Harmonizing across environmental nanomaterial testing media for increased comparability of nanomaterial datasets†
Nicholas K.
Geitner
 ab,
Christine
Ogilvie Hendren
ab,
Christine
Ogilvie Hendren
 ab,
Geert
Cornelis
ab,
Geert
Cornelis
 c,
Ralf
Kaegi
c,
Ralf
Kaegi
 d,
Jamie R.
Lead
ef,
Gregory V.
Lowry
d,
Jamie R.
Lead
ef,
Gregory V.
Lowry
 bg,
Iseult
Lynch
bg,
Iseult
Lynch
 h,
Bernd
Nowack
h,
Bernd
Nowack
 i,
Elijah
Petersen
i,
Elijah
Petersen
 j,
Emily
Bernhardt
bk,
Scott
Brown
l,
Wei
Chen
j,
Emily
Bernhardt
bk,
Scott
Brown
l,
Wei
Chen
 m,
Camille
de Garidel-Thoron
n,
Jaydee
Hanson
o,
Stacey
Harper
p,
Kim
Jones
bq,
Frank
von der Kammer
r,
Alan
Kennedy
s,
Justin
Kidd
t,
Cole
Matson
m,
Camille
de Garidel-Thoron
n,
Jaydee
Hanson
o,
Stacey
Harper
p,
Kim
Jones
bq,
Frank
von der Kammer
r,
Alan
Kennedy
s,
Justin
Kidd
t,
Cole
Matson
 bu,
Chris D.
Metcalfe
v,
Joel
Pedersen
bu,
Chris D.
Metcalfe
v,
Joel
Pedersen
 w,
Willie J. G. M.
Peijnenburg
w,
Willie J. G. M.
Peijnenburg
 xy,
Joris T. K.
Quik
xy,
Joris T. K.
Quik
 z,
Sónia M.
Rodrigues
z,
Sónia M.
Rodrigues
 aa,
Jerome
Rose
aa,
Jerome
Rose
 ab,
Phil
Sayre
ac,
Marie
Simonin
ab,
Phil
Sayre
ac,
Marie
Simonin
 bk,
Claus
Svendsen
ad,
Robert
Tanguay
ae,
Nathalie
Tefenkji
bk,
Claus
Svendsen
ad,
Robert
Tanguay
ae,
Nathalie
Tefenkji
 af,
Tom
van Teunenbroek
agah,
Gregory
Thies
ai,
Yuan
Tian
ab,
Jacelyn
Rice
an,
Amalia
Turner
ab,
Jie
Liu
af,
Tom
van Teunenbroek
agah,
Gregory
Thies
ai,
Yuan
Tian
ab,
Jacelyn
Rice
an,
Amalia
Turner
ab,
Jie
Liu
 baj,
Jason
Unrine
baj,
Jason
Unrine
 bak,
Marina
Vance
bak,
Marina
Vance
 al,
Jason C.
White
al,
Jason C.
White
 am and
Mark R.
Wiesner
*ab
am and
Mark R.
Wiesner
*ab
aDepartment of Civil and Environmental Engineering, Duke University, Durham, NC, USA. E-mail: wiesner@duke.edu
bCenter for the Environmental Implications of NanoTechnology (CEINT), USA
cDepartment of Soil and Environment, Swedish University of Agricultural Sciences, Uppsala, Sweden
dEawag, Swiss Federal Institute of Aquatic Science and Technology, Dübendorf, Switzerland
eSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham, UK
fCenter for Environmental Nanoscience and Risk, University of South Carolina, Columbia, SC, USA
gDepartment of Civil and Environmental Engineering, Carnegie Mellon University, Pittsburgh, PA 15213, USA
hDepartment of Earth and Environmental Sciences, School of Geography, University of Birmingham, Birmingham, UK
iEMPA, Swiss Federal Laboratories for Materials Science and Technology, Technology and Society Laboratory, Lerchenfeldstrasse 5, Gallen, Switzerland
jBiosystems and Biomaterials Division, National Institute of Standards and Technology, Gaithersburg, Maryland, USA
kDepartment of Biology, Duke University, Durham, NC, USA
lThe Chemours Company, Wilmington, DE, USA
mCollege of Environmental Science and Engineering, Nankai University, China
nCNRS, Aix-Marseille Univ, IRD, INRA, Coll France, CEREGE, Europole Arbois, Aix en Provence, France
oCenter for Food Safety and International Center for Technology Assessment, USA
pEnvironmental and Molecular Toxicology, School of Chemical, Biological, and Environmental Engineering, Oregon State University, Corvallis, OR, USA
qDepartment of Civil and Environmental Engineering, Howard University, Washington, DC, USA
rDepartment of Environmental Geosciences and Environmental Science Research Network, University of Vienna, Vienna, Austria
sEnvironmental Laboratory, U.S. Army Engineer Research and Development Center, Vicksburg, VA, USA
tNanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment, Arizona State University, School of Sustainable Engineering and the Built Environment, Tempe, AZ, USA
uCenter for Reservoir and Aquatic Systems Research, and Department of Environmental Science, Baylor University, Waco, TX, USA
vInstitute for Watershed Science, Trent University, Peterborough, ON, Canada
wDepartments of Soil Science, Chemistry, and Civil & Environmental Engineering, University of Wisconsin-Madison, Madison, WI, USA
xNational Institute for Public Health and the Environment (RIVM), Centre for Safety of Substances and Products, Bilthoven, The Netherlands
yLeiden University, Center for Environmental Sciences, Leiden, The Netherlands
zNational Institute for Public Health and the Environment (RIVM), Centre for Safety of Substances and Products, Bilthoven, The Netherlands
aaCentre for Environmental and Marine Studies (CESAM), Department of Chemistry, University of Aveiro, Aveiro, Portugal
abAix Marseille Univ, CNRS, IRD, INRA, Coll France, CEREGE, Aix-en-Provence, France
acnanoRisk Analytics, LLC, Auburn, CA, USA
adCentre for Ecology and Hydrology, Wallingford, Oxfordshire, UK
aeDepartment of Environmental and Molecular Toxicology, Environmental Health Sciences Center, Marine and Freshwater Biomedical Sciences Center, Oregon State University, Corvallis, OR, USA
afDepartment of Chemical Engineering, McGill University, Montreal, QC, Canada
agMinistry of Infrastructure and the Environment, The Hague, The Netherlands
ahProject Office ProSafe PB 73, Bilthoven, BA, Netherlands
aiCGI, Montreal, Canada
ajDepartment of Chemistry, Duke University, Durham, USA
akDepartment of Plant and Soil Sciences, University of Kentucky, Lexington, KY, USA
alDepartment of Mechanical Engineering, University of Colorado Boulder, Boulder, CO, USA
amDepartment of Analytical Chemistry, The Connecticut Agricultural Experiment Station, New Haven, CT, USA
anDepartment of Engineering Technology and Construction Management, University of North Carolina at Charlotte, Charlotte, NC, USA
First published on 8th November 2019
Abstract
The chemical composition and properties of environmental media determine nanomaterial (NM) transport, fate, biouptake, and organism response. To compare and interpret experimental data, it is essential that sufficient context be provided for describing the physical and chemical characteristics of the setting in which a nanomaterial may be present. While the nanomaterial environmental, health and safety (NanoEHS) field has begun harmonization to allow data comparison and re-use (e.g. using standardized materials, defining a minimum set of required material characterizations), there is limited guidance for standardizing test media. Since most of the NM properties driving environmental behaviour and toxicity are medium-dependent, harmonization of media is critical. A workshop in March 2016 at Duke University identified five categories of test media: aquatic testing media, soil and sediment testing media, biological testing media, engineered systems testing media and product matrix testing media. For each category of test media, a minimum set of medium characteristics to report in all NM tests is recommended. Definitions and detail level of the recommendations for specific standardized media vary across these media categories. This reflects the variation in the maturity of their use as a test medium and associated measurement techniques, variation in utility and relevance of standardizing medium properties, ability to simplify standardizing reporting requirements, and in the availability of established standard reference media. Adoption of these media harmonization recommendations will facilitate the generation of integrated comparable datasets on NM fate and effects. This will in turn allow testing of the predictive utility of functional assay measurements on NMs in relevant media, support investigation of first principles approaches to understand behavioral mechanisms, and support categorization strategies to guide research, commercial development, and policy.
Environmental significanceTo support regulatory and other environmental evaluation of nanomaterials, efficient generation of large arrays of standardized and comparable data are vital. To fulfill these requirements, the selected set of parameters must allow investigation of possible effects of both the surrounding medium and the nanomaterial properties on various endpoints. The authors propose minimum sets of parameters needed to provide such context in five system categories, providing a rationale for their designation. Adherence to adequate media characterization provides a basis for comparison even while allowing researchers to employ preferred media of choice in terms of specific parameter values. In some cases standard media are also proposed, calling for specific parameter values that allow studies to be directly compared and benchmarked. |
1. Introduction
The chemical composition and properties of environmental media play defining roles in determining nanomaterial (NM) transport, fate, biouptake, and even organismal response. To compare and interpret experimental data, it is therefore essential that sufficient context be provided describing the chemical dimensions of the setting in which a nanomaterial may be present. The nanomaterial environment, health and safety (nanoEHS) field has agreed upon the need for standardized NMs,1 that a minimum set of NM characterization is required (although the specifics are still debated),1 and has even agreed upon specific assays for key parameters.2–4 Similarly, standard tests in a set of consistent media are also needed since most of the NM properties driving environmental behavior and toxicity are context-dependent. Here we use NM to refer to nanomaterials generally, since the advancement of nanoEHS has brought increasing understanding of the relevance of nanoscale materials of engineered, incidental, and natural origin, and the testing of all categories of NMs benefits from efforts to harmonize datasets.Consistency across testing is already appreciated within nanoEHS communities. For example, it has been proposed that aggregated semi-empirical parameters called functional assays (FAs) be investigated for their utility in predicting nanomaterial behavior in complex systems.5 Functional assays to test dissolution rate,6 attachment efficiencies,7 and reactivities of NM would help to categorize these materials at various stages of their lifecycles in a way that is meaningfully predictive of their potential subsequent transport and impact. Besides capturing the functional behaviors most useful for forecasting impacts, the parameters generated by FAs, describing where NMs go and what they do, are inherently inclusive of phenomena that derive from initial nanomaterial characteristics (e.g., primary particle size) as well as those controlled by surrounding media (e.g. zeta potential, aggregation state). As a next step, the nanomaterial testing community must adopt a suite of relevant media in which to conduct functional assays and other studies. Establishing a set of consistently defined media will not only serve to build in a measure of quality assurance and interoperability to the resulting data but will make resulting datasets more conducive to modeling and cross-study analyses.
To address this need for media harmonization, an expert workshop was convened in February 29 through March 2 of 2016 entitled “Environmental Nano Testing Media (ENTM) Harmonization”. This Perspective presents the resulting recommendations for relevant and consistently characterized medium types in which nanomaterial characterization and testing should be carried out, along with proposed media characterization parameters that should be consistently measured and reported. The intent is to collectively endorse a handful of high priority media and matrices. Where appropriate, we also propose the adoption of some defined values for those parameters in common systems to support maximum comparability in emerging datasets. Consistent characterization allows researchers to employ any medium of choice, whereas media standards allow studies to be directly compared and benchmarked. It should be clearly stated that these media, though proposed for NM testing, are defined here without any NMs in them.
1.1. On community-generated harmonization guidance
These recommendations represent a user-community consensus on approaches for harmonization on behalf of a collaborative sub-set of the nanoEHS field. This is distinct from products of official standards organizations and processes (e.g., ASTM, ISO, or OECD). This framework and the recommended media, if adopted into future experimental plans, will support downstream data integration and provide helpful community-based input to discussions in the US and EU regarding more formal standardization processes for generating and storing integrated data. In the broader arena of nanomaterial testing and data integration, there have been early successes with the model of community-initiated or -facilitated harmonization that supports cross-disciplinary translation and education on appropriate method applications and standards adoption. Two examples include the development of the ISA-TAB-nano data sharing file format extension by the open National Cancer Institute Nanotechnology Working Group (NCI NanoWG)8 and the community-developed guidance on the proper execution and interpretation of zeta potential measurements.9 Such harmonization is an acknowledgment of a philosophical and cultural shift required to address interlinked problems with the size and complexity of forecasting the behaviors and impacts of NMs. Community convergence on minimum reporting requirements for relevant media in which to test nanomaterials can foster shared, integrated research strategies. Adoption of such requirements will provide guidance and feedback to official standardization processes concerning current research consensus that can feed into standard test guidelines and guidance documents, and highlight research needs and emerging datasets to facilitate building of weight of evidence arguments through enhanced inter-comparability.1.2. Workshop methods
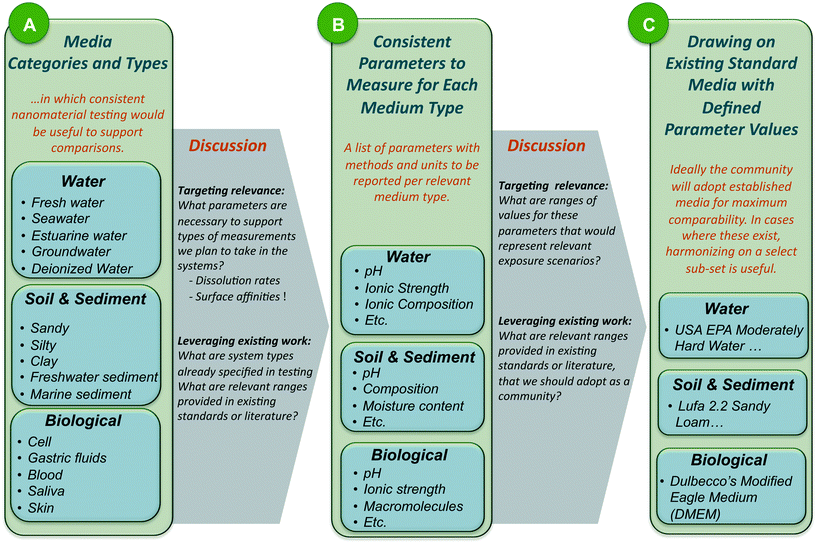
A working group of forty-one experts representing different disciplines, geographies and sectors was convened at Duke University. Attendees were asked to provide input prior to the workshop regarding classes of media that were important to address, any specific defined media they recommended for consideration, and critical parameters necessary to describe these media types. Breakout groups were organized to address five categories of communally-defined priority systems, and workshop activities were designed to move each breakout group through the process of: A) reaching consensus on media types within the category, B) arrive at consistent parameters that must be measured for each media type, and C) draw upon existing standard systems wherever appropriate to define values for the consistently defined parameters. Workshop process and outputs are depicted in Fig. 1 generally; more detail on the workshop method is available in the ESI† section of this Perspective. Fig. S1† provides additional detail on the workshop decision-making process.2. Harmonized media recommendations
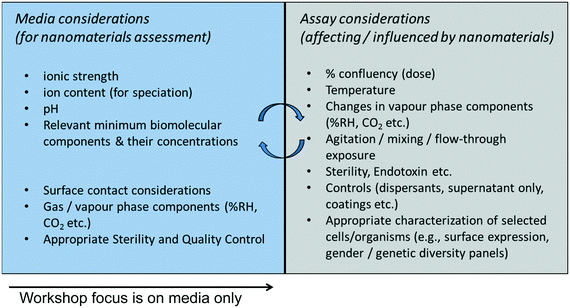
Recommendations are presented here for harmonizing across five broad categories of testing media, all identified as important for testing of nanomaterial behavior in terms of both exposure and hazard endpoints. The media considered are aqueous and solid phase in nature; atmospheric media are not considered in this Perspective.The broad categories presented here include:
• Aquatic testing media (section 2.1).
