Emerging investigator series: polymeric nanocarriers for agricultural applications: synthesis, characterization, and environmental and biological interactions
Sheyda
Shakiba
a,
Carlos E.
Astete
b,
Sachin
Paudel
a,
Cristina M.
Sabliov
b,
Debora F.
Rodrigues
 a and
Stacey M.
Louie
a and
Stacey M.
Louie
 *a
*a
aDepartment of Civil & Environmental Engineering, University of Houston, 4726 Calhoun Rd., Engineering Building 1, Rm. N107, Houston, TX 77204, USA. E-mail: slouie@uh.edu
bDepartment of Biological and Agricultural Engineering and LSU AgCenter, Louisiana State University, 141 E. B. Doran Building, Baton Rouge, Louisiana 70803, USA
First published on 9th December 2019
Abstract
Polymeric nanoparticles represent one major class of nanomaterials that has been proposed to improve the sustainability of agricultural operations by delivering organic agrochemicals such as pesticides more efficiently. Polymeric nanoparticles can improve efficiency through improved targeting and uptake, slow release, and lower losses of the chemicals, while also conferring the benefits of biodegradability and biocompatibility. This review provides a tutorial to environmental nanotechnology researchers interested in initiating research on the development and application of polymeric nanocarriers for delivery of agrochemicals, including pesticides and growth promoters for crops and antibiotics for livestock. In particular, this review covers the wider suite of methods that will be required beyond those typically used for inorganic metal or metal oxide nanoparticles, including synthesis of custom polymeric nanocarriers and characterization and tuning of agrochemical loading and release profiles. Benefits of polymeric nanocarriers are then discussed in terms of the physicochemical properties and fate and transport behaviors that contribute to higher efficiency and lesser environmental impacts compared to traditional (non-nano) formulations. Finally, opportunities for environmental nanotechnology researchers to collaborate with material scientists, microbiologists, and agricultural scientists to optimize the development of polymeric nanocarriers for agriculture are discussed.
Environmental significanceSustainable nanotechnology for agriculture encourages the development of nanomaterials that will reduce the reliance on traditional chemicals, such as pesticides for crops or antibiotics for livestock, or the environmental impact of agrochemicals. While the environmental nanotechnology community has developed significant knowledge on the applications and impacts of inorganic nanoparticles, an opportunity exists to expand upon the applications of polymeric nanocarriers that can improve the efficiency of delivery of traditional agrochemicals while also providing advantages of biocompatibility and biodegradability. This Tutorial Review provides an overview of the synthesis, characterization, and fate and transport of polymeric nanocarriers as alternatives to inorganic nanoparticles, along with the potential benefits of polymeric nanocarriers over traditional agrochemicals. |
1. Introduction
Nanotechnology is emerging as a means to improve the sustainability of agricultural operations. The general use of nanomaterials (both inorganic and organic or polymeric) for agriculture has recently been reviewed to provide a general understanding of the opportunities and research priorities,1–5 as well as a critical evaluation of the efficacy of nano-enabled pesticides and fertilizers relative to conventional formulations.6 Here, this review focuses specifically on polymeric “nanocarriers,” in which active ingredients are loaded into or onto a polymeric nanoparticle. While polymeric nanocarriers have extensively been considered for human drug delivery applications, this review highlights the agricultural applications of polymeric nanocarriers for crops (pesticides, plant growth promoters, etc.) and livestock (specifically, antibiotic delivery). In these applications, polymer nanoparticles can improve the efficiency of application of active ingredients by enhancing the aqueous dispersibility and bioavailability of hydrophobic active ingredients, conferring targeting properties, and extending the effective lifetime of the active ingredient (e.g. via slow release, enhanced adhesion to leaves or roots, or protection from degradation). Polymeric nanomaterials can also serve as more sustainable alternatives to inorganic nanoparticles when biocompatible and biodegradable polymers are selected that are expected to minimize the potential for ecotoxicity.This tutorial review aims to serve as a primer for environmental researchers to initiate new research on the application and development of polymeric nanocarriers for agricultural applications. Given the extensive experience developed in the environmental nanotechnology community with inorganic nanoparticles, special considerations that are required for the study of polymeric nanomaterials as compared to inorganic nanoparticles are emphasized. First, methods for synthesis of polymeric nanocarriers and approaches to optimize the synthesis are presented. Then, important characterization needs for polymeric nanoparticles loaded with active ingredients are discussed. Finally, mechanisms for the delivery or release of active ingredients, environmental fate, and biological effects of polymers nanocarriers, and how these mechanisms inform the design of the nanoparticles, are presented. The integration of knowledge on synthesis, characterization, behavior, and effects is expected to lead to advances in the development of polymeric nanocarriers as a beneficial technology for agriculture and the environment.
2. Synthesis of polymeric nanocarriers
Research and development teams in both industry and academia will likely need to develop expertise in synthesizing materials in-house during the development of new polymeric nanocarriers for ultimate application by farmers. Currently, polymeric nanoparticles have limited commercial availability, with those available for purchase limited to a few common synthetic polymer types (e.g. polystyrene and poly(lactic-co-glycolic acid) (PLGA)). Furthermore, the pure polymeric nanoparticle typically does not serve as an “active ingredient.” Rather, an active ingredient (e.g., a pesticide, hormone, or antibiotic) must be loaded into the nanoparticle during the synthesis of the particle. Hence, considering the number of polymer types multiplied by the number of agrochemical types that may be of interest, new materials for research and development purposes will require custom syntheses. This issue is in contrast to the relatively widespread commercial availability of inorganic (metal and metal oxide) nanoparticles, where the nanoparticle itself confers the “active” properties (e.g., fungicidal copper nanoparticles) and does not need to be loaded during the synthesis with an active ingredient. Here, we introduce common materials and synthesis methods for polymeric nanoparticles, as well as approaches to optimize the synthesis parameters to obtain desired nanoparticle properties.2.1. Common polymeric materials for agricultural applications
Research published over the last 10 years indicates that the preferred natural polymers for food and agricultural applications are chitosan,7,8 zein,9–14 and alginic acid.15–17 Also, some biocompatible and biodegradable synthetic polymers, such as PLGA, are of interest to confer new properties to the delivery systems, and as a platform to develop new biomaterials, for example by linking synthetic and natural polymers. In addition to the active ingredient that will be loaded into the nanoparticle, other ingredients that are frequently added include surfactants (e.g., poly(vinyl alcohol) or Tween) to impart colloidal stability to the nanoparticles, as well as a cryoprotectant (e.g. mannitol or trehalose) to preserve material integrity during lyophilization. Finally, the addition of an oil can be used to form a “nanocapsule” structure, where the oil forms a liquid core at room temperature surrounded by a polymer shell and can be used to carry poorly soluble active ingredients.2.2. General approaches for optimization of nanocarrier synthesis
Optimization of the synthesis of polymeric nanocarriers typically revolves around obtaining a desired particle size with low polydispersity, good colloidal stability, and high loading or entrapment efficiency of the active ingredient. Entrapment efficiency is defined as the percentage of active ingredient added in the synthesis that is incorporated into the nanoparticles, while loading refers to the concentration of active ingredient in the nanoparticles (typically expressed as a weight percent). Smaller sized nanoparticles with narrow size distributions can be achieved by tuning the ratio of ingredients (polymers, surfactants, and active ingredients)18–23 and/or the forces imparted (e.g. by shear, impact, sonication, or high pressure homogenization) during or immediately after the synthesis.19,22,24,25 Colloidal stability is determined by Derjaguin Landau Verwey Overbeek (DLVO) interaction energies, similarly to inorganic nanoparticles, and hence charged polymers or surfactants can be utilized to confer electrostatic stabilization.Entrapment efficiency and loading are optimized by selection of materials that favor incorporation of the active ingredient into the nanoparticle during synthesis. Optimal loading conditions can be identified experimentally by varying ingredient concentrations, e.g. using factorial design.20,21,26 Specific interactions, such as electrostatic complexation interactions27–30 or covalent bonding (conjugation) of the active ingredient to the polymer,31 can be used to increase loading. However, loading is more typically achieved through partitioning of the active ingredient into the polymer phase versus the solvent, e.g. based on the hydrophobicity or polarity of the active ingredient and polymer.32 Models have hence been developed to explain or predict a priori the active ingredient loading based on thermodynamic parameters such as the Flory–Huggins interaction parameter33 or Hansen solubility parameter,34 or using universal functional activity coefficient (UNIFAC) methods that account for the chemical structure of the active ingredient, polymer properties (including the glass transition temperature), and partitioning of active ingredient into surfactant micelles.35 However, agreement between experimental data and these models is rarely evaluated and would be useful in future studies.
2.3. Synthesis methods for polymeric nanocarriers
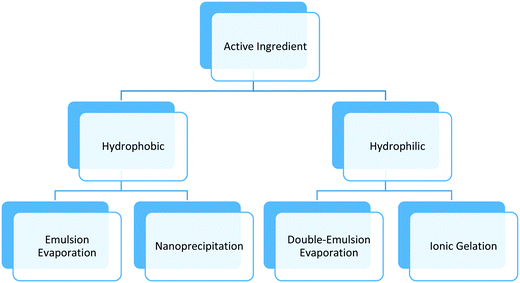
Synthesis methods for polymeric nanoparticles can be divided into two categories: bottom-up techniques that involve in situ polymerization, and top-down techniques that involve steps such as mixing or emulsification with external energy input. The first approach involves organic chemical synthesis in the presence of solvents, initiators, and other potentially toxic agents. The separation and purification steps add extra cost that limit its uses in food and agriculture. The top-down techniques use natural or synthetic polymers to form particles in the nanometer size range and surfactants, needed to stabilize the system. The active components are entrapped in the core of the polymeric matrix, adsorbed on the surface, or both depending on the chemical nature of the polymer, surfactant, active component, and other additives. The top-down techniques require less solvents and chemicals in general, and have been adopted for various food and agricultural applications based on the safety of materials, versatility offered in delivery of both hydrophobic and hydrophilic bioactives, and ease of scale up.This section will focus on top-down techniques used to make biocompatible, biodegradable polymeric nanoparticles, which can be easily functionalized as required by the application, using low cost, versatile and scalable processes (Table 1). The method chosen to synthesize polymeric nanoparticles depends on the type of polymer, surfactant, and active component. Usually, nanoprecipitation or emulsion evaporation techniques are preferred for hydrophobic polymers; these techniques call for the use of organic solvents such as alcohol, acetone, or ethyl acetate. Other techniques such as ionic gelation, e.g. attraction between oppositely-charged amine and carboxylic groups of two polymers, or double-emulsion evaporation are employed for more hydrophilic polymers and bioactives ingredients. Fig. 1 and Table 1 show a summary of techniques used in the agricultural nanotechnology literature, chemicals needed, and the main characteristics of the synthesized polymeric nanoparticles. Notably, the majority of studies reporting polymeric nanoformulations produced particles with diameters between 100 nm to 1000 nm rather than the generally accepted size definition of nanoparticles having sizes from 1 nm to 100 nm. Here, we follow the convention of the literature in using the “nanoparticle” terminology and discuss the effect of size in subsequent sections.
| Methods | Polymer | Active ingredient | Solvent | Other comp. | Size (nm) | Zeta (mV) | PDI | pH | EE (%) | Application | Process | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emulsion–evaporation | PCL | Atrazine | Dichloromethane | Myritol, PVA | 365 to 520 | −23 to −26 | >0.200 | NR | 93% | Increase efficiency of A.I. tested in Brassica sp. and Zea mays | Sonication | 37 |
| PCL | Carbendazim and tebuconazole | Acetone, chloroform | Myritol, PVA | 300 to 700 | −20 to −30 | 0.120–0.200 | NR | >99% | Controlled release of A.I. tested in P. vulgaris seeds | Sonication | 53 | |
| Double-emulsion | Carboxymethyl cellulose | Clodinafop-propargyl | Dichloromethane | Sodium dioctyl sulfosuccinate, PVA | 150 to 350 | −37.4 | NR | NR | 90% | Reduce toxicity of A.I. tested in wheat | Sonication | 49 |
| Nanoprecipitation | Zein | NA | Acetone | DMAB | 100 to 300 | +35 | 0.205 | 6.2 | NA | Biodistribution of nanoparticles in soybeans and sugarcane | Microfluidizer | 10, 11 |
| PCL | Essential oils | Acetone | Span 60, Tween 80 | 450 to 460 | −23 to −26 | NA | 4.5–5 | 96 to 99% | Increase solubility and protection of A.I. tested in tomatoes | Mixing, evaporation | 38 | |
| PCL | Atrazine, ametryn | Oil, alcohol | Tween 80, Span 60, Myritol 318 | 200 to 300 | NA | NA | NA | 83% | Delivery and toxicity to algae and microcrustacean | Mixing, evaporation | 39 | |
| Ionic gelation | Chitosan | NA | Water | STPP, acetic acid | 181 | −23 to −26 | 0.31 | 45 | NA | Inhibition against F. Graminearum in wheat | Mixing | 48 |
| Sodium alginate | Gibberrellic acid plant hormone | Water | CaCl2 | 392 to 545 | −27 to −31 | 0.26–0.36 | 4–5 | 100% | Stabilization and increase efficiency of A.I. | Mixing | 42 | |
| Chitosan | Gibberrellic acid plant hormone | Water | STPP | 188 to 430 | +17 to +27 | 0.3–0.4 | 4–5 | 97% | Increases release times of A.I. compared to sodium alginate nanoparticles | Mixing | 42 | |
| Chitosan | Hexaconazole | Water, ethanol | STPP, Tween 80 | 100 to 600 | +35 | 0.3–0.6 | 4.9 | 73% | Less toxic and more efficient A.I. in nanoparticles compared to commercial formulation | Mixing | 7 | |
| Chitosan | NA | Water | STPP | 233 | +21 | 0.3 | 4–5 | NA | Concentration dependent inhibition of germination and plant growth | Mixing | 47 | |
| Other techniques, e.g. crosslink | Carboxymethyl chitosan, 93% DA | Methomyl | Water | Azidobenzaldehyde | 78 to 99 | −17 to −23 | 0.101–0.124 | 4–6 | 94 to 97% | Better stability and controlled release of A.I. tested against armyworm on red kidney beans foliage | Mixing | 50 |
Several examples of applications of this method to produce polymeric nanocarriers for agriculture are available in the literature. For example, polycaprolactone (PCL) polymer was used to entrap essential oils from Zanthoxylum rhoifolium (Rutaceae) as a pesticide.38 In another approach, the herbicides atrazine and ametryn were entrapped in PCL nanocapsules.39 Capric and caprylic acid oils (Myritol 318) were dissolved in the organic phase together with the herbicides and the hydrophobic surfactant Span 60, while the surfactant Tween 80 was dissolved in the aqueous phase.39–41
Other studies reported on the formation of zein nanoparticles capable to deliver pesticides for soybeans and sugarcane using the same technique.10,11 A cationic surfactant was used to promote ionic interaction between the polymeric nanoparticle and the plant tissue, especially with the roots, imparting a positive zeta potential of +81 ± 4 mV at pH 6.10,11
An alternative polymer suitable for formation of nanoparticles by ionic gelation is alginic acid. Alginic acid is negatively charged and can be crosslinked by calcium ions or alternatively used in combination with cationic chitosan. For example, Kumar et al. studied the entrapment of water-soluble a neonicotinoid insecticide (acetamiprid) in sodium alginate.46 Similarly, alginic acid was used to entrap GA3 hormone,42 and polylysine in chitosan alginate particles.44
An interesting new star amphiphilic copolymer was formed from poly(aspartic acid) and polysuccinimide (PSI). The amphiphilic properties of the copolymer allow its self-assembly in water and entrapment of the synthetic plant hormone naphthaleneacetic acid (NAA). The copolymer degrades at basic pH, providing pH-controlled release properties, of importance considering the basic environment of plant phloem (pH 8 to 8.5). The release profiles confirmed that a minimum amount of NAA (<20%) was released at pH 7 compared to almost 75% of NAA at pH 8.5 in 24 hours.51 Alternatively, PSI nanoparticles can be prepared by dispersing PSI polymer in dimethylformamide and 2-aminoethoxyethanol, and dialyzing against DI water to precipitate the nanoparticles, followed by freeze-drying.51,52 The polymeric nanoparticles showed a mean size of 20.6 nm and minimal toxic effects on plant tissue with no negative effect on soil microbial growth.52
3. Characterization of polymeric nanocarriers
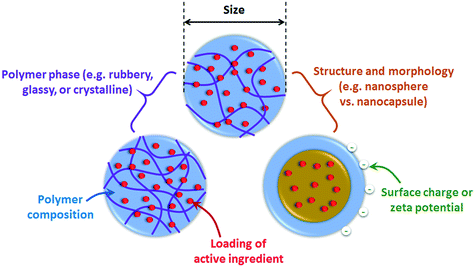
Comprehensive characterization is a critical need to explain or predict the behavior and efficiency of nano-enabled agrochemicals.6Fig. 2 summarizes important properties to characterize. Notably, additional characterization will be needed beyond what has been specified in previous “minimum characterization” guidance that was developed for inorganic nanoparticles.54–57 In particular, the loading and release behavior of active ingredients within the polymer matrix,58 as well as the composition and phase of the polymer itself, are needed. Furthermore, the internal structure of the polymeric nanoparticle will be important to explain the release or retention of active ingredients within the particle under environmental conditions. These special considerations are emphasized hereafter.3.1. Size, morphology, internal structure, and surface charge
Particle size and surface charge are well known to be key factors in the fate and biological interactions of nanoparticles. Following the same methods of surface charge evaluation for inorganic nanoparticles, electrophoretic light scattering (ELS) is typically used to determine the electrophoretic mobility, which is converted to zeta potential using the Smoluchowski, Hückel, or Henry equations.A tutorial review by Patterson et al.59 covers the application of scattering techniques and microscopy to characterize the size and morphology or structure of self-assembled polymeric nanomaterials, which is also generally relevant to other polymer nanomaterials. Briefly, morphology or structural information can be acquired using a combination of dynamic light scattering (DLS) to obtain the hydrodynamic radius, Rh, together with static light scattering (SLS) for the radius of gyration, Rg. The relationship between Rg and Rh depends on particle morphology and can hence be used to deduce the shape (e.g. rod or spherical) or structure (e.g., hollow or filled spheres) of the nanoparticles.59 Microscopy techniques, including transmission electron microscopy (TEM), scanning electron microscopy (SEM), and atomic force microscopy (AFM), can also determine both size and important structural characteristics. For example, Ye et al. developed photolabile 2-nitrobenzyl succinate (NBS) – carboxymethyl chitosan (CMCS) micellar nanoparticles for pesticide delivery, in which the NBS forms a photodegradable core within a crosslinked CMCS shell.60 Using TEM imaging, photodegradation of the NBS core could be deduced by the observed transformation of the micellar structures to hollow nanocapsules.
