accounts for the increase of acidic species and the decrease of adsorbed water on the particle surface, thus elevating the particle acidity. Under these circumstances, the dissolution of zinc oxide (ZnO) takes place, and results in diverse principal products varying with the illumination condition, e.g. ZnSO3 under weak sunlight while ZnSO4 under strong irradiation. In general, photochemistry has profound impacts on atmospheric sulfur cycling, as well as the cycle of metal elements. The irradiation intensity dependent effects imply the raising uncertainty in the current air quality models.
Environmental significancePhotochemistry plays an important role in haze formation. However, the irradiation intensity dependence, especially the photochemistry under weak sunlight during heavy haze events, is not known. In this study, we firstly investigated the irradiation-intensity-dependence of the heterogeneous reaction of SO2 on a mineral dust surrogate (ZnO nanoparticles). The sulfate formation rate grows rapidly from the dark state to weak irradiation, while it becomes insensitive to the irradiation intensity variation as the illumination becomes stronger. This interesting phenomenon is explained by the presence of excess SO2 under weak sunlight and sufficient photoinduced active species under strong illumination. Meanwhile, the dissolution of ZnO was observed, and the Zn2+ generation on the particle surface depends on the acidic products and adsorbed water. ZnSO3 and ZnSO4 become the primary products under weak and strong sunlight, respectively. This work provides new insights into understanding the irradiation intensity dependent effects on the secondary formation of sulfate and the accompanying dissolution of mineral dust nanoparticles. |
1 Introduction
Atmospheric sulfate, originating mainly from the oxidation of sulfur dioxide (SO2), greatly contributes to the persistent haze events in Chinese megacities.1,2 Current field observations highlight that the high sulfate production during severe haze events cannot be reproduced by atmospheric models. After rounds of discussion on the reaction mechanism, a variety of sulfate formation pathways have been suggested to narrow this gap, while their relative importance remains uncertain.3 Recent studies have proved that the heterogeneous reaction of SO2 on solid or liquid particles is an underlying important pathway of sulfate formation based on various strong pieces of evidence from model, field, and laboratory measurements.4For this reason, the heterogeneous reaction of SO2 on primary particles provides an opportunity to explain the discrepancy between field and model measurements in the estimation of atmospheric sulfate.5 Mineral dust is constantly selected as the proxy of primary aerosols in numerous laboratory studies due to its wide existence in the troposphere.6,7 Mineral dust acts as a sink for many trace gases during their global journey from arid or semi-arid areas to humid urban regions. This distinctive pathway has been introduced into atmospheric models and triggers improvements.8–10 Hence, the research on the heterogeneous process on mineral dust has received worldwide interest especially in East Asia due to the frequent occurrence of sand storms.11 Some pioneers investigated the synergistic effects between trace gases or particle components, and provided valuable knowledge to models.12–18 Meanwhile, various influential factors, including moisture,19–22 temperature,23–26 particle size,27 crystal structure,28 and exposed facet,15 were frequently studied. More importantly, irradiation was considered as an essential factor in the heterogeneous uptake of SO2 on primary aerosols. Yet, most studies highlighted the photocatalytic surfaces of mineral dust,29,30 while ignoring how the reaction kinetics and product compositions change with the variations of irradiation intensity. Until recently, very limited studies have investigated irradiation intensity dependent atmospheric processes.31,32
As mentioned above, we attempted to explore the heterogeneous reaction of SO2 on mineral dust influenced by irradiation intensity. Commonly, photoactive components (e.g. TiO2, Fe2O3) were considered when discussing the photochemistry of mineral dust. Herein, ZnO (zinc oxide) is selected as the model particle for its ubiquitous existence and rich abundance in the atmospheric environment.33 ZnO is a component employed in various industries, among which ceramic industries can be highlighted, as well as in lubricants, paints, electronics, etc.34,35 High levels of atmospheric Zn were also found in urban areas, mainly attributed to traffic emission, as well as abrasion of brakes and tires.36 However, for the heterogeneous process on ZnO, only moisture dependence was considered.37 More significantly, little attention was devoted to exploring how the particle changes after being in close contact with gaseous reactants. While the dissolution of iron oxide was studied due to its link with the sulfur cycle,38–40 few attempts have been done on other atmospheric metal oxides. Moreover, the dissolution of iron oxides was mostly performed in aqueous media,40–42 making the particle variations during the atmospheric heterogeneous process a problem that needs to be solved urgently.
Our work aims to investigate the heterogeneous formation of secondary inorganic components (sulfur-containing species) influenced by the intensity of sunlight. Both in situ and ex situ experiments were designed for the discussions on sulfur oxidation and particle dissolution. The uptake coefficients (γ-values) for sulfate formation were calculated and compared. The dissolution of ZnO was observed and the generated zinc ions (Zn2+) were quantified. The influence of irradiation intensity on the photoinduced heterogeneous uptake of SO2 on mineral dust was systematically investigated.
2 Experimental
2.1 Materials
All chemicals are of analytical grade and purchased from Aladdin Chemical Reagent Co., Ltd. Ultrapure water (specific resistance ≧ 18.2 MΩ cm) produced from a deionizer (Direct-Q5-UV, MERCK, Germany) was used throughout the research process. Applied gaseous reactants include high-purity air (99.999% purity) and 2.46 × 1015 molecules per cm3 SO2 (N2 dilution).2.2 In situ measurements
The in situ DRIFTS (diffuse reflectance infrared Fourier transform spectroscopy) experiments were performed on an FTIR spectrometer (Tracer-100, Shimadzu, Japan) equipped with a liquid-nitrogen-cooled mercury–cadmium–telluride (MCT) detector. The general features of this equipment are described in Fig. S1.† Herein, a xenon lamp (CEL-TCX250, Beijing Ceaulight Co., LTD, China) was shined on the particles20 to provide simulated sunlight (Fig. S2†).Before the reaction started, the particles were purged in high-purity air (300 ml min−1) for 30 min, and then saturated with water vapor at the relative humidity (RH) of 40% for another 30 min. In this case, the particles can be covered with ∼2 monolayers of water.43 After that, a background spectrum was recorded, followed by the introduction of SO2 (4.08 ml min−1) into the DRIFTS chamber. The SO2 concentration is calculated to be 1.00 × 1014 molecules per cm3. Calibration gases with SO2 concentrations of 5.01 × 1013 and 1.43 × 1014 molecules per cm3 were also involved in this work for the concentration dependence experiments. Ten irradiation intensity (I) levels (0.0, 0.71, 1.86, 4.30, 7.50, 19.4, 36.2, 73.3, 105.7, 125.7, 145.0 and 160.0 mW cm−2) were used. Three intensities higher than ∼100 mW cm−2 were used to explore the general rules of the illumination effects. During the 60 min experiment, a series of spectra were recorded22 every 5 min with a resolution of 4 cm−1 for 100 scans.