• Soil and sediment testing media (section 2.2).
• Biological testing media (section 2.3).
• Engineered systems testing media (section 2.4).
• Product matrix testing media (section 2.5).
Different medium categories present different challenges. Some (e.g., soils) have an enormous number of competing standards from which to select the most critical representative systems and parameters. Others do not have any competing standards (e.g., product testing media) yet are nevertheless essential for key measurements across material life-cycles. Therefore, the content and detail level of the harmonization recommendations vary accordingly among categories.
For each of the systems, we propose two tiers of key characteristics in terms of primary (absolutely required) and secondary (required in cases where these characteristics are available and/or pertinent to the specific measurement being taken). The hypothesis for encouraging measurement of primary, and when possible secondary, characteristics is that knowing these attributes will enable comparability among studies when different media are used.
2.1. Aquatic testing media
Aquatic systems can be broadly differentiated based on levels of salinity, ranging from marine to soft freshwater ecosystems. Low salinity freshwater includes lakes, rivers and streams, urban runoff, groundwater, rain water, ephemeral pools, and tap water; all of which have a wide range of possible water quality characteristics. Representative parameters are given in Table S1.† The characteristics of estuarine systems are also highly variable, as well as dynamic, depending on tidal cycles, freshwater discharge and Coriolis effects. The characteristics of marine systems close to land are similarly dynamic. Open ocean ecosystems are less variable, yet are influenced by the degree of biological productivity, which in turn is governed by nutrient inputs, temperature, and other factors. Therefore, there is a wide range of test systems that could be developed to represent the range of scenarios where nanomaterials may be present in an aquatic system.
Other factors may well be strong determinants of NM behavior outcomes depending on the scenario, including light, temperature, and well-defined natural organic matter (NOM). For a streamlined first step toward harmonization, we focus discussion on the chemical composition of aquatic media rather than the entirety of parameters required to define aquatic systems for experimental design that would additionally include conditions of energy flux (e.g., light, mixing, heat) and biotic composition.
Dissolution and agglomeration are perhaps the most important transformation processes affecting the fate and toxicity of NMs within aquatic systems, although many others exist.12 Understanding the extent of NM dissolution and agglomeration in aquatic test systems is therefore essential for estimating environmental risks. The extent of these transformations in a test system will depend on physicochemical conditions, such as concentrations of dissolved organic carbon (DOC), pH, ionic strength and redox conditions (i.e., oxidizing or reducing environment), in addition to NM specific properties and concentration.5,13,14
| Media type | Primary parameters | Secondary parameters |
|---|---|---|
| Freshwater | pH | Specific anionic ligands (e.g., sulfate, chloride) |
| Ionic strength | Key nutrients (e.g., nitrogen & phosphorus species) | |
| Major cations (e.g., hardness) | Redox potential | |
| Major anions (e.g., alkalinity) | Particulate matter: organic and inorganic | |
| Dissolved organic matter (e.g., DOC) | ||
| Estuarine | pH | Major anions (e.g., alkalinity) |
| Ionic strength | Key nutrients (e.g., nitrogen & phosphorus species) | |
| Dissolved organic matter (e.g., DOC) | Dissolved organic matter (e.g., DOC) | |
| Particulate matter: organic and inorganic | ||
| Marine | pH | Key nutrients (e.g., nitrogen & phosphorus species) |
| Ionic strength | Particulate matter: organic and inorganic | |
| Dissolved organic matter (e.g., DOC) | ||
| Deionized water | pH | |
| Resistivity |
Discussion of primary parameters. The minimum specifications considered to be adequate for evaluation of NM data in all aquatic systems were quantification of pH, ionic strength, and a measure of DOC (typically total organic carbon). Additional characterization of the composition of ionic components, such as hardness and alkalinity, was also considered to be essential for freshwater systems. Hardness and alkalinity are important as indicators of ionic composition beyond ionic strength; differences in ionic composition, such as the presence of divalent cations, are known to have significant impact on nanomaterial stability. The relative uniformity of seawater composition and their low DOC concentrations suggest that characterization of DOC may be a secondary consideration in marine systems. However, they should still be reported when possible for comparability with freshwater systems.
Discussion of secondary parameters. In addition to the parameters considered to be essential for minimum characterization of aqueous media, several additional parameters were identified as being highly desirable. In some cases, synthetic laboratory media include detailed analysis or complete specification of the medium content. In these cases, a reference for these media should be provided that will allow readers to obtain the full details of media composition. However, in many cases, such as where experiments are performed in field or mesocosm conditions, the complete composition of the medium will be unknown. In these cases, measurement of specific divalent ions (Ca2+ and Mg2+) are among the most important secondary measures for characterizing ionic content beyond total hardness and conductivity. Measurement of nitrogen and phosphorus species are highly desirable from the perspective of their potential impact on biotic components in a given experiment. These species, while likely to be present in low concentrations, are also potentially important in determining surface properties of NMs due to their propensity for inner sphere coordination with NM surfaces resulting in effects on NM surface charge. Alkalinity was also deemed to be an important parameter as a partial characterization of the anionic composition of aqueous solutions.
Most aquatic solutions include complex mixtures of dissolved organic matter and naturally occurring particulates that may merit additional characterization that, in the simplest instance, include measurements of total organic carbon (TOC) in unfiltered samples and in samples that have been membrane filtered (typically, 0.2 μm or 0.45 μm membranes). The selection of filter cut-off size may be influenced by what specific information is sought about the system; if it is desirable to capture agglomerates of nanoscale particles, 0.45 μm may be preferable. The materials retained by such membranes are commonly used to characterize the mass concentration of total suspended solids, which may give a very approximate estimate of the potential for NM heteroaggregation. Measures of organic carbon in the unfiltered (TOC) and filtered (DOC) samples additionally yield estimates of the percent organic carbon averaged over all suspended particulates, which may play a role in determining NM stability and affinity of these background particulates for heteroaggregation with engineered NMs.
Measurement of the UV absorbance at 254 nm in filtered samples provides additional information regarding the quality of the organic matter in terms of aromaticity, often normalized by the DOC concentration and reported as the specific UV absorbance.15 More detailed analysis of suspended particulates in terms of particle size distributions and the electrophoretic mobility of suspended particulates are also among the secondary parameters that may be important in specific experimental settings. Though measurements of naturally occurring background particulate size distributions and concentrations would be considered required input for modeling NM heteroagglomeration, persistence and transport, it is recognized that many tests seek to evaluate NM toxicity or dissolution in the absence of heteroagglomeration. Therefore, characterization of the naturally occurring background particulate phases present in these systems was considered to be a secondary consideration in these instances. Table 2 provides standard units and references for standard methods to measure each of the parameters listed in Table 1.
| Parameters | Units | Rationale | Ref. |
|---|---|---|---|
| pH | −logM | Assay independent indication of water pH, good for comparability | 16, 17 |
| Ionic strength | mmol L−1 | Calculate from either reagent addition or from conductivity measurements | 18 |
| Major cations | mmol L−1 | Importance in NM stability and properties of organic matter | 19 |
| Major anions | mmol L−1 | Importance in NM stability and properties of organic matter | 19 |
| Dissolved organic carbon (DOC) | mg L−1 | Importance in cation binding and NM stability | 20 |
| Specific anionic ligands (e.g., sulfate, chloride) | mmol L−1 | Importance in NM stability and properties of organic matter | 19, 21 |
| Redox potential | mV | Possible electron sources/sinks | 22 |
| Particulate matter: organic and inorganic | mg L−1 | Importance in heteroaggregation | 23, 24 |
| Parameters | VSW | MHW | Units |
|---|---|---|---|
| pH | 6.4 | 7.4–7.8 | — |
| Hardness | 10–13 | 80–100 | mg CaCO3 L−1 |
| Alkalinity | 10–13 | 60–70 | mg CaCO3 L−1 |
| Deionized water (DIW) | |||
| NaHCO3 | 12.0 | 96.0 | mg L−1 |
| MgSO4 | 7.5 | 60.0 | mg L−1 |
| KCl | 0.5 | 4.0 | mg L−1 |
| CaSO4·2H20 | 7.5 | 60.0 | mg L−1 |
In addition to the three environmental categories of aqueous systems, deionized water (DIW) is one of the media options that many NM research laboratories have already converged on. Although not representative of a natural water system, DIW is a medium that is frequently and easily implemented for comparison across laboratories. It is used for the synthesis of many NMs as well as the dilution of media.
Rationale for very soft water (VSW) recommendation. As discussed above, the USEPA reconstituted freshwaters may be formulated to have a range of hardness values. For functional assays5 and toxicity bioassays, lower Ca2+ concentration/hardness may be advantageous for maintaining more stable dispersions in the case of charge-stabilized NMs, although the impacts on animal health in this diluted media must be considered.26–28 The lowest hardness standard water (10 to 13 mg L−1 as CaCO3), which has relevance to alpine streams and e.g. Canadian Shield lakes, is very soft water (VSW). This test water has the lowest Ca2+ content (0.044 mmol L−1) of any available test standardized test water.29 Relative to higher Ca2+ concentration/hardness media, VSW and comparable low hardness media have been shown to enable higher stability of carbon NMs,30,31 nanoAg (ref. 27 and 32–34) and other metal NMs35 during fate and toxicity testing. Other coatings using steric stabilization methods show less sensitivity to ion concentration and composition.36,37
Rationale for moderately hard water (MHW) recommendation. The USEPA has standardized a series of reconstituted freshwaters for toxicity (hazard) testing. Regulatory testing programs in the USA, under the umbrella of the Clean Water Act, that require use of these test waters include industrial effluent testing38 and open water dredged material placement operations. In addition, regulatory program testing for superfund toxic substances and pesticides39 use USEPA test waters. These standard test waters range in hardness from 10 mg L−1 to 320 mg L−1 as CaCO3, (10−2 mmol L−1 to 3.2 mmol L−1 of Ca2+) to allow selection based on site-specific relevance.
Moderately hard water (MHW), with an acceptable hardness range of 80 mg L−1 to 100 mg L−1 as CaCO3 (or 0.8 mmol L−1 to 1 mmol L−1) is the most commonly applied test water for hazard assessment. MHW is a reconstituted water, designed generically to satisfy the basic requirements of taxonomically diverse freshwater organisms (algae, invertebrates, fish) that are used in standardized acute and chronic toxicity test methods.40,41 The recipe for MHW is freely available and consists of four simple salts (NaHCO3, CaSO4, MgSO4, KCl) dissolved in DI water. There is a very large database of standardized toxicity tests using this test system for both traditional substances and NMs;26,42 because of this coalescence around MHW use for toxicity tests, it makes sense to adopt similar media for exposure research as well.
Limitations are also recognized in the MHW recommendation. While MHW was the standardized test media that most closely represented the ideal parameters for NM hazard testing, it must be recognized that it is not ideal for NM dispersion and stability investigations. It is a USA-centric test water that may be used less frequently relative to OECD and ISO test media in laboratories outside of the USA and is not used in OECD testing standards (which are internationally recognized). Further, its Ca–Mg ratio is not representative of surface waters under certain conditions.29 However, this is balanced by the reproducibility and ease of synthesis of the water with appropriate hardness, pH, and ionic strength.
Rationale for artificial seawater (ASW) recommendation. The primary parameters of concern when attempting to model seawater are ionic composition, and to a lesser extent trace elements. As is the case for most of the media categories and types covered in this paper, multiple competing seawaters have been defined without consensus adoption to date. Many studies have been conducted with filtered natural seawater43 or a mixture of natural and artificial seawater,44 which can vary temporally and spatially, necessitating extensive characterization of each batch collected for site specific accuracy. However, compared to terrestrial waters, seawaters are relatively homogenous, and for the purposes of enabling cross-comparison of data we propose agreement on a harmonized recipe. There are numerous commercially available ASW alternatives, several of which have been used extensively in scientific research (e.g. Instant Ocean and Crystal Sea Marine Mix).45 The ionic and elemental compositions of these are quite similar to actual seawater,46 but quality control and batch variability are a significant concern. Alternatively, several artificial seawater standards are commonly used and can be made with reagent-grade chemicals. EPA synthetic seawater,41 ASTM D1141-98,47 and Marine Biological Laboratory (MBL)48 are a few examples.
