Environmental occurrence, fate, effects, and remediation of halogenated (semi)volatile organic compounds
Paul G.
Tratnyek
 *a,
Elizabeth
Edwards
*a,
Elizabeth
Edwards
 b,
Lucy
Carpenter
b,
Lucy
Carpenter
 c and
Sarah
Blossom
c and
Sarah
Blossom
 d
d
aOHSU-PSU School of Public Health, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA. E-mail: tratnyek@ohsu.edu
bDepartment of Chemical Engineering and Applied Chemistry, University of Toronto, Ontario, Canada
cWolfson Atmospheric Chemistry Laboratories, Department of Chemistry, University of York, YO10 5DD, UK
dDepartment of Pediatrics, University of Arkansas for Medical Sciences, Arkansas Children’s Research Institute, Little Rock, AR 72202, USA
 Paul G. Tratnyek | Paul Tratnyek is a Professor in the OHSU-PSU School of Public Health, at the Oregon Health & Science University, in Portland, Oregon. |
 Elizabeth Edwards | Elizabeth Edwards is a Professor of Chemical Engineering and Applied Chemistry at the University of Toronto. |
 Lucy Carpenter | Lucy Carpenter is a Professor of Atmospheric Chemistry at the University of York. |
 Sarah Blossom | Sarah Blossom is an Associate Professor of Pediatrics at the University of Arkansas for Medical Sciences. |
Note from the Editor’s deskThis issue on halogenated volatile and semivolatile organic compounds, X(S)VOCs, is the latest in an ongoing series of themed issues that we have put together at Environmental Science: Processes & Impacts (ESPI) over the past few years, which are meant to highlight exciting and important areas within the scope of our journal. I am particularly happy with the collection of articles in this issue for a few reasons. First and foremost, the papers are excellent from top to bottom. Hats off to the authors for their fine work and a hearty thanks from my side for choosing to publish in ESPI. A personal favorite of mine in this issue is the retrospective piece by Craig Criddle, Timothy Vogel, and Perry McCarty, reflecting on the early days of halogenated VOC research. Second, the guest editor team of Elizabeth Edwards, Lucy Carpenter, Sarah Blossom, and Associate Editor Paul Tratnyek did a wonderful job through their leadership to stretch the scientific scope of the issue to touch on far-reaching aspects of environmental chemistry and environmental science. I want to take the opportunity to personally thank them here for their hard work on the issue. Finally, the topic of this issue has great resonance for me because the papers, taken together, are a fresh look at an “old” set of compounds, like trichloroethylene. I know I speak for many of us when I say that our tendency to focus on the newest contaminants and contaminant classes, while exciting, can feel a bit like chasing fashion trends. What is the molecule of the month? So, it is refreshing to revisit some of the classics, leveraging what we have known for years and decades to reveal new insights with great science. I hope you enjoy this issue as much as I do.
Kristopher McNeill Editor-in-Chief, Environmental Science: Processes & Impacts |
Environmental science is a field so broad that it overlaps with all the classical science disciplines, many major applied disciplines (e.g., geology, toxicology, epidemiology), and has applications ranging from engineering to public policy. This breadth and diversity presents both challenges and opportunities for a journal such as Environmental Science: Processes & Impacts (ESPI), which aims to cover all aspects of the environmental chemical sciences.
Ideally, the balance of topics covered by ESPI should reveal cross-cutting and over-arching themes that advance understanding of the field and influence its future directions. To encourage such consilience, ESPI has published several themed issues that explore major cross-cutting themes through strategic selection of invited and contributed manuscripts. This effort is exemplified by both the 2018 themed issue on application of modeling to chemical fate and exposure, which covered scales from molecular to global,1 and the 2017 themed issue on in silico environmental chemical science, which juxtaposed theoretical, statistical, and hybrid methods.2