Polymeric particles can present new challenges to microscopy characterization methods relative to inorganic nanoparticles. Notably, organic nanomaterials will show lower contrast relative to the background, so the nanoparticles may need to be stained for improved imaging by TEM.59,61 The use of high energy microscopy techniques such as TEM is also prone to cause beam damage to polymeric nanoparticles that must be considered.61 For example, a diminishment in the measured size of latex particles of up to 29% over time in TEM measurements was attributed to degradation under the high energy electron beam.62 Drying artifacts will also be particularly significant for polymeric nanoparticles in conventional TEM, SEM, or AFM analysis, where sample dehydration can result in shrinking of the nanoparticles or bursting of hollow nanocapsules. Advanced methods such as cryo-TEM may be required to preserve the hydrated structure,63 which can be particularly useful to visualize swelling and shrinking of polymer nanoparticles, e.g. for thermoresponsive polymers.64 While AFM imaging can be performed in a liquid cell, the nanoparticles must be firmly attached to the substrate such that they are not removed by contact with the AFM tip.59 Furthermore, since the forces imparted by the AFM tip during contact mode can deform soft polymeric materials, intermittent contact or tapping modes may be required.65,66
Direct characterization of the volume density profile of the polymer matrix typically requires the use of advanced methods. Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) are useful to determine the internal radial structure of polymeric nanoparticles, as well as the core–shell structure of polymeric nanoparticles comprised of two polymers or block copolymers, as reviewed by Ballauff.67,68 For nanoparticles with multiple components, either a combination of SAXS and SANS or contrast matching of each polymeric component in SANS (by deuteration of the polymers) can be applied to better distinguish the structure of each individual component.67 Such detailed characterization can be important to understand the encapsulation and release of active compounds in the polymer particles, as has been demonstrated for drug delivery nanoparticles.69 Overall, while each sizing method has advantages and limitations, the combined analysis of information from several different sizing methods (more than may be required for inorganic nanoparticles) is recommended to acquire a complete understanding of not only the size but also the structure of polymeric nanocarriers.
3.2. Phase and phase transitions of the polymeric matrix
The phase (e.g. rubbery, glassy, or crystalline) and phase transition temperatures of the polymer matrix can be critical to characterize for polymeric nanocarriers, because a phase change will strongly affect the release rate of active ingredients from the matrix, as discussed in section 4. Crystallinity can be evaluated by X-ray diffraction (XRD) and has previously been applied to confirm crosslinking in polymeric nanoparticles, e.g., for chitosan nanoparticles after binding of cyclodextrin (which was used to enhance loading of hydrophobic pesticides),27 or alginate nanoparticles crosslinked with calcium for pesticide delivery.70 Differential scanning calorimetry (DSC) provides further information on the glass transition temperature (Tg) and melting temperatures of the polymer and the active ingredient. Finally, thermogravimetric analysis (TGA) provides the thermal degradation profile of the nanoparticles as well as quantitative information on the mass composition, provided the degradation temperatures of different components are distinct and represent a significant mass percent of the particle.Strong interactions between active ingredients and the polymer can result in shifts or disappearance of phase transition or thermal degradation temperatures in the loaded nanoparticles compared to the individual components. For example, a change in the melting temperature of the pure active ingredient has been suggested to indicate successful dispersion of antibiotics71,72 and herbicides30,31,73 in an amorphous state throughout the nanoparticles. Tg of the polymeric matrix can also be affected by the presence of the active ingredient, depending on the size, structure, hydrophilicity, and amount of loaded ingredient.18,74 For example, Stloukal et al. reported that Tg decreased with increasing loading of an herbicide, metazachlor, in poly(lactic acid) nanoparticles.18 Therefore, it will be important to evaluate phase transition temperatures on each specific sample, rather than relying on reference data for bulk materials, to predict temperature-dependent release behavior for polymeric nanocarriers.
3.3. Chemical composition of polymer and active ingredients
Spectroscopic methods, particularly attenuated total reflectance-Fourier-transform infrared (ATR-FTIR) spectroscopy, are frequently performed to confirm the polymer composition, as well as the presence of active ingredient if the loading is above the detection limit and has distinct spectral features from the polymer. A strong interaction between the polymer and active ingredient may also be deduced from changes in the peak intensity, peak location, or peak broadening of functional groups participating in the interaction. For this analysis, the spectrum of the loaded nanoparticle should be compared to not only the “empty” nanoparticle and pure active ingredient controls, but also a “physical mixture” of the active ingredient and empty nanoparticles to confirm whether or not spectral changes are attributable to entrapment within the nanoparticle.Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and proton and carbon nuclear magnetic resonance spectroscopy (1H NMR and 13C NMR, respectively) are less commonly applied but can provide further information beyond ATR-FTIR spectroscopy. An advantage of Raman over ATR-FTIR spectroscopy is the significant reduction in interferences from liquid water;75 hence, Raman spectroscopy has recently been shown to be capable of obtaining spectra of individual drug-loaded PLGA nanoparticles in combination with optical trapping.76 XPS and NMR can further provide information on structure: for example, Celasco et al. reported the use of depth profiling XPS and angle-resolved XPS to distinguish the organization of poly(ethylene glycol) copolymers in nanosphere versus nanocapsule structures,22 and 1H NMR has been applied to understand the mobility of drug molecules in liposomes or solid lipid nanoparticles.77,78
Additional research is needed that applies these methods not only to the as-synthesized nanocarriers, but also after exposing the nanoparticles to environmental conditions, such as light exposure or biodegradation. For example, Chen et al. applied FTIR and 1H NMR spectroscopy to confirm the proposed pH-dependent hydrolysis of polysuccinimide (PSI) groups for targeted plant phloem delivery of plant hormones.51 Ye et al. also demonstrated the use of 1H NMR to confirm the formation of photolytic products in micellar carboxymethyl chitosan nanoparticles with 2-nitrobenzyl modification for photo-responsiveness.60 Mass spectrometry is also applied to identify polymer degradation products, e.g. for PLGA.79In situ (flow cell) ATR-FTIR methods have previously been used to monitor adsorption80–86 and chemical reactions or degradation87–89 of organic coatings on inorganic nanoparticles; these methods would be interesting to apply for polymeric nanocarriers to further evaluate the kinetics of transformation or degradation and hence understand their long-term fate and interactions in the environment.
3.4. Quantification of the loading and release of active ingredients in simple media
The loading and release rate of active ingredients from nanocarriers are key factors in assessing or predicting their efficiency. Two approaches can be used (Fig. 3): either the concentration of ingredients remaining inside the polymeric matrix is measured, or the released ingredients are quantified. Regardless of the chosen approach, separation of nanoparticles from the matrix (which includes the released ingredients) is required.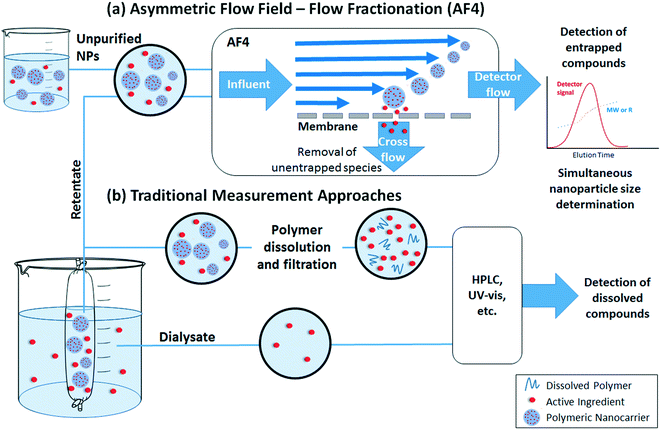 | ||
| Fig. 3 Methods for measurement of active ingredient loading and release by (a) asymmetric flow field – flow fractionation (AF4) or (b) traditional measurement approaches. | ||
Traditional quantification of loading or release involves separation of the nanoparticles and dissolved materials prior to measurement (Fig. 3b). In some separation methods (e.g., ultracentrifugation or centrifugal ultrafiltration), release may be overestimated due to the force applied during the separation process or time required to process the sample.90 If the nanoparticles are needed for further analysis, another drawback is the possibility for poor recovery. Dialysis is a gentler separation process, but slow diffusion of dissolved ingredients through the dialysis membrane may result in underestimation of the true release rate.90 In addition, the released compounds are significantly diluted in the dialysate, which may require the use of high nanoparticle concentrations to achieve measurable results (however, if “sink” conditions are maintained on the dialysate side, release rates are still representative of diluted conditions e.g. as would occur when diluting a formulation for use in the field). After separation, released ingredients in the filtrate, dialysate, or supernatant can be easily quantified by high performance liquid chromatography (HPLC) or batch UV-vis spectrophotometry. To quantify the entrapped ingredient, addition of an organic solvent is often required to extract compounds from the polymeric matrix or dissolve the polymeric nanoparticle. In both measurements, the presence of dissolved polymer or other reagents can interfere with the analysis, and hence it is important that high recovery is confirmed in spike recovery tests or appropriate corrections are made, e.g. by the method of standard additions instead of quantifying against external standards.
Direct quantification of entrapped ingredients within an intact nanoparticle without a need for pre-separation can provide advantages to the traditional approach described above. However, such approach requires the compound of interest to have a distinct property (e.g. fluorescence or UV-vis absorbance) from the polymer and minimal matrix interference. Asymmetric flow field – flow fractionation (AF4) (Fig. 3a) is a relatively new approach that can eliminate sample processing steps and provide simultaneous particle characterization together with quantification of loading. In this method, the nanoparticles are focused in an AF4 channel at the beginning of the analysis; incidentally, the nanoparticles are also separated from dissolved species (which pass through a cross-flow membrane) during this step. Hence, no pre-separation steps are required as in traditional measurement approaches. Thereafter, the nanoparticles are separated by size (diffusion coefficient) in the AF4 channel, enabling size-resolved detection and characterization by downstream detectors. Based on the choice of detectors, quantitative information about the loading (e.g., using the UV-vis absorbance or fluorescence of the active ingredient), as well as the concentration and size distribution of the nanoparticles, can be obtained. Sources of error include the potential for entrapped ingredients to be washed out during the focus step,91–93 the need to correct for any particle scattering contributions to the signal used,94,95 as well as the possibility for interactions of the active ingredient within the nanoparticle to change its spectral properties.96 Despite these issues, AF4 with online UV-vis detection has successfully been applied by Hinna et al. to quantify a porphyrin drug within liposomal nanocarriers,94,97,98 by Iavicoli et al. to quantify the binding of antimicrobial peptides to liposomes,99 and by Fraunhofer et al. to quantify oligonucleotide loading on gelatin nanoparticles.100
Dialysis and AF4 can be successfully performed in aqueous matrices containing dissolved humic substances or biomolecules as well as other ingredients that may comprise the matrix of a commercial formulation. Chromatographic methods such as AF4 can even probe interactions between the nanoparticles and matrix components. For example, Holzschuh et al. have applied A4F to separate liposomes from human plasma and to evaluate lipid and drug transfer from the liposomes.93 However, to our knowledge, most studies evaluate release in only simplified media (deionized water at a specified pH, possibly with a background electrolyte). Interactions with natural molecules present in soil porewaters, as well as other solutions that may be co-applied (e.g. fertilizer solutions101) should be considered in future studies.
3.5. Detection and characterization in complex matrices
The application of polymeric nanoparticles in soils, plants, and animals introduces the significant challenge of finding a carbon-based material in a highly complex matrix full of other organic carbon species and solid or particulate material. Measuring active ingredient release rates will also be highly challenging. Incorporation of a probe compound, such as a fluorescent tag10,11,102 or radiolabeled polymers, in the nanoparticle is often used to identify the particle by imaging or other methods. Otherwise, the nanoparticles would need to be isolated from the media due to the severe interferences. However, extraction processes are likely to be either ineffective or likely to disrupt the nanomaterial or the partitioning of the active ingredient. Kah et al. highlight the difficulty in measuring release in soils and suggest that release may only be possible to evaluate through indirect methods.6 One such method that has been applied for pesticides103,104 is to assume that degradation of the active ingredient occurs only upon release. Then, the total remaining (undegraded) active ingredient in the soil can be extracted into organic solvent at several time points for measurement by HPLC, and the rate of degradation is measured for the pure (unentrapped) pesticide and the nano-formulation. Models that incorporate both the release rate and degradation rate of released compound can then be fitted to estimate the release rate.4. Mechanisms for release of active ingredients
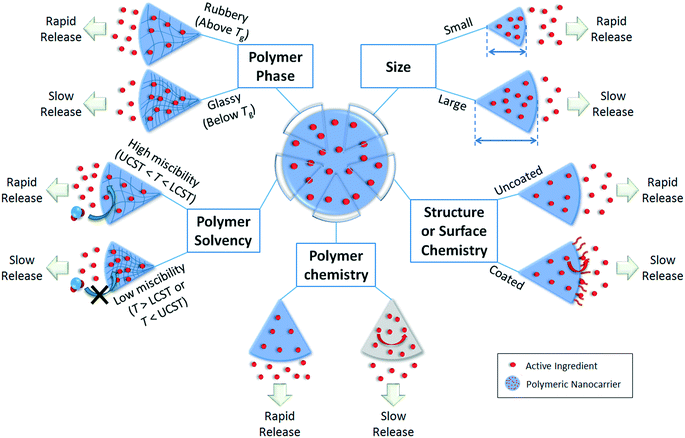
The release profile of the active ingredient from the polymer matrix will be critical in designing or predicting the behavior of the overall nanoparticle, e.g. controlled, slow release for prolonged application, or stimuli-responsive release for timed or targeted delivery of active compounds.1 Release can occur by Fickian diffusion, swelling or relaxation of the polymer (promoting more rapid diffusion), and surface or bulk erosion (degradation) of the nanoparticle.105 An initial “burst” release is also commonly observed. Major factors affecting the release rate are illustrated in Fig. 4 for the diffusion and relaxation mechanisms (which do not involve decomposition of the polymeric nanoparticle) and Fig. 5 for the erosion mechanisms (in which polymer degradation leads to release).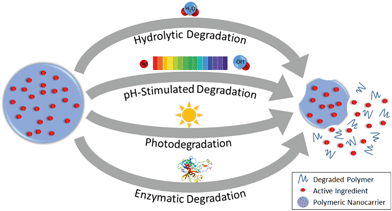 | ||
| Fig. 5 Release of active ingredients from polymeric nanocarriers by degradation of the polymeric matrix. | ||
4.1. “Burst” release
Burst release refers to the phenomenon in which an initial rapid release of active ingredient occurs prior to slow release, and can be undesirable if an initially high concentration of active ingredient is not tolerable for the application of interest.105 A burst release phenomenon would indicate a higher concentration of active ingredient residing on or near the surface of the nanoparticles after synthesis, with smaller nanoparticles (higher surface area to volume ratio) demonstrating more significant burst releases, as shown by Stloukal et al. for poly(lactic acid) (PLA) nanoparticles loaded with an herbicide, metazachlor.18 The use of a nanocapsule structure or a coating around the surface of the nanoparticles has been suggested to suppress the rapid initial “burst” release that is often observed for nanospheres.1064.2. Release by diffusion through the polymer matrix and nanoparticle swelling/relaxation
In Fickian diffusion, active ingredients will diffuse from regions of high concentration inside the nanoparticle to low concentration outside the nanoparticle following Fick's second law. Because of the dependence of release rate on the concentration gradient, release would occur more rapidly when the nanocarriers are diluted, e.g. upon dilution of a solid or concentrated formulation by growers prior to application, or during rainfall or irrigation events. Release by Fickian diffusion can be slowed by increasing the nanoparticle size (i.e. increasing the distance across which the active ingredient must diffuse). For example, in addition to a reduced burst release, Stloukal et al. also observed slower release of metazachlor by diffusion from PLA nanoparticles as the size increased.18 Increased cross-linking has also been reported as a successful strategy to delay diffusion by decreasing the porosity or increasing the tortuosity through the polymer matrix, as shown for a methomyl pesticide loaded into azidobenzaldehyde–carboxymethyl chitosan nanocapsules before and after crosslinking of the polymer.50Swelling or relaxation of the polymeric nanoparticle will cause faster release of active ingredients as they dissolve into the infiltrating solvent (typically an aqueous medium) and transport more rapidly out of the relaxed polymer matrix through the solvent-filled pores. This mechanism is referred to as “Case II” transport, and can be distinguished from Fickian diffusion by modeling the release profile. For example, the empirical Korsmeyer–Peppas model107 (eqn (1)) is frequently applied to distinguish release mechanisms:
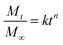 | (1) |
Polymer swelling and relaxation can be strongly affected by environmental factors, such as temperature, and hence be exploited to achieve triggered or stimuli-responsive release in agricultural applications.109 Important temperatures of note are the upper critical solution temperature (UCST) and lower critical solution temperature (LCST), between which the polymer is miscible with the solvent. For example, poly-N-isopropyl acrylamide (PNIPAm) is a well-known temperature-sensitive polymer that swells at temperatures below its LCST of 32 °C. Grafting of the PNIPAm polymer onto polydopamine (PDA) nanoparticles has hence been shown to lead to temperature-dependent release of a pesticide, emamectin benzoate, with faster release at lower temperature attributable to swelling of the PNIPAm below the LCST.110
Another important thermal property is the glass transition temperature (Tg), describing the phase transition of the polymer from glassy (rigid) below Tg to rubbery (flexible) above Tg. Lappe et al.74 showed that for DL-PLA, L-PLA, and PLGA nanocarriers, primarily burst release of adsorbed drugs on the nanoparticle surface occurred at temperatures below Tg. On the other hand, at temperatures higher than Tg, higher release of the entrapped drugs occurred.74
To further slow the release of an active compound below the rate of Fickian diffusion or the swelling/relaxation rate, materials can be selected such that the active ingredients have more favorable interactions with the components of the nanoparticle matrix relative to the solvent. For example, when Campos et al. compared the release of two pesticides, carvacrol and linalool, co-loaded in β-cyclodextrin-functionalized chitosan nanoparticles, faster and more extensive release of the more hydrophilic linalool ingredient was observed.111 Grillo et al. compared the release rates and profiles of three herbicides, ametryn, atrazine, simazine, from nanocapsules with a PCL shell and an oil core.41 The slower release of ametryn compared to atrazine was attributed to the higher affinity of ametryn with either the PCL shell or oily interior of the nanocapsule. The release was slowest for simazine, which was proposed to occur because of hydrogen bonding between simazine and the PCL shell of the nanoparticles, which is blocked by the methyl groups present on atrazine and ametryn.41
Similarly, different structures or compositions of the nanoparticle have been proposed to tune the release kinetics. Nanocapsules or vesicles comprised of a shell surrounding a core of a different composition have been suggested to provide slower release profiles than homogeneous nanospheres;106 however, release profiles were similar for atrazine loaded into PCL nanocapsules compared to PCL nanospheres.37 Therefore, tuning the chemistry of the coating or shell around the nanoparticle may be a more promising strategy to delay release, as opposed to developing nanoparticles comprised of the same material in different nanocapsule or nanosphere structures. For example, for pesticide delivery, the addition of a polyurea coating onto imidacloprid-loaded PDA microcapsules112 or a chitosan coating onto deltamethrin-loaded beeswax solid lipid nanoparticles113 delayed the release relative to the uncoated nanoparticles. Sun et al. also reported that high entrapment of a pesticide, methomyl, in carboxymethyl chitosan nanocapsules was primarily attributable to adsorption of the methomyl to the polymer, rather than partitioning into the aqueous interior of the nanocapsules.50 Additional characterization and predictive models to localize interactions between the active ingredient and the specific components of the nanoparticle would be useful to better predict a priori materials that can be used to develop nanoparticles with a desired release rate.