Given the need for quality control and reproducibility, there are significant concerns about recommending commercially available ASW mixtures, or filtered natural seawater. Based primarily on batch-to-batch variability of commercially available artificial seawaters, we recommend using EPA artificial seawater (EPA ASW), made with reagent-grade chemicals, at 35‰ salinity in accordance with the EPA protocol. Ionic composition and the trace element complexity of EPA ASW are similar to ASTM D1141-98 Substitute Ocean Water. We are recommending EPA ASW as the recipe is freely available. The inclusion of trace elements along with the major ionic components allows EPA ASW to be used for both acute and chronic experiments,40 whereas standard MBL does not contain trace elements (although several variants exist for MBL with increased complexity).49
For estuarine subsystems, we recommend using 3.5‰ salinity EPA ASW, although species' physiological requirements may prevent the usage of this salinity. Alternative dilutions of EPA ASW may be used where necessary to accommodate differing species' requirements. In all cases, full documentation is needed. Further recommendations for experiment-specific amendments to these media, including natural organic matter and inorganic colloids, may be found in the ESI.†
2.2. Soils, sediments and other important solid phases
| Media type | Primary measurements | Secondary measurements |
|---|---|---|
| Agriculture and forestry soils | Soil pH | Dissolved organic carbon (DOC) |
| Total organic carbon (TOC) | Porewater pH | |
| Texture | ||
| Acid extractable Si, Ca, Mg, Fe, Al, Mn | ||
| Redox potential | ||
| Specific conductance | ||
| Biosolids | All agriculture soil parameters except texture | All of the above |
| Acid extractable C, S, P, K, N and potentially toxic metals | ||
| Acid volatile sulfide (AVS) | ||
| Subaquatic sediments | All agriculture soil parameters | All of the above |
| AVS | ||
| Unconsolidated aquifers | All agriculture soil parameters | All of the above |
| Effective porosity | ||
| Grain size | ||
| Dispersivity | ||
| Consolidated aquifers | All agriculture soil parameters | All of the above |
| Equivalent aperture of fractures | ||
| Coefficient of variation of fractures |
| Parameters | Units | Rationale | Ref. |
|---|---|---|---|
| pH in 1 M KCl or 0.01 M CaCl2 extract | Assay independent indication of pore water pH good for comparability | 55 | |
| Total organic carbon (TOC) | mg kg−1 | Immobile OM to which NM can attach, contributes to structure, indicator of biological activity | 56, 57 |
| Texture (clay, silt, sand) | % | Indicator of % mineralogical clays and or oxides and thus of quantity of favorable deposition sites for NMs, contributes to structure, indicator of straining potential and permeability, nutrient retention capacity, water holding capacity, dispersible clays | 58 |
| Redox potential | V to SHE | Master variable for speciation, indicator of microbial activity, controls extent of dissolution of redox-sensitive NPs (Ag, Fe, Al, …), especially in sludges | 59 |
| Water extractable Ca, Al, P, Mn, Fe, Si, SO4, Mg, K, Na, NO3−, Cl− | mg L−1 | Calculate ionic strength/divalent versus monovalent to determine colloidal stability, specific adsorption to NM and soil surfaces (PO4, Ca), nutrient availability, metal speciation | 60 |
| Dissolved organic carbon (DOC) | mg C L−1 | Sterically stabilizes NMs, usually increases pore water residence times | 20 |
| Porewater pH | Master variable for dissolution, surface charge, speciation, … | 61, 62 | |
| Specific conductance | μS cm−1 | Affects NM (homo- and hetero-) aggregation and deposition | 63 |
| Total C, S, P, K, N | mg kg−1 | Nutrient status of sludge, potential for sulfidisation and phosphatization of NMs | 64 |
| Total toxic metals | mg kg−1 | Accounting for toxic effects of metals | 64 |
| Acid volatile sulfide | mg kg−1 | Sulfidation potential of NMs | 65 |
| Effective porosity | Important transport parameter in aquifers | 66 | |
| Dispersivity | m | Important transport parameter in aquifers | 67 |
| Grain size | μm | Important transport parameter in aquifers | 58 |
| Equivalent aperture of fractures | μm | Accounting for preferential flow in consolidated aquifers | 68 |
| Coefficient of variation of fractures | μm | Important transport parameter in consolidated aquifers | 68 |
| Tortuosity | Important transport parameter in consolidated aquifers | 69 |
Soils. A large variety of soils have been used as reference materials in environmental studies. Some are too simplistic for use in studies of NM transport, fate and effects, including the OECD standard soil70 as outlined below. Several well-characterized reference materials soils are being or have been sold by the National Institute of Science and Technology (NIST) (e.g., San Joaquin Soil, NIST 2709a) in quantities of up to 50 g per unit. However, these soils are intended for use as standard reference materials for chemical analysis and as such they are characterized with respect to composition of certain chemical analytes, not parameters like texture, cation exchange capacity, and organic matter content. Most soils are not readily available in sufficient quantities to support widespread adoption as reference soils for some functional assays of NM fate and effects (up to 1 kg), although the required quantity of soil would depend upon the assay. For the purpose of harmonizing studies of NM transport, a commercially available natural sandy soil may be a good choice. One example of a commercially available natural sandy soil that is widely available is Lufa 2.1 (Speyer).71 However, restrictions on importing soils may make this a challenge for some researchers. The choice of a sandy soil was driven by the need for a soil allowing measurable NM transport parameters in column studies. For NM transformation, bioaccumulation, and toxicity studies, a natural sandy loam soil may be a better choice, because it represents a textural class common among agricultural soils. One example of a commercially available soil that provides consistent soil characteristics, has been used extensively in studies of NMs and other soil contaminants,72,73 and can support the organisms used in bioaccumulation and toxicity studies (e.g., plants, earthworms) is Lufa 2.2 (Speyer). The properties of these soils are provided in the ESI.† Note that not all the recommended parameters mentioned are supplied by the provider, so additional characterization will be required for NM exposure, fate and behavior studies.
Benchmark sediments. In contrast to soil, few examples of reference subaquatic sediments are available. Natural freshwater sediment from West Bearskin Lake, MN, USA has been used as a control sediment by the USEPA for the development of benthic invertebrate toxicity assays74 and subsequently used by other researchers.75 However, the physico-chemical properties for West Bearskin Lake sediment reported by different authors vary considerably so its immediate use as a benchmark sediment may not be advisable.76,77 NIST sells estuarine sediment78 and river sediment79 at up to 70 g quantities per unit; however, like the NIST soil SRMs, these SRMs are intended for use in chemical analysis. Identification of a suitable reference material for sediments should be based on finding a material in sufficiently large supply that can be considered relevant in terms of the properties outlined in Table 4. This table highlights that redox-sensitive properties of porous materials are relevant for the fate of NM, particularly for dissolution and transformation, imposing additional requirements on the anoxic storage and testing of any reference material.
Sludge. Treated sludges, also called biosolids, are an important sink for a large proportion of NMs entering wastewater streams. Sludges are often applied to agricultural lands as a soil amendment. Reference sludges only exist as analytical chemistry standard reference materials (SRMs), such as SRM 2781 (NIST, Gaithersburg, Maryland, USA). This material is neither intended for, nor suitable for, use as a medium in fate, transport or toxicity studies due to its complexity and variability. A number of recent studies have operated pilot scale wastewater treatment systems, utilizing local primary sludge as a feed.80,81 This approach will not be feasible for routine studies and local primary sludge and wastewater treatment processes vary considerably from location to location. Municipal biosolids are commercially available and could possibly be used as reference media. For example, Milorganite®, which has been produced for over 90 years by heat treatment of sewage sludge from the municipal sewerage district of Milwaukee, Wisconsin, USA,82 has been used in a variety of studies concerning behavior of contaminants, such as metals, after land application of sludges.83–86
2.3. Biological media
| Media type | Primary measurements | Secondary measurements | |
|---|---|---|---|
| Liquid-surface exposure scenarios | Mammalian culture medium | pH | Relative humidity |
| Ionic strength | % CO2 | ||
| Ionic content | |||
| % serum | |||
| Source of serum (bovine calf, mouse, rabbit, human etc.) | |||
| Whether serum was heat inactivated or not | |||
| Aquatic organism culture media | pH | Ionic content (e.g. monovalent, divalent, Cl−, sulfate, etc.) | |
| Ionic strength | Food type and amount | ||
| Major cations (e.g. hardness) | Natural organic matter (NOM) or other biomolecules added – concentration, source | ||
| Major anions (e.g. alkalinity) | |||
| Dissolved oxygen | |||
| Microbial medium | pH | Major cations (e.g. hardness) | |
| Ionic strength | Major anions (e.g. alkalinity) | ||
| Rhizosomal system (roots) – see soil pore extracts in section 2.2 | Soil pH | Dissolved organic carbon (DOC) | |
| Total organic carbon (TOC) | Porewater pH | ||
| Texture | |||
| Extractable Si, Ca, Mg, Fe, Al, Mn | |||
| Redox potential | |||
| Specific conductance | |||
| Simulated digestive fluids | pH | Ion content (towards speciation) | |
| Humans | Ionic strength | ||
| Organisms | Enzymes or other additives | ||
| Insects | |||
| Artificial lysosomal fluid | pH | Ion content (towards speciation) | |
| Ionic strength | |||
| Enzymes or other additives | |||
| Plant transport mimics | pH | Ion content (towards speciation) | |
| Ionic strength | |||
| Air-surface exposure scenarios | Simulated surface contact media | pH | Viscosity |
| Dermal | Ionic strength | ||
| Lung | Biological molecules (proteins) | ||
| Ocular | |||
| Leaves e.g. epicuticular wax | pH | Hydrophobicity | |
| Alkane chain length | Viscosity | ||
| Fluidity | |||
| Parameters | Units | Rationale | Media types | Ref. |
|---|---|---|---|---|
| pH | Many biomolecules are pH sensitive; different biological compartments have specific pHs for optimal functionality | All buffers and liquid media for toxicity & NM fate assessment | 17 | |
| Ionic strength | mmol L−1 | Calculate from either reagent addition or from conductivity measurements | All liquid media | 18 |
| CO2 concentration | % | Tissue/cell culture | 95 | |
| Dissolved oxygen (DO) concentration | % | Important for both biological growth and NM behavior | All liquid media | 20 |
| Relevant minimum biomolecular components & relative concentration | % | Relative concentration (e.g. concentration to surface area) | Tissue/cell culture | |
| Major cations | mmol L−1 | Hardness – needs to be suitable for the organism being tested, affects NM stability and size distribution | Aquatic & soil organism test media | 19 |
| Major anions | mmol L−1 | Alkalinity – needs to be suitable for the organism being tested, affects NM stability and size distribution | Aquatic & soil organism test media | 19 |
| Dissolved organic carbon (DOC) | mg L−1 | Important for both biological growth and NM behavior | All liquid media | 20 |
| Growth factors | mg L−1 | Modulators and antibiotics/antimiotics | Cell culture |
Discussion of primary parameters.
pH. Most normal mammalian cell lines grow well at pH 7.4, with very little variability among different cell strains. However, some transformed cell lines have been shown to grow better in slightly more acidic environments (pH 7.0 to 7.4), and some normal fibroblast cell lines prefer a slightly more basic environments (pH 7.4 to 7.7). Insect cell lines such as Sf9 and Sf21 grow optimally at pH 6.2.87 Bacterial testing is also routinely performed at pH 7.4, with OCED 301 stating an optimal pH of 7.4 ± 0.2. Similarly, most organisms have an optimal pH range, and media derived for these usually sit in physiological ranges. For example, Daphnia have been found to have optimal survival, growth and reproduction at pHs in the range 7.9 to 8.3.88 Thus, pH is an essential parameter for all liquid media types, as physiological changes related to environmental stress will arise when organisms are outside of their optimal pH range, which will compromise any subsequent exposure or hazard assessments. In the case of simulated fluids, such as digestive fluids, or artificial lysosomal fluids (ALF), these are specific fluids where an unusual pH is required to achieve a specific function, and thus assessment of the effectiveness of this function can only be done at the relevant pH.
Ionic strength and ionic content. The physiological ionic strength is between 100 mmol L−1 to 200 mmol L−1 KCl or NaCl. The growth medium controls the pH of the culture and buffers the cells in culture against changes in the pH. Usually, this buffering is achieved by including an organic (e.g., HEPES) or CO2− bicarbonate based buffer. Note that ionic strength and pH may both impact nanomaterial properties, and are therefore essential parameters to report. Biomolecule protonation and deprotonation further depend on the ionic composition of the surrounding medium. At pH 7.5, phosphate buffers add approximately 7× more ions to the medium than zwitterionic Tricine (nitrogen containing) buffers.89
Serum percentage, source of serum, and whether serum was heat inactivated. This consideration is quite specific to mammalian cell culture, where serum is routinely utilized as a food source for cells. Depending on the cell type, the amount of serum ranges between 2% (e.g. for blood–brain barrier cells) to 20% often recommended to speed up the growth of Caco-2 cells, with 10% being a common standard. However, with NMs, the ratio of the NM surface to the amount of proteins present can have an important role in terms of proteins bound in the corona: for some NMs more proteins available results in thicker coronas of the same composition, while for others quite different coronas occur at different surface area: FBS ratios.90,91
The source of the serum (fetal bovine, fetal calf, or other animal sera such as mouse, rat, rabbit or horse, as well as human) is an important consideration, as the different sera have been shown to result in quite different corona compositions and differential cellular uptake of the particles and thus differential toxicity induction in cells.91,92 For example, lower rates of uptake of the same NMs by the same cells were observed with the NMs/cells incubated in human serum compared to bovine serum (for 50 nm amine-modified polystyrene NMs in A549 cells).91 Heat inactivation of the FBS has also been shown to affect the NM corona composition,93 and thus should be reported.
Discussion of secondary parameters.