2.3 Ion analysis
The samples after DRIFTS measurements were extracted in 10 ml water by 5 min oscillation. The leaching solution that was obtained through a 0.22 μm PTFE membrane filter was analyzed by ion chromatography (IC, 883 Basic, Metrohm, Switzerland). For SO42− analysis, the detection was conducted by using 3.2 mmol L−1 Na2CO3 and 1.0 mmol L−1 NaHCO3 at a flow rate of 0.70 ml min−1. Sulfite was not analyzed because the analytical column (A5-250) is not able to analyze the SO32− ion. For Zn2+ analysis, the eluent was replaced by 2.5 mmol oxalic acid. Standard solutions were used for multipoint calibration curves (R2 > 0.998).An ultraviolet-visible (UV-vis) spectrophotometer (UV-2600, Shimadzu, Japan) was used to quantify Zn2+ in the extraction solution (Fig. S3†). The color development reagent is 2-(1-(2-hydroxy-5-sulfophenyl)-3-phenyl-5-formazano) benzoic acid (C20H15N4NaO6S) with a concentration of 0.125 mmol L−1. The buffer solution, with a pH level of 8.30, comprises 0.4 mol L−1 H3BO3, 0.4 mol L−1 KCl, and 0.2 mol L−1 NaOH. The calibration curve was established using a Zn(CH3COO)2 solution with R2 > 0.999.
2.4 X-ray photoelectron spectroscopy (XPS) test
Actually, the particles in the bottom of the DRIFTS sample cup may not be fully exposed to the reactant gases. Hence, a customized quartz chamber (Fig. S4†) can be used for ex situ measurements. Approximately 100 mg particles were put into the reactor. After reaching moisture saturation (see section 2.2), the particles were exposed to SO2 (1.00 × 1014 molecules per cm3) for 60 min in the presence or absence of irradiation. The reacted sample was used to analyze the atomic states of the surface adsorbed species by means of X-ray photoelectron spectroscopy (XPS, ESCALAB 250XI, Thermo, the USA) with Al Kα radiation (1486.7 eV). The C 1s peak at 284.6 eV was used as an internal standard for the calibration of binding energies.2.5 Photo-electrochemical test
To study the influence of irradiation intensity on the charge separation and transfer efficiency, the photocurrent response was measured on a CHI-660D workstation (Shanghai Chenhua Co., LTD, China). ZnO was deposited on fluorine-tin-oxide glass with an effective surface area of 2 cm2, which was used as the working electrode. A platinum wire and an Ag/AgCl electrode were implemented as the counter and reference electrodes, respectively.44 The electrolyte was 0.5 mol L−1 Na2SO4. A xenon lamp (CEL-S500, Beijing Ceaulight Co., LTD, China) was used as the sunlight source. This lamp has the same spectral distribution as that used for the in situ DRIFTS experiments.2.6 Kinetics evaluation
The reactive uptake coefficient (γ) is an essential indicator to quantify the surface collision efficiency between gas molecules and particles. Assuming that the rate of reactive uptake of SO2 is equivalent to the sulfate formation rate (d[SO42−]/dt), the γ-values can be calculated by the following equations.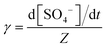 | (1) |
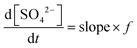 | (2) |
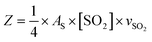 | (3) |
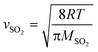 | (4) |
The conversion factor (f) is derived from a calibration plot with the integrated areas for sulfate species versus the number of SO42− ions at the end of each test. Herein, the factor is (1.17 ± 0.03) × 1015 (ion K-M per unit) (Fig. S5†). The integrated area for all the sulfur products is included in the conversion to reflect the highest sulfate production in ambient air, where S(IV) species would be rapidly oxidized to S(IV) products by the ubiquitous atmospheric oxidants. Normally, As is classified into the geometric surface area (Ageo) and BET surface area (ABET). If the reaction rate is high, SO2 has no chance to diffuse into the particles, making Ageo the effective surface in estimating the upper limit of the γ-values (denoted as γgeo). When the reaction rate is low, it is possible for SO2 to diffuse into the entire sample and thus ABET is more suitable to calculate the lower limit of the γ-values (denoted as γBET).
Monte Carlo simulation was used to quantify the uncertainty and its impact on the kinetics assessment.45,46 Each independent variable was determined via four or more replication measurements and assumed to follow a normal distribution. We performed independent runs at 1500, 3000, 5000, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 iterations with each parameter. The results showed that 5000 iterations are sufficient to ensure the stability of the results.
000 iterations with each parameter. The results showed that 5000 iterations are sufficient to ensure the stability of the results.