4.3. Degradation of nanoparticles
Release can be accelerated or triggered by chemical, physical, or biological degradation of the nanoparticle. This degradation can proceed by hydrolysis with water, or it can require a specific stimulus, such as a change in pH or temperature, light exposure, or enzymatic activity, to occur (Fig. 5).109In hydrolytic degradation, water participates in a cleavage reaction of vulnerable bonds such as esters, degrading the polymer chains and then leading to loss of mass from the nanoparticle.114 For instance, PLGA nanoparticles show slow degradation that occurs by bulk erosion via hydrolysis of ester bonds; after the initial hydrolysis, faster degradation is catalyzed by the increasing water penetration and formation of carboxylic groups.115,116 Nano-sized PLGA shows faster hydrolytic degradation than micro-sized PLGA because of the higher surface area to volume ratio (i.e. higher accessibility to water), as well as the greater ease for polymer degradation products to diffuse out through the polymer matrix.117 The degradation rate can also be tuned by adjusting the composition of a nanocarrier such that the proportion or accessibility of labile bonds is modified. For example, the rate of hydrolysis of nanoparticles composed of mixtures of PLGA and poly(L-lactic acid) or solely of PLGA with different ratios of lactic acid to glycolic acid, decreases with increasing lactic acid content: the methyl side groups on the lactic acid impart steric hindrance inhibiting the hydrolysis of the ester bonds118 while the glycolic acid groups have higher bound, reactive water content.119 On the other hand, incorporating methoxy poly(ethylene glycol) (mPEG) in PLGA nanoparticles leads to faster degradation of the nanoparticles,120 since the mPEG increases the hydrophilicity of the nanoparticle and hence accessibility for hydrolysis.121
Polymer degradation can be acid- or base-catalyzed, enabling pH-responsive release. For example, solid lipid nanoparticles have been synthesized with acetal groups that are cleavable under acidic conditions (e.g., pH 6.5) for targeted release of vancomycin antibiotics at acidic infection sites.122 In plants, the pH is higher in the phloem than other regions,123 and hence pH-sensitive PSI-based nanoparticles have been proposed for triggered release of active compounds in the phloem. For example, Chen et al.51 suggested the use of poly(aspartic acid-co-succinimide) polymeric nanoparticles for targeted delivery of a synthetic plant hormone, naphthaleneacetic acid (NAA), to the phloem of plants. These nanoparticles are stable under neutral conditions. In contrast, at pH 8.5, the PSI units of the nanoparticles are hydrolyzed to polyaspartate, resulting in more rapid release of the NAA.51 Similarly, the release of two model compounds, coumarin 6 (ref. 52) and Nile Red,124 from PSI-based nanocarriers occurs more rapidly at basic pH, with slightly faster release of Nile Red under hydrolytic conditions for smaller nanoparticles with higher surface area.124 Functionalization of the PSI with hydrophobic hexylamine was able to prevent base hydrolysis and dye release,124 providing another option to tune the release behavior by tuning the penetration of solvent carrying reactive species into the polymer matrix.
The pH can also affect the physical stability of the nanoparticle when the polymer is a weak acid or base, such that the charge and electrostatic interactions will depend on pH. For example, Lin et al. developed nanoparticles from feather keratin and carboxymethyl cellulose (CMC) loaded with a pesticide, avermectin.28 While diffusion was Fickian at lower pH, the release rate became faster and non-Fickian transport at higher pH. The faster release was proposed to be caused by the transition of the keratin to negative charge at higher pH, resulting in electrostatic repulsion with the negatively-charged CMC and dissociation of the nanoparticles.
Stimuli-responsive release can also be achieved using photosensitive polymers. For example, UV-labile core–shell or micellar nanoparticles were developed by conjugating nitrobenzyl compounds to carboxymehtyl chitosan60 and poly(ethylene glycol) (PEG)125 polymers. These nanoparticles were loaded with diuron and 2,4-dichlorophenoxyacetic acid (2,4-D) herbicides, respectively, and demonstrated to exhibit UV-triggered release. Further study on light-activated nanoparticles would be interesting for applications of nanoparticles in sunlit environments, such as foliar delivery of agrochemicals.
Finally, the activity of enzymes such as proteases, glycosidases and phosphatases can induce the degradation of nanoparticles. For example, Chawla et al. found that the degradation of PCL nanoparticles increases dramatically in the presence of lipase enzyme in comparison with enzyme-free phosphate buffered saline.126 They proposed that the hydrophilicity of the enzyme prohibits movement into the hydrophobic interior of the nanoparticle, so enzymatic hydrolysis occurs at the surface of nanoparticle where the enzyme adsorbs.126 Another study by Fu et al. showed more rapid and extensive degradation of zein nanoparticles and release of an entrapped antibiotic, ciprofloxacin, in the presence of trypsin than collagenase or enzyme-free phosphate buffered saline.127In vitro enzymatic degradation of chitosan nanoparticles by lysozyme was also reported by Hou et al.128 Akagi et al. demonstrated that the enzyme-mediated degradation of poly(γ-glutamic acid) (γ-PGA) nanoparticles by γ-glutamyl transpeptidase (γ-GTP), which is a common enzyme found in wide range of organisms, is more rapid than hydrolytic degradation.129 In addition, enzymes such as pronase, protease, cathepsin B, and lipase, all of which may be present in in vivo systems, have also been reported to induce degradation of γ-PGA by cleaving the amide bond of the polymer.130 Given the wide variety of enzymes present in in vivo systems and the variety of enzymatic activities demonstrated in these studies, additional research is needed to fully understand and develop a generic mechanism to predict the enzymatic degradation behavior of polymeric nanoparticles.
5. Environmental fate and biological effects
The fate, transport, bio-uptake, and biological effects of the polymeric nanoparticles and their associated active ingredients must all ultimately be optimized in order to develop a successful technology that improves the desired function of the active ingredient (compared to non-nano formulations) while having minimal adverse effects in the environment. Potential mechanisms for polymeric nanocarriers to play this role are highlighted below.5.1. Fate, transport, and uptake of polymeric nanocarriers and their associated active ingredients
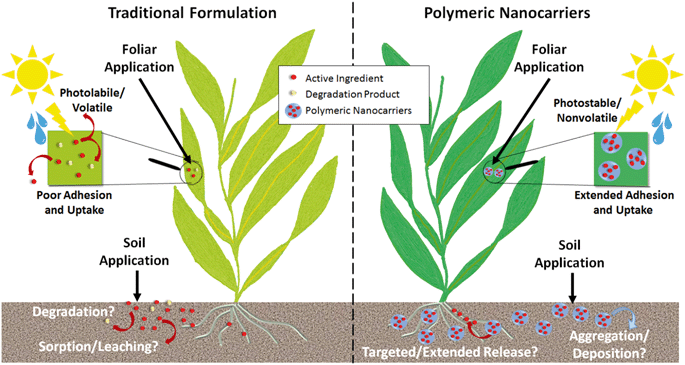
For agricultural applications, the goal of using a polymeric nanocarrier is often to reduce the overall quantity of agrochemicals needed, which can be achieved by improved targeting or uptake of the active ingredient or protecting the active ingredient from degradation (Fig. 6).Enhanced photostability and reduced volatility of the active ingredient have been demonstrated across a variety of polymer types, as summarized in Table 2, and would reduce the quantities of pesticides required as well as the need for reapplication over time. Furthermore, the enhanced stability afforded by the nanoparticles enables the use of more sustainable active ingredients, such as botanical oils, that would be prone to degradation or volatilization in their unentrapped form.21,38,131,132 Polymeric nanoparticles can also be designed to enhance the adhesion or uptake of agrochemicals, particularly for foliar applications (Table 2). For example, bio-inspired polydopamine and polycatechol-coated nanoparticles have been proposed for enhanced adhesion of pesticides to plant leaves.133,134 Few studies are available that directly demonstrate plant uptake, likely due to the challenges in detecting polymeric nanoparticles within plants, but recent studies using fluorescently-labeled nanocarriers have shown promising results for foliar uptake of PCL nanoparticles (up to 345 nm in diameter) and root uptake of zein nanoparticles (135 nm).10,102 For comparison, the typical upper size limits summarized by Lv et al. for inorganic nanoparticles are up to 140 nm for root uptake, with foliar uptake by stomatal pathways having a largely unknown size limit with uptake of up to ≈50 nm reported thus far.135 Additional uptake studies on the variety of other polymer types that have been proposed as well as across a range of sizes are needed to identify the ideal nanoparticles for agrochemical delivery.
| Function | Polymer (nanoparticle diameter in parentheses) | Active ingredient | Benefits conferred by polymeric nanoformulations | Ref. |
|---|---|---|---|---|
| Notes: A.I.: active ingredient; PCL: poly(ε-caprolactone); PLA: poly(lactic acid): PLGA: poly(lactic-co-glycolic acid). | ||||
| Photostability of active ingredients | PCL (450 to 465 nm by DLS) | Essential oils (insecticides) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 7 h exposure to UV-A and UV-C light | 38 |
| Chitosan/gum arabic (≈200 nm) | Geraniol (insecticide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 7 d exposure to UV (365 nm) light | 21 | |
| Zein (143 to 172 nm by DLS) | R-Citronellal, geraniol (insecticide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 7 d exposure to UV (365 nm) light; photoprotection more apparent for geraniol than citronella | 131 | |
| Polydopamine (215 nm by TEM) | Avermectin (insecticide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 78 h exposure to UV light | 133 | |
| Poly(styrene-co-methacrylic acid) – polycatechol (102 to 122 nm by DLS) | Avermectin (insecticide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 96 h exposure to UV light | 134 | |
| Feather keratin – carboxymethylcellulose (≈390 nm by DLS) | Avermectin (insecticide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 30 h exposure to UV light | 28 | |
| PLA (680 to 4600 nm by DLS) | λ-Cyhalothrin (insecticide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 72 h exposure to UV (365 nm) light | 138 | |
| Beeswax solid lipid nanoparticles, with or without chitosan coating (≈200 to 230 nm by DLS) | Deltamethrin (insecticide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 72 h exposure to UV-B light | 113 | |
| Polyacrylate (≈80 nm by DLS) | Emamectin benzoate (insecticide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 9 h exposure to simulated sunlight | 139 | |
| PLGA (600 nm by laser particle size distribution analysis) | Pyraclostrobin (fungicide) | Enhanced photostability compared to unentrapped A.I., evaluated over up to 1 h exposure to UV light | 140 | |
| Volatility of active ingredients | Zein (234 to 282 nm by DLS) | Cinnamaldehyde, eugenol, and geraniol (insecticides) | Reduced volatility compared to unentrapped A.I., evaluated for 120 d storage duration | 132 |
| Adhesion and uptake by plants | Polydopamine (215 nm by TEM) | Avermectin (insecticide) | Attachment to cotton and corn leaves from aqueous suspension, with and without water washing | 133 |
| Poly(styrene-co-methacrylic acid) – polycatechol (102 to 122 nm by DLS) | Avermectin (insecticide) | Attachment to cucumber and broccoli leaves after spraying, drying, and washing | 134 | |
| PCL (256 to 345 nm) | Atrazine (herbicide) | Uptake through stomata, particularly in hydathode regions, and vascular transport in Brassica juncea | 102 | |
| Zein (135 nm) | None | Root uptake and translocation in sugar cane plants | 10 | |
| Zein (135 nm) | None | Association of nanoparticles with roots, with possible uptake and translocation, in soybean plants | 11 | |
Subsequent to field application, the effect of the polymeric nanoparticles on the transport of the agrochemicals from soils is also of interest, given the problems of surface water and groundwater pollution from agricultural runoff. Varying results have been observed in the literature regarding whether entrapment or encapsulation enhances or reduces release of the agrochemicals from soils. For example, loading of carbendazim and tebuconazole fungicides into polymeric and PCL nanocapsules and solid lipid nanoparticles resulted in diminished leaching from soils compared to commercial, non-nano formulations;53 on the contrary, Grillo et al. and Silva et al. reported lower sorption of paraquat to soils when loaded into chitosan/tripolyphosphate and alginate/chitosan nanoparticles, respectively,30,45 and Pereira et al. reported deeper penetration of atrazine into soil columns when loaded into PCL nanocapsules and nanospheres.37 Chen et al., Kah et al., and Petosa et al. have each found that the transport or deposition of polymeric nanocarriers and their associated active ingredients (e.g. drugs or herbicides) varies widely with the type of polymer as well as the environmental conditions (e.g., water chemistry and soil type).104,136,137 The possibility for naturally occurring macromolecules such as natural organic matter, proteins, and polysaccharides to adsorb to the nanoparticles and change their transport behavior should also be considered. While Grillo et al. reported that aquatic humic substances did not affect the colloidal stability of paraquat-loaded chitosan nanoparticles,45 Chen et al. observed a significant effect of the interaction of negatively-charged humic acids on the deposition of positively-charged poly(caprolactone-b-ethylenimine) (PCL-PEI) nanoparticles onto silica surfaces, consistent with charge neutralization and reversal.136
In summary, to fully describe the transport behavior of active ingredients carried by polymer nanoparticles, not only the aggregation and deposition behavior of the nanoparticle, but also the kinetics of release and the sorption behavior of the active ingredient, must all be taken into account. Hence, transport models can be more complex than those previously developed for inorganic nanoparticles (without an active ingredient loading), and a large suite of additional studies will likely be needed to develop such models.