CO2 levels. Because the pH of the medium is dependent on the delicate balance of dissolved carbon dioxide (CO2) and bicarbonate (HCO3−), changes in the atmospheric CO2 can alter the pH of the medium. Therefore, it is necessary to use exogenous CO2 when using media buffered with a CO2-bicarbonate based buffer, especially if the cells are cultured in open dishes or transformed cell lines are cultured at high concentrations. Most researchers use 5% to 7% CO2 in air, however, each medium has a recommended CO2 tension and bicarbonate concentration to achieve the correct pH.
Relative humidity. Using an incubator humidity of 85% to 95% limits evaporation of water from cell culture media. Evaporation can substantially raise the media concentrations of salts, minerals, etc., potentially resulting in toxicity and cell death. High humidity is the most difficult condition to maintain but is critically important, as evaporation is 4 times faster at 80% humidity than at >93%.94
Tissue medium (submerged culture).
Mammalian culture medium. FBS is a ubiquitously used essential supplement in cell culture media, for both human and mammalian cells. However, there are serious scientific and ethical concerns about the use of FBS regarding its harvest and production.96 Efforts are underway to develop serum-free cell cultures in other fields, mostly as means to harmonize or reduce the inherent variability from animal-derived compounds. There are various degrees of chemical definition, e.g. serum-free (SF), animal-derived component free or chemically defined, and the type of medium, e.g. basal media, medium supplements, or full replacement media.96 However, these are far from being standardized, and are thus not discussed further here.
The large reactive surface area of NMs makes the addition of biomolecules to the medium essential in order to avoid physical damage of membranes. Thus, SF conditions may not be feasible for use with NMs, unless other synthetic macromolecules can be substituted instead to bind to the NMs and passivate their surface. Further potential complications with biomolecule addition include the potential for non-self immune recognition.97
In light of these and other considerations, our recommendation for cell culture medium is a commonly applied medium, Dulbecco's modified Eagle's medium (DMEM), which is a modification of basal medium Eagle (BME) that contains a four-fold higher concentration of amino acids and vitamins, as well as additional supplementary components. DMEM requires supplementation with 1% to 5% FBS and 4 mmol L−1L-glutamine supplement. The FBS concentration must be optimized for each cell line to obtain maximum serum reduction. DMEM uses a sodium bicarbonate buffer system (3.7 g L−1), and therefore requires a 5% to 10% CO2 environment to maintain physiological pH. See Table 8 for full details.
| Parameters | Value (range) | Units |
|---|---|---|
| Chemical | ||
| Sodium bicarbonate buffer | 3.7 | mg L−1 |
| Phenol red | 15.0 | mg L−1 |
| D-Glucose (dextrose) 180.0 | 4500.0 | mg L−1 |
| Inorganic salts | mg L−1 | |
| CaCl2 (ANHYD.) | 200.0 | |
| Fe(NO3)3·9H2O | 0.1 | |
| MgSO4 (ANHYD.) | 97.67 | |
| KCl | 400.0 | |
| NaHCO3 | 3700.0 | |
| NaCl | 6400.0 | |
| NaH2PO4–H2O | 125.0 | |
| Physical | ||
| Requires CO2 to maintain physiological pH | 5–10 | % |
| Biological | ||
| L-Glutamine | 584.0 | mg L−1 |
| Amino acids (14 in addition to the L-glutamine) | 30–584 | mg L−1 |
| Vitamins (8 different) | 0.4–7.2 | mg L−1 |
| Requires supplementation with e.g. 10% fetal bovine serum (FBS) | 5–10 | % |
Simulated human saliva. Recent studies that have simulated saliva fluid lack cohesiveness of background media constituents. It is challenging to duplicate human saliva because (a) it is excreted from several different glands at different volumes, (b) its contents can vary at different times during the day, and (c) it can be influenced by the diet.98 Based on a critical review of reported synthetic saliva used for in vitro studies between 1931 and 1996 (n = 60),99 a standard saliva gastric fluid medium has been proposed. We endorse this as a model simulated saliva (Table 9). Depending upon the assay purpose, it may relevant to also test the saliva with slight modifications such as additional biomolecules (i.e. mucin, amylase) or an altered pH.
| Parameters | Value (range) | Units |
|---|---|---|
| Chemical | ||
| pH | 6.4–6.8 | — |
| Deionized water (DIW) | ||
| Urea (CH4N2O) | 0.200 | g L−1 |
| Sodium chloride (NaCl) | 0.126 | g L−1 |
| Ammonium chloride (NH4Cl) | 0.178 | g L−1 |
| Potassium chloride (KCl) | 0.964 | g L−1 |
| Potassium thiocyanate (KSCN) | 0.189 | g L−1 |
| Monopotassium phosphate (KH2PO4) | 0.654 | g L−1 |
| Sodium sulfate (Na2SO4, 10 H2O) | 0.763 | g L−1 |
| Calcium chloride (CaCl2, 2 H2O) | 0.228 | g L−1 |
| Sodium bicarbonate (NaHCO3) | 0.631 | g L−1 |
| Physical | ||
| Biological | ||
| Appropriate sterility | — | — |
| Enzymes/proteins | ||
Simulated human gastric fluids. Here we define a specific model simulated gastric fluid (Table 10), closely resembling the fluids found in the stomach of mammals.100 In the development of a model gastric fluid, careful consideration must be given to the behavior of NMs in such a medium. For that reason, we decided that the frequently used 0.07 mol L−1 HCl solution is insufficient to accurately capture NM behaviors such as aggregation state, deposition kinetics, and transformation among others, all of which will strongly impact nearly any nanoparticle assay run in the medium.75 Therefore, the current proposed standard medium includes a small number of gastric proteins in addition to the proper ionic strength expected in a gastric fluid. This has been adapted from model gastric fluids reported previously with some adaptation in order to include a more complete picture of relevant biomolecules in a fasted state. Adjustments for fed states include higher pH and ionic strength.101
| Parameters | Value (range) | Units |
|---|---|---|
| Chemical | ||
| pH | 1.6 | — |
| NaCl | mmol L−1 | |
| KCl | 50 | |
| Deionized water (DIW) | ||
| Physical | ||
| Biological | ||
| Pepsin | 0.2 | g L−1 |
| Mucin | 1.5 | g L−1 |
| Lecithin | 0.02 | g L−1 |
Simulated digestive fluids for fish and other organisms. Simulated gut or digestive fluids are significantly less widely used in environmental organisms. Only a small number of recipes for simulated fish (carp) digestive fluid have been reported.102–104 However, closer inspection of these recipes revealed that they were intended to simulate human digestive fluids. Non-human digestive fluids would thus seem like an area where more research is needed, and as such as we are not making any recommendations regarding simulated digestive fluids for non-human organisms at this time.
Simulated surface contact media. Simulated biological fluids have been used traditionally in the pharmaceutical and biomedical industries for testing and defining the dosage of drugs.101 We recommend the use of these established media to investigate the physico-chemical behavior of NMs at the point of contact with biological systems and for quantifying the potential absorption of NMs by those systems.
Pulmonary fluids. Here we endorse an existing simulated pulmonary surfactant fluid105 for use as a model medium in NM inhalation exposure scenarios (Table 11). As with the other proposed simulated biological fluids, the composition was chosen for its ability to capture NM behavior in complex biological systems, which will depend on not just the quantity of surfactant in the fluid but on its composition and relative concentrations. This has been observed in several studies, in which protein or natural amphiphiles exhibited differential binding for NM surfaces.106,107 While the entire spectrum of possible pulmonary proteins and surfactants is not included here for the sake of simplicity in preparation and analysis, we believe it to be a representative minimum set of relevant biomolecules.
| Parameters | Value (range) | Units |
|---|---|---|
| Chemical | ||
| pH | 7.4 | — |
| Ionic strength | 122 | mmol L−1 |
| Ion content | mmol L−1 | |
| NaCl | 103 | |
| KCl | 4 | |
| CaCl2 | 2.3 | |
| Na2HPO4 | 1 | |
| NaHCO3 | 32 | |
| MgCl2 | 2.1 | |
| Physical | ||
| Biological | ||
| Relevant minimum biomolecular components | mg L−1 | |
| Albumin | 260 | |
| Ascorbic acid | 18 | |
| Cysteine | 122 | |
| DPPC | 100 | |
| Glutathione | 30 | |
| Glycine | 376 | |
| Mucin | 500 | |
| Uric acid | 8 | |
Artificial lysosomal fluid (ALF). When considering the interactions of NMs with mammalian and other living cells, lysosomes are frequent endpoints of interest. This environment has a particular impact on the dissolution and speciation of NMs within cells.108,109 Our recommended ALF composition is given in Table 12.
| Parameters | Value (range) | Units |
|---|---|---|
| Chemical | ||
| pH | 4.5 | — |
| Ionic strength | Media specific | Total mmol L−1 |
| Ion content (towards speciation) | Media specific | Composition of each component in mmol L−1 |
| Deionized water (DIW) | ||
| Sodium chloride (NaCl) | 3.21 | g L−1 |
| Sodium hydroxide | 6 | g L−1 |
| Citric acid | 20.8 | g L−1 |
| Calcium chloride (CaCl·2H2O) | 0.128 | g L−1 |
| NaHPO4 dibasic | 0.071 | g L−1 |
| MgCl2 hexahydrate | 0.106 | g L−1 |
| Sodium sulfate (Na2SO4) | 0.039 | g L−1 |
| Glycerol or glycerine (C3H8O3) | 0.059 | g L−1 |
| Glycine (NH2CH2COOH) | 30.3 | g L−1 |
| C6H5Na3O7·2H2O (Na3 citrate·2H2O) | 0.077 | g L−1 |
| C4H4O6Na2·2H2O (Na2 tartrate·2H2O) | 0.09 | g L−1 |
| C3H5NaO3 (Na lactate, 60% in water) | 0.065 | mL |
| C3H5O3Na (Na pyruvate) | 0.086 | g L−1 |
| Formaldehyde | 1 | mL |
| Physical | ||
| N/A | ||
| Biological | ||
| N/A | ||
Simulated leaf surfaces (e.g. for nanopesticide formulations110). This is an emerging area for NMs with no studies found to date, however simulated leaf surfaces have been prepared for chemical testing. For example, hydrocarbon wax and beeswax were compared by forming surfaces on stainless steel plates, with a target wax coverage of 1 mg cm−2.110 Some additional work is needed in order to assess the suitability of this approach for assessing NM interactions, and thus no recommendation is made at this point.
2.4. Engineered waste systems
Incineration is a very important treatment process, which affects the form and availability of NMs. Werther and Ogada112 defined three categories of thermal sludge treatment: i) mono-incineration ii) co-combustion and iii) alternative thermal processes (pyrolysis, gasification). Each thermal process will produce ash with different chemical and physical properties. Thermal processes are prone to modify the physical state of NMs as a function of the temperature and the thermal stability of the NMs. Carbon NMs are of particular concern as the temperature reached during incineration may lead to only partial combustion and transformation of carbon-based NMs.113–116 Due to the relatively small variations in sewage sludge ash, these ashes may be appropriate for standardization purpose, but no standardized sewage sludge ash yet exists. However, using the sewage sludge ash as a medium to conduct experiments addressing the fate of NM (e.g., released in column experiments) is not particularly meaningful, as directly adding the NMs to sewage sludge ash omits the high temperature process leading to a fundamentally different incorporation of NMs into the ash matrix. We therefore did not consider sewage sludge ash as a useful medium in the context of this Perspective. Nevertheless, the use of ashes should include characterization of both the source material (Tables 7 and 8) and the process by which it was produced. Relevant media associated with wastewater treatment and landfilling are defined in Table 13. Definitions, rationale, and references for the measurement of each parameter are shown in Table 14.
| Media type | Primary measurements | Secondary measurements |
|---|---|---|
| Activated sludge | pH | Total organic carbon (TOC) |
| Total suspended solids (TSS) | O2 | |
| Dissolved organic carbon (DOC) | Major cations (e.g. K+, Na+, Ca2+, Mg2+) | |
| Electrical conductivity | Major anions (e.g., Cl−, SO42−) | |
| Sludge volume index (SVI) | Nitrate (NO3−) | |
| Ammonium (NH4+) | ||
| Treated wastewater | pH | TOC |
| TSS | Major cations (e.g. K+, Na+, Ca2+, Mg2+) | |
| DOC | Major anions (e.g., Cl−, SO42−) | |
| Electrical conductivity | Nitrate (NO3−) | |
| Landfill leachate | pH | O2 |
| TSS | TOC | |
| DOC | Major cations (Ca2+, Mg2+) | |
| Electrical conductivity | Major anions (SO42−, Cl−, NO3−) | |
| Redox potential |
| Parameters | Units | Rationale | Media types | Ref. |
|---|---|---|---|---|
| a Activated sludge. b Treated wastewater. c Landfill leachate. Values in bracket refer to secondary measurements for the different media types. | ||||
| pH | Indication of the state of the associated wastewater treatment plant.a,b It is important to assess conditions in the landfill and the stability of inorganic compounds (e.g. dissolution or precipitation of mineral phases)c | 1, 2, 3 | 55, 62 | |
| Total suspended solids (TSS) | mg L−1 | Indication of the state of the associated wastewater treatment plant.a,b Useful for comparability between different field sites/synthetic mixturesc | 1, 2, 3 | 24 |
| Dissolved organic carbon (DOC) | mg L−1 | Influence on colloidal stabilities of NMsa–c | 1, 2, 3 | 20, 57 |
| Electrical conductivity | μS cm−1 | Allows estimation of total dissolved solids and ionic strength (key parameter for stability calculations)a–c | 1, 2, 3 | 18 |
| Redox potential | mV | Important for speciation calculations of mineral phases (and selected MNs)c | 3 | 22, 59 |
| Sludge volume index | mg g−1 | Informs about the ‘quality’ of the sewage sludgea | 1 | 119 |
| Major cations | mg L−1 | Required for the accurate calculation of the ionic strength (key parameter for stability calculations)a–c | (1, 2, 3) | 19 |
| Major anions | mg L−1 | Required for the accurate calculation of the ionic strength (key parameter for stability calculations)a–c | (1, 2, 3) | 19 |
| Total organic carbon (TOC) | mg L−1 | Informs about the state/performance of the wastewater treatment process,a,b gives an indication of the biological activity in the landfill. | (1, 2, 3) | 20, 56, 57 |
| O2 | mg L−1 | Provides information about the wastewater treatment process (nitrification, denitrification) and characterizes the respective sludge (oxic/anoxic),a informs about the conditions and processes in the landfill (influences mineral/NM stability)c | (1, 3) | 120 |
| Nitrate | mg L−1 | Key parameter used to evaluate the performance of the wastewater treatment processa,b | (1, 2) | 121 |
| Ammonium | mg L−1 | Important to assess the performance of the wastewater treatment process (nitrification)a | (1) | 122 |
Discussion of primary parameters. For all three media, pH, TSS, DOC and the electrical conductivity were considered key parameters which should be reported. In addition, the redox potential should be reported in landfill leachates. From the measurement of the electrical conductivity, the ionic strength and the total dissolved solids can be estimated based on well-established correlations between these parameters.117 The redox potential is a crucial parameter for predicting the speciation of metals in aqueous environments (see soils and sediments section discussion). Therefore, the redox potential will be particularly important to assess the fate Cu, Zn, and Ag NMs. It should be noted that speciation calculations are only applicable under thermodynamic equilibrium conditions, which may not be reached in the media described in this section or elsewhere in this Perspective.