3 Results and discussion
3.1 Particle characterization
As an example of the widespread mineral dust suspended in dust storms, ZnO crystals were employed in this work. Based on the X-ray diffraction (XRD, S4EXPLORER, BRUKER, Germany) analysis, the sample is pure ZnO (Fig. 1A). The transmission electron microscopy (TEM, Nova NanoSem 450, FEI, Japan) image shows the morphology of ZnO (Fig. 1B). These particles possess a nanorod morphology, with breadth from 30 to 100 nm and length from 80 to 1000 nm. As shown in Fig. 1C, the average length of ZnO is 827.6 nm (ZS90, Malvern Panalytical, UK), comparable to those of authentic zinc-rich aerosols.33,47 Additionally, the Brunauer–Emmett–Teller (BET) specific surface area (SBET) of ZnO is determined to be 5.91 ± 0.28 m2 g−1 (TriStarII3020, Micromeritics Instrument Co., USA). | ||
| Fig. 1 (A) XRD pattern of the commercial ZnO particles. (B) Transmission electron microscopy (TEM) image of the ZnO nanoparticles. (C) Particle size distribution of the ZnO sample. | ||
3.2 Oxidation of sulfite intermediates
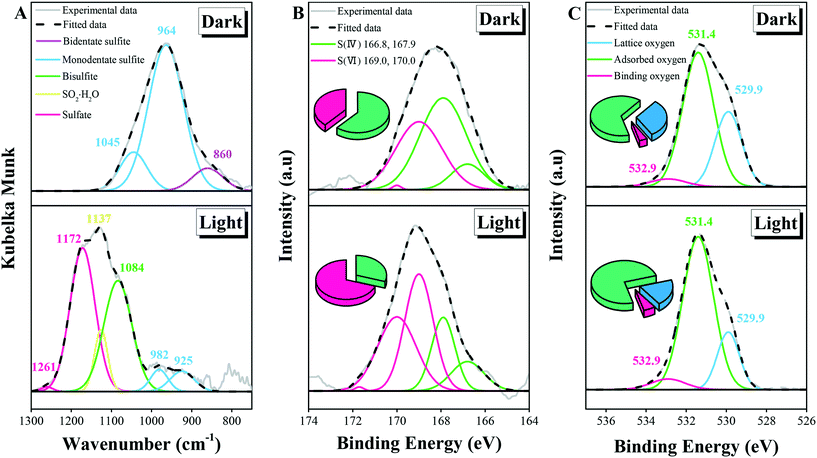
DRIFTS spectra are conductive to combing through the surface products after different processes (Fig. 2A). Under dark conditions, the observed bands were totally assigned to sulfite, which can be further classified into monodentate sulfite species (1045 and 964 cm−1) and bidentate ones (860 cm−1).48,49 Under irradiation, S(IV) species were oxidized to sulfate products, along with the physical adsorption of SO2 and the further ionization of H2SO3. Products like bisulfite, hydrated SO2 and sulfate became more dominant as evidenced by the peaks around 1084 cm−1, 1137 cm−1, and 1172 and 1261 cm−1, respectively.13,17,50–52 Sulfate behaved as the principal product for the light reaction, while it was not observed under dark conditions, highlighting the photoinduced oxidation capacity.29 Interestingly, the main S(IV) product was sulfite in the absence of irradiation, while hydrated SO2 and bisulfite were dominant under illumination, indicating the increased particle acidity after the accumulation of photoinduced sulfate.53XPS spectra are helpful in identifying the chemical speciation of surface-adsorbed species (Fig. 2B). The S(IV) species were observed at 166.8 and 167.9 eV for the S2p3/2 and S2p1/2 transitions, respectively. Correspondingly, the S(VI) ones characterized by S2p3/2 and S2p1/2 transitions could be identified at 169.0 and 170.0 eV, respectively.54 Analysis of pure ZnSO4 and ZnSO3 supports the above results (section S3†). The tiny peak at the higher binding energy (171.7 eV) is assigned to bisulfate.54 The occurrence of bisulfate suggested the increased particle acidity under irradiation compared with that in the dark process.18,55 The bisulfate was not included in the following discussion because of its small fraction among the products. The SO32−/SO42− ratios were studied using curve-fitting procedures, with values of 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 for the dark reaction and 30
39 for the dark reaction and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 for the light process, suggesting the increased proportion of S(VI) species after illumination.
70 for the light process, suggesting the increased proportion of S(VI) species after illumination.
The three peaks centered at 529.9, 531.4, and 532.9 eV in the O1s region (Fig. 2C) can be assigned to lattice oxygen, adsorbed oxygen, and binding oxygen, respectively.28,56 In detail, the 531.4 eV peak is ascribed to surface hydroxyl groups, or weakly adsorbed sulfite species. For the proportion of lattice oxygen, a decreased trend can be found from the unreacted sample (55.2%) to the particles after the dark reaction (30.9%) and illumination process (19.9%), suggesting the variations of surface oxygen species accompanied by the heterogeneous uptake of SO2. Compared to lattice oxygen, the amount of adsorbed oxygen and binding oxygen increased after SO2 exposure, which can be attributed to the production of sulfite and the formation of oxygen coordinated sulfate, respectively.
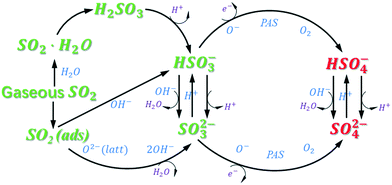
The heterogeneous uptake of SO2 on mineral dust is a classic topic in the field of atmospheric chemistry (Scheme 1). On dry surfaces, after the physical adsorption of gaseous SO2 (R.S1†), some adsorbed SO2 converts to chemisorbed sulfite/bisulfite species by reaction with lattice oxygen (O2−) (R.S2†) or surface hydroxyl groups (OH−), respectively (R.S3 and S4†). Meanwhile, originating from molecular oxygen (O2) on an oxygen vacancy site (R.S5 and S6†), surface active oxygen (O−) promotes the oxidation of sulfite to sulfate (R.S7†). Upon moisture absorption, H2SO3 appears in a large amount from hydrated SO2 (SO2·H2O) (R.S8†) and then ionizes into bisulfite (R.S9†) and sulfite (R.S10 and S11†). Accordingly, the S(IV) intermediates would be oxidized into S(VI) products with the aid of O− and dissolved oxygen (R.S12–S15†). When ZnO is excited with sunlight (wavelength ≤ 380 nm), electron–hole pairs are generated (R.S16†). These holes and electrons would react with H2O and O2, and then form hydroxyl radicals (˙OH) and reactive oxygen radicals (O2˙−), respectively (R.S17 and S18†). Hydrogen peroxide (H2O2) and superoxide hydrogen radical (HO2˙) may produce extra OH˙ (R.S19–S22†). These free radicals (e.g. ˙OH, O2˙−, and HO2˙), which can be defined as photoinduced active species (PAS), favor the formation of sulfate. Noticeably, due to the accumulation of sulfur products on the particle, the surface acidity increases as the reaction prolongs or upon the participation of irradiation. Under these circumstances, the sulfite and sulfate may transform into bisulfite (R.S23†) and bisulfate (R.S24†), respectively.11,18,55
Generally, the whole heterogeneous process can be classified into the initial physical adsorption and the subsequent chemical oxidation. The O−, O2, and PAS serve as the oxidants in the transformation of S(IV) species into S(VI) products. In contrast to iron oxides56,57 and manganese oxides,28 ZnO exhibits no sulfate formation in the DRIFTS spectra under dark conditions, in good agreement with a previous result.37 The phenomenon suggests that the adsorption of O2 is much more difficult to occur on ZnO. The difference can be explained by the different surface properties of these particles. Accordingly, PAS may act as the leading oxidants in the generation of S(VI) products under irradiation.