5.2. Effectiveness for agricultural applications
For crop growth and protection, polymeric nanoparticles have been proposed to deliver plant growth promoters and pesticides, including insecticides, herbicides, and fungicides. For livestock and aquaculture, polymeric nanocarriers may also be used to deliver antibiotics. As summarized in Table 3, many types of polymeric nanoparticles or nanocapsules have been developed using biocompatible or biodegradable materials (e.g., alginate, chitosan, zein, PEG, and PCL) to deliver both conventional synthetic herbicides, insecticides, and fungicides as well as unconventional, botanically derived oils as more sustainable alternatives.| Purpose | Polymer (nanoparticle diameter in parentheses) | Active ingredient (A.I.) | Target species | Activity against target species | Representative concentration | Cytotoxic, phytotoxic, or ecotoxicological effects | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Free A.I. | NP A.I. | |||||||
| Notes: A.I.: active ingredient; PCL: poly(ε-caprolactone); (m)PEG: (methoxy)poly(ethylene glycol); PLA: poly(lactic acid): PLGA: poly(lactic-co-glycolic acid); SLNs: solid lipid nanoparticles. a Indicates the concentration where a significantly improved effect was reported relative to the unentrapped A.I. or commercial formulation. b Indicates the lethal concentration to achieve 50% mortality (LC50) of the A.I. c Indicates the minimum inhibitory concentration (MIC) of the A.I. d Indicates the A.I. concentration resulting in a measurable zone of inhibition. | ||||||||
| Plant growth hormones | γ-Polyglutamic acid/chitosan (134 nm by DLS) | Gibberellic acid | Phaseolus vulgaris | Enhanced germination and development compared to unentrapped A.I. | 0.7 and 2.1 μg g−1 of seeds | Not evaluated | 177 | |
| Alginate/chitosan (450 nm by DLS); chitosan/tripolyphosphate (195 nm by DLS) | Gibberellic acid | Phaseolus vulgaris | Increased leaf area only for alginate/chitosan nanoparticles compared to unentrapped A.I.; shoot and root growth similar | 0.037% and 0.05% | Not evaluated | 42 | ||
| Alginate/chitosan (450 nm by DLS); chitosan/tripolyphosphate (195 nm by DLS) | Gibberellic acid | Solanum lycopersicum | Enhanced root/shoot growth and fruit production compared to unentrapped A.I., particularly for the alginate/chitosan nanoparticles | 0.0005 to 0.005 mg ml−1 | Not evaluated | 165 | ||
| Herbicide | Alginate/chitosan (378 nm by DLS) and chitosan/tripolyphosphate (479 nm by DLS) | Imazapic and imazapyr (co-loaded) | Bidens pilosa | Similar herbicidal activity to the unentrapped A.I. (evaluated at 400 g ha−1) | n/a (no significant difference) | Lower cytotoxicity and genotoxicity to Chinese hamster ovary cells and Allium cepa seedlings, compared to unentrapped A.I. | 169 | |
| Chitosan/tripolyphosphate (300 nm by DLS) | Paraquat | Brassica sp. | Similar herbicidal activity to the unentrapped A.I. (evaluated at 2 kg ha−1) | n/a (no significant difference) | Less pronounced phytotoxicity to non-target Zea mays plants; lower cytotoxicity and genotoxicity to Chinese hamster ovary cells and Allium cepa seedlings, compared to unentrapped A.I. | 45 | ||
| Chitosan/tripolyphosphate (282 nm by DLS) | Paraquat | n/a | n/a | n/a | n/a | Lower toxicity to Pseudokirchneriella subcapitata (algae) than unentrapped A.I. | 43 | |
| Alginate/chitosan (200 to 1000 nm by DLS) | Clomazone | n/a | n/a | n/a | n/a | Similar hepatotoxicity to Lithobates catesbeianus (bullfrog tadpoles) compared to unentrapped A.I. | 178 | |
| Lignin (150 to 190 nm by NTA) | Diuron | Brassica rapa | Similar or greater leaf chlorosis and mortality compared to unentrapped A.I.; similar efficiency to commercial formulation | 2.5 mg/pot compared to unentrapped A.I. | Not evaluated | 19 | ||
| Pectin nanocapsules (164 nm by DLS) | Metsulfuron methyl | Chenopodium album in wheat crop (T. aestivum) | Reduced weed biomass compared to commercial formulation | 50 mg L−1 (with 6.3% herbicide loading); total dose not reported | Lower cytotoxicity to Vero cell lines, compared to commercial formulation | 20 | ||
| PCL nanocapsules (241 nm by DLS) | Atrazine | Brassica juncea | Similar reduction in shoot growth compared to commercial formulation (evaluated at 0.1 and 1 mg mL−1) | n/a (no significant difference) | Not evaluated | 145 | ||
| PCL nanocapsules (483 nm by DLS) and wfi 1nanospheres (409 nm by DLS) | Atrazine | Brassica sp. | Greater inhibition of seedling emergence by nanocapsules and nanospheres compared to unentrapped A.I. | 2.5 kg ha−1 | No effect on non-target crops (Zea mays); reduced genotoxicity to Allium cepa at some concentrations | 37 | ||
| PCL nanocapsules (size not reported) | Atrazine | Bidens pilosa | Higher mortality of weeds at lower dose compared to commercial formulation | 200 g ha−1 | Higher short-term (17 d) toxicity to non-target Glycine max (soybean) plants, but gradual recovery over 60 d | 179 | ||
| PCL nanocapsules (260 nm by DLS) | Atrazine | Amaranthus viridis and Bidens pilosa | Inhibition of root and shoot growth at lower dose compared to commercial formulation | 200 and 2000 g ha−1 | Not evaluated | 180 | ||
| PCL nanocapsules (size not reported) | Atrazine | n/a | n/a | n/a | n/a | No effect of atrazine (commercial or nanocapsule) to Zea mays L. at 0.1 mg mL−1; reduction in photosynthesis for 1 to 2 days with nanocapsule at 1 mg mL−1 but recovery by 4 days after application | 181 | |
| PCL nanocapsules (260 nm by DLS) | Ametryn, atrazine, and simazine | n/a | n/a | n/a | n/a | Reduced DNA damage in Comet assay, reduced genotoxicity to Allium cepa compared to unentrapped A.I. | 41 | |
| PCL nanocapsules (200 to 300 nm by DLS) | Ametryn, or atrazine | n/a | n/a | n/a | n/a | Lower toxicity to algae, higher toxicity to Daphnia similis, and lower cytotoxicity to lymphocytes than unentrapped A.I. | 39 | |
| PCL nanocapsules, chitosan/tripolyphosphate, and SLNs (≈250 to 370 nm) | Atrazine, simazine, and/or paraquat | n/a | n/a | n/a | n/a | Higher toxicity to C. elegans for both empty and loaded nanocarriers compared to unentrapped A.I. | 182 | |
| mPEG-PLGA (≈90 nm by DLS) | Metolachlor | Oryza sativa, Digitaria sanguinalis, Arabidopsis thaliana | Inhibited seedling growth (unentrapped A.I. was not evaluated) | Not evaluated | 0.1 mg L−1 | Lower cytotoxicity to MC3T3 preosteoblast cells than unentrapped A.I. | 183 | |
| Insecticide or insect repellent | Alginate (150 nm by DLS) | Imidacloprid | Leafhoppers | Lesser efficacy in reducing pest population over short duration (7 d), but improved efficacy at longer durations (9 to 15 d), compared to commercial formulation | 0.145 mg L−1 (0.02 mg m−2 total dose) | Lower cytotoxicity to Vero cell lines than commercial formulation | 142 | |
| Carboxymethyl chitosan nanocapsules with aqueous core (90 to 99 nm by DLS) | Methomyl | Armyworm larvae | Higher larvicidal activity compared to the unentrapped A.I. | 50 and 100 mg L−1 in spray | Not evaluated | 50 | ||
| Chitosan with β-cyclodextrin functionalization (175 to 246 nm by DLS) | Carvacrol or linalool (separately loaded) | Tetranychus urticae | Higher repellency, and higher acaricidal activity and hindrance of oviposition, compared to the unentrapped A.I. | 1.56 mg cm−2 of leaf area | Not evaluated | 27 | ||
| Chitosan/gum arabic with β-cyclodextrin functionalization (226 nm by DLS) | Carvacrol and linalool (co-loaded) | Helicoverpa armigera, Tetranychus urticae | Higher insecticidal activity, and higher acaricidal activity and hindrance of oviposition for T. urticae, compared to the unentrapped A.I. | 1.25 mg ml−1 | Lower cytotoxicity to pulmonary (v79) and mouse fibroblast (Balb C-33) cell lines, and lower phytotoxicity to Zea mays, than unentrapped A.I. | 111 | ||
| Polyacrylate (≈80 nm by DLS) | Emamectin benzoate | Helicoverpa armigera | Improved efficacy for larva mortality over 72 h compared to unentrapped A.I. | 1% | Not evaluated | 139 | ||
| PCL (450 to 465 nm by DLS) | Essential oils | Bemisia tabaci | Reduction in eggs and nymphs compared to pyriproxyfen 1% insecticide (unentrapped essential oils not evaluated) | Not evaluated | 1% | Not evaluated | 38 | |
| PEG (≈230 nm by DLS) | Garlic oil | Tribolium castaneum | Improved insecticidal activity over 5 month duration compared to unentrapped A.I. | 640 mg kg−1 of rice for 5 months | Not evaluated | 144 | ||
| PEG copolymer (initial size 10 to 20 nm by TEM, followed by formation of microcapsules) | Imidacloprid | Glyphodes pyloalis | Higher efficiency for larva mortality compared to unentrapped A.I., especially over longer durations (2 to 5 d) | Time- and assay-dependent (e.g. 60 mg L−1 at 5 d) | Time- and assay-dependent (e.g. 7 mg L−1 at 5 d) | Not evaluated | 143 | |
| PEG–PLA (150 nm by DLS) | λ-Cyhalothrin | Aphis craccivora | Similar aphid mortality compared to commercial emulsion or microemulsion | 0.27 mg L−1 | 0.26 mg L−1 | Not evaluated | 25 | |
| Unknown polymer (commercial formulation separated into ≈250 nm and ≈2200 nm fractions) | λ-Cyhalothrin | n/a | n/a | n/a | n/a | Lesser tremors in embryonic Danio rerio for unentrapped A.I. compared to all polymeric formulations; otherwise similar sublethal impacts and mortality for all A.I. exposures | 184 | |
| Poly(styrene-co-methacrylic acid) – polycatechol (102 to 122 nm by DLS) | Avermectin | Aphids | Improved efficiency with adhesive polycatechol functionalization compared to commercial emulsification and water-dispersible granule formulations; nanoformulations without polycatechol showed similar or lower efficiency than commercial formulations | 10.1 to 12.4 mg L−1 on cucumber; 124.6 to 150.3 mg L−1 on broccoli for commercial formulations | 4.3 mg L−1 on cucumber; 55.4 mg L−1 on broccoli for catechol-functionalized NPs | Not evaluated | 134 | |
| Zein (143 to 172 nm by DLS) | Geraniol or R-citronellal | Tetranychus urticae | Better insect repellent activity for geraniol nanoformulation compared to unentrapped A.I. at shorter times (e.g. 8 h and 24 h) | 0.5 and 5 mg ml−1 | Similar or lower cytotoxicity and phyotoxicity to pulmonary fibroblast permanent cell line (v79) and fibroblast cell line (3 T3) and Phaseolus vulgaris, respectively, than unentrapped A.I. | 131 | ||
| Zein (234 to 282 nm by DLS) | Cinnamaldehyde, eugenol, or geraniol | Tetranychus urticae, Chrysodeixis includes | Lesser insect repellency to T. urticae at short time (2 h) compared to unentrapped A.I., but improved repellency after longer times because of sustained release; lower mortality and sublethal effects to C. includes | 5 mg ml−1 | Lower cytotoxicity to pulmonary fibroblast permanent cell line (v79) and fibroblast cell line (3 T3) than unentrapped A.I. | 132 | ||
| Zein (288 nm by DLS) | Neem oil | n/a | n/a | n/a | n/a | Lower chromosomal damage to Allium cepa and lower toxicity to C. elegans than commercial formulation; no significant long-term effect on soil bacterial community for N cycling | 170 | |
| Fungicide | Chitosan/tripolyphosphate (100 nm by DLS) | Hexaconazole | Rhizoctonia solani | Better antifungal activity at moderate concentration compared to commercial formulation | 1 mg L−1 | Similar or lower cytotoxicity to Vero cell lines than commercial formulation | 7 | |
| Chitosan/pectin (129 nm by DLS) | Carbendazim | Aspergillus parasiticus, Fusarium oxysporum | Better antifungal activity compared to both unentrapped A.I. and commercial formulation | 0.5 mg L−1 | Lower phytotoxicity to Zea mays, Cannabis sativa, and Lycopersicon esculentum than unentrapped A.I.; no bacterial inhibition against E. coli or S. aureus for both nano- and unentrapped A.I. | 23 | ||
| PCL nanocapsules; SLNs (479 to 472 nm by DLS) | Carbendazim and tebuconazole (co-loaded) | n/a | Not evaluated | n/a | n/a | Lower phytotoxicity to Phaseolus vulgaris for PCL nanocapsules than for SLNs or commercial formulation; cytotoxicity can be lower or higher than commercial formulation, depending on cell line (3T3, MC3T3, or HeLa) | 53 | |
| Antibiotic | O-Carboxymethyl chitosan (200 nm by DLS) | Tetracycline | Staphylococcus aureus | Higher survival of S. aureus-infected THP-1 and HEK-293 cells in vitro, compared to unentrapped A.I., but similar MIC for S. aureus in broth culture | 0.2 to 0.4 mg L−1 | 0.3 to 0.6 mg L−1 | No significant cytotoxicity to NIH-3T3, L-929 and HEK-293 epithelial cell lines or THP-1 monocytic cells | 185 |
| Chitosan/tripolyphosphate (≈20 to 50 nm by SEM) | Cefazolin | Multi drug resistant Klebsiella pneumoniae, Pseudomonas aeruginosa & extended spectrum beta lactamase (ESBL) positive Escherichia coli | Improved inhibition in vitro compared to unentrapped A.I. | n/a (no zone of inhibition observed) | 200 μg mL−1 for all species | Not evaluated | 163 | |
| Chitosan/tripolyphosphate (220 nm) | Ceftriaxone | Ceftriaxone-resistant strains of Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA) | Improved antibacterial efficacy compared to unentrapped A.I. in vitro and in vivo (neutropenic mouse thigh model) | n/a (no zone of inhibition observed) | 0.1 mg mL−1 for both species | >80% viability of MCF-7 cells | 164 | |
| Chitosan/tripolyphosphate (≈200 to 220 nm by DLS) | Ceftriaxone | Salmonella typhimurium | Improved antibacterial efficacy to S. typhimurium-infected Caco-2 and J774.2 cells compared to unentrapped A.I. | 50 μg mL−1 | Reduced hemolysis compared to unentrapped A.I. | 158 | ||
| Chondroitin sulfate (CS)/chitosan; dextran sulfate (DS)/chitosan (180 nm by DLS) | Chloramphenicol | Salmonella paratyphi A | Lower antibacterial activity in vitro than unentrapped A.I., but improved intracellular efficacy for DS nanoformulation in RAW 264.7 macrophage cells and ex vivo efficacy in chicken intestine model | 3 μg mL−1 | 120 μg mL−1 and 80 μg mL−1 for CS and DS, respectively; a4 × MIC used in ex vivo tests | Minimal hemolysis and cytotoxicity to IEC-6, VERO, and NIH-3T3 cell lines | 159 | |
| Polyacrylate (25 to 40 nm by DLS) | Penicillin | Staphylococcus aureus & methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MSSA and MRSA, respectively) | Antibacterial activity maintained against S. aureus and MRSA | 0.012 μg mL−1 for S. aureus and 16 μg mL−1 for MRSA | 2 μg mL−1 for S. aureus and 2 μg mL−1 for MRSA | No significant cytotoxicity to human dermal fibroblast cells | 162 | |
| PLGA (130 to 353 nm by DLS) | Ciprofloxacin | Escherichia coli | Similar or slightly lower antibacterial activity in vitro against E. coli than unentrapped A.I.; significantly improved activity in in vivo model (dialysis tubing) because of reduced drug washout | 0.05 μg mL−1 | 0.05 μg mL−1; a25 mg kg−1 for in vivo test | Not evaluated | 186 | |
| PLGA (300 nm by DLS) | Ciprofloxacin | Staphylococcus aureus & Pseudomonas aeruginosa | Similar efficacy of single dose of nanoformulation to repeated doses of unentrapped A.I. over 6 d because of sustained release | 0.5 μg mL−1 for S. aureus and 0.25 μg mL−1 for P. aeruginosa | 0.5 μg mL−1 for S. aureus and 0.125 μg mL−1 for P. aeruginosa | Not evaluated | 161 | |
| PLGA (102 nm by DLS) | Enrofloxacin | Escherichia coli & Staphylococcus aureus | Similar or slightly lower antibacterial activity in vitro than unentrapped A.I. | 0.031 mg L−1 for E. coli and 0.083 mg L−1 for S. aureus | 0.024 mg L−1 for E. coli and 0.128 mg L−1 for S. aureus | Significantly reduced cytotoxicity to IPEC-J2 cells | 187 | |
| PLGA (289 to 299 nm by DLS) | Gentamicin (modified with anionic surfactant) | Brucella melitensis | Improved inhibition in in vitro macrophage infection test and significantly better reduction of infection in mice, compared to unentrapped A.I. | 1 mg L−1 for in vitro macrophage test and 100 μg per mouse for in vivo test | No observed toxicity to mice | 188 | ||
| PLGA (240 to 360 nm by DLS) | Gentamicin | Pseudomonas aeruginosa | Poorer efficiency in vitro, but improved efficiency at extended duration against biofilms (36 h) and in vivo (96 h), relative to unentrapped A.I. | 1.5 μg mL−1 | 3 μg mL−1; a0.08 mg mL−1 for 36 h biofilm test and 0.4 mg kg−1 dose for mouse studies | Not evaluated | 160 | |
| PLGA (230 nm by DLS) | Rifampicin | Methicillin-resistant Staphylococcus aureus (MRSA), Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli | Improved antibacterial activity against MRSA and similar activity against B. subtilis compared to unentrapped A.I.; no improved activity against P. aeruginosa or E. coli | 0.008 μg mL−1 for S. aureus, 0.06 μg mL−1 for B. subtilis | 0.002 μg mL for S. aureus, 0.06 μg mL−1 for B. subtilis | Not evaluated | 189 | |
| PLGA (243 nm by DLS); mPEG-PLGA (150 nm by DLS) | Ofloxacin | Escherichia coli, Proteus vulgaris, Salmonella typhimurium, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, Bacillus subtilis | Improved bacterial uptake and antibacterial activity compared to unentrapped A.I.; inhibition of antibiotic resistance development in B. subtilis | 25 μg per agar plate – smaller zone of inhibition (19.2 mm) with growth of resistant colonies by 60 h | 25 μg per agar plate – larger zone of inhibition (22.8 mm) with no growth of resistant colonies by 60 h | Not evaluated | 190 | |
PLGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCL (80 PCL (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) (230 to 360 nm by DLS) 20) (230 to 360 nm by DLS) |
Doxycycline | Escherichia coli (DH5α) | Improved antibacterial efficiency compared to unentrapped A.I. | 6 mg L−1 | 4 mg L−1 | Not evaluated | 191 | |
| Zein (500 to 2000 nm) | Ciprofloxacin | Escherichia coli, Staphylococcus aureus | Lower bacterial adhesion compared to empty zein particles or glass surface; continuous and slow release of drug to inhibit bacterial growth over 6 d | Not evaluated | Not measured (particle film was deposited for evaluation) | Not evaluated | 127 | |
For plant growth promoters, herbicides, insecticides, and fungicides, the nano-formulations typically show similar to improved efficiency compared to the unentrapped active ingredient. A possible mechanism for the improved efficiency is the ability for the nanoparticles to provide targeted or enhanced delivery, e.g. by designing nanoparticles that encourage the adhesion or uptake of the active ingredients by the target organism.133,134 In contrast, hydrophobic insecticides can have potential flaws of poor solubility, which can reduce their targeting efficiency to less than 1%.141 Nanoparticles have also been highlighted to perform particularly well over extended time durations,132,142–144e.g. by stabilizing the active ingredient against degradation or providing slow release properties, which reduces the overall quantity of pesticides required.
A limitation of the currently available data is that only ten of twenty-six studies identified in the literature compare the activity of the nano-formulation to a commercial formulation. Of these studies, three report no significant difference for the nano-formulation,19,25,145 and two report improvement only under specific conditions (e.g. at longer durations142 or with an adhesive coating on the nanoparticles134). As noted by Kah et al.,6 to truly demonstrate an advantage of the nano-formulation over currently available alternatives, several commercial formulations that may differ in composition (e.g. solution, suspension, or emulsion; with or without polymeric ingredients) should be compared. The majority of nanocarrier studies available report “nano”-formulations with diameters between 100 nm and 1000 nm, and more information is also needed to evaluate whether particle sizes <1000 nm truly confer additional benefits (e.g. enhanced uptake or targeting) that would justify their use over micron-sized polymeric particles (which would be expected to provide slower and more extended release). The two studies that compare microemulsions or water-dispersible granules report no improvement using the nano-formulation25 or improvement only after adding an adhesive surface coating on the nanoparticles.134 Additional studies providing side-by-side characterization of particle size (for suspensions or emulsions) and release rates for both the commercial and nano-formulations are needed to better understand the benefits or lack thereof of the nano-formulation.