Discussion of secondary parameters. Apart from the primary parameters listed above, we have identified a set of secondary parameters which should be reported if possible. Nitrate and ammonia concentrations are of key importance for activated sludge media and provide information about the performance of a wastewater treatment plant. Furthermore, the oxygen concentration informs about processes (nitrification/denitrification) that are occurring within the sludge. Although it is possible to calculate the ionic strength based on empirical correlations with the electrical conductivity, measurements of major cations and anions will provide more reliable data on the ionic strength of the respective media. TOC content provides information about the condition of the sewage sludge, and when measured in the treated water can be used as a measure of the performance of the wastewater treatment plant. Oxygen concentrations measured in landfill leachates are useful to assess the conditions in the landfill (oxic vs. anoxic), which will have a direct impact on NM transformations including dissolution, oxidation, and redox activity.118 For example, anoxic conditions will limit dissolution of some NMs (e.g., AgNPs), while the redox activity will also impact the extent to which the speciation of other NMs such as cerium oxide NMs occurs.12
Sludge. Depending on the process design of a wastewater treatment plant and on the specific requirements, different types of sludge are used/produced. Examples include primary sludge, activated sludge, granular sludge and digested sludge. A typical sludge, as summarized from various sources123 is the primary solid-containing residual produced from the separation of water and solids in the primary, secondary and tertiary wastewater treatment processes. Activated sludge mainly consists of bacteria and protozoa that form biological flocs. Activated sludge must be biologically active to allow degradation of wastes and could be affected by NMs. The partitioning of NMs to sludge indicates the amounts of NMs that may pass through sewage treatment processes and enter receiving soils, sediments, and surface waters. Sewage sludge is expected to represent a major sink for many NMs124,125 and waste water treatment plants will therefore be central to decipher the fate of NMs after their use.
A standardized activated sludge is not available. For analytical purposes, a powered sludge is available as standard reference material (e.g. SRM 2781, NIST, Gaithersburg, Maryland, USA), but powdered sludge cannot be used as an analog for activated sludge for the purpose outlined in this Perspective. Several authors have used sewage sludge collected from field- or pilot-scale wastewater treatment plants to study the behavior of NMs in sludge medium.80,81,126 We argue that the general properties of sewage sludge resulting from the activated sludge process are broadly comparable. Therefore, we recommend use of activated sludge collected from local sewage treatment plants as sludge media. By reporting the key parameters described above (Table 8), differences in the general properties of the sludge are revealed and can be compared.
Treated wastewater. Engineered NMs are released into municipal wastewater streams towards the end of the lifetime of the materials, where the NM will partition into the solid phase (sludge, or biosolids) or aqueous phase (effluent). A majority (>95%) of NMs tend to be attached to the heterogeneous, dense bacterial communities found in biological wastewater treatment processes.127–129 Nevertheless, despite the efficient removal of NMs during the wastewater treatment, a small fraction still escapes the treatment and is discharged into surface waters. Therefore, we consider treated wastewater as an important medium to assess potential exposure routes for NMs.
A standardized treated wastewater does not exist; however, wastewater effluents need to fulfill certain quality criteria before being discharged into surface waters. Although these criteria can vary from country to country, they set a general baseline for the quality of treated wastewater. In addition to variations caused by different influent waters, the contents of dissolved components are further influenced by the local geological environment. Thus, the natural variability caused by the geological settings may lead to considerable differences of dissolved components in treated wastewater. We suggest the use of ‘Moderately Hard Reconstituted Water (MHRW)’ described in the aqueous media section (section 2.1.3), with a few modifications as an analog for treated wastewater. The most important modification recommendations are strongly elevated concentrations of Na+ and Cl−, and possibly HCO3−, affecting the ionic strength of the treated wastewater and thereby the colloidal stability of NMs. Furthermore, increased concentrations of DOC in treated wastewater are conceivable, which may stabilize NMs against agglomeration. Thus, we suggest modifying the MHRW by increasing the Na+ and Cl− concentrations to 1000 mg L−1 each. The DOC can be adjusted to 10 mg L−1, representing effluent values of proper operating WWTPs, by adding humic acid. In agreement with recommendations for freshwater and estuarian systems, we recommend the use of Suwannee River humic acid.
Landfill effluent. Four different types of landfills (sanitary, municipal solid waste (MSW), construction and demolition, and industrial waste landfills) are generally distinguished, each of which receive different kinds of wastes. Increasingly important categories of landfill materials include MSW, which are either directly landfilled or are incinerated and landfilled mainly as bottom ash. In developed counties, incineration of municipal waste is most popular, but the disposal of MSW in landfill remains the most important waste management strategy worldwide.130 The properties of the landfill effluents strongly vary with: i) the type of landfill (and thus the kinds of materials that are deposited); ii) the operation principles; and iii) the age of the landfill.131 The most important parameters determining the composition of the landfill leachates (MSW) is the age of the landfill which is related to the respective landfill fermentation stage.132 The following four phases are typically described: aerobic, acid, initial methanogenic, stable methanogenic. Over extended periods of time (hundreds to thousands of years), other phases have been postulated, however, the composition of the respective leachates are still very speculative as no experimental data are available for such systems.131
We therefore identified the leachate originating from a landfill under the stable methanogenic phase, which lasts longest (of the three phases for which experimental data are available) and extends over several decades, as the most relevant and suitable for harmonization purposes. As no reference or standardized landfill leachate compositions are available, we recommend average values reported in the literature131 to define an average landfill leachate which can be used for NM testing purposes. Cl− and Na+ (both up to 1 to 2 g L−1) are much higher than in treated wastewater, but considerably lower compared to ASW (see water media) and also K+ concentration can reach 1 g L−1. Furthermore, Ca and sulfate concentrations can be as high as a few hundred mg L−1. The considerably high ionic strength may strongly affect the agglomeration behavior of NMs in landfill effluents. Furthermore, DOC (extrapolated from BOD and COD values reported by Kjeldsen131) can range from a few tens to a few thousands mg L−1. This large variation makes a selection of one specific value rather arbitrary. However, for a worst-case scenario, we recommend using elevated DOC concentrations added in the form of Suwannee River or other appropriately characterized humic acid. Following this reasoning, we recommend modifying the MHRW medium by adjusting the Cl− and Na+ concentrations to 1000 mg L−1 and a Ca concentration of 100 mgL−1. Furthermore, DOC concentrations of 1000 mgL−1 should be adjusted by adding respective amounts of humic acid.
2.5. Product matrix media
- surface bound,
- suspended in liquids,
- suspended in solids,
- free (the scope of this Perspective which excludes consideration of air, “free” includes intentionally directly released nanomaterial products into media other than air).
For each category, a large number of potential matrices with very different chemical–physical behavior could be chosen. In the solid medium for example, the range of matrices could go from polyethylene to concrete and it has been shown that the potential for release of NMs from these matrices varies by five orders of magnitude.135 Moreover, for a given matrix, different additives/surface coatings have to be used depending on the NM to be incorporated in order to allow NM dispersion and facilitate NM/Matrix compatibility.
Most published studies on release of NMs from a product matrix have used commercially available products with only limited description of the matrix and only in a few studies has a more defined matrix been used.136 To study NM release from paints, standard paint formulations have been described in the NanoHouse project.137,138 For polymer nanocomposites, standard materials have been used in inter-laboratory comparisons.139 Also for NM release from textiles, materials with full characterization of the fabrics and the methods to produce them have been described.140 A number of case studies have emerged including methods to generate and characterize releases from matrix-embedded materials.141 These few studies with materials that are relatively well described are clearly not sufficient to allow the proposition of standard testing materials for product matrices.
The recommended product matrix types are shown in Table 15. Two are solid matrices with NMs embedded within or with surface-bound NMs, and one is a colloidal suspension of NMs (e.g., a cream). The primary measurements that are required are the NM concentration inside the matrix and the composition of the matrix. In the case of nano-enabled products, NM concentrations may be significant due to potential impacts on the matrix structure itself in addition to a direct impact on release rates.142 Depending on whether the NM/matrix is obtained from a commercial source or is produced in-house, more or less information is available about the matrix and the embedded NMs. The characterization of the identity of the NM and the chemical composition of the matrix is more challenging in the case of a commercial product and often relies on manufacturer's information only. It is therefore recommended to produce the matrix in-house to allow a full control over its composition and the type of NM added. A very close collaboration with manufacturers and a full disclosure of all ingredients of the matrix is also a preferred option because it ensures that the matrix is relevant from a real-world perspective but still allows full knowledge about the matrix composition. The use of “generic” formulations that combine the requirement of both scientists and industry has been shown to be a good compromise.143
| Media type | Primary measurements (matrix) | Secondary measurements (material release) |
|---|---|---|
| Solid matrix with nanomaterials embedded | Nanomaterial concentration | Changes in product matrix over time |
| Composition of product matrix | ||
| Solid matrix with surface bound nanomaterials | Composition of product matrix | Changes in product matrix over time |
| Colloidal suspension of nanomaterials | Composition of product suspension | Changes in product matrix over time |
A secondary set of measurements should deal with the changes in product matrix over time when added to an environmental medium. The product matrix ages over time when present in an environmental medium and these changes drive the behavior of the NM. The characterization of the “matrix in the matrix”, i.e. the product matrix that is present in the environmental matrix, is also necessary. These environmental matrices should follow the recommendations in the previous sections of this Perspective.
The recommendations for harmonizing across this medium are therefore not equivalent to selecting a particular representative matrix in the way that aqueous, soils, biological or even engineered matrices may present. In this case, the recommendations are limited to conditional lists of parameters that are appropriate to report for comparison across the broadly varying set of product matrices into which NMs will be incorporated.
Solid matrix with NMs embedded. This type of matrix is one of the most frequently used product matrices135,136 and also constitutes the major type of matrix reported in release studies.133 Not all NM/matrix combinations make sense from the point of view of actual product use and therefore for each combination another test material might be needed. The chemical ‘compatibility’ between matrix and NM must be taken into account. This means that different to the environmental testing media, where all NMs can enter the same system, in the case of the product matrix, different test media need to be prepared for each NM and each product type (e.g., polymer nanocomposite) that exists. In that specific case, the application domain, what NMs are used in which types of solid matrices, needs to be taken into account. The matrix composition needs to be known or has to be determined analytically regardless if a commercially available matrix or one produced in-house is used. It has been shown that it is mainly the type of matrix that determined the release of materials.135
Solid matrix with surface bound NMs. The general issues discussed for NMs embedded in a solid matrix also apply to surface-bound NMs. The choice of this matrix is justified by the much higher release potential of NMs when bound onto the surface than when incorporated into/embedded in the matrix.144 The technology used to bind the NMs onto the surface is a crucial determinant of the system and needs to be known so that the behavior can be linked to composition.
Colloidal suspension of NMs. This matrix represents the simplest form of a product matrix because the NMs are present suspended in a liquid (or gel) matrix, therefore being similar to the pristine NMs that are usually used in experiments. Nevertheless, the additional presence of matrix materials influences the behavior of the NMs in the system and therefore the detailed characterization of the matrix and knowledge about the major constituents is necessary. While there have been a number of studies, there is not convergence on a reference matrix that can span across groupings or even phases. It may be that more specialized groupings can be developed based in part on use and in part on phase (e.g. liquid foods require a particular set of characteristics to harmonize reporting).