It is worth noting that, while sulfite was not detected in most aerosol samples, it still behaves as an important atmospheric intermediate in various homogeneous/heterogeneous processes. For instance, it has been documented that sulfite and bisulfite in aqueous media could generate the sulfite radical, which accelerates the degradation of organic compounds.58,59 More importantly, sulfite was proved to be beneficial for some reduction processes such as the transformation of NO2 into HONO.52
3.3 Kinetics for sulfate formation
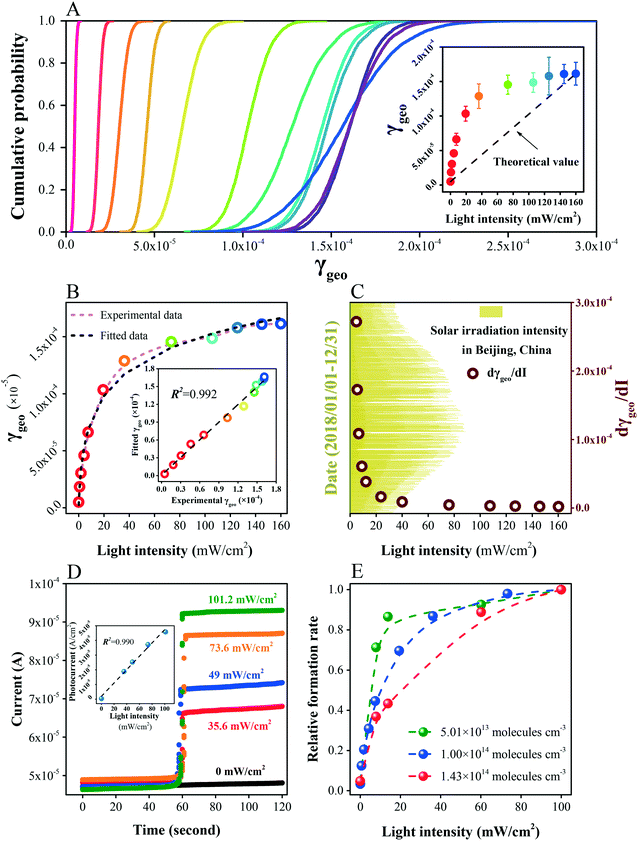
Based on the Monte Carlo simulation, γ-values were calculated to assess the photoinduced sulfate formation with cumulative probability distributions, as depicted in Fig. 3A. The variation of γBET is similar to that of γgeo (Fig. S6†). Percentile values and arithmetic mean ones are summarized in Table S2.† Because the reaction is first order with respect to SO2 concentration, the γ-values remain valuable for atmospheric processes at lower SO2 concentrations.28,60 Interestingly, with the increase of irradiation intensity, the growth of γ-values is rapid in the initial period and then tends to be slow. For example, the γgeo under 19.4 mW cm−2 exceeds the half of that under 160 mW cm−2. For better comparisons of these estimated results, theoretical γ-values were linearly calculated using the results under 0 and 160 mW cm−2. The actual γ-values under 0.71, 1.86, 4.30, 7.50, 19.4, 36.2, 73.3, 105.7, 125.7, and 145.0 mW cm−2 are 227.1%, 351.0%, 401.9%, 439.7%, 332.6%, 219.4%, 89.9%, 23.5% and 9.7% greater than the corresponding theoretical ones, respectively. The ‘fast–slow’ uptrend suggests that the γ-values measured at designed light intensities cannot be linearly extrapolated to those atmospheric processes.For the fast heterogeneous reaction in the DRIFTS chamber, only the topmost layers of the particle sample are accessible to the reactant gases, while more underlying layers are available for slower uptake processes.5,60 Hence, the γgeo calculated using the projected area of the dust (geometric area of the sample holder) is supposed to be closer to the actual situation and more suitable for atmospheric models. For this purpose, we conducted regression analysis on the obtained values and further predicted the results for relevant reactions. A polynomial regression model (eqn (5)) is employed to describe the ‘fast–slow’ uptrend.
γf,geo = a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(I + b) + c ln(I + b) + c | (5) |
| γf,geo = [0.336 × ln(I + 1.236) − 0.044] × 10−4 | (6) |
| dγgeo/dI = 3.63 × 10−4 × (I + 1.236)−1 | (7) |
What is the immanent cause of this variation? The photocurrent of ZnO is linearly correlated with irradiation intensity, suggesting that the electron–hole pairs generate evenly with increasing intensity (Fig. 3D). Since no saturation effect was observed during the in situ experiments, the adsorption rate of SO2 is considered constant throughout each test. When the sunlight is weak, the adsorbed SO2 is in excess in comparison to the generated PAS, making illumination the rate-limiting factor. Under these circumstances, most PAS are involved in the oxidation of S(IV) species. Under strong irradiation, the PAS are in excess compared to the adsorbed SO2. In this situation, the increase of irradiation intensity exhibits limited impacts on the promotion of adsorption capacity and SO2 adsorption becomes the rate-limiting factor. In general, it can be concluded that the balance between SO2 adsorption and PAS generation accounts for the nonlinear uptrend of γ-values.
Concentration dependence was further studied. The product formation rates under given conditions were normalized by the corresponding results under the highest irradiation (Fig. 3E). At low concentration (5.01 × 1013 molecules per cm3), the formation rate is light-dependent under weak illumination, while it appears to be steady as the irradiation intensity becomes stronger, suggesting an excess of PAS under strong illumination. In contrast, at high concentration (1.43 × 1014 molecules per cm3), the sulfate formation rate increases continuously from moderate illumination to strong irradiation, implying sufficient SO2 adsorption under relatively high intensity. Hence, greater SO2 concentrations broaden the influence scope of sunlight. In other words, strong SO2 pollution may increase the participation of solar irradiation in the formation of secondary aerosols.
The proposed mechanism is shown in Scheme 2. The balance between SO2 adsorption and PAS generation is responsible for the irradiation intensity dependent sulfate formation. Light-dependent and SO2-dependent processes should be highlighted under weak and strong sunlight, with S(IV) and S(VI) compounds being the principal surface products, respectively (section 3.2).