Polymeric nanoparticles have also been proposed and developed to deliver antimicrobial agents.146–148 Livestock have particularly been highlighted as a reservoir for the development of antibiotic-resistant microorganisms, such as methicillin-resistant Staphylococcus aureus (MRSA),149 that could potentially be transmissible to humans.150,151 Nanoparticles have been suggested as a possible route to mitigate the proliferation of such antibiotic-resistant microorganisms by reducing the overall quantities of antibiotics required by improving the efficiency of delivery or by restoring the efficacy of the antibiotic.152–157 While few studies are currently available that specifically target development of antibiotic-loaded nanoparticles for livestock and aquaculture applications, research on the development of nanoparticles loaded with antibiotics relevant to livestock are summarized in Table 3. Similarly to the agrochemicals, mechanisms for improved antibiotic efficiency include their targeting properties or enhanced uptake, leading to improved intracellular activity toward pre-infected cells,158,159 as well as sustained release allowing for single doses to be effective over long durations (e.g., several days)127,160 and potentially eliminate the need for repeated doses of traditional (non-nano) formulations.161 Interestingly, polymeric nanocarriers have also been found to restore the effectiveness of antibiotics against antibiotic-resistant organisms. For example, drug resistance to β-lactam drugs, such as penicillin and methicillin, is conferred by the production of β-lactamase enzymes that degrade the antibiotics. By incorporating these drugs into polymeric nanoparticles, e.g. penicillin-loaded polyacrylate nanoparticles,162 cefazolin-loaded chitosan nanoparticles,163 or ceftriaxone-loaded chitosan nanoparticles,164 the drug is effectively shielded from enzymatic degradation to restore its antibiotic efficacy against resistant strains such as methicillin resistant Staphylococcus aureus (MRSA).
Finally, we note that a major hurdle that must be overcome for commercialization of the nanocarriers is the implementation of larger scale field trials that evaluate the ultimate improvements in endpoints of interest to farmers (e.g. crop or animal yield). The survey of literature in Table 3 shows that a variety of endpoints are evaluated across different studies, including seedling emergence, root and shoot growth, weed growth or mortality, and insect deterrence or mortality for crop applications, or minimum inhibitory concentrations, cell survival, or antibiotic resistance development for antibiotics. One recent study showed improved crop productivity (tomato production) in a field study for plant growth hormones delivered by nanocarrier,165 and additional field studies are needed to make a convincing case for investments leading to commercialization of nanocarrier formulations.
5.3. Environmental and biological implications
The use of agrochemicals has spurred concerns over potential hazards associated with their application, e.g. cytotoxicity against nontarget species or ecotoxicity. Therefore, many studies on the development of nanocarriers have also investigated whether nano-formulations would exacerbate the cytotoxicity, phytotoxicity, or ecotoxicity compared to traditional formulations, or whether these side effects will be mitigated.Cytotoxicity can be triggered upon penetration of cell membranes and leakage of important intracellular components and generation of reactive oxygen species (ROS), which eventually leads to oxidative stress, cell inflammation, and damage to intracellular components like mitochondria, protein, and DNA.166 While cytotoxicity of polymeric nanoparticles has been reported, e.g. for smaller nanoparticles higher surface area and bioavailability167 or PLGA-PEG nanoparticles with needle-shaped morphologies that could disrupt the lipid bilayer membrane,168 polymeric nanoparticles typically have low cytotoxicity and furthermore, polymeric nanocarriers are often reported to minimize the cytotoxicity of agrochemicals and antibiotics (Table 3), hence providing a substantial benefit in improving not only the efficiency of the active ingredient but also improving their safe use.
Several studies have also demonstrated benefits of polymeric nanocarriers to reduce the toxicity of synthetic pesticides toward nontarget crop species (e.g., Zea mays or Phaseolus vulgaris)23,45,53,111,131 or environmental test organisms such as C. elegans, Allium cepa, and Pseudokirchneriella subcapitata (algae).41,43,169,170 For applications in complex matrices, effects of nano-formulations on the microbiome are also of interest, given the key role of the microbial community in carbon and nutrient cycling in soils or the utilization of food and regulation of gastrointestinal diseases for oral drug delivery in animal health applications.171 Nano-microbiota interactions have been studied for metal and metal oxide nanoparticles such as silver nanoparticles172,173 or zinc oxide (ZnO), cerium oxide (CeO2), and titanium dioxide (TiO2) nanoparticles,174 where the nanoparticles did not significantly impact the microbiome composition. However, new studies may be required for polymeric nanocarriers, particularly those carrying active ingredients with known microbial activity, e.g. antibiotics. In such cases, the use of a nanoformulation compared to traditional formulations may change the nano-microbiota interaction due to the changes in the site of the gastrointestinal tract in which the antibiotics are delivered, and hence differences in the types of gut microbiota impacted.175 In soils, recent work conducted by Maruyama et al.169 showed a slight change in the soil microbiome when chitosan/tripolyphosphate nanoparticles loaded with imazapic and imazapyr herbicides were applied,169 where the ratios of nitrogen-fixing bacteria may be reduced and denitrifying bacteria may increase. Pascoli et al. reported that the application of neem oil-loaded zein nanoparticles as pesticides did not significantly change the relative number of genes associated with nitrogen-fixing or denitrifying bacteria after 30 d.170
The currently available studies generally suggest that short-term environmental hazards posed by polymeric nanocarriers can be minimal or even alleviated relative to unentrapped or commercial formulations of active ingredients. Some commonly used polymers such as PLGA/PLA are FDA-approved for human drug delivery and expected to pose minimal environmental risk. However, studies are needed to evaluate the rate of polymer degradation in agricultural applications, the recalcitrance and accumulation of the polymer and any additives or byproducts, and potential toxicity of degradation products. For example, PVA (often used as a surfactant) has been reported to have limited degradation only by specific microorganisms despite being considered “biodegradable”, and hence its longevity in soils is unknown.176 To our knowledge, long-term soil studies of polymeric nanocarriers have not been conducted thus far to evaluate the consequences of repeated applications over longer durations.
6. Challenges and opportunities for future research
This review has demonstrated the potential environmental benefits of polymeric nanocarriers in agricultural applications, as well as many examples thus far of the successful synthesis of these materials and methods to characterize these materials in order to understand their behavior and effectiveness for the desired application. As shown in the literature, the function of the polymeric carrier to provide targeting or enhanced uptake, protect the active ingredient until it is delivered to its target, and slowly release the active ingredient over extended durations can be key to the improved efficiency of these nanomaterials compared to traditional formulations.Based on the current literature, several major challenges and research questions can be identified to develop polymeric nanocarriers with optimal effectiveness. First, while extended release is one of the main benefits of nano-formulations, a more quantitative or systematic consideration of extended release has yet to be achieved. For example, a better consensus or practical guidance on the duration of release that would be desirable for various applications (e.g. crop protection, antibiotic delivery, etc.) will be critical for researchers to develop materials with appropriate release profiles. Alternatively, studies that specifically evaluate or report the dose and duration at which a single application of slow release nano-formulation is equivalent to repeated applications of non-nano-formulations will be useful to better quantify the benefits of using nano-formulations over current practices. Life-cycle analyses (LCA) have been proposed192 and can incorporate information when available on the tradeoff between upstream resource costs to produce the nano-formulations and benefits of reducing the overall amount of active ingredients needed or improving agricultural yield. However, the potential ecological and safety benefits of nano-formulations conferred through the reduction in cytotoxicity or ecotoxicity of the active ingredient or reduced proliferation of antibiotic resistant organisms should also be considered and will be difficult to incorporate in LCA approaches.
Quantitative structure–function relationships to predict biological responses of polymeric nanocarriers from their physicochemical properties and other key phenomena, such as release or degradation rates, are also needed. Prior studies have postulated that enhanced efficiency of the nanocarriers is tied to their slow release, targeting, and protective capabilities of the active ingredient. Hence, new models that correlate spatial distribution or temporal release profiles of the nanocarrier and active ingredient to the biological effects (e.g. pesticide or antibiotic efficiency) are expected to be extremely useful to understand how to better design the nanocarriers. However, gathering experimental data to parameterize these models is non-trivial because of the variety of tools needed to comprehensively characterize polymeric nanocarriers, as well as a lack of satisfactory methods to directly measure the localization of the nanocarriers and release of their active ingredients in vivo or in the field. Machine learning represents an alternative “black-box” approach to correlate nanocarrier properties to biological endpoints. However, as discussed in a review by Jones et al. for biomedical effects of drug delivery nanoparticles,193 machine learning approaches are currently challenged by limitations in the quantity and completeness of data relative to the high number of potential predictive parameters, as well as an imbalance in the types of nanocarriers (e.g., PLGA) with available data. Hence, these is a higher risk for the models to be overfitted or biased toward the samples in the training data, which would result in poorer predictive capability for other nanocarriers.
Finally, once a design goal has been defined based on properties of the nanocarriers needed to achieve the desired efficiency of the active ingredient, synthesis of nanomaterials that meet the design goal may be non-trivial. Again, a major challenge is presented by the large number of experimental factors that contribute to the nanoparticle properties, including size, structure (e.g. phase and solvency), and loading capacity, as well as the release behavior (rate and mechanism) of the active ingredient from the nanoparticle. Optimization of the synthesis can successfully be conducted on an individual basis for each polymer and active ingredient type, as in factorial design studies,20,21,26 but this approach requires significant time and effort. Predictive approaches have been proposed: either first principles approaches to predict particle properties and release rates from thermodynamic models33–35 and molecular dynamics simulations,194–197 or machine learning approaches to develop correlations from existing data sets.194,198–201 In both cases, studies have been limited to either modeling a limited number of polymers for several types of active ingredients, or vice versa. Experimental validation studies are needed to evaluate whether the proposed first principles tools can be applied a broader set of combinations of material types and experimental conditions. Machine learning approaches also require larger data sets with thorough characterization, as discussed above. Therefore, it is currently unknown whether any single tool will successfully predict the synthesis materials and conditions that are most likely to provide a favorable outcome across a broad variety of polymers and active ingredients. Furthermore, the limited accessibility of predictive modeling tools to experimentalists hinders progress toward validation against new experimental data, updating the tools to incorporate new data, or application of the tools to test their capabilities for design of new nanocarriers with a desired set of properties.
Considering the integrated nature of these challenges, the development of polymeric nanocarriers presents a great opportunity for multi-disciplinary collaborations between synthetic and analytical chemists, environmental engineers and microbiologists, and agricultural scientists and engineers. Such collaborations will advance our understanding of how environmental nanotechnology can enhance the portfolio of technologies for agricultural applications and spur the development of new materials and predictive tools to achieve the maximum benefit from these technologies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge A. Hakimian for contributions to draft figures. This work was supported by the USDA National Institute of Food and Agriculture (NIFA) under AFRI Grant No. 2018-67022-27969 (Project No. 2017-07878), the National Science Foundation under NSF EPSCoR Track 2 RII, OIA 1632854, the USDA NIFA (Hatch Project #1008750), and the Welch Foundation under Grant No. E-2011-20190330. Thanks also go to the Louisiana Soybean and Grain Research Board and the University of Houston Grants to Enhance and Advance Research (GEAR) Award for funding this research.References
- S. M. Rodrigues, P. Demokritou, N. Dokoozlian, C. O. Hendren, B. Karn, M. S. Mauter, O. A. Sadik, M. Safarpour, J. M. Unrine and J. Viers, Nanotechnology for Sustainable Food Production: Promising Opportunities and Scientific Challenges, Environ. Sci.: Nano, 2017, 4, 767–781 RSC.
- C. O. Dimkpa and P. S. Bindraban, Nanofertilizers: New Products for the Industry?, J. Agric. Food Chem., 2018, 66, 6462–6473 CrossRef CAS PubMed.
- H. Guo, J. C. White, Z. Wang and B. Xing, Nano-enabled Fertilizers to Control the Release and Use Efficiency of Nutrients, Curr. Opin. Environ. Sci. Health, 2018, 6, 77–83 CrossRef.
- M. Kah and T. Hofmann, Nanopesticide Research: Current Trends and Future Priorities, Environ. Int., 2014, 63, 224–235 CrossRef CAS PubMed.
- R. Raliya, V. Saharan, C. Dimkpa and P. Biswas, Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives, J. Agric. Food Chem., 2018, 66, 6487–6503 CrossRef CAS PubMed.
- M. Kah, R. S. Kookana, A. Gogos and T. D. Bucheli, A Critical Evaluation of Nanopesticides and Nanofertilizers Against Their Conventional Analogues, Nat. Nanotechnol., 2018, 13, 677–684 CrossRef CAS PubMed.
- N. Chauhan, N. Dilbaghi, M. Gopal, R. Kumar, K. H. Kim and S. Kumar, Development of Chitosan Nanocapsules for the Controlled Release of Hexaconazole, Int. J. Biol. Macromol., 2017, 97, 616–624 CrossRef CAS PubMed.
- A. Murugeshu, C. Astete, C. Leonardi, T. Morgan and C. M. Sabliov, Chitosan/PLGA Particles for Controlled Release of α-Tocopherol in the GI Tract via Oral Administration, Nanomedicine, 2011, 6, 1513–1528 CrossRef CAS PubMed.
- T. Kacsó, I. O. Neaga, A. Erincz, C. E. Astete, C. M. Sabliov, R. Oprean and E. Bodoki, Perspectives in the Design of Zein-Based Polymeric Delivery Systems with Programmed Wear Down for Sustainable Agricultural Applications, Polym. Degrad. Stab., 2018, 155, 130–135 CrossRef.
- A. Prasad, C. E. Astete, A. E. Bodoki, M. Windham, E. Bodoki and C. M. Sabliov, Zein Nanoparticles Uptake and Translocation in Hydroponically Grown Sugar Cane Plants, J. Agric. Food Chem., 2018, 66, 6544–6551 CrossRef CAS PubMed.
- K. D. Ristroph, C. E. Astete, E. Bodoki and C. M. Sabliov, Zein Nanoparticles Uptake by Hydroponically Grown Soybean Plants, Environ. Sci. Technol., 2017, 51, 14065–14071 CrossRef CAS PubMed.
- T. Chuacharoen and C. M. Sabliov, Zein Nanoparticles as Delivery Systems for Covalently Linked and Physically Entrapped Folic Acid, J. Nanopart. Res., 2017, 19, 81 CrossRef.
- T. Chuacharoen and C. M. Sabliov, Stability and Controlled Release of Lutein Loaded in Zein Nanoparticles With and Without Lecithin and Pluronic F127 Surfactants, Colloids Surf., A, 2016, 503, 11–18 CrossRef CAS.
- T. Chuacharoen and C. M. Sabliov, The Potential of Zein Nanoparticles to Protect Entrapped β-Carotene in the Presence of Milk under Simulated Gastrointestinal (GI) Conditions, LWT--Food Sci. Technol., 2016, 72, 302–309 CrossRef CAS.
- F. Ye, C. E. Astete and C. M. Sabliov, Entrapment and Delivery of α-Tocopherol by a Self-Assembled, Alginate-Conjugated Prodrug Nanostructure, Food Hydrocolloids, 2017, 72, 62–72 CrossRef CAS.
- C. E. Astete, C. M. Sabliov, F. Watanabe and A. Biris, Ca2+ Cross-Linked Alginic Acid Nanoparticles for Solubilization of Lipophilic Natural Colorants, J. Agric. Food Chem., 2009, 57, 7505–7512 CrossRef CAS PubMed.
- C. H. Goh, P. W. S. Heng and L. W. Chan, Alginates as a Useful Natural Polymer for Microencapsulation and Therapeutic Applications, Carbohydr. Polym., 2012, 88, 1–12 CrossRef CAS.
- P. Stloukal, P. Kucharczyk, V. Sedlarik, P. Bazant and M. Koutny, Low Molecular Weight Poly(lactic acid) Microparticles for Controlled Release of the Herbicide Metazachlor: Preparation, Morphology, and Release Kinetics, J. Agric. Food Chem., 2012, 60, 4111–4119 CrossRef CAS PubMed.
- S. R. Yearla and K. Padmasree, Exploitation of Subabul Stem Lignin as a Matrix in Controlled Release Agrochemical Nanoformulations: A Case Study with Herbicide Diuron, Environ. Sci. Pollut. Res., 2016, 23, 18085–18098 CrossRef CAS PubMed.
- S. Kumar, G. Bhanjana, A. Sharma, N. Dilbaghi, M. C. Sidhu and K.-H. Kim, Development of Nanoformulation Approaches for the Control of Weeds, Sci. Total Environ., 2017, 586, 1272–1278 CrossRef CAS PubMed.
- J. L. de Oliveira, E. V. R. Campos, A. E. S. Pereira, L. E. S. Nunes, C. C. L. da Silva, T. Pasquoto, R. Lima, G. Smaniotto, R. A. Polanczyk and L. F. Fraceto, Geraniol Encapsulated in Chitosan/Gum Arabic Nanoparticles: A Promising System for Pest Management in Sustainable Agriculture, J. Agric. Food Chem., 2018, 66, 5325–5334 CrossRef PubMed.
- E. Celasco, I. Valente, D. L. Marchisio and A. A. Barresi, Dynamic Light Scattering and X-ray Photoelectron Spectroscopy Characterization of PEGylated Polymer Nanocarriers: Internal Structure and Surface Properties, Langmuir, 2014, 30, 8326–8335 CrossRef CAS PubMed.
- Sandhya, S. Kumar, D. Kumar and N. Dilbaghi, Preparation, Characterization, and Bio-Efficacy Evaluation of Controlled Release Carbendazim-Loaded Polymeric Nanoparticles, Environ. Sci. Pollut. Res., 2017, 24, 926–937 CrossRef CAS PubMed.
- P. Severino, M. V. Chaud, A. Shimojo, D. Antonini, M. Lancelloti, M. H. A. Santana and E. B. Souto, Sodium Alginate-Cross-Linked Polymyxin B Sulphate-Loaded Solid Lipid Nanoparticles: Antibiotic Resistance Tests and HaCat and NIH/3T3 Cell Viability Studies, Colloids Surf., B, 2015, 129, 191–197 CrossRef CAS PubMed.
- K. Chen, Z. Fu, M. Wang, Y. Lv, C. Wang, Y. Shen, Y. Wang, H. Cui and X. Guo, Preparation and Characterization of Size-Controlled Nanoparticles for High-Loading λ-Cyhalothrin Delivery through Flash Nanoprecipitation, J. Agric. Food Chem., 2018, 66, 8246–8252 CrossRef CAS PubMed.