3. Discussion and conclusions
A number of consistently observed tensions were encountered in selecting and compromising on harmonized media, including the desire to propose a minimum set of characteristics that would be required for comparison without overburdening researchers. The separation of recommended measurements into primary and secondary parameters addresses that tension. Another significant difficulty was encountered in separating media conditions from broader assay conditions; the scope of this effort was limited to characterizing the media in which NMs are tested. Integrated data and cross-study comparison will be enabled by a combination of the harmonized media parameters proposed here, together with standard material characterizations and assays. This was especially challenging for the biological fluids, waste water treatment and product categories, where several iterations were required to tease out the boundaries between medium and assay. Fig. 2 shows the split that was agreed for the biological fluids parameters as a representative example.We know the media surrounding NMs are key determinants of the transformations those NMs will undergo, and of the ultimate characteristics of the resulting materials that will be moving through environments and taken up into biota. Because the characteristics and effects of the material are actually a function of the combined system of the material and the media in which it is tested, data characterizing both the material and the media must be reported together to facilitate meaningful analysis. We also know that integrating and comparing multiple datasets is necessary to make progress on understanding behavior and effects of NMs, given the infinite variety of materials and media and the limits of any one individual project. To enable this, we must harmonize data reporting not only on NM characteristics but also of media; and to start toward harmonization, we must select some sample media which are expected to be of particular relevance to guidance and decision making on the part of risk assessors, regulators, and manufacturers. Prior calls have been made for consolidating testing efforts around key functional assays that deliver empirical measurements of how nanomaterials behave in particular systems (e.g. attachment efficiency, dissolution). If such tests are carried out in consistent reference media, the resulting datasets will be comparable, and can propel the nanoEHS community toward both directional guidance for risk purposes, as well as provide a growing mass of meta-data to back out the mechanistic interactions between particle and medium property that governed the FA result.5
The hope is that the proposals in this Perspective of primary and secondary parameters to be consistently reported for several key classes of media will be adopted by the broad array of communities engaged in NM testing. In studies where a standard or synthesized medium is relevant, the suggested standardized media should be used wherever possible. The resulting potential for comparison of datasets will be particularly fruitful when data are then entered, as is increasingly the goal, into shared databases (e.g. NanoInformatics Knowledge Commons, eNanoMapper, the developing NanoCommons and EUON).
For several of the more complex media categories here, there are important next steps to be realized in analysis and detection (e.g. characterization of NMs in situ within product matrices), and in agreement of most relevant systems (e.g. insights from life cycle analysis to align product matrices with environmental compartments of their likely release). As these insights emerge, continued harmonization of environmental media for NM testing will be improved. Work in the US-EU nanoEHS Communities of Research to coordinate and harmonize efforts in multiple projects and regions may serve as a platform for continued development and discussion.
List of abbreviations
| ALI | Air–liquid interfaces |
| ASTM | American Society for Testing and Materials |
| ASW | Artificial seawater |
| AVS | Acid volatile sulfide |
| BOD | Biological oxygen demand |
| CNTs | Carbon nanotubes |
| COD | Chemical oxygen demand |
| DIW | Deionized water |
| DMEM | Dulbecco's modified Eagle's medium |
| DOC | Dissolved organic carbon |
| EHS | Environmental health and safety |
| ENTM | Environmental nano testing media |
| EU | European Union |
| FAs | Functional assays |
| FBS | Foetal bovine serum |
| ISA-TAB-nano | Investigation-study-assay data capture approach using delimited tabs |
| ISO | International Standardization Organization |
| MBL | Marine Biological Laboratory |
| MHW | Moderately hard water |
| MHRW | Moderately hard reconstituted water |
| MSW | Municipal solid waste |
| NCI NanoWG | National Cancer Institute Nanotechnology Working Group |
| NM | Nanomaterial |
| NOM | Natural organic matter |
| NZVI | Nano zero-valent iron |
| OECD | Organization for Economic Cooperation and Development |
| SF | Serum-free |
| SRMs | Standard reference materials |
| SVI | Volume index |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| US EPA | United States Environmental Protection Agency |
| VSW | Very soft water |
| WWTP | Wastewater treatment plant |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
N. K. Geitner and C. Ogilvie Hendren contributed equally to the organization and preparation of the Perspective. G. Cornelis, R. Kaegi, J. Lead, G. V. Lowry, I. Lynch, B. Nowack, and E. Petersen, and M. R. Wiesner were workshop group leaders and primary contributors to the major sections of the Perspective; they are listed alphabetically after the two lead authors. The remaining authors took part in the workshop and contributed material directly to the final Perspective; they are listed alphabetically after the core contributors. C. Ogilvie Hendren led the design and facilitation of the workshop. M. R. Wiesner convened the workshop and serves as corresponding author. This material is based upon work supported by the National Science Foundation (NSF) and the Environmental Protection Agency (EPA) under NSF Cooperative Agreement EF-0830093 and DBI-1266252, Center for the Environmental Implications of NanoTechnology (CEINT). The work was also supported in part via cooperative agreement W912HZ-17-2-0002 between the US Army Corps of Engineer Research and Development Center (USACE ERDC) and the US Consumer Product Safety Commission. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF, the EPA, the USACE ERDC or the US CPSC. This work has not been subjected to EPA review and no official endorsement should be inferred. Certain commercial products or equipment are described in this paper in order to specify adequately the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that it is necessarily the best available for the purpose. The contributions of C. S., I. L., G. C., J. Q., R. K., B. N., F. v. d. K. and W. P., were supported through the “NanoFASE” project funded by the European Union's Horizon 2020 research and innovation programme under grant agreement number 642007. S. M. Rodrigues acknowledges the financial support of CESAM (UID/AMB/50017/2019) by FCT/MCTES through national funds, and the co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020. This is a contribution to Projects SIINN/0001/2014 (NanoFarm) and POCI-01-0145-FEDER-016749-PTDC/AGR-PRO/6262/2014 (NanoFertil) funded by FEDER, through COMPETE2020 – Programa Operacional Competitividade e Internacionalização (POCI), and by national funds (OE), through FCT/MCTES. C. G.-T. acknowledges funding from Excellence Initiative of Aix-Marseille University – A*MIDEX, a French “Investissements d'Avenir” program, through its associated Labex SERENADE project. J. H. was funded by the CSFund and the Park Foundation for work on the environmental and societal implications of nanotechnologies. S. L. H. would like to acknowledge funding support from the National Science Foundation (NSF Grant #1438165) and the National Institutes of Health (Grant #ES017552). A. K. was funded by the US Army Environmental Quality and Installation Research Program (Dr. Elizabeth Ferguson, Technical Director). J. A. P. acknowledges support from the National Science Foundation under the Center for Sustainable Nanotechnology, CHE-1503408. N. T. acknowledges the support of the Canada Research Chairs program.References
- A. B. Stefaniak, V. A. Hackley, G. Roebben, K. Ehara, S. Hankin, M. T. Postek, I. Lynch, W. E. Fu, T. P. Linsinger and A. F. Thunemann, Nanoscale reference materials for environmental, health and safety measurements: needs, gaps and opportunities, Nanotoxicology, 2013, 7(8), 1325–1337 CrossRef PubMed.
- C. O. Hendren, C. M. Powers, M. D. Hoover and S. L. Harper, The Nanomaterial Data Curation Initiative: A collaborative approach to assessing, evaluating, and advancing the state of the field, Beilstein J. Nanotechnol., 2015, 6, 1752–1762 CrossRef CAS PubMed.
- V. Stone, B. Nowack, A. Baun, N. van den Brink, F. Kammer, M. Dusinska, R. Handy, S. Hankin, M. Hassellov, E. Joner and T. F. Fernandes, Nanomaterials for environmental studies: classification, reference material issues, and strategies for physico-chemical characterisation, Sci. Total Environ., 2010, 408(7), 1745–1754 CrossRef CAS PubMed.
- S. L. Harper, J. L. Carriere, J. M. Miller, J. E. Hutchison, B. L. Maddux and R. L. Tanguay, Systematic evaluation of nanomaterial toxicity: utility of standardized materials and rapid assays, ACS Nano, 2011, 5(6), 4688–4697 CrossRef CAS PubMed.
- C. O. Hendren, G. V. Lowry, J. M. Unrine and M. R. Wiesner, A functional assay-based strategy for nanomaterial risk forecasting, Sci. Total Environ., 2015, 536, 1029–1037 CrossRef CAS.
- B. E. Vencalek, S. N. Laughton, E. Spielman-Sun, S. M. Rodrigues, J. M. Unrine, G. V. Lowry and K. B. Gregory, In Situ Measurement of CuO and Cu(OH)2 Nanoparticle Dissolution Rates in Quiescent Freshwater Mesocosms, Environ. Sci. Technol. Lett., 2016, 3(10), 375–380 CrossRef CAS.
- N. K. Geitner, N. J. O'Brien, A. A. Turner, E. J. Cummins and M. R. Wiesner, Measuring Nanoparticle Attachment Efficiency in Complex Systems, Environ. Sci. Technol., 2017, 51(22), 13288–13294 CrossRef CAS.
- D. G. Thomas, S. Gaheen, S. L. Harper, M. Fritts, F. Klaessig, E. Hahn-Dantona, D. Paik, S. Pan, G. A. Stafford, E. T. Freund, J. D. Klemm and N. A. Baker, ISA-TAB-Nano: a specification for sharing nanomaterial research data in spreadsheet-based format, BMC Biotechnol., 2013, 13(1), 2 CrossRef.
- G. V. Lowry, R. J. Hill, S. Harper, A. F. Rawle, C. O. Hendren, F. Klaessig, U. Nobbmann, P. Sayre and J. Rumble, Guidance to improve the scientific value of zeta-potential measurements in nanoEHS, Environ. Sci.: Nano, 2016, 3(5), 953–965 RSC.
- M. R. Wiesner, G. V. Lowry, K. L. Jones, J. Hochella, F. Michael, R. T. Di Giulio, E. Casman and E. S. Bernhardt, Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials, ACS Publications, 2009 Search PubMed.
- B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urrea, C. Metcalfe, J. Rose, N. Horne, A. A. Koelmans and S. J. Klaine, Potential scenarios for nanomaterial release and subsequent alteration in the environment, Environ. Toxicol. Chem., 2012, 31(1), 50–59 CrossRef CAS.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46(13), 6893–6899 CrossRef CAS.
- G. Metreveli, B. Frombold, F. Seitz, A. Grun, A. Philippe, R. R. Rosenfeldt, M. Bundschuh, R. Schulz, W. Manz and G. E. Schaumann, Impact of chemical composition of ecotoxicological test media on the stability and aggregation status of silver nanoparticles, Environ. Sci.: Nano, 2016, 3(2), 418–433 RSC.
- A. L. Dale, E. A. Casman, G. V. Lowry, J. R. Lead, E. Viparelli and M. Baalousha, Modeling nanomaterial environmental fate in aquatic systems, ACS Publications, 2015 Search PubMed.
- J. L. Weishaar, G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii and K. Mopper, Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon, Environ. Sci. Technol., 2003, 37(20), 4702–4708 CrossRef CAS.
- USEPA, EPA-RCA: 9040B: pH in Water by Electrometric Measurement, ed. Waste, O. o. S., SW-846 Online: Test Methods, 1995 Search PubMed.
- International, A., ASTM D1293-18, Standard Test Methods for pH of Water, ASTM International, West Conshohocken, PA, 2018 Search PubMed.
- T. E. Baxter and R. B. Baird, Method 2510: Conductivity, in Standard Methods For the Examination of Water and Wastewater, 2017 Search PubMed.
- J. D. Pfaff and D. P. Hautman, EPA Method 300.1: Determination of Inorganic Anions in Drinking Water by Ion Chromatography, ed. Laboratory, N. E. R., 1999 Search PubMed.
- B. B. Potter and J. C. Wimsatt, Method 415.3- Measurement of Total Organic Carbon, Dissolved Organic Carbon and Specific UV Absorbance at 254 nm in Source Water and Drinking Water, ed. Development, O. o. R. a., Science Inventory, 2005 Search PubMed.
- EPA Method 375.2: Determination of Sulfate by Automated Colorimetry, ed. J. W. O'Dell, 1993 Search PubMed.
- B. Striggow, SESDPROC-113-R2: Field Measurement of Oxidation-Reduction Potential, 2017 Search PubMed.
- EPA Method 180.1: Determination of Turbidity by Nephelometry, ed. J. W. O'Dell, 1993 Search PubMed.
- International, A., ASTM D5907-18: Standard Test Methods for Filterable Matter (Total Dissolved Solids) and Nonfilterable Matter (Total Suspended Solids) in Water, ASTM International, West Conshohocken, PA, 2018, vol. ASTM D5907-18 Search PubMed.
- C. Weber, Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and marine Organisms, ed. E. M. S. Laboratory, Office of Research and Development, 1993 Search PubMed.
- E. J. Petersen, S. A. Diamond, A. J. Kennedy, G. G. Goss, K. Ho, J. Lead, S. K. Hanna, N. B. Hartmann, K. Hund-Rinke, B. Mader, N. Manier, P. Pandard, E. R. Salinas and P. Sayre, Adapting OECD Aquatic Toxicity Tests for Use with Manufactured Nanomaterials: Key Issues and Consensus Recommendations, Environ. Sci. Technol., 2015, 49(16), 9532–9547 CrossRef CAS.
- A. R. Harmon, A. J. Kennedy, A. R. Poda, A. J. Bednar, M. A. Chappell and J. A. Steevens, Determination of nanosilver dissolution kinetics and toxicity in an environmentally relevant aqueous medium, Environ. Toxicol. Chem., 2014, 33(8), 1783–1791 CrossRef CAS.
- J. Fabrega, S. R. Fawcett, J. C. Renshaw and J. R. Lead, Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter, Environ. Sci. Technol., 2009, 43(19), 7285–7290 CrossRef CAS.
- A. Potasznik and S. Szymczyk, Magnesium and Calcium Concentrations in the Surface Water and Bottom Deposits of a River-Lake System, J. Elem., 2015, 20(3), 677–692 Search PubMed.
- J. Brant, H. Lecoanet and M. R. Wiesner, Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems, J. Nanopart. Res., 2005, 7(4–5), 545–553 CrossRef CAS.
- Y. Su, G. Yang, K. Lu, E. J. Petersen and L. Mao, Colloidal properties and stability of aqueous suspensions of few-layer graphene: Importance of graphene concentration, Environ. Pollut., 2017, 220, 469–477 CrossRef CAS.
- J. G. Coleman, A. J. Kennedy, A. J. Bednar, J. F. Ranville, J. G. Laird, A. R. Harmon, C. A. Hayes, E. P. Gray, C. P. Higgins, G. Lotufo and J. A. Steevens, Comparing the effects of nanosilver size and coating variations on bioavailability, internalization, and elimination, using Lumbriculus variegatus, Environ. Toxicol. Chem., 2013, 32(9), 2069–2077 CrossRef CAS.