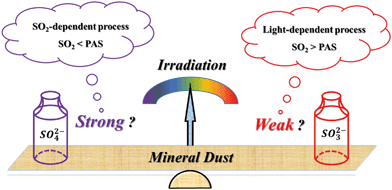 | ||
| Scheme 2 Heterogeneous reaction of SO2 on mineral dust influenced by the intensity of solar irradiation. PAS: photoinduced active species. | ||
3.4 Particle dissolution
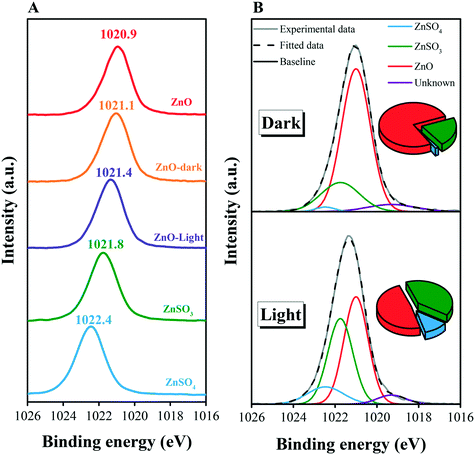
Samples with different chemical compositions or after different exposure processes were further studied by XPS. The spectra in the Zn2p region exhibit different peak locations, indicating diverse binding modes between Zn element and others on the particle surface (Fig. 4A). According to the peak assignments of pure ZnO, ZnSO3, and ZnSO4, curve fitting procedures were carried out to quantify the surface products (Fig. 4B). Briefly, illumination greatly contributes to the heterogeneous uptake of SO2, resulting in the increased proportions of ZnSO4 and ZnSO3. Noticeably, the ratio of ZnSO4/ZnSO3 was 0.078 for the dark reaction, while it increased up to 0.290 after illumination exposure, in accordance with the results from DRIFTS spectra (section 3.2), suggesting significant oxidation processes caused by the simulated sunlight.These results provide a new insight into the characterization of heterogeneous surface reactions, during which the mineral particles undergo huge changes. Hence, the dissolution of mineral dust may also take place in the particle surface media, in nice agreement with the processes in aqueous environments.38,39,61 The process is significant because of its great influence on aerosol chemistry. Additionally, it is notable that dissolved Zn2+ released from ZnO particles has been proved to induce adverse biological effects in organisms.62
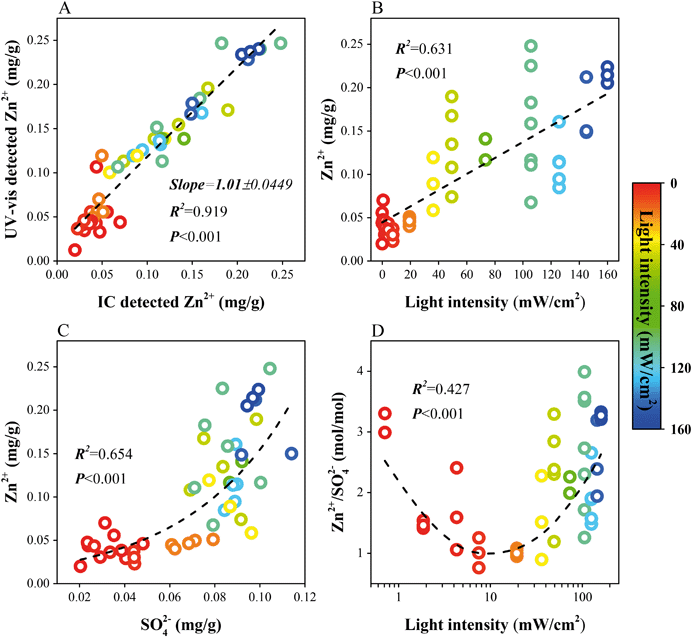
To quantitatively investigate the dissolution course, both IC and UV-vis were used to determine the dissolved Zn2+ concentrations. A good linear correlation was found with a slope of 1.01, indicating the stability and accuracy of the measuring methods (Fig. 5A). IC results were used for further discussion. Differing from the variation of sulfate formation, the Zn2+ concentrations increase linearly as the illumination becomes stronger (Fig. 5B). Hence, the nonlinear correlation between SO42− and Zn2+ can be described by an exponential-like function (Fig. 5C). That is, ZnO dissolution is still in progress when the sulfate formation rate tends to be stable under strong illumination.
Could we assess the dissolution process by the amount of acidic products? For the Zn2+/SO42− ratio, these values present a parabola-like variation with the lowest value around 1 and the highest around 4 (Fig. 5D). Under weak irradiation, sulfate outpaces Zn2+ in formation, making the ratios decrease with increasing irradiation intensity. The dissolution of ZnO by the surface S(IV) compounds accounts for the high values under dark conditions or weak sunlight. It is worth noting that, when the irradiation intensity exceeds 10 mW cm−2, although S(IV) compounds become the main promoters influencing the ZnO dissolution, Zn2+ seems to appear at a higher rate compared to the formation of sulfate. Separate ex situ experiments confirmed that irradiation would not directly trigger the dissolution of ZnO nanoparticles in the absence of SO2.
The particles in this work were in a hygroscopic saturation state before SO2 exposure. Note that sulfate has been proved to show a deliquescence point up to 80% RH at room temperature, indicating low hygroscopicity under most humid conditions.57,63,64 Compared to fresh particles, sulfated particles reveal a weaker capacity in moisture absorption, which thus leads to the decrease of surface water along with SO2 uptake.57 Therefore, the heterogeneous uptake of SO2 results in the increase of solute (sulfur products) while the decrease of solvent (H2O) in the water layers of the particle surface, which thus greatly elevates the particle acidity. In other words, the continuous dissolution of ZnO under strong irradiation can be further interpreted by the weak increase of sulfate products accompanied by the decrease of surface adsorbed water. Accordingly, the acidic products on atmospheric metal oxides are not sufficient to evaluate the dissolution process. On the other hand, the dissolved Zn2+ may exist in different forms. In this case, ZnO dissolution takes place in H2SO3 medium under weak sunlight and triggers the production of ZnSO3. Correspondingly, ZnSO4 becomes dominant after the interaction between ZnO and H2SO4 under strong sunlight.
4 Conclusions and environmental implications
Ubiquitous sulfate in the troposphere has a myriad of impacts and is of great interest. The formation of sulfur components on mineral dust is widely recognized as a source of secondary sulfate aerosols, as well as a sink of atmospheric SO2. Herein, how the intensity of sunlight affects the heterogeneous reaction of SO2 was firstly investigated by means of in situ and ex situ experiments. The sulfate formation rate speeds up with the increase of irradiation intensity. Nevertheless, the role of irradiation intensity variation in the heterogeneous process varies with the illumination condition: being the protagonist under weak illumination while a co-star under strong irradiation. This uneven illumination effect, which can be interpreted by the balance between SO2 adsorption and formation of photoinduced active species, provides a new perspective to explain the sulfate burst during heavy haze events under low visibility.Moreover, the dissolution of mineral dust during photoinduced reactions, which appears to be meaningful and complex, has attracted little attention. Our results proved the dissolution of ZnO accompanied by the heterogeneous uptake of SO2. Influenced by either the surface acidic products or the surface adsorbed water, the dissolution process associates closely with the particle acidity. The nonlinear link between dissolved Zn2+ and sulfate products implies that the dissolution may not be simply estimated by the measured acidic components. The dissolution of atmospheric metal oxides results in the production of a variety of surface coordinated species, and may further affect the geochemistry cycle and human health.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The authors gratefully acknowledge financial support from the Ministry of Science and Technology of the People's Republic of China (2016YFE0112200, 2016YFC0202700), National Natural Science Foundation of China (No. 21976030 and No. 21677037), and the Natural Science Foundation of Shanghai (No. 19ZR1471200 and No. 17ZR1440200).References
- G. Wang, R. Zhang, M. E. Gomez, L. Yang, M. Levy Zamora, M. Hu, Y. Lin, J. Peng, S. Guo, J. Meng, J. Li, C. Cheng, T. Hu, Y. Ren, Y. Wang, J. Gao, J. Cao, Z. An, W. Zhou, G. Li, J. Wang, P. Tian, W. Marrero-Ortiz, J. Secrest, Z. Du, J. Zheng, D. Shang, L. Zeng, M. Shao, W. Wang, Y. Huang, Y. Wang, Y. Zhu, Y. Li, J. Hu, B. Pan, L. Cai, Y. Cheng, Y. Ji, F. Zhang, D. Rosenfeld, P. S. Liss, R. A. Duce, C. E. Kolb and M. J. Molina, Persistent sulfate formation from London Fog to Chinese haze, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(48), 1–6 CrossRef PubMed.