- Y. Wang, Z. Gao, F. Shen, Y. Li, S. Zhang, X. Ren and S. Hu, Physicochemical Characteristics and Slow Release Performances of Chlorpyrifos Encapsulated by Poly(butyl acrylate-co-styrene) with the Cross-Linker Ethylene Glycol Dimethacrylate, J. Agric. Food Chem., 2015, 63, 5196–5204 CrossRef CAS PubMed.
- E. V. R. Campos, P. L. F. Proença, J. L. Oliveira, C. C. Melville, J. F. Della Vechia, D. J. de Andrade and L. F. Fraceto, Chitosan Nanoparticles Functionalized with β-Cyclodextrin: A Promising Carrier for Botanical Pesticides, Sci. Rep., 2018, 8, 2067 CrossRef PubMed.
- G. Lin, X. Chen, H. Zhou, X. Zhou, H. Xu and H. Chen, Elaboration of a Feather Keratin/Carboxymethyl Cellulose Complex Exhibiting pH Sensitivity for Sustained Pesticide Release, J. Appl. Polym. Sci., 2019, 136, 47160 CrossRef.
- N. Günday Türeli, A. E. Türeli and M. Schneider, Counter-ion Complexes for Enhanced Drug Loading in Nanocarriers: Proof-of-concept and Beyond, Int. J. Pharm., 2016, 511, 994–1001 CrossRef PubMed.
- M. D. S. Silva, D. S. Cocenza, R. Grillo, N. F. S. D. Melo, P. S. Tonello, L. C. D. Oliveira, D. L. Cassimiro, A. H. Rosa and L. F. Fraceto, Paraquat-Loaded Alginate/Chitosan Nanoparticles: Preparation, Characterization and Soil Sorption Studies, J. Hazard. Mater., 2011, 190, 366–374 CrossRef CAS PubMed.
- Z. Shen, X. Zhou, X. Sun, H. Xu, H. Chen and H. Zhou, Preparation of 2,4-Dichlorophenoxyacetic Acid Loaded on Cysteamine-Modified Polydopamine and Its Release Behaviors, J. Appl. Polym. Sci., 2019, 136, 47469 CrossRef.
- J. A. S. Ritsema, E. M. A. Herschberg, S. E. Borgos, C. Løvmo, R. Schmid, Y. M. te Welscher, G. Storm and C. F. van Nostrum, Relationship between Polarities of Antibiotic and Polymer Matrix on Nanoparticle Formulations Based on Aliphatic Polyesters, Int. J. Pharm., 2018, 548, 730–739 CrossRef CAS PubMed.
- A. Jäger, E. Jäger, F. C. Giacomelli, F. Nallet, M. Steinhart, J.-L. Putaux, R. Konefał, J. Spěváček, K. Ulbrich and P. Štěpánek, Structural Changes on Polymeric Nanoparticles Induced by Hydrophobic Drug Entrapment, Colloids Surf., A, 2018, 538, 238–249 CrossRef.
- K. Vay, S. Scheler and W. Frieß, Application of Hansen Solubility Parameters for Understanding and Prediction of Drug Distribution in Microspheres, Int. J. Pharm., 2011, 416, 202–209 CrossRef CAS PubMed.
- G. Tse, D. Blankschtein, A. Shefer and S. Shefer, Thermodynamic Prediction of Active Ingredient Loading in Polymeric Microparticles, J. Controlled Release, 1999, 60, 77–100 CrossRef CAS PubMed.
- M. L. Hans and A. M. Lowman, Biodegradable Nanoparticles for Drug Delivery and Targeting, Curr. Opin. Solid State Mater. Sci., 2002, 6, 319–327 CrossRef CAS.
- A. E. S. Pereira, R. Grillo, N. F. S. Mello, A. H. Rosa and L. F. Fraceto, Application of Poly(epsilon-caprolactone) Nanoparticles Containing Atrazine Herbicide as an Alternative Technique to Control Weeds and Reduce Damage to the Environment, J. Hazard. Mater., 2014, 268, 207–215 CrossRef CAS PubMed.
- M. Christofoli, E. C. C. Costa, K. U. Bicalho, V. D. Domingues, M. F. Peixoto, C. C. F. Alves, W. L. Araujo and C. D. Cazal, Insecticidal Effect of Nanoencapsulated Essential Oils from Zanthoxylum rhoifolium (Rutaceae) in Bemisia tabaci Populations, Ind. Crops Prod., 2015, 70, 301–308 CrossRef CAS.
- Z. Clemente, R. Grillo, M. Jonsson, N. Z. P. Santos, L. O. Feitosa, R. Lima and L. F. Fraceto, Ecotoxicological Evaluation of Poly(epsilon-Caprolactone) Nanocapsules Containing Triazine Herbicides, J. Nanosci. Nanotechnol., 2014, 14, 4911–4917 CrossRef CAS PubMed.
- R. Grillo, A. H. Rosa and L. F. Fraceto, Poly(epsilon-caprolactone) Nanocapsules Carrying the Herbicide Atrazine: Effect of Chitosan-Coating Agent on Physico-chemical Stability and Herbicide Release Profile, Int. J. Environ. Sci. Technol., 2014, 11, 1691–1700 CrossRef CAS.
- R. Grillo, N. Z. P. dos Santos, C. R. Maruyama, A. H. Rosa, R. de Lima and L. F. Fraceto, Poly(epsilon-caprolactone)nanocapsules as Carrier Systems for Herbicides: Physico-chemical Characterization and Genotoxicity Evaluation, J. Hazard. Mater., 2012, 231, 1–9 CrossRef PubMed.
- A. E. S. Pereira, P. M. Silva, J. L. Oliveira, H. C. Oliveira and L. F. Fraceto, Chitosan Nanoparticles as Carrier Systems for the Plant Growth Hormone Gibberellic Acid, Colloids Surf., B, 2017, 150, 141–152 CrossRef CAS PubMed.
- R. Grillo, Z. Clemente, J. L. de Oliveira, E. V. R. Campos, V. C. Chalupe, C. M. Jonsson, R. de Lima, G. Sanches, C. S. Nishisaka, A. H. Rosa, K. Oehlke, R. Greiner and L. F. Fraceto, Chitosan Nanoparticles Loaded the Herbicide Paraquat: The Influence of the Aquatic Humic Substances on the Colloidal Stability and Toxicity, J. Hazard. Mater., 2015, 286, 562–572 CrossRef CAS PubMed.
- J. H. Liu, J. Xiao, F. Li, Y. Shi, D. P. Li and Q. R. Huang, Chitosan-Sodium Alginate Nanoparticle as a Delivery System for Epsilon-Polylysine: Preparation, Characterization and Antimicrobial Activity, Food Control, 2018, 91, 302–310 CrossRef CAS.
- R. Grillo, A. E. S. Pereira, C. S. Nishisaka, R. de Lima, K. Oehlke, R. Greiner and L. F. Fraceto, Chitosan/Tripolyphosphate Nanoparticles Loaded with Paraquat Herbicide: An Environmentally Safer Alternative for Weed Control, J. Hazard. Mater., 2014, 278, 163–171 CrossRef CAS PubMed.
- S. Kumar, N. Chauhan, M. Gopal, R. Kumar and N. Dilbaghi, Development and Evaluation of Alginate-Chitosan Nanocapsules for Controlled Release of Acetamiprid, Int. J. Biol. Macromol., 2015, 81, 631–637 CrossRef CAS PubMed.
- D. Y. Nakasato, A. E. S. Pereira, J. L. Oliveira, H. C. Oliveira and L. F. Fraceto, Evaluation of the Effects of Polymeric Chitosan/Tripolyphosphate and Solid Lipid Nanoparticles on Germination of Zea mays, Brassica rapa and Pisum sativum, Ecotoxicol. Environ. Saf., 2017, 142, 369–374 CrossRef CAS PubMed.
- A. Kheiri, S. A. M. Jorf, A. Malihipour, H. Saremi and M. Nikkhah, Application of Chitosan and Chitosan Nanoparticles for the Control of Fusarium Head Blight of Wheat (Fusarium graminearum) in vitro and Greenhouse, Int. J. Biol. Macromol., 2016, 93, 1261–1272 CrossRef CAS PubMed.
- S. Kumar, G. Bhanjana, A. Sharma, Sarita, M. C. Sidhu and N. Dilbaghi, Herbicide Loaded Carboxymethyl Cellulose Nanocapsules as Potential Carrier in Agrinanotechnology, Sci. Adv. Mater., 2015, 7, 1143–1148 CrossRef CAS.
- C. X. Sun, K. Shu, W. Wang, Z. Ye, T. Liu, Y. X. Gao, H. Zheng, G. H. He and Y. H. Yin, Encapsulation and Controlled Release of Hydrophilic Pesticide in Shell Cross-Linked Nanocapsules Containing Aqueous Core, Int. J. Pharm., 2014, 463, 108–114 CrossRef CAS PubMed.
- M. S. Chen, S. P. Jensen, M. R. Hill, G. Moore, Z. L. He and B. S. Sumerlin, Synthesis of Amphiphilic Polysuccinimide Star Copolymers for Responsive Delivery in Plants, Chem. Commun., 2015, 51, 9694–9697 RSC.
- X. P. Xin, Z. L. He, M. R. Hill, R. P. Niedz, X. J. Jiang and B. S. Sumerlin, Efficiency of Biodegradable and pH-Responsive Polysuccinimide Nanoparticles (PSI-NPs) as Smart Nanodelivery Systems in Grapefruit: In Vitro Cellular Investigation, Macromol. Biosci., 2018, 18, 1800159 CrossRef PubMed.
- E. V. R. Campos, J. L. D. Oliveira, C. M. G. da Silva, M. Pascoli, T. Pasquoto, R. Lima, P. C. Abhilash and L. Fernandes Fraceto, Polymeric and Solid Lipid Nanoparticles for Sustained Release of Carbendazim and Tebuconazole in Agricultural Applications, Sci. Rep., 2015, 5, 13809 CrossRef PubMed.
- OECD Working Party on Manufactured Nanomaterials, List of Manufactured Nanomaterials and List of Endpoints for Phase One of the Sponsorship Programme for the Testing of Manufactured Nanomaterials: Revision, Report No. 27, 2010 Search PubMed.
- M. E. Pettitt and J. R. Lead, Minimum Physicochemical Characterisation Requirements for Nanomaterial Regulation, Environ. Int., 2013, 52, 41–50 CrossRef PubMed.
- K. C. Mills, D. Murry, K. A. Guzan and M. L. Ostraat, Nanomaterial Registry: Database that Captures the Minimal Information about Nanomaterial Physico-chemical Characteristics, J. Nanopart. Res., 2014, 16, 2219 CrossRef.
- M. J. McCall, V. A. Coleman, J. Herrmann, J. K. Kirby, I. R. Gardner, P. J. Brent and C. M. Johnson, A Tiered Approach, Nat. Nanotechnol., 2013, 8, 307 CrossRef CAS PubMed.
- M. Faria, M. Björnmalm, K. J. Thurecht, S. J. Kent, R. G. Parton, M. Kavallaris, A. P. R. Johnston, J. J. Gooding, S. R. Corrie, B. J. Boyd, P. Thordarson, A. K. Whittaker, M. M. Stevens, C. A. Prestidge, C. J. H. Porter, W. J. Parak, T. P. Davis, E. J. Crampin and F. Caruso, Minimum Information Reporting in Bio–Nano Experimental Literature, Nat. Nanotechnol., 2018, 13, 777–785 CrossRef CAS PubMed.
- J. P. Patterson, M. P. Robin, C. Chassenieux, O. Colombani and R. K. O'Reilly, The Analysis of Solution Self-Assembled Polymeric Nanomaterials, Chem. Soc. Rev., 2014, 43, 2412–2425 RSC.
- Z. Ye, J. Guo, D. Wu, M. Tan, X. Xiong, Y. Yin and G. He, Photo-Responsive Shell Cross-Linked Micelles Based on Carboxymethyl Chitosan and Their Application in Controlled Release of Pesticide, Carbohydr. Polym., 2015, 132, 520–528 CrossRef CAS PubMed.
- L. Sawyer, D. T. Grubb and G. F. Meyers, Polymer Microscopy, Springer Science & Business Media, 2008 Search PubMed.
- G. Claver and W. Farnham, Polymer Particle Damage in the Electron Microscope—A Practical Problem, Powder Technol., 1972, 6, 313–316 CrossRef CAS.
- J. Kuntsche, J. C. Horst and H. Bunjes, Cryogenic Transmission Electron Microscopy (Cryo-TEM) for Studying the Morphology of Colloidal Drug Delivery Systems, Int. J. Pharm., 2011, 417, 120–137 CrossRef CAS PubMed.
- J. J. Crassous, M. Ballauff, M. Drechsler, J. Schmidt and Y. Talmon, Imaging the Volume Transition in Thermosensitive Core−Shell Particles by Cryo-Transmission Electron Microscopy, Langmuir, 2006, 22, 2403–2406 CrossRef CAS PubMed.
- J. Sitterberg, A. Özcetin, C. Ehrhardt and U. Bakowsky, Utilising Atomic Force Microscopy for the Characterisation of Nanoscale Drug Delivery Systems, Eur. J. Pharm. Biopharm., 2010, 74, 2–13 CrossRef CAS PubMed.
- A. Z. Mühlen, E. Z. Mühlen, H. Niehus and W. Mehnert, Atomic Force Microscopy Studies of Solid Lipid Nanoparticles, Pharm. Res., 1996, 13, 1411–1416 CrossRef PubMed.
- M. Ballauff, Nanoscopic Polymer Particles with a Well-Defined Surface: Synthesis, Characterization, and Properties, Macromol. Chem. Phys., 2003, 204, 220–234 CrossRef CAS.
- M. Ballauff, SAXS and SANS Studies of Polymer Colloids, Curr. Opin. Colloid Interface Sci., 2001, 6, 132–139 CrossRef CAS.
- B. Yang, J. P. Lowe, R. Schweins and K. J. Edler, Small Angle Neutron Scattering Studies on the Internal Structure of Poly(lactide-co-glycolide)-block-poly(ethylene glycol) Nanoparticles as Drug Delivery Vehicles, Biomacromolecules, 2015, 16, 457–464 CrossRef CAS PubMed.
- S. Patel, J. Bajpai, R. Saini, A. K. Bajpai and S. Acharya, Sustained Release of Pesticide (Cypermethrin) from Nanocarriers: An Effective Technique for Environmental and Crop Protection, Process Saf. Environ. Prot., 2018, 117, 315–325 CrossRef CAS.
- G. Mohammadi, H. Valizadeh, M. Barzegar-Jalali, F. Lotfipour, K. Adibkia, M. Milani, M. Azhdarzadeh, F. Kiafar and A. Nokhodchi, Development of Azithromycin–PLGA Nanoparticles: Physicochemical Characterization and Antibacterial Effect Against Salmonella typhi, Colloids Surf., B, 2010, 80, 34–39 CrossRef CAS PubMed.
- X. Liu, W. Sun, B. Zhang, B. Tian, X. Tang, N. Qi, H. He, H. Li and X. Jin, Clarithromycin-Loaded Liposomes Offering High Drug Loading and Less Irritation, Int. J. Pharm., 2013, 443, 318–327 CrossRef CAS PubMed.
- J. L. de Oliveira, E. V. R. Campos, C. M. Gonçalves da Silva, T. Pasquoto, R. Lima and L. F. Fraceto, Solid Lipid Nanoparticles Co-loaded with Simazine and Atrazine: Preparation, Characterization, and Evaluation of Herbicidal Activity, J. Agric. Food Chem., 2015, 63, 422–432 CrossRef CAS PubMed.
- S. Lappe, D. Mulac and K. Langer, Polymeric Nanoparticles–Influence of the Glass Transition Temperature on Drug Release, Int. J. Pharm., 2017, 517, 338–347 CrossRef CAS PubMed.
- S. Wartewig and R. H. Neubert, Pharmaceutical Applications of Mid-IR and Raman Spectroscopy, Adv. Drug Delivery Rev., 2005, 57, 1144–1170 CrossRef CAS PubMed.
- H. Yan, Y.-F. Hou, P.-F. Niu, K. Zhang, T. Shoji, Y. Tsuboi, F.-Y. Yao, L.-M. Zhao and J.-B. Chang, Biodegradable PLGA Nanoparticles Loaded with Hydrophobic Drugs: Confocal Raman Microspectroscopic Characterization, J. Mater. Chem. B, 2015, 3, 3677–3680 RSC.
- K. Westesen, H. Bunjes and M. H. J. Koch, Physicochemical Characterization of Lipid Nanoparticles and Evaluation of Their Drug Loading Capacity and Sustained Release Potential, J. Controlled Release, 1997, 48, 223–236 CrossRef CAS.
- N. Maurer, K. F. Wong, M. J. Hope and P. R. Cullis, Anomalous Solubility Behavior of the Antibiotic Ciprofloxacin Encapsulated in Liposomes: A 1H-NMR Study, Biochim. Biophys. Acta, Biomembr., 1998, 1374, 9–20 CrossRef CAS.
- J. Li, P. Nemes and J. Guo, Mapping Intermediate Degradation Products of Poly(Lactic-co-Glycolic Acid) in vitro, J. Biomed. Mater. Res., Part B, 2018, 106, 1129–1137 CrossRef CAS PubMed.
- I. A. Mudunkotuwa, A. A. Minshid and V. H. Grassian, ATR-FTIR Spectroscopy as a Tool to Probe Surface Adsorption on Nanoparticles at the Liquid–Solid Interface in Environmentally and Biologically Relevant Media, Analyst, 2014, 139, 870–881 RSC.
- I. A. Mudunkotuwa and V. H. Grassian, Biological and Environmental Media Control Oxide Nanoparticle Surface Composition: The Roles of Biological Components (Proteins and Amino Acids), Inorganic Oxyanions and Humic Acid, Environ. Sci.: Nano, 2015, 2, 429–439 RSC.
- D.-H. Tsai, F. W. DelRio, A. M. Keene, K. M. Tyner, R. I. MacCuspie, T. J. Cho, M. R. Zachariah and V. A. Hackley, Adsorption and Conformation of Serum Albumin Protein on Gold Nanoparticles Investigated Using Dimensional Measurements and in Situ Spectroscopic Methods, Langmuir, 2011, 27, 2464–2477 CrossRef CAS PubMed.