In this themed issue, we aimed to combine multiple cross-cutting themes by focusing on a class of environmental chemicals that overlaps with most aspects of environmental chemical science. As a class, the halogenated volatile and semivolatile organic compounds—precisely abbreviated as X(S)VOCs, and loosely equated with halocarbons or organohalides throughout this issue—have played an outsized role in the development of many aspects of environmental science. The influence of X(S)VOCs ranges from motivating the development of modern methods in gas chromatography (including James Lovelock’s electron capture detector3); to mediating the depletion of stratospheric ozone4 and the global tropospheric ozone budget;5 to driving seminal policy developments such as the Montreal Protocol6 and Superfund law.7
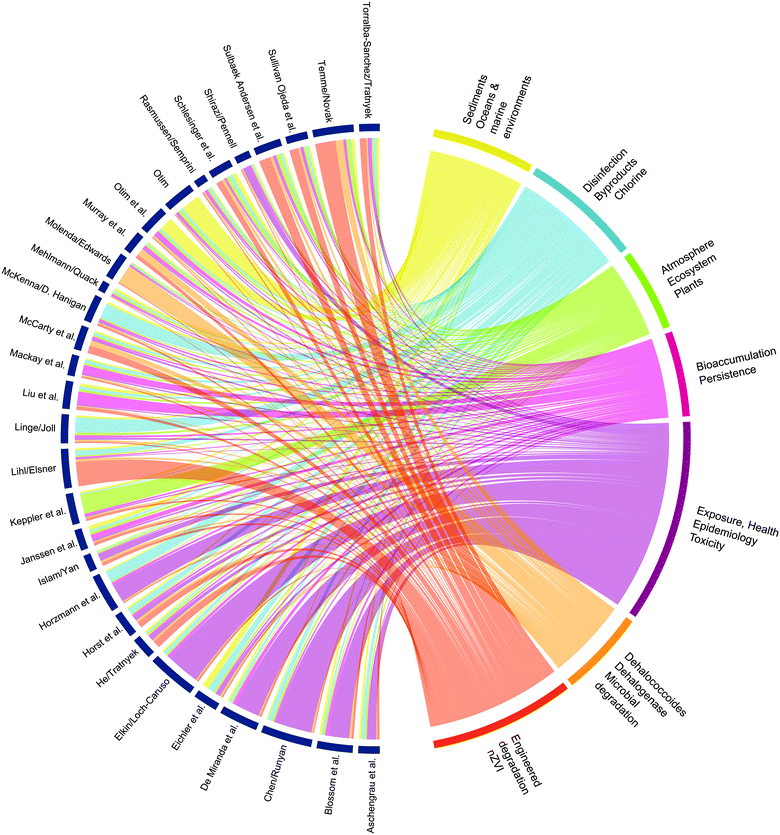
The long history of prior research on environmental aspects of X(S)VOCs makes them legacy contaminants compared with more recently emerging contaminants such as the per- and poly-fluorinated alkyl substances (PFAS). However, the X(S)VOCs include newly emerging contaminants such as 1,2,3-trichloropropane8 and legacy X(S)VOCs like trichloroethene (TCE) that can re-emerge due to new information that alters their risk assessment.9 Furthermore, the legacy of research on X(S)VOCs has contributed to development of core concepts that apply to assessment of the fate, effects, and treatment options of most past, present, and future environmental contaminants. These core concepts include fugacity models of contaminant fate,10 microbial respiration of xenobiotics,11 reactive halogen species-mediated oxidation,12 and epigenetics as biomarkers for toxicant exposures.13 These core concepts and other high-level cross-cutting themes are evident in Fig. 1, which shows connections between keywords in the papers included in this issue.
Focusing on X(S)VOCs also provides broad coverage of another dimension of environmental science: the various media, or compartments, and their corresponding spheres (air/atmosphere, soil/pedosphere, etc.). To ensure balanced coverage of this range of media, co-editors were selected to cover all of the major compartments (air, water, soil, biota, and built/engineered systems). This order of compartments is roughly how the papers are organized in the summary below. However, many of the papers apply to multiple compartments—or interfaces between compartments—and these connections can be hard to track, as evidenced by the complex pattern of chords in Fig. 1. Nevertheless, we have tried to review the strongest connections, along with highlights of each individual paper.
Air, atmosphere, ocean, and global
Natural sources of chlorine and bromine comprise a significant and increasing part of the halogens entering the stratosphere (currently more than half of stratospheric bromine and more than 15% of stratospheric chlorine14), while the atmospheric abundances of anthropogenic ozone-depleting chlorine and bromine-containing gases continue to decline as a consequence of Montreal Protocol controls. There is a need to better quantify these natural emissions and how they might evolve in the future with climate change.Chloromethane (methyl chloride, CH3Cl) is the most abundant naturally-occurring chlorinated compound. Its natural sources are similar to those of bromomethane (methyl bromide, CH3Br), and include the ocean, biomass burning, fungi, salt marshes, wetlands, rice paddies, mangroves, and tropical forests. Even after accounting for the relatively-minor anthropogenic sources of CH3Cl (such as biomass burning and industry), the overall atmospheric budget for CH3Cl has a significant imbalance, with known sinks outweighing sources.15 Here, Keppler et al. (DOI: 10.1039/c9em00540d) quantify CH3Cl production and consumption of a salt marsh ecosystem (atmosphere–plant–soil), using a range of techniques including monitoring stable hydrogen and carbon isotope ratios of CH3Cl. They show that cycling of CH3Cl in such ecosystems is a result of several biotic and abiotic processes occurring simultaneously, all of which are temperature dependent and therefore will be affected by environmental change. Schlesinger and Myneni (DOI: 10.1039/c9em00604d) demonstrate there is a coupling between loss of organochlorines from wetland soils and reactions involving bromine species.