- R. J. Huang, Y. Zhang, C. Bozzetti, K. F. Ho and J. J. Cao, High secondary aerosol contribution to particulate pollution during haze events in China, Nature, 2014, 514(7521), 218–222 CrossRef CAS PubMed.
- J. Xue, Z. Yuan, S. M. Griffith, X. Yu, A. K. H. Lau and J. Z. Yu, Sulfate Formation Enhanced by a Cocktail of High NOx, SO2, Particulate Matter, and Droplet pH during Haze-Fog Events in Megacities in China: An Observation-Based Modeling Investigation, Environ. Sci. Technol., 2016, 50(14), 7325–7334 CrossRef CAS PubMed.
- Z. An, R. Huang, R. Zhang, X. Tie, G. Li, J. Cao, W. Zhou, Z. Shi, Y. Han, Z. Gu and Y. Ji, Severe haze in northern China: A synergy of anthropogenic emissions and atmospheric processes, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(18), 8657–8666 CrossRef CAS PubMed.
- M. Tang, X. Huang, K. Lu, M. Ge, Y. Li, P. Cheng, T. Zhu, A. Ding, Y. Zhang, S. Gligorovski, W. Song, X. Ding, X. Bi and X. Wang, Heterogeneous reactions of mineral dust aerosol: implications for tropospheric oxidation capacity, Atmos. Chem. Phys., 2017, 17(19), 11727–11777 CrossRef CAS.
- H. Chen, C. E. Nanayakkara and V. H. Grassian, Titanium dioxide photocatalysis in atmospheric chemistry, Chem. Rev., 2012, 112, 5919–5948 CrossRef CAS PubMed.
- C. R. Usher, A. E. Michel and V. H. Grassian, Reactions on mineral dust, Chem. Rev., 2003, 103(12), 4883–4940 CrossRef CAS PubMed.
- G. Li, N. Bei, J. Cao, R. Huang and J. Wu, A possible pathway for rapid growth of sulfate during haze days in China, Atmos. Chem. Phys., 2017, 17, 3301–3316 CrossRef CAS.
- Y. Wang, Q. Zhang, J. Jiang, W. Zhou, B. Wang, K. He, F. Duan, Q. Zhang, S. Philip and Y. Xie, Enhanced sulfate formation during China's severe winter haze episode in January 2013 missing from current models, J. Geophys. Res.: Atmos., 2014, 119, 10425–10440 Search PubMed.
- X. Huang, Y. Song, C. Zhao, M. Li, T. Zhu, Q. Zhang and X. Zhang, Pathways of sulfate enhancement by natural and anthropogenic mineral aerosols in China, J. Geophys. Res.: Atmos., 2014, 119(24), 14165–14179 CAS.
- R. Zhang, G. Wang, S. Guo, M. L. Zamora, Q. Ying, Y. Lin, W. Wang, M. Hu and Y. Wang, Formation of Urban Fine Particulate Matter, Chem. Rev., 2015, 115(10), 3803–3855 CrossRef CAS PubMed.
- Q. Ma, Y. Liu and H. He, Synergistic effect between NO2 and SO2 in their adsorption and reaction on γ-Alumina, J. Phys. Chem. A, 2008, 112(29), 6630–6635 CrossRef CAS PubMed.
- C. Liu, Q. Ma, Y. Liu, J. Ma and H. He, Synergistic reaction between SO2 and NO2 on mineral oxides: a potential formation pathway of sulfate aerosol, Phys. Chem. Chem. Phys., 2012, 14(5), 1668–1676 RSC.
- H. He, Y. Wang, Q. Ma, J. Ma, B. Chu, D. Ji, G. Tang, C. Liu, H. Zhang and J. Hao, Mineral dust and NOx promote the conversion of SO2 to sulfate in heavy pollution days, Sci. Rep., 2014, 4(1), 4172 CrossRef PubMed.
- W. Yang, M. Chen, W. Xiao, Y. Guo, J. Ding, L. Zhang and H. He, Molecular Insights into NO-Promoted Sulfate Formation on Model TiO2 Nanoparticles with Different Exposed Facets, Environ. Sci. Technol., 2018, 52(24), 14110–14118 CrossRef CAS PubMed.
- W. Yang, Q. Ma, Y. Liu, J. Ma, B. Chu, L. Wang and H. He, Role of NH3 in the Heterogeneous Formation of Secondary Inorganic Aerosols on Mineral Oxides, J. Phys. Chem. A, 2018, 122(30), 6311–6320 CrossRef CAS PubMed.
- W. Yang, H. He, Q. Ma, J. Ma, Y. Liu, P. Liu and Y. Mu, Synergistic formation of sulfate and ammonium resulting from reaction between SO2 and NH3 on typical mineral dust, Phys. Chem. Chem. Phys., 2015, 18(2), 956–964 RSC.
- W. Yang, Q. Ma, Y. Liu, J. Ma, B. Chu and H. He, The effect of water on the heterogeneous reactions of SO2 and NH3 on the surfaces of α-Fe2O3 and γ-Al2O3, Environ. Sci.: Nano, 2019, 6, 2749–2758 RSC.
- L. Huang, Y. Zhao, H. Li and Z. Chen, Kinetics of Heterogeneous Reaction of Sulfur Dioxide on Authentic Mineral Dust: Effects of Relative Humidity and Hydrogen Peroxide, Environ. Sci. Technol., 2015, 49(18), 10797–10805 CrossRef CAS PubMed.