- A. J. Kennedy, M. A. Chappell, A. J. Bednar, A. C. Ryan, J. G. Laird, J. K. Stanley and J. A. Steevens, Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles, Environ. Sci. Technol., 2012, 46(19), 10772–10780 CrossRef CAS.
- I. Romer, T. A. White, M. Baalousha, K. Chipman, M. R. Viant and J. R. Lead, Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests, J. Chromatogr. A, 2011, 1218(27), 4226–4233 CrossRef.
- R. Ma, C. Levard, S. M. Marinakos, Y. Cheng, J. Liu, F. M. Michel, G. E. Brown and G. V. Lowry, Size-controlled dissolution of organic-coated silver nanoparticles, Environ. Sci. Technol., 2012, 46(2), 752–759 CrossRef CAS.
- M. Tejamaya, I. Romer, R. C. Merrifield and J. R. Lead, Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media, Environ. Sci. Technol., 2012, 46(13), 7011–7017 CrossRef CAS.
- A. Hitchman, G. H. Smith, Y. Ju-Nam, M. Sterling and J. R. Lead, The effect of environmentally relevant conditions on PVP stabilised gold nanoparticles, Chemosphere, 2013, 90(2), 410–416 CrossRef CAS PubMed.
- Agency, U. S. E. P., Watershed-based National Pollutant Discharge Elimination System (NPDES) Permitting Technical Guidance, USEPA, 2007 Search PubMed.
- Agency, U. S. E. P., Analytical Methods and Precedures for Pesticides. https://www.epa.gov/pesticide-analytical-methods.
- C. Weber, Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, ed. Agency, U. S. E. P., Washington, D.C Search PubMed.
- Agency, U. S. E. P., Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, Washington, D.C., 4th edn, 2002 Search PubMed.
- A. J. Kennedy, M. S. Hull, S. Diamond, M. Chappell, A. J. Bednar, J. G. Laird, N. L. Melby and J. A. Steeyens, Gaining a Critical Mass: A Dose Metric Conversion Case Study Using Silver Nanoparticles, Environ. Sci. Technol., 2015, 49(20), 12490–12499 CrossRef CAS.
- E. A. Fairbairn, A. A. Keller, L. Madler, D. Zhou, S. Pokhrel and G. N. Cherr, Metal oxide nanomaterials in seawater: linking physicochemical characteristics with biological response in sea urchin development, J. Hazard. Mater., 2011, 192(3), 1565–1571 CrossRef CAS.
- B. D. Johnson, S. L. Gilbert, B. Khan, D. L. Carroll and A. H. Ringwood, Cellular responses of eastern oysters, Crassostrea virginica, to titanium dioxide nanoparticles, Mar. Environ. Res., 2015, 111, 135–143 CrossRef CAS.
- M. Auffan, C. W. Matson, J. Rose, M. Arnold, O. Proux, B. Fayard, W. Liu, P. Chaurand, M. R. Wiesner, J. Y. Bottero and R. T. Di Giulio, Salinity-dependent silver nanoparticle uptake and transformation by Atlantic killifish (Fundulus heteroclitus) embryos, Nanotoxicology, 2014, 8(sup1), 167–176 CrossRef CAS.
- M. Atkinson and C. Bingman, Elemental composition of commercial seasalts, J. Aquaric. Aquat. Sci., 1997, 8(2), 39–43 Search PubMed.
- ASTM, Standard Practice for the Preparation of Substitute Ocean Water. ASTM, 2013.
- G. M. Cavanaugh, Formulae and methods VI of the Marine Biological Laboratory Chemical Room, ed. G. M. Cavanaugh, Woods Hole, Mass, 1975 Search PubMed.
- Compendia, B. B. Artificial Seawaters - Recipes. http://comm.archive.mbl.edu/BiologicalBulletin/COMPENDIUM/CompTab3.html#3A (accessed February 5, 2019).
- G. Cornelis, K. Hund-Rinke, T. Kuhlbusch, N. Van den Brink and C. Nickel, Fate and Bioavailability of Engineered Nanoparticles in Soils: A Review, Crit. Rev. Environ. Sci. Technol., 2014, 44(24), 2720–2764 CrossRef CAS.
- E. M. Hotze, T. Phenrat and G. V. Lowry, Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment, J. Environ. Qual., 2010, 39(6), 1909–1924 CrossRef CAS PubMed.
- J. T. Quik, M. C. Stuart, M. Wouterse, W. Peijnenburg, A. J. Hendriks and D. van de Meent, Natural colloids are the dominant factor in the sedimentation of nanoparticles, Environ. Toxicol. Chem., 2012, 31(5), 1019–1022 CrossRef CAS PubMed.
- A. R. Petosa, F. Rajput, O. Selvam, C. Ohl and N. Tufenkji, Assessing the transport potential of polymeric nanocapsules developed for crop protection, Water Res., 2017, 111, 10–17 CrossRef CAS.
- R. S. Kookana, A. B. Boxall, P. T. Reeves, R. Ashauer, S. Beulke, Q. Chaudhry, G. Cornelis, T. F. Fernandes, J. Gan, M. Kah, I. Lynch, J. Ranville, C. Sinclair, D. Spurgeon, K. Tiede and P. J. Van den Brink, Nanopesticides: guiding principles for regulatory evaluation of environmental risks, J. Agric. Food Chem., 2014, 62(19), 4227–4240 CrossRef CAS PubMed.
- EPA, U., Method 9045D: Soil and Waste pH, in Soil and Waste pH, U S Environmental Protection Acency, epa.gov, 2004, vol. 9045D, pp. 1–5 Search PubMed.
- International, A., Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils, ASTM INTERNATIONAL, West Conshockocken, PA, 2014, vol. D2974-14 Search PubMed.
- E. E. Schulte and B. Hoskins, Recommended Soil Organic Matter Tests, in Recommended Soil Testing Procedures for the Northeastern United States, ed. J. T. Sims and A. Wolf, Agricultural Experiment Station, University of Deleware, Newark, DE, 2011, vol. 3, pp. 63–74 Search PubMed.
- G. M. Bowman and J. Hutka, Particle Size Analysis, in Soil Physical Measurement and Interpretation for Land Evaluation, ed. N. McKenzie, K. Coughlan and H. Cresswell, CSIRO, Victoria, 2002, pp. 224–239 Search PubMed.
- International, A., ASTM Method G200-09 Standard Test Method for Measurement of Oxidation-Reduction Potential (ORP) of Soil. https://www.astm.org/Standards/G200.htm Search PubMed.
- A. Wolf and D. Beegle, Recommended Soil Tests for Macro and Micronutrients, in Recommended Soil Testing Procedures for the Northeastern United States, ed. J. T. Sims and A. Wolf, Agricultural Experiment Station, University of Deleware, Newark, DE, 2011, vol. 3, pp. 39–47 Search PubMed.
- P. M. Chapman, F. Wang, J. D. Germano and G. Batley, Pore water testing and analysis: the good, the bad, and the ugly, Mar. Pollut. Bull., 2002, 44(5), 359–366 CrossRef CAS.
- International, A., ASTM D4972-18. Standard Test Methods for pH of Soils, ASTM International, West Conshohocken, PA, 2018 Search PubMed.
- EPA, U., EPA Method 120.1 – Conductivity, ed. Development, O. o. R. a., 1983 Search PubMed.
- A. L. Page, Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. American Society of Agronomy, Soil Science Society of America, Madison, WI, 1965 Search PubMed.
- H. E. Allen, G. Fu, W. Boothman, D. M. DiToro and J. D. Mahony, EPA Method 376.3 - Analytical Method For Determination of Acid Volatile Sulfide in Sediment, ed. Technology, O. o. S. a., 1991 Search PubMed.
- International, A., ASTM D7063/D7063M-18, Standard Test Method for Effective Porosity and Effective Air Voids of Compacted Asphalt Mixture Samples, ASTM International, West Conshohocken, PA, 2018, vol. ASTM D7063/D7063M-18 Search PubMed.
- International, A., ASTM D4647/D4647M-13, Standard Test Methods for Identification and Classification of Dispersive Clay Soils by the Pinhole Test, ASTM International, West Conshohocken, PA, 2013, vol. ASTM D4647/D4647M-13 Search PubMed.
- International, A., Standard Practice for Active Soil Gas Sampling in the Vadose Zone for Vapor Intrusion Evaluations. ASTM International, West Conshohocken, PA, 2018, vol. D7663-12 Search PubMed.
- J. L. BRAGASSA, Method 3.1 Determining Tortuosity, IR/IR, ed. Administrative Record and Information Repository, 2008 Search PubMed.
- OECD, Test No. 207: Earthworm, Acute Toxicity Tests, 1984 Search PubMed.
- Speyer, L. Standard Soils. http://www.lufa-speyer.de/index.php/dienstleistungen/standardboeden/8-dienstleistungen/artikel/57-standard-soils.
- P. S. Tourinho, C. A. van Gestel, S. Lofts, A. M. Soares and S. Loureiro, Influence of soil pH on the toxicity of zinc oxide nanoparticles to the terrestrial isopod Porcellionides pruinosus, Environ. Toxicol. Chem., 2013, 32(12), 2808–2815 CrossRef CAS PubMed.
- P. L. Waalewijn-Kool, S. Rupp, S. Lofts, C. Svendsen and C. A. van Gestel, Effect of soil organic matter content and pH on the toxicity of ZnO nanoparticles to Folsomia candida, Ecotoxicol. Environ. Saf., 2014, 108, 9–15 CrossRef CAS PubMed.
- G. T. Ankley, D. A. Benoit, R. A. Hoke, E. N. Leonard, C. W. West, G. L. Phipps, V. R. Mattson and L. A. Anderson, Development and Evaluation of Test Methods for Benthic Invertebrates and Sediments - Effects of Flow-Rate and Feeding on Water-Quality and Exposure Conditions, Arch. Environ. Contam. Toxicol., 1993, 25(1), 12–19 CrossRef CAS.
- J. M. Besser, W. G. Brumbaugh, N. E. Kemble, C. D. Ivey, J. L. Kunz, C. G. Ingersoll and D. Rudel, Toxicity of nickel-spiked freshwater sediments to benthic invertebrates—Spiking methodology, species sensitivity, and nickel bioavailability. US Geological Survey 2011 Search PubMed.
- C. G. Ingersoll, J. L. Kunz, J. P. Hughes, N. Wang, D. S. Ireland, D. R. Mount, J. R. Hockett and T. W. Valenti, , Jr., Relative sensitivity of an amphipod Hyalella azteca, a midge Chironomus dilutus, and a unionid mussel Lampsilis siliquoidea to a toxic sediment, Environ. Toxicol. Chem., 2015, 34(5), 1134–1144 CrossRef CAS.
- D. J. Call, C. N. Polkinghorne, T. P. Markee, L. T. Brooke, D. L. Geiger, J. W. Gorsuch and K. A. Robillard, Toxicity of silver in water and sediment to the freshwater amphipod Hyalella azteca, Environ. Toxicol. Chem., 2006, 25(7), 1802–1808 CrossRef CAS PubMed.
- NIST SRM Order Request System 1646a - Estuarine Sediment. https://www-s.nist.gov/srmors/view_detail.cfm?srm=1646a.
- NIST SRM Order Request System RM 8704 - Buffalo River Sediment. https://www-s.nist.gov/srmors/view_detail.cfm?srm=8704.
- R. Ma, C. Levard, J. D. Judy, J. M. Unrine, M. Durenkamp, B. Martin, B. Jefferson and G. V. Lowry, Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processed biosolids, Environ. Sci. Technol., 2014, 48(1), 104–112 CrossRef CAS.
- R. Kaegi, A. Voegelin, B. Sinnet, S. Zuleeg, H. Hagendorfer, M. Burkhardt and H. Siegrist, Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant, Environ. Sci. Technol., 2011, 45(9), 3902–3908 CrossRef CAS PubMed.
- V. H. Kadish, Milorganite–A New Fertilizer Material1, Ind. Eng. Chem., 1928, 20(1), 9–10 CrossRef CAS.
- R. L. Chaney, G. S. Stoewsand, C. A. Bache and D. J. Lisk, Cadmium deposition and hepatic microsomal induction in mice fed lettuce grown on municipal sludge-amended soil, J. Agric. Food Chem., 1978, 26(4), 992–994 CrossRef CAS PubMed.
- M. Anderson and R. G. Skerratt, Variability study of incinerated sewage sludge ash in relation to future use in ceramic brick manufacture, Br. Ceram. Trans., 2003, 102(3), 109–113 CrossRef CAS.
- N. J. McNab, J. C. Hughes and J. R. Howard, Pollution effects of wastewater sludge application to sandy soils with particular reference to the behaviour of mercury, Appl. Geochem., 1997, 12(3), 321–325 CrossRef CAS.
- S. D. Young, H. Zhang, A. M. Tye, A. Maxted, C. Thums and I. Thornton, Characterizing the availability of metals in contaminated soils. I. The solid phase: sequential extraction and isotopic dilution, Soil Use Manage., 2005, 21(s2), 450–458 CrossRef.
- T. F. Scientific pH and CO2 Levels, https://www.thermofisher.com/us/en/home/references/gibco-cell-culture-basics/cell-culture-environment/ph-co2-levels.html (accessed February 12, 2019).
- M. M. El-Deeb Ghazy, M. M. Habashy and E. Y. Mohammady, Effects of pH on Survival, Growth and Reproduction Rates of The Crustacean, Daphnia Magna, Aust. J. Basic Appl. Sci., 2011, 5, 1–10 CAS.
- N. E. Good and S. Izawa, Hydrogen ion buffers, in Methods in enzymology, Elsevier, 1972, vol. 24, pp. 53–68 Search PubMed.
- M. P. Monopoli, D. Walczyk, A. Campbell, G. Elia, I. Lynch, F. B. Bombelli and K. A. Dawson, Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles, J. Am. Chem. Soc., 2011, 133(8), 2525–2534 CrossRef CAS.