Isotope fractionation is used in multiple manuscripts in this issue (DOI: 10.1039/c9em00540d, DOI: 10.1039/c9em00498j, DOI: 10.1039/c9em00583h, DOI: 10.1039/c9em00503j), including Horst et al. who present a detailed study of how partitioning between water and air can induce isotopic changes. They use two volatile organochlorine compounds (trichloroethene, TCE, and trichloromethane, TCM) as a model system to show that isotope effects during volatilization of organics from water are caused by transport inhibition in the aqueous phase, and then provide a critical overview of where isotopic changes might be expected, or neglected, during phase transfer processes.
On a per-atom basis, bromine is 50–60 times more effective than chlorine in destroying stratospheric ozone.15 CH3Br is the single largest natural source of bromine to the stratosphere. While small anthropogenic emissions (allowed under the Montreal Protocol) remain, atmospheric CH3Br is believed to have now declined close to natural levels.14 However, the CH3Br budget—like that of CH3Cl—has a significant imbalance between sources and sinks, with the known sinks outweighing sources by 20–25%.15 Liu et al. (DOI: 10.1039/c9em00473d) identify a new photochemical process involving ferric ions and dissolved organic matter for the formation of CH3Br in natural seawater.
Halocarbons with atmospheric lifetimes of less than about 6 months are commonly termed very short lived substances (VSLS), and the combined VSLS bromocarbons—primarily tribromomethane (bromoform, CHBr3) and dibromomethane (CH2Br2)—together contribute similar quantities of bromine to the stratosphere as the longer-lived CH3Br.14 The VSLS bromocarbons are thought to be predominantly from natural oceanic sources, but here Mehlmann et al. (DOI: 10.1039/c9em00599d) show that the sea–air fluxes of CHBr3 can be dominated in some areas by anthropogenic sources such as water treatment plants, where it is produced as a disinfection byproduct (DBP), and which could increase with improvements in water treatment infrastructure in the developing world. The authors also suggest that CH2Br2 may be produced from heterotrophy by microorganisms, which is expected to increase in a warming, deoxygenated ocean. Another organohalogen species with short atmospheric lifetimes is 2,2-dichloroethanol, due to the fast kinetics of its reaction with Cl and OH radicals in the gas phase (DOI: 10.1039/c9em00581a).
Soil and water microbiology
Many volatile and semi-volatile contaminants are found predominantly in the atmosphere, but their overall environmental fate involves partitioning from air to the aqueous phase, and this partitioning can be predicted using well-established theory based on fugacity. The power of relatively simple fugacity calculations was shown by Mackay more than 40 years ago,10 but applications of this theory continue to expand, so Celsie et al. (DOI: 10.1039/c9em00496c) show that fugacity and activity can be used as predictors of other contaminant traits in addition to partitioning, including toxicity and bioaccumulation, which in turn make fugacity calculations essential for risk assessment.Just as fugacity, and related descriptors of interphase partitioning like Henry’s law constants, are powerful predictors of contaminant distribution among environmental compartments (soil, water, air, biota etc.), contaminant transformation within compartments can be described, even predicted, using quantitative indicators that can account for variations in environmental conditions. One such indicator is isotope fractionation of the parent molecules or metabolites, which can provide definitive proof of transformation even in the presence of other sources/sinks (DOI: 10.1039/c9em00498j, DOI: 10.1039/c9em00503j, DOI: 10.1039/c9em00557a). Several articles in this issue use various biological indicators to provide new insight into the enzymes that catalyze both hydrolytic dechlorination (DOI: 10.1039/c9em00499h) and strictly anaerobic reductive dechlorination reactions (DOI: 10.1039/c9em00544g, DOI: 10.1039/c9em00605b). Such chemical and biological biomarkers of transformation are also vital tools for risk assessment, providing multiple lines of evidence to help decide on a course of action at a contaminated site, or to understand chemical fate in the environment.
Microbial biomarkers for dehalogenation reactions include the remarkable organohalide respiring bacteria that are now routinely monitored at contaminated sites where bioremediation is a favored remedy. These specialized organisms have unique sets of genes that encode for (confirmed and putative) reductive dehalogenase enzymes with the ability to channel the chemical energy from carbon–halogen bond breakage into their metabolism for growth. Several papers in this issue present aspects of these organisms and their enzymes (DOI: 10.1039/c9em00499h, DOI: 10.1039/c9em00544g, DOI: 10.1039/c9em00605b, DOI: 10.1039/c9em00575g). Modern genetic methods of microbial characterization have repeatedly uncovered surprising diversity and capabilities among natural dechlorinators (e.g., Rasmussen et al. (DOI: 10.1039/c9em00607a)); so much so that identifying and utilizing these natural capabilities might be more promising than genetic engineering in the foreseeable future (DOI: 10.1039/c9em00601j).
Currently, the bioremediation industry is