- Y. Zhang, S. Tong, M. Ge, B. Jing, S. Hou, F. Tan, Y. Chen, Y. Guo and L. Wu, The influence of relative humidity on the heterogeneous oxidation of sulfur dioxide by ozone on calcium carbonate particles, Sci. Total Environ., 2018, 633, 1253–1262 CrossRef CAS PubMed.
- L. Huang, Y. Zhao, H. Li and Z. Chen, Hydrogen peroxide maintains the heterogeneous reaction of sulfur dioxide on mineral dust proxy particles, Atmos. Environ., 2016, 141, 552–559 CrossRef CAS.
- Q. Ma, L. Wang, B. Chu, J. Ma and H. He, Contrary Role of H2O and O2 in the Kinetics of Heterogeneous Photochemical Reactions of SO2 on TiO2, J. Phys. Chem. A, 2018, 123(7), 1311–1318 CrossRef PubMed.
- L. Wu, S. Tong and M. Ge, Heterogeneous Reaction of NO2 on Al2O3: The Effect of Temperature on the Nitrite and Nitrate Formation, J. Phys. Chem. A, 2013, 117, 4937–4944 CrossRef CAS PubMed.
- L. Wu, S. Tong, S. Hou and M. Ge, Influence of Temperature on the Heterogeneous Reaction of Formic Acid on α-Al2O3, J. Phys. Chem. A, 2012, 116, 10390–10396 CrossRef CAS PubMed.
- L. W. Wu, S. R. Tong, W. G. Wang and M. F. Ge, Effects of temperature on the heterogeneous oxidation of sulfur dioxide by ozone on calcium carbonate, Atmos. Chem. Phys., 2011, 11(13), 6593–6605 CrossRef CAS.
- I. W. M. Smith, Laboratory Studies of Atmospheric Reactions at Low Temperatures, Chem. Rev., 2003, 103(12), 4549–4564 CrossRef CAS PubMed.
- Y. Zhang, S. R. Tong and M. F. Ge, A study about the influence of the size of CaCO3 on the heterogeneous oxidation of sulfur dioxide by ozone, Spectrosc. Spectral Anal., 2016, 36(10), 126–127 Search PubMed.
- W. Yang, J. Zhang, Q. Ma, Y. Zhao, Y. Liu and H. He, Heterogeneous Reaction of SO2 on Manganese Oxides: the Effect of Crystal Structure and Relative Humidity, Sci. Rep., 2017, 7(1), 4550 CrossRef.
- J. Park, M. Jang and Z. Yu, Heterogeneous Photo-oxidation of SO2 in the Presence of Two Different Mineral Dust Particles: Gobi and Arizona Dust, Environ. Sci. Technol., 2017, 51(17), 9605–9613 CrossRef CAS.
- J. Y. Park and M. Jang, Heterogeneous photooxidation of sulfur dioxide in the presence of airborne mineral dust particles, RSC Adv., 2016, 6(63), 58617–58627 RSC.
- C. George, M. Ammann, B. D. Anna, D. J. Donaldson and S. A. Nizkorodov, Heterogeneous Photochemistry in the Atmosphere, Chem. Rev., 2015, 115(10), 4218–4258 CrossRef CAS PubMed.
- T. Wang, Y. Liu, Y. Deng, H. Cheng, Y. Yang, Y. Feng, M. A. Tahir, X. Fang, X. Dong, K. Li, S. Ajmal, A. Bacha, I. Nabi, H. Fu, L. Zhang and J. Chen, Is the photochemistry activity weak during haze events? A novel exploration on the photoinduced heterogeneous reaction of NO2 on mineral dust, Atmos. Chem. Phys., 2019, 1–20 Search PubMed.
- L. T. González, F. E. Longoria Rodríguez, M. Sánchez-Domínguez, A. Cavazos, C. Leyva-Porras, L. G. Silva-Vidaurri, K. A. Askar, B. I. Kharissov, J. F. Villarreal Chiu and J. M. Alfaro Barbosa, Determination of trace metals in TSP and PM2.5 materials collected in the Metropolitan Area of Monterrey, Mexico: A characterization study by XPS, ICP-AES and SEM-EDS, Atmos. Res., 2017, 196, 8–22 CrossRef.
- E. Manoli, D. Voutsa and C. Samara, Chemical characterization and source identification/apportionment of fine and coarse air particles in Thessaloniki, Greece, Atmos. Environ., 2002, 36(6), 949–961 CrossRef CAS.
- R. Vedantham, M. S. Landis, D. Olson and J. P. Pancras, Source Identification of PM2.5 in Steubenville, Ohio Using a Hybrid Method for Highly Time-Resolved Data, Environ. Sci. Technol., 2014, 48(3), 1718–1726 CrossRef CAS PubMed.
- M. C. Minguillón, M. Cirach, G. Hoek, B. Brunekreef, M. Tsai, K. de Hoogh, A. Jedynska, I. M. Kooter, M. Nieuwenhuijsen and X. Querol, Spatial variability of trace elements and sources for improved exposure assessment in Barcelona, Atmos. Environ., 2014, 89, 268–281 CrossRef.
- J. Li, J. Shang and T. Zhu, Heterogeneous reactions of SO2 on ZnO particle surfaces, Sci. China: Chem., 2010, 54(1), 161–166 CrossRef.
- G. Zhuang, Z. Yi, R. A. Duce and P. R. Brown, Link between iron and sulphur cycles suggested by detection of Fe(II) in remote marine aerosols, Nature, 1992, 355(6360), 537–539 CrossRef CAS.
- H. Fu, J. Lin, G. Shang, W. Dong, V. H. Grassian, G. R. Carmichael, Y. Li and J. Chen, Solubility of iron from combustion source particles in acidic media linked to iron speciation, Environ. Sci. Technol., 2012, 46, 11119–11127 CrossRef CAS PubMed.
- Z. Shi, M. D. Krom, S. Bonneville and L. G. Benning, Atmospheric Processing Outside Clouds Increases Soluble Iron in Mineral Dust, Environ. Sci. Technol., 2015, 49, 1472–1477 CrossRef CAS.
- H. Fu, D. M. Cwiertny, G. R. Carmichael, M. M. Scherer and V. H. Grassian, Photoreductive dissolution of Fe-containing mineral dust particles in acidic media, J. Geophys. Res.: Atmos., 2010, 115, D11304 CrossRef.
- Z. Wang, T. Wang, H. Fu, L. Zhang, M. Tang, C. George, V. H. Grassian and J. Chen, Enhanced heterogeneous uptake of sulfur dioxide on mineral particles through modification of iron speciation during simulated cloud processing, Atmos. Chem. Phys., 2019, 19(19), 12569–12585 CrossRef CAS.