- J. A. Kim, A. Salvati, C. Aberg and K. A. Dawson, Suppression of nanoparticle cytotoxicity approaching in vivo serum concentrations: limitations of in vitro testing for nanosafety, Nanoscale, 2014, 6(23), 14180–14184 RSC.
- S. Schottler, K. Klein, K. Landfester and V. Mailander, Protein source and choice of anticoagulant decisively affect nanoparticle protein corona and cellular uptake, Nanoscale, 2016, 8(10), 5526–5536 RSC.
- A. Lesniak, A. Campbell, M. P. Monopoli, I. Lynch, A. Salvati and K. A. Dawson, Serum heat inactivation affects protein corona composition and nanoparticle uptake, Biomaterials, 2010, 31(36), 9511–9518 CrossRef CAS PubMed.
- P. Esser and L. Weitzmann, Evaporation from cell culture plates, ed. T. Scientific, 2011, vol. TILSPNUNCBU02 0111 Search PubMed.
- International, A., ASTM D 513-88: Standard Test Methods for Total and Dissolved Carbon Dioxide in Water, ASTM International, Philadelphia, PA, 1992, vol. ASTM D 513-88 Search PubMed.
- Repetitive flaws, Nature, 2016, 529(7586), 256, DOI:10.1038/529256a.
- C. Pisani, E. Rascol, C. Dorandeu, J. C. Gaillard, C. Charnay, Y. Guari, J. Chopineau, J. Armengaud, J. M. Devoisselle and O. Prat, The species origin of the serum in the culture medium influences the in vitro toxicity of silica nanoparticles to HepG2 cells, PLoS One, 2017, 12(8), e0182906 CrossRef.
- G. S. Duffo and E. Q. Castillo, Development of an artificial saliva solution for studying the corrosion behavior of dental alloys, Corrosion, 2004, 60(6), 594–602 CrossRef CAS.
- J. Y. Gal, Y. Fovet and M. Adib-Yadzi, About a synthetic saliva for in vitro studies, Talanta, 2001, 53(6), 1103–1115 CrossRef CAS.
- M. R. C. Marques, R. Loebenberg and M. Almukainzi, Simulated biological fluids with possible application in dissolution testing, Dissolution Technol., 2011, 18, 15–28 CrossRef CAS.
- M. R. C. Marques, R. Loebenberg and M. Almukainzi, Simulated Biological Fluids with Possible Application in Dissolution Testing, Dissolution Technol., 2011, 18(3), 15–28 CrossRef CAS.
- S. Tao, Y. Lu, D. Y. Zhang, Y. F. Yang, Y. Yang, X. X. Lu and D. J. Sai, Assessment of Oral Bioaccessibility of Organochlorine Pesticides in Soil Using an In Vitro Gastrointestinal Model, Environ. Sci. Technol., 2009, 43(12), 4524–4529 CrossRef CAS PubMed.
- Z. Y. Wang, J. Zhao, L. Song, H. Mashayekhi, B. Chefetz and B. S. Xing, Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids, Environ. Sci. Technol., 2011, 45, 6018–6024 CrossRef CAS PubMed.
- H. Sun, Y. Ruan, H. Zhu, Z. Zhang, Y. Zhang and L. Yu, Enhanced bioaccumulation of pentachlorophenol in carp in the presence of multi-walled carbon nanotubes, Environ. Sci. Pollut. Res., 2014, 21(4), 2865–2875 CrossRef CAS PubMed.
- N. Boisa, N. Elom, J. R. Dean, M. E. Deary, G. Bird and J. A. Entwistle, Development and application of an inhalation bioaccessibility method (IBM) for lead in the PM10 size fraction of soil, Environ. Int., 2014, 70, 132–142 CrossRef CAS PubMed.
- T. Cedervall, I. Lynch, S. Lindman, T. Berggard, E. Thulin, H. Nilsson, K. A. Dawson and S. Linse, Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(7), 2050–2055 CrossRef CAS.
- S. Radic, N. K. Geitner, R. Podila, A. Kakinen, P. Chen, P. C. Ke and F. Ding, Competitive binding of natural amphiphiles with graphene derivatives, Sci. Rep., 2013, 3, 2273 CrossRef PubMed.
- W. S. Cho, R. Duffin, S. E. Howie, C. J. Scotton, W. A. Wallace, W. Macnee, M. Bradley, I. L. Megson and K. Donaldson, Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes, Part. Fibre Toxicol., 2011, 8, 27 CrossRef CAS.
- A. Fashina, E. Antunes and T. Nyokong, Silica nanoparticles grafted with phthalocyanines: photophysical properties and studies in artificial lysosomal fluid, New J. Chem., 2013, 37(9), 2800–2809 RSC.
- R. E. Stark and S. Tian, The Cutin Biopolymer Matrix, in Biology of the Plant Cuticle, 2006, pp. 126–144 Search PubMed.
- OECD, Nanomaterials in Waste Streams: Current Knowledge on Risks and Impacts, OECD Publishing, 2016 Search PubMed.
- J. Werther and T. Ogada, Sewage sludge combustion, Prog. Energy Combust. Sci., 1999, 25(1), 55–116 CrossRef CAS.
- G. A. Sotiriou, D. Singh, F. Zhang, M. G. Chalbot, E. Spielman-Sun, L. Hoering, I. G. Kavouras, G. V. Lowry, W. Wohlleben and P. Demokritou, Thermal decomposition of nano-enabled thermoplastics: Possible environmental health and safety implications, J. Hazard. Mater., 2016, 305, 87–95 CrossRef CAS.
- D. Singh, G. A. Sotiriou, F. Zhang, J. Mead, D. Bello, W. Wohlleben and P. Demokritou, End-of-life thermal decomposition of nano-enabled polymers: effect of nanofiller loading and polymer matrix on by-products, Environ. Sci.: Nano, 2016, 3(6), 1293–1305 RSC.
- G. A. Sotiriou, D. Singh, F. Zhang, W. Wohlleben, M. G. Chalbot, I. G. Kavouras and P. Demokritou, An integrated methodology for the assessment of environmental health implications during thermal decomposition of nano-enabled products, Environ. Sci.: Nano, 2015, 2(3), 262–272 RSC.
- D. G. Goodwin, A. S. Adeleye, L. Sung, K. T. Ho, R. M. Burgess and E. J. Petersen, Detection and Quantification of Graphene-Family Nanomaterials in the Environment, Environ. Sci. Technol., 2018, 52(8), 4491–4513 CrossRef CAS PubMed.
- A. K. Alva and J. P. Syvertsen, Irrigation Water Salinity Affects Soil Nutrient Distribution, Root Density, and Leaf Nutrient Levels of Citrus under Drip Fertigation, J. Plant Nutr., 1991, 14(7), 715–727 CrossRef CAS.
- S. S. Wijesekara, B. F. Basnayake and M. Vithanage, Organic-coated nanoparticulate zero valent iron for remediation of chemical oxygen demand (COD) and dissolved metals from tropical landfill leachate, Environ. Sci. Pollut. Res., 2014, 21(11), 7075–7087 CrossRef CAS PubMed.
- I. Yousuf, Methods for Estimation and Comparison of Activated Sludge Settleability, in 38th Annual WIOA Qld Water Industry Operations Conference, Parklands, Gold Coast, 2013, pp. 95–101 Search PubMed.
- 4500-O OXYGEN (DISSOLVED), in Standard Methods For the Examination of Water and Wastewater, ed. A. P. H. Association, American Public Health Association, American Water Works Association, 2017 Search PubMed.
- EPA, U., Method 1686: Nitrate/Nitrite-N in Water and Biosolids by Manual Colorimetry, US Environmental Protection Agency, Washington, DC, 2001, vol. Method 1686 Search PubMed.
- EPA, U., Method 1690: Ammonia-N in Water and Biosolids by Automated Colorimetry with Preliminary Distillation, US Environmental Protection Agency, Washington, DC, 2001, vol. Method 1690 Search PubMed.
- D. Fytili and A. Zabaniotou, Utilization of sewage sludge in EU application of old and new methods - A review, Renewable Sustainable Energy Rev., 2008, 12(1), 116–140 CrossRef CAS.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Modeled environmental concentrations of engineered nanomaterials (TiO(2), ZnO, Ag, CNT, Fullerenes) for different regions, Environ. Sci. Technol., 2009, 43(24), 9216–9222 CrossRef CAS PubMed.
- P. Westerhoff, S. Lee, Y. Yang, G. W. Gordon, K. Hristovski, R. U. Halden and P. Herckes, Characterization, Recovery Opportunities, and Valuation of Metals in Municipal Sludges from US Wastewater Treatment Plants Nationwide, Environ. Sci. Technol., 2015, 49(16), 9479–9488 CrossRef CAS PubMed.
- C. L. Doolette, M. J. McLaughlin, J. K. Kirby, D. J. Batstone, H. H. Harris, H. Ge and G. Cornelis, Transformation of PVP coated silver nanoparticles in a simulated wastewater treatment process and the effect on microbial communities, Chem. Cent. J., 2013, 7(1), 46 CrossRef PubMed.
- L. K. Limbach, R. Bereiter, E. Muller, R. Krebs, R. Galli and W. J. Stark, Removal of oxide nanoparticles in a model wastewater treatment plant: influence of agglomeration and surfactants on clearing efficiency, Environ. Sci. Technol., 2008, 42(15), 5828–5833 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Fate and transformation of silver nanoparticles in urban wastewater systems, Water Res., 2013, 47(12), 3866–3877 CrossRef CAS PubMed.
- P. K. Westerhoff, M. A. Kiser and K. Hristovski, Nanomaterial removal and transformation during biological wastewater treatment, Environ. Eng. Sci., 2013, 30(3), 109–117 CrossRef CAS.
- J. Scott, D. Beydoun, R. Amal, G. Low and J. Cattle, Landfill management, leachate generation, and leach testing of solid wastes in Australia and overseas, Crit. Rev. Environ. Sci. Technol., 2005, 35(3), 239–332 CrossRef CAS.
- P. Kjeldsen, M. A. Barlaz, A. P. Rooker, A. Baun, A. Ledin and T. H. Christensen, Present and long-term composition of MSW landfill leachate: A review, Crit. Rev. Environ. Sci. Technol., 2002, 32(4), 297–336 CrossRef CAS.
- M. El-Fadel, A. Findikakis and J. Leckie, Modeling leachate generation and transport in solid waste landfills, Environ. Technol., 1997, 18(7), 669–686 CrossRef.
- S. J. Froggett, S. F. Clancy, D. R. Boverhof and R. A. Canady, A review and perspective of existing research on the release of nanomaterials from solid nanocomposites, Part. Fibre Toxicol., 2014, 11(1), 17 CrossRef PubMed.
- S. Foss Hansen, B. H. Larsen, S. I. Olsen and A. Baun, Categorization framework to aid hazard identification of nanomaterials, Nanotoxicology, 2007, 1(3), 243–250 CrossRef.
- W. Wohlleben and N. Neubauer, Quantitative rates of release from weathered nanocomposites are determined across 5 orders of magnitude by the matrix, modulated by the embedded nanomaterial, NANO, 2016, 1, 39–45 Search PubMed.
- A. J. Koivisto, A. C. O. Jensen, K. I. Kling, A. Norgaard, A. Brinch, F. Christensen and K. A. Jensen, Quantitative material releases from products and articles containing manufactured nanomaterials: Towards a release library, NANO, 2017, 5, 119–132 Search PubMed.
- A. Al-Kattan, A. Wichser, R. Vonbank, S. Brunner, A. Ulrich, S. Zuin and B. Nowack, Release of TiO 2 from paints containing pigment-TiO 2 or nano-TiO 2 by weathering, Environ. Sci.: Processes Impacts, 2013, 15(12), 2186–2193 RSC.
- S. Zuin, A. Massari, A. Ferrari and L. Golanski, Formulation effects on the release of silica dioxide nanoparticles from paint debris to water, Sci. Total Environ., 2014, 476-477, 298–307 CrossRef CAS PubMed.
- W. Wohlleben, G. Vilar, E. Fernandez-Rosas, D. Gonzalez-Galvez, C. Gabriel, S. Hirth, T. Frechen, D. Stanley, J. Gorham, L. P. Sung, H. C. Hsueh, Y. F. Chuang, T. Nguyen and S. Vazquez-Campos, A pilot interlaboratory comparison of protocols that simulate aging of nanocomposites and detect released fragments, Environ. Chem., 2014, 11(4), 402–418 CrossRef CAS.
- D. M. Mitrano, P. Limpiteeprakan, S. Babel and B. Nowack, Durability of nano-enhanced textiles through the life cycle: releases from landfilling after washing, Environ. Sci.: Nano, 2016, 3(2), 375–387 RSC.
- B. Nowack, A. Boldrin, A. Caballero, S. F. Hansen, F. Gottschalk, L. Heggelund, M. Hennig, A. Mackevica, H. Maes and J. Navratilova, Meeting the Needs for Released Nanomaterials Required for Further Testing-The SUN Approach, ACS Publications, 2016 Search PubMed.
- A. Vílchez, E. Fernández-Rosas, D. González-Gálvez and S. Vázquez-Campos, Nanomaterials Release from Nano-Enabled Products, in Indoor and Outdoor Nanoparticles, ed. M. V. Viana, Springer, 2015, vol. 48, pp. 127–158 Search PubMed.
- R. Hischier, B. Nowack, F. Gottschalk, I. Hincapie, M. Steinfeldt and C. Som, Life cycle assessment of façade coating systems containing manufactured nanomaterials, J. Nanopart. Res., 2015, 17(2), 68 CrossRef.
- D. M. Mitrano, E. Rimmele, A. Wichser, R. Erni, M. Height and B. Nowack, Presence of nanoparticles in wash water from conventional silver and nano-silver textiles, ACS Nano, 2014, 8(7), 7208–7219 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: ESI includes additional rationale for recommendations made in this perspective, additional recommendations for amendments to media, and noted challenges and tradeoffs for guiding benchmark suggestions. See DOI: 10.1039/c9en00448c |
| This journal is © The Royal Society of Chemistry 2020 |