- D.-H. Tsai, M. Davila-Morris, F. W. DelRio, S. Guha, M. R. Zachariah and V. A. Hackley, Quantitative Determination of Competitive Molecular Adsorption on Gold Nanoparticles Using Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy, Langmuir, 2011, 27, 9302–9313 CrossRef CAS PubMed.
- S. Shakiba, A. Hakimian, L. R. Barco and S. M. Louie, Dynamic Intermolecular Interactions Control Adsorption from Mixtures of Natural Organic Matter and Protein onto Titanium Dioxide Nanoparticles, Environ. Sci. Technol., 2018, 52, 14158–14168 CrossRef CAS PubMed.
- H. Wu, N. I. Gonzalez-Pech and V. H. Grassian, Displacement Reactions Between Environmentally and Biologically Relevant Ligands on TiO2 Nanoparticles: Insights Into the Aging of Nanoparticles in the Environment, Environ. Sci.: Nano, 2019, 6, 489–504 RSC.
- J. Kim and K. Doudrick, Emerging Investigator Series: Protein Adsorption and Transformation on Catalytic and Food-Grade TiO2 Nanoparticles in the Presence of Dissolved Organic Carbon, Environ. Sci.: Nano, 2019, 6, 1688–1703 RSC.
- S. M. Louie, J. M. Gorham, J. Tan and V. A. Hackley, Ultraviolet Photo-Oxidation of Polyvinylpyrrolidone (PVP) Coatings on Gold Nanoparticles, Environ. Sci.: Nano, 2017, 4, 1866–1875 RSC.
- S. M. Louie, J. M. Gorham, E. A. McGivney, J. Liu, K. B. Gregory and V. A. Hackley, Photochemical Transformations of Thiolated Polyethylene Glycol Coatings on Gold Nanoparticles, Environ. Sci.: Nano, 2016, 3, 1090–1102 RSC.
- T. J. Cho, J. M. Pettibone, J. M. Gorham, T. M. Nguyen, R. I. MacCuspie, J. Gigault and V. A. Hackley, Unexpected Changes in Functionality and Surface Coverage for Au Nanoparticle PEI Conjugates: Implications for Stability and Efficacy in Biological Systems, Langmuir, 2015, 31, 7673–7683 CrossRef CAS PubMed.
- L. Nothnagel and M. G. Wacker, How to Measure Release from Nanosized Carriers?, Eur. J. Pharm. Sci., 2018, 120, 199–211 CrossRef CAS PubMed.
- M. Wagner, S. Holzschuh, A. Traeger, A. Fahr and U. S. Schubert, Asymmetric Flow Field-Flow Fractionation in the Field of Nanomedicine, Anal. Chem., 2014, 86, 5201–5210 CrossRef CAS PubMed.
- J. Kuntsche, C. Decker and A. Fahr, Analysis of Liposomes Using Asymmetrical Flow Field-Flow Fractionation: Separation Conditions and Drug/Lipid Recovery, J. Sep. Sci., 2012, 35, 1993–2001 CrossRef CAS PubMed.
- S. Holzschuh, K. Kaeß, A. Fahr and C. Decker, Quantitative In Vitro Assessment of Liposome Stability and Drug Transfer Employing Asymmetrical Flow Field-Flow Fractionation (AF4), Pharm. Res., 2016, 33, 842–855 CrossRef CAS PubMed.
- A. Hinna, F. Steiniger, S. Hupfeld, M. Brandl and J. Kuntsche, Asymmetrical Flow Field-Flow Fractionation with On-line Detection for Drug Transfer Studies: A Feasibility Study, Anal. Bioanal. Chem., 2014, 406, 7827–7839 CrossRef CAS PubMed.
- S. Hupfeld, D. Ausbacher and M. Brandl, Asymmetric Flow Field-Flow Fractionation of Liposomes: 2. Concentration Detection and Adsorptive Loss Phenomena, J. Sep. Sci., 2009, 32, 3555–3561 CrossRef CAS PubMed.
- J. Ehrhart, A.-F. Mingotaud and F. Violleau, Asymmetrical Flow Field-Flow Fractionation with Multi-Angle Light Scattering and Quasi Elastic Light Scattering for Characterization of Poly(ethyleneglycol-b-ε-caprolactone) Block Copolymer Self-Assemblies Used as Drug Carriers for Photodynamic Therapy, J. Chromatogr. A, 2011, 1218, 4249–4256 CrossRef CAS PubMed.
- A. H. Hinna, S. Hupfeld, J. Kuntsche and M. Brandl, The Use of Asymmetrical Flow Field-Flow Fractionation with On-line Detection in the Study of Drug Retention within Liposomal Nanocarriers and Drug Transfer Kinetics, J. Pharm. Biomed. Anal., 2016, 124, 157–163 CrossRef CAS PubMed.
- A. H. Hinna, S. Hupfeld, J. Kuntsche, A. Bauer-Brandl and M. Brandl, Mechanism and Kinetics of the Loss of Poorly Soluble Drugs from Liposomal Carriers Studied by a Novel Flow Field-Flow Fractionation-Based Drug Release−/Transfer-Assay, J. Controlled Release, 2016, 232, 228–237 CrossRef CAS PubMed.
- P. Iavicoli, P. Urbán, A. Bella, M. G. Ryadnov, F. Rossi and L. Calzolai, Application of Asymmetric Flow Field-Flow Fractionation Hyphenations for Liposome–Antimicrobial Peptide Interaction, J. Chromatogr. A, 2015, 1422, 260–269 CrossRef CAS PubMed.
- W. Fraunhofer, G. Winter and C. Coester, Asymmetrical Flow Field-Flow Fractionation and Multiangle Light Scattering for Analysis of Gelatin Nanoparticle Drug Carrier Systems, Anal. Chem., 2004, 76, 1909–1920 CrossRef CAS PubMed.
- M. Kah, H. Walch and T. Hofmann, Environmental Fate of Nanopesticides: Durability, Sorption and Photodegradation of Nanoformulated Clothianidin, Environ. Sci.: Nano, 2018, 5, 882–889 RSC.
- A. B. Bombo, A. E. S. Pereira, M. G. Lusa, E. de Medeiros Oliveira, J. L. de Oliveira, E. V. R. Campos, M. B. de Jesus, H. C. Oliveira, L. F. Fraceto and J. L. S. Mayer, A Mechanistic View of Interactions of a Nanoherbicide with Target Organism, J. Agric. Food Chem., 2019, 67, 4453–4462 CrossRef CAS PubMed.
- M. Kah, P. Machinski, P. Koerner, K. Tiede, R. Grillo, L. F. Fraceto and T. Hofmann, Analysing the Fate of Nanopesticides in Soil and the Applicability of Regulatory Protocols Using a Polymer-Based Nanoformulation of Atrazine, Environ. Sci. Pollut. Res., 2014, 21, 11699–11707 CrossRef CAS PubMed.
- M. Kah, A. K. Weniger and T. Hofinann, Impacts of (Nano)formulations on the Fate of an Insecticide in Soil and Consequences for Environmental Exposure Assessment, Environ. Sci. Technol., 2016, 50, 10960–10967 CrossRef CAS PubMed.
- N. Kamaly, B. Yameen, J. Wu and O. C. Farokhzad, Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release, Chem. Rev., 2016, 116, 2602–2663 CrossRef CAS PubMed.
- J. H. Lee and Y. Yeo, Controlled Drug Release from Pharmaceutical Nanocarriers, Chem. Eng. Sci., 2015, 125, 75–84 CrossRef CAS PubMed.
- R. W. Korsmeyer, R. Gurny, E. Doelker, P. Buri and N. A. Peppas, Mechanisms of Solute Release from Porous Hydrophilic Polymers, Int. J. Pharm., 1983, 15, 25–35 CrossRef CAS.
- P. L. Ritger and N. A. Peppas, A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices, J. Controlled Release, 1987, 5, 37–42 CrossRef CAS.
- B. N. Huang, F. F. Chen, Y. Shen, K. Qian, Y. Wang, C. J. Sun, X. Zhao, B. Cui, F. Gao, Z. H. Zeng and H. X. Cui, Advances in Targeted Pesticides with Environmentally Responsive Controlled Release by Nanotechnology, Nanomaterials, 2018, 8, 102 CrossRef PubMed.
- Y. Shen, Y. Wang, X. Zhao, C. Sun, B. Cui, F. Gao, Z. Zeng and H. Cui, Preparation and Physicochemical Characteristics of Thermo-Responsive Emamectin Benzoate Microcapsules, Polymers, 2017, 9, 418 CrossRef.
- E. V. R. Campos, P. L. F. Proença, J. L. Oliveira, A. E. S. Pereira, L. N. de Morais Ribeiro, F. O. Fernandes, K. C. Gonçalves, R. A. Polanczyk, T. Pasquoto-Stigliani, R. Lima, C. C. Melville, J. F. Della Vechia, D. J. Andrade and L. F. Fraceto, Carvacrol and Linalool Co-Loaded in β-Cyclodextrin-Grafted Chitosan Nanoparticles as Sustainable Biopesticide Aiming Pest Control, Sci. Rep., 2018, 8, 7623 CrossRef PubMed.
- Z. Gao, L. Pang, H. Feng, S. Wang, Q. Wang, M. Wang, Y. Xia and S. Hu, Preparation and Characterization of a Novel Imidacloprid Microcapsule via Coating of Polydopamine and Polyurea, RSC Adv., 2017, 7, 15762–15768 RSC.
- H. M. Nguyen, I.-C. Hwang, J.-W. Park and H.-J. Park, Photoprotection for Deltamethrin Using Chitosan-Coated Beeswax Solid Lipid Nanoparticles, Pest Manage. Sci., 2012, 68, 1062–1068 CrossRef CAS PubMed.
- E. Marin, M. I. Briceño and C. Caballero-George, Critical Evaluation of Biodegradable Polymers Used in Nanodrugs, Int. J. Nanomed., 2013, 8, 3071–3091 Search PubMed.
- H. K. Makadia and S. J. Siegel, Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier, Polymers, 2011, 3, 1377–1397 CrossRef CAS PubMed.
- B. S. Zolnik and D. J. Burgess, Effect of Acidic pH on PLGA Microsphere Degradation and Release, J. Controlled Release, 2007, 122, 338–344 CrossRef CAS PubMed.
- J. Panyam, M. M. Dali, S. K. Sahoo, W. Ma, S. S. Chakravarthi, G. L. Amidon, R. J. Levy and V. Labhasetwar, Polymer Degradation and in vitro Release of a Model Protein from Poly (D, L-lactide-co-glycolide) Nano-and Microparticles, J. Controlled Release, 2003, 92, 173–187 CrossRef CAS PubMed.
- H. Y. Tan, E. Widjaja, F. Boey and S. C. J. Loo, Spectroscopy Techniques for Analyzing the Hydrolysis of PLGA and PLLA, J. Biomed. Mater. Res., Part B, 2009, 91, 433–440 CrossRef PubMed.
- E. A. Schmitt, D. R. Flanagan and R. J. Linhardt, Importance of Distinct Water Environments in the Hydrolysis of Poly(DL-lactide-co-glycolide), Macromolecules, 1994, 27, 743–748 CrossRef CAS.
- A. Kumari, S. K. Yadav and S. C. Yadav, Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems, Colloids Surf., B, 2010, 75, 1–18 CrossRef CAS PubMed.
- K. Avgoustakis, A. Beletsi, Z. Panagi, P. Klepetsanis, A. Karydas and D. Ithakissios, PLGA–mPEG Nanoparticles of Cisplatin: In vitro Nanoparticle Degradation, In vitro Drug Release and In vivo Drug Residence in Blood Properties, J. Controlled Release, 2002, 79, 123–135 CrossRef CAS.
- R. S. Kalhapure, D. R. Sikwal, S. Rambharose, C. Mocktar, S. Singh, L. Bester, J. K. Oh, J. Renukuntla and T. Govender, Enhancing Targeted Antibiotic Therapy via pH Responsive Solid Lipid Nanoparticles from an Acid Cleavable Lipid, Nanomed.: Nanotechnol., Biol. Med., 2017, 13, 2067–2077 CrossRef CAS PubMed.
- D. Vreugdenhil and E. A. M. Kootgronsveld, Measurements of Ph, Sucrose and Potassium-Ions in the Phloem Sap of Castor Bean (Ricinus-Communis) Plants, Physiol. Plant., 1989, 77, 385–388 CrossRef CAS.
- M. R. Hill, E. J. MacKrell, C. P. Forsthoefel, S. P. Jensen, M. S. Chen, G. A. Moore, Z. L. L. He and B. S. Sumerlin, Biodegradable and pH-Responsive Nanoparticles Designed for Site-Specific Delivery in Agriculture, Biomacromolecules, 2015, 16, 1276–1282 CrossRef CAS PubMed.
- K. Ding, L. Shi, L. Zhang, T. Zeng, Y. Yin and Y. Yi, Synthesis of Photoresponsive Polymeric Propesticide Micelles Based on PEG for the Controlled Release of a Herbicide, Polym. Chem., 2016, 7, 899–904 RSC.
- J. S. Chawla and M. M. Amiji, Biodegradable Poly (ε-caprolactone) Nanoparticles for Tumor-Targeted Delivery of Tamoxifen, Int. J. Pharm., 2002, 249, 127–138 CrossRef CAS PubMed.
- J.-X. Fu, H.-J. Wang, Y.-Q. Zhou and J.-Y. Wang, Antibacterial Activity of Ciprofloxacin-Loaded Zein Microsphere Films, Mater. Sci. Eng., C, 2009, 29, 1161–1166 CrossRef CAS.
- Y. Hou, J. Hu, H. Park and M. Lee, Chitosan Based Nanoparticles as a Sustained Protein Release Carrier for Tissue Engineering Applications, J. Biomed. Mater. Res., Part A, 2012, 100, 939–947 CrossRef PubMed.
- T. Akagi, M. Higashi, T. Kaneko, T. Kida and M. Akashi, In vitro Enzymatic Degradation of Nanoparticles Prepared from Hydrophobically-Modified Poly(γ-glutamic acid), Macromol. Biosci., 2005, 5, 598–602 CrossRef CAS PubMed.
- T. Akagi, M. Higashi, T. Kaneko, T. Kida and M. Akashi, Hydrolytic and Enzymatic Degradation of Nanoparticles Based on Amphiphilic Poly(γ-glutamic acid)-graft-l-Phenylalanine Copolymers, Biomacromolecules, 2006, 7, 297–303 CrossRef CAS PubMed.
- J. L. De Oliveira, E. V. R. Campos, A. E. S. Pereira, T. Pasquoto, R. Lima, R. Grillo, D. J. D. Andrade, F. A. D. Santos and L. F. Fraceto, Zein Nanoparticles as Eco-Friendly Carrier Systems for Botanical Repellents Aiming Sustainable Agriculture, J. Agric. Food Chem., 2018, 66, 1330–1340 CrossRef CAS PubMed.
- J. L. de Oliveira, E. V. R. Campos, T. Germano-Costa, R. Lima, J. F. D. Vechia, S. T. Soares, D. J. de Andrade, K. C. Gonçalves, J. do Nascimento, R. A. Polanczyk and L. F. Fraceto, Association of Zein Nanoparticles with Botanical Compounds for Effective Pest Control Systems, Pest Manage. Sci., 2019, 75, 1855–1865 CrossRef CAS PubMed.
- X. Jia, W.-B. Sheng, W. Li, Y.-B. Tong, Z.-Y. Liu and F. Zhou, Adhesive Polydopamine Coated Avermectin Microcapsules for Prolonging Foliar Pesticide Retention, ACS Appl. Mater. Interfaces, 2014, 6, 19552–19558 CrossRef CAS PubMed.
- J. Liang, M. Yu, L. Guo, B. Cui, X. Zhao, C. Sun, Y. Wang, G. Liu, H. Cui and Z. Zeng, Bioinspired Development of P(St–MAA)–Avermectin Nanoparticles with High Affinity for Foliage To Enhance Folia Retention, J. Agric. Food Chem., 2018, 66, 6578–6584 CrossRef CAS PubMed.
- J. Lv, P. Christie and S. Zhang, Uptake, Translocation, and Transformation of Metal-Based Nanoparticles in Plants: Recent Advances and Methodological Challenges, Environ. Sci.: Nano, 2019, 6, 41–59 RSC.
- I.-C. Chen, M. Zhang, B. Teipel, I. S. de Araujo, Y. Yegin and M. Akbulut, Transport of Polymeric Nanoparticulate Drug Delivery Systems in the Proximity of Silica and Sand, Environ. Sci. Technol., 2015, 49, 3575–3583 CrossRef CAS PubMed.
- A. R. Petosa, F. Rajput, O. Selvam, C. Öhl and N. Tufenkji, Assessing the Transport Potential of Polymeric Nanocapsules Developed for Crop Protection, Water Res., 2017, 111, 10–17 CrossRef CAS PubMed.
- B. Liu, Y. Wang, F. Yang, X. Wang, H. Shen, H. Cui and D. Wu, Construction of a Controlled-Release Delivery System for Pesticides using Biodegradable PLA-Based Microcapsules, Colloids Surf., B, 2016, 144, 38–45 CrossRef CAS PubMed.
- Q. Shang, Y. Shi, Y. Zhang, T. Zheng and H. Shi, Pesticide-Conjugated Polyacrylate Nanoparticles: Novel Opportunities for Improving the Photostability of Emamectin Benzoate, Polym. Adv. Technol., 2013, 24, 137–143 CrossRef CAS.
- M.-M. Yin, Y. Zheng and F.-L. Chen, Pyraclostrobin-Loaded Poly (lactic-co-glycolic acid) Nanospheres: Preparation and Characteristics, J. Integr. Agric., 2018, 17, 1822–1832 CrossRef CAS.
- S. Reichenberger, M. Bach, A. Skitschak and H.-G. Frede, Mitigation Strategies to Reduce Pesticide Inputs into Ground- and Surface Water and Their Effectiveness; A Review, Sci. Total Environ., 2007, 384, 1–35 CrossRef CAS PubMed.
- S. Kumar, G. Bhanjana, A. Sharma, M. C. Sidhu and N. Dilbaghi, Synthesis, Characterization and On Field Evaluation of Pesticide Loaded Sodium Alginate Nanoparticles, Carbohydr. Polym., 2014, 101, 1061–1067 CrossRef CAS PubMed.