- Q. Ma, H. He and Y. Liu, In situ DRIFTS study of hygroscopic behavior of mineral aerosol, J. Environ. Sci., 2010, 22(4), 555–560 CrossRef CAS.
- Y. Yang, F. Teng, Y. Kan, L. Yang, Z. Liu, W. Gu, A. Zhang, W. Hao and Y. Teng, Investigation of the charges separation and transfer behavior of BiOCl/BiF3 heterojunction, Appl. Catal., B, 2017, 205, 412–420 CrossRef CAS.
- Z. Xia, X. Duan, S. Tao, W. Qiu, D. Liu, Y. Wang, S. Wei, B. Wang, Q. Jiang, B. Lu, Y. Song and X. Hu, Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China, Environ. Pollut., 2013, 173, 150–156 CrossRef CAS PubMed.
- K. Chiang, C. Chio, Y. Chiang and C. Liao, Assessing hazardous risks of human exposure to temple airborne polycyclic aromatic hydrocarbons, J. Hazard. Mater., 2009, 166, 676–685 CrossRef CAS.
- L. F. Pineda-Martínez, Dispersion of atmospheric coarse particulate matter in the San Luis Potosí, Mexico, urban area, Atmosfera, 2014, 27(1), 5–19 CrossRef.
- C. E. Nanayakkara, J. Pettibone and V. H. Grassian, Sulfur dioxide adsorption and photooxidation on isotopically-labeled titanium dioxide nanoparticle surfaces: roles of surface hydroxyl groups and adsorbed water in the formation and stability of adsorbed sulfite and sulfate, Phys. Chem. Chem. Phys., 2012, 14(19), 6957–6966 RSC.
- Y. Zhao, Y. Liu, J. Ma, Q. Ma and H. He, Heterogeneous reaction of SO2 with soot: The roles of relative humidity and surface composition of soot in surface sulfate formation, Atmos. Environ., 2017, 152, 465–476 CrossRef CAS.
- L. Wu, S. Tong, L. Zhou, W. Wang and M. Ge, Synergistic Effects between SO2 and HCOOH on α-Fe2O3, J. Phys. Chem. A, 2013, 117, 3972–3979 CrossRef CAS PubMed.
- X. Zhao, L. Kong, Z. Sun, X. Ding, T. Cheng, X. Yang and J. Chen, Interactions between heterogeneous uptake and adsorption of sulfur dioxide and acetaldehyde on hematite, J. Phys. Chem. A, 2015, 119(17), 4001–4008 CrossRef CAS PubMed.
- Q. Ma, T. Wang, C. Liu, H. He, Z. Wang, W. Wang and Y. Liang, SO2 Initiates the Efficient Conversion of NO2 to HONO on MgO Surface, Environ. Sci. Technol., 2017, 51(7), 3767–3775 CrossRef CAS PubMed.
- W. M. Haynes, Handbook of Chemistry and Physics, 2014, CRC Press Search PubMed.
- J. Baltrusaitis, D. M. Cwiertny and V. H. Grassian, Adsorption of sulfur dioxide on hematite and goethite particle surfaces, Phys. Chem. Chem. Phys., 2007, 9, 5542–5554 RSC.
- P. Persson and L. L. Vgren, Potentiometric and spectroscopic studies of sulfate complexation at the goethite-water interface, Geochim. Cosmochim. Acta, 1996, 60(15), 2789–2799 CrossRef CAS.
- H. Fu, X. Wang, H. Wu, Y. Yin and J. Chen, Heterogeneous uptake and oxidation of SO2 on iron oxides, J. Phys. Chem. C, 2007, 111(16), 6077–6085 CrossRef CAS.
- T. Wang, Y. Liu, Y. Deng, H. Fu, L. Zhang and J. Chen, Adsorption of SO2 on mineral dust particles influenced by atmospheric moisture, Atmos. Environ., 2018, 191, 153–161 CrossRef CAS.
- A. Bacha, H. Cheng, J. Han, I. Nabi, K. Li, T. Wang, Y. Yang, S. Ajmal, Y. Liu and L. Zhang, Significantly Accelerated PEC Degradation of Organic Pollutant with Addition of Sulfite and Mechanism Study, Appl. Catal., B, 2019, 248, 441–449 CrossRef CAS.
- W. Deng, H. Zhao, F. Pan, X. Feng, B. Jung, A. Abdel-Wahab, B. Batchelor and Y. Li, Visible-Light-Driven Photocatalytic Degradation of Organic Water Pollutants Promoted by Sulfite Addition, Environ. Sci. Technol., 2017, 51(22), 13372–13379 CrossRef CAS PubMed.
- L. D. Kong, X. Zhao, Z. Y. Sun, Y. W. Yang, H. B. Fu, S. C. Zhang, T. T. Cheng, X. Yang, L. Wang and J. M. Chen, The effect of nitrate on the heterogeneous uptake of sulfur dioxide on hematite, Atmos. Chem. Phys., 2014, 14, 9451–9467 CrossRef CAS.
- Z. Shi, S. Bonneville, M. D. Krom, K. S. Carslaw, T. D. Jickells, A. R. Baker and L. G. Benning, Iron dissolution kinetics of mineral dust at low pH during simulated atmospheric processing, Atmos. Chem. Phys., 2011, 11, 995–1007 CrossRef CAS.
- P. Wu, P. Cui, H. Du, M. E. Alves, C. Liu, D. Zhou and Y. Wang, Dissolution and Transformation of ZnO Nano- and Microparticles in Soil Mineral Suspensions, ACS Earth Space Chem., 2018, 3(4), 495–502 CrossRef.
- T. B. Onasch, R. L. Siefert, S. D. Brooks, A. J. Prenni, M. Benjamin, M. A. Wilson and M. A. Tolber, Infrared spectroscopic study of the deliquescence and efflorescence of ammonium sulfate aerosol as a function of temperature, J. Geophys. Res.: Atmos., 1999, 104(17), 21317–21326 CrossRef CAS.
- M. Tang, C. K. Chan, Y. J. Li, H. Su, Q. Ma, Z. Wu, G. Zhang, Z. Wang, M. Ge, M. Hu, H. He and X. Wang, A review of experimental techniques for aerosol hygroscopicity studies, Atmos. Chem. Phys., 2019, 19(19), 12631–12686 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Section S1. Experimental; section S2. Kinetics evaluation; section S3. XPS evidence; section S4. Detailed reactions in the photocatalytic process. See DOI: 10.1039/c9en01148j |
| This journal is © The Royal Society of Chemistry 2020 |