- N. Memarizadeh, M. Ghadamyari, M. Adeli and K. Talebi, Preparation, Characterization and Efficiency of Nanoencapsulated Imidacloprid under Laboratory Conditions, Ecotoxicol. Environ. Saf., 2014, 107, 77–83 CrossRef CAS PubMed.
- F.-L. Yang, X.-G. Li, F. Zhu and C.-L. Lei, Structural Characterization of Nanoparticles Loaded with Garlic Essential Oil and Their Insecticidal Activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae), J. Agric. Food Chem., 2009, 57, 10156–10162 CrossRef CAS PubMed.
- H. C. Oliveira, R. Stolf-Moreira, C. B. R. Martinez, R. Grillo, M. B. de Jesus and L. F. Fraceto, Nanoencapsulation Enhances the Post-Emergence Herbicidal Activity of Atrazine against Mustard Plants, PLoS One, 2015, 10, e0132971 CrossRef PubMed.
- L. Zhang, D. Pornpattananangkul, C.-M. J. Hu and C.-M. Huang, Development of Nanoparticles for Antimicrobial Drug Delivery, Curr. Med. Chem., 2010, 17, 585–594 CrossRef CAS PubMed.
- S. Xie, Y. Tao, Y. Pan, W. Qu, G. Cheng, L. Huang, D. Chen, X. Wang, Z. Liu and Z. Yuan, Biodegradable Nanoparticles for Intracellular Delivery of Antimicrobial Agents, J. Controlled Release, 2014, 187, 101–117 CrossRef CAS PubMed.
- E. K. Hill and J. Li, Current and Future Prospects for Nanotechnology in Animal Production, J. Anim. Sci. Biotechnol., 2017, 8, 26 CrossRef PubMed.
- M. Wulf and A. Voss, MRSA in Livestock Animals—An Epidemic Waiting to Happen?, Clin. Microbiol. Infect., 2008, 14, 519–521 CrossRef CAS PubMed.
- A. G. Mathew, R. Cissell and S. Liamthong, Antibiotic Resistance in Bacteria Associated with Food Animals: A United States Perspective of Livestock Production, Foodborne Pathog. Dis., 2007, 4, 115–133 CrossRef CAS PubMed.
- M. Woolhouse, M. Ward, B. van Bunnik and J. Farrar, Antimicrobial Resistance in Humans, Livestock and the Wider Environment, Philos. Trans. R. Soc. London, Ser. B, 2015, 370, 20140083 CrossRef PubMed.
- A. M. Allahverdiyev, K. V. Kon, E. S. Abamor, M. Bagirova and M. Rafailovich, Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents, Expert Rev. Anti-infect. Ther., 2011, 9, 1035–1052 CrossRef CAS PubMed.
- B. D. Brooks and A. E. Brooks, Therapeutic strategies to combat antibiotic resistance, Adv. Drug Delivery Rev., 2014, 78, 14–27 CrossRef CAS PubMed.
- R. Y. Pelgrift and A. J. Friedman, Nanotechnology as a therapeutic tool to combat microbial resistance, Adv. Drug Delivery Rev., 2013, 65, 1803–1815 CrossRef CAS PubMed.
- S. C. Abeylath and E. Turos, Drug delivery approaches to overcome bacterial resistance to beta-lactam antibiotics, Expert Opin. Drug Delivery, 2008, 5, 931–949 CrossRef CAS PubMed.
- A. J. Huh and Y. J. Kwon, "Nanoantibiotics": a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era, J. Controlled Release, 2011, 156, 128–145 CrossRef CAS PubMed.
- S. J. Lam, E. H. H. Wong, C. Boyer and G. G. Qiao, Antimicrobial Polymeric Nanoparticles, Prog. Polym. Sci., 2018, 76, 40–64 CrossRef CAS.
- N. M. Zaki and M. M. Hafez, Enhanced Antibacterial Effect of Ceftriaxone Sodium-Loaded Chitosan Nanoparticles Against Intracellular Salmonella typhimurium, AAPS PharmSciTech, 2012, 13, 411–421 CrossRef CAS PubMed.
- V. Kiruthika, S. Maya, M. K. Suresh, V. Anil Kumar, R. Jayakumar and R. Biswas, Comparative Efficacy of Chloramphenicol Loaded Chondroitin Sulfate and Dextran Sulfate Nanoparticles to Treat Intracellular Salmonella Infections, Colloids Surf., B, 2015, 127, 33–40 CrossRef CAS PubMed.
- S. M. Abdelghany, D. J. Quinn, R. J. Ingram, B. F. Gilmore, R. F. Donnelly, C. C. Taggart and C. J. Scott, Gentamicin-Loaded Nanoparticles Show Improved Antimicrobial Effects Towards Pseudomonas aeruginosa Infection, Int. J. Nanomed., 2012, 7, 4053–4063 CAS.
- N. Thomas, C. Thorn, K. Richter, B. Thierry and C. Prestidge, Efficacy of Poly-Lactic-Co-Glycolic Acid Micro- and Nanoparticles of Ciprofloxacin Against Bacterial Biofilms, J. Pharm. Sci., 2016, 105, 3115–3122 CrossRef CAS PubMed.
- E. Turos, G. S. K. Reddy, K. Greenhalgh, P. Ramaraju, S. C. Abeylath, S. Jang, S. Dickey and D. V. Lim, Penicillin-Bound Polyacrylate Nanoparticles: Restoring the Activity of β-Lactam Antibiotics Against MRSA, Bioorg. Med. Chem. Lett., 2007, 17, 3468–3472 CrossRef CAS PubMed.
- B. Jamil, H. Habib, S. Abbasi, H. Nasir, A. Rahman, A. Rehman, H. Bokhari and M. Imran, Cefazolin Loaded Chitosan Nanoparticles to Cure Multi Drug Resistant Gram-Negative Pathogens, Carbohydr. Polym., 2016, 136, 682–691 CrossRef CAS PubMed.
- S. Mushtaq, J. A. Khan, F. Rabbani, U. Latif, M. Arfan and M. A. Yameen, Biocompatible Biodegradable Polymeric Antibacterial Nanoparticles for Enhancing the Effects of a Third-Generation Cephalosporin Against Resistant Bacteria, J. Med. Microbiol., 2017, 66, 318–327 CrossRef CAS PubMed.
- A. D. E. S. Pereira, H. C. Oliveira and L. F. Fraceto, Polymeric Nanoparticles as an Alternative for Application of Gibberellic Acid in Sustainable Agriculture: A Field Study, Sci. Rep., 2019, 9, 7135 CrossRef PubMed.
- W. S. Shin, H. I. Song and H. S. Um, Role of Physicochemical Properties in Nanoparticle Toxicity, Nanomaterials, 2015, 5, 1351–1365 CrossRef PubMed.
- S. Bhattacharjee, D. Ershov, K. Fytianos, J. van der Gucht, G. M. Alink, I. M. C. M. Rietjens, A. T. M. Marcelis and H. Zuilhof, Cytotoxicity and Cellular Uptake of Tri-Block Copolymer Nanoparticles with Different Size and Surface Characteristics, Part. Fibre Toxicol., 2012, 9, 11 CrossRef CAS PubMed.
- B. Zhang, P. Sai Lung, S. Zhao, Z. Chu, W. Chrzanowski and Q. Li, Shape Dependent Cytotoxicity of PLGA-PEG Nanoparticles on Human Cells, Sci. Rep., 2017, 7, 7315 CrossRef PubMed.
- C. R. Maruyama, M. Guilger, M. Pascoli, N. Bileshy-José, P. C. Abhilash, L. F. Fraceto and R. de Lima, Nanoparticles Based on Chitosan as Carriers for the Combined Herbicides Imazapic and Imazapyr, Sci. Rep., 2016, 6, 19768 CrossRef CAS PubMed.
- M. Pascoli, M. T. Jacques, D. A. Agarrayua, D. S. Avila, R. Lima and L. F. Fraceto, Neem Oil Based Nanopesticide as an Environmentally-Friendly Formulation for Applications in Sustainable Agriculture: An Ecotoxicological Perspective, Sci. Total Environ., 2019, 677, 57–67 CrossRef CAS PubMed.
- M. Karavolos and A. Holban, Nanosized Drug Delivery Systems in Gastrointestinal Targeting: Interactions with Microbiota, Pharmaceuticals, 2016, 9, 62 CrossRef PubMed.
- N. Hadrup, K. Loeschner, A. Bergström, A. Wilcks, X. Gao, U. Vogel, H. L. Frandsen, E. H. Larsen, H. R. Lam and A. Mortensen, Subacute Oral Toxicity Investigation of Nanoparticulate and Ionic Silver in Rats, Arch. Toxicol., 2012, 86, 543–551 CrossRef CAS PubMed.
- L. A. Wilding, C. M. Bassis, K. Walacavage, S. Hashway, P. R. Leroueil, M. Morishita, A. D. Maynard, M. A. Philbert and I. L. Bergin, Repeated Dose (28-Day) Administration of Silver Nanoparticles of Varied Size and Coating Does Not Significantly Alter the Indigenous Murine Gut Microbiome, Nanotoxicology, 2016, 10, 513–520 CrossRef CAS PubMed.
- A. A. Taylor, I. M. Marcus, R. L. Guysi and S. L. Walker, Metal Oxide Nanoparticles Induce Minimal Phenotypic Changes in a Model Colon Gut Microbiota, Environ. Eng. Sci., 2015, 32, 602–612 CrossRef CAS.
- E. E. Fröhlich and E. Fröhlich, Cytotoxicity of Nanoparticles Contained in Food on Intestinal Cells and the Gut Microbiota, Int. J. Mol. Sci., 2016, 17, 509 CrossRef PubMed.
- E. Chiellini, A. Corti, S. D'Antone and R. Solaro, Biodegradation of Poly (Vinyl Alcohol) Based Materials, Prog. Polym. Sci., 2003, 28, 963–1014 CrossRef CAS.
- A. E. S. Pereira, I. E. Sandoval-Herrera, S. A. Zavala-Betancourt, H. C. Oliveira, A. S. Ledezma-Pérez, J. Romero and L. F. Fraceto, γ-Polyglutamic Acid/Chitosan Nanoparticles for the Plant Growth Regulator Gibberellic Acid: Characterization and Evaluation of Biological Activity, Carbohydr. Polym., 2017, 157, 1862–1873 CrossRef CAS PubMed.
- C. R. D. Oliveira, L. F. Fraceto, G. M. Rizzi, R. F. Salla, F. C. Abdalla, M. J. Costa and E. C. M. Silva-Zacarin, Hepatic Effects of the Clomazone Herbicide in Both Its Free Form and Associated with Chitosan-Alginate Nanoparticles in Bullfrog Tadpoles, Chemosphere, 2016, 149, 304–313 CrossRef PubMed.
- A. C. Preisler, A. E. S. Pereira, E. V. R. Campos, G. Dalazen, L. F. Fraceto and H. C. Oliveira, Atrazine Nanoencapsulation Improves Pre-Emergence Herbicidal Activity Against Bidens pilosa without Enhancing Long-Term Residual Effect on Glycine max, Pest Manage. Sci., 2020, 76, 141–149 CrossRef CAS PubMed.
- G. F. M. Sousa, D. G. Gomes, E. V. R. Campos, J. L. Oliveira, L. F. Fraceto, R. Stolf-Moreira and H. C. Oliveira, Post-Emergence Herbicidal Activity of Nanoatrazine Against Susceptible Weeds, Front. Environ. Sci., 2018, 6, 12 CrossRef.
- H. C. Oliveira, R. Stolf-Moreira, C. B. R. Martinez, G. F. M. Sousa, R. Grillo, M. B. de Jesus and L. F. Fraceto, Evaluation of the Side Effects of Poly(epsilon-caprolactone) Nanocapsules Containing Atrazine Toward Maize Plants, Front. Chem., 2015, 3, 61 Search PubMed.
- M. T. Jacques, J. L. Oliveira, E. V. R. Campos, L. F. Fraceto and D. S. Ávila, Safety assessment of nanopesticides using the roundworm Caenorhabditis elegans, Ecotoxicol. Environ. Saf., 2017, 139, 245–253 CrossRef CAS PubMed.
- Y. Tong, Y. Wu, C. Zhao, Y. Xu, J. Lu, S. Xiang, F. Zong and X. Wu, Polymeric Nanoparticles as a Metolachlor Carrier: Water-Based Formulation for Hydrophobic Pesticides and Absorption by Plants, J. Agric. Food Chem., 2017, 65, 7371–7378 CrossRef CAS PubMed.
- A. N. Meredith, B. Harper and S. L. Harper, The Influence of Size on the Toxicity of an Encapsulated Pesticide: A Comparison of Micron- and Nano-Sized Capsules, Environ. Int., 2016, 86, 68–74 CrossRef CAS PubMed.
- S. Maya, S. Indulekha, V. Sukhithasri, K. T. Smitha, S. V. Nair, R. Jayakumar and R. Biswas, Efficacy of Tetracycline Encapsulated O-Carboxymethyl Chitosan Nanoparticles Against Intracellular Infections of Staphylococcus aureus, Int. J. Biol. Macromol., 2012, 51, 392–399 CrossRef CAS PubMed.
- Y.-I. Jeong, H.-S. Na, D.-H. Seo, D.-G. Kim, H.-C. Lee, M.-K. Jang, S.-K. Na, S.-H. Roh, S.-I. Kim and J.-W. Nah, Ciprofloxacin-Encapsulated Poly(dl-Lactide-co-Glycolide) Nanoparticles and its Antibacterial Activity, Int. J. Pharm., 2008, 352, 317–323 CrossRef CAS PubMed.
- S. Paudel, C. Cerbu, C. E. Astete, S. M. Louie, C. Sabliov and D. F. Rodrigues, Enrofloxacin-Impregnated PLGA Nanocarriers for Efficient Therapeutics and Diminished Generation of Reactive Oxygen Species, ACS Appl. Nano Mater., 2019, 2, 5035–5043 CrossRef CAS.
- E. Imbuluzqueta, C. Gamazo, H. Lana, M. Á. Campanero, D. Salas, A. G. Gil, E. Elizondo, N. Ventosa, J. Veciana and M. J. Blanco-Prieto, Hydrophobic Gentamicin-Loaded Nanoparticles Are Effective Against Brucella melitensis Infection in Mice, Antimicrob. Agents Chemother., 2013, 57, 3326 CrossRef CAS PubMed.
- F. Esmaeili, M. Hosseini-Nasr, M. Rad-Malekshahi, N. Samadi, F. Atyabi and R. Dinarvand, Preparation and Antibacterial Activity Evaluation of Rifampicin-Loaded Poly Lactide-co-Glycolide Nanoparticles, Nanomed.: Nanotechnol., Biol. Med., 2007, 3, 161–167 CrossRef CAS PubMed.
- G. Marslin, A. M. Revina, V. K. M. Khandelwal, K. Balakumar, C. J. Sheeba and G. Franklin, PEGylated Ofloxacin Nanoparticles Render Strong Antibacterial Activity Against Many Clinically Important Human Pathogens, Colloids Surf., B, 2015, 132, 62–70 CrossRef CAS PubMed.
- R. Misra, S. Acharya, F. Dilnawaz and S. K. Sahoo, Sustained Antibacterial Activity of Doxycycline-Loaded Poly(D,L-lactide-co-glycolide) and Poly(ε-caprolactone) Nanoparticles, Nanomedicine, 2009, 4, 519–530 CrossRef CAS PubMed.
- L. Pourzahedi, M. Pandorf, D. Ravikumar, J. B. Zimmerman, T. P. Seager, T. L. Theis, P. Westerhoff, L. M. Gilbertson and G. V. Lowry, Life Cycle Considerations of Nano-Enabled Agrochemicals: Are Today's Tools Up to the Task?, Environ. Sci.: Nano, 2018, 5, 1057–1069 RSC.
- D. E. Jones, H. Ghandehari and J. C. Facelli, A Review of the Applications of Data Mining and Machine Learning for the Prediction of Biomedical Properties of Nanoparticles, Comput. Methods Programs Biomed., 2016, 132, 93–103 CrossRef PubMed.
- A. A. Metwally and R. M. Hathout, Computer-Assisted Drug Formulation Design: Novel Approach in Drug Delivery, Mol. Pharmaceutics, 2015, 12, 2800–2810 CrossRef CAS PubMed.
- A. O. Kasimova, G. M. Pavan, A. Danani, K. Mondon, A. Cristiani, L. Scapozza, R. Gurny and M. Möller, Validation of a Novel Molecular Dynamics Simulation Approach for Lipophilic Drug Incorporation into Polymer Micelles, J. Phys. Chem. B, 2012, 116, 4338–4345 CrossRef CAS PubMed.
- S. M. Loverde, M. L. Klein and D. E. Discher, Nanoparticle Shape Improves Delivery: Rational Coarse Grain Molecular Dynamics (rCG-MD) of Taxol in Worm-Like PEG-PCL Micelles, Adv. Mater., 2012, 24, 3823–3830 CrossRef CAS PubMed.
- C. Forrey, D. M. Saylor, J. S. Silverstein, J. F. Douglas, E. M. Davis and Y. A. Elabd, Prediction and Validation of Diffusion Coefficients in a Model Drug Delivery System Using Microsecond Atomistic Molecular Dynamics Simulation and Vapour Sorption Analysis, Soft Matter, 2014, 10, 7480–7494 RSC.
- C. Rodrigues de Azevedo, M. von Stosch, M. S. Costa, A. M. Ramos, M. M. Cardoso, F. Danhier, V. Préat and R. Oliveira, Modeling of the Burst Release from PLGA Micro- and Nanoparticles as Function of Physicochemical Parameters and Formulation Characteristics, Int. J. Pharm., 2017, 532, 229–240 CrossRef CAS PubMed.
- J. Szlęk, A. Pacławski, R. Lau, R. Jachowicz and A. Mendyk, Heuristic Modeling of Macromolecule Release from PLGA Microspheres, Int. J. Nanomed., 2013, 8, 4601 Search PubMed.
- H. M. Zawbaa, J. Szlȩk, C. Grosan, R. Jachowicz and A. Mendyk, Computational Intelligence Modeling of the Macromolecules Release from PLGA Microspheres—Focus on Feature Selection, PLoS One, 2016, 11, e0157610 CrossRef PubMed.
- J. Youshia, M. E. Ali and A. Lamprecht, Artificial Neural Network Based Particle Size Prediction of Polymeric Nanoparticles, Eur. J. Pharm. Biopharm., 2017, 119, 333–342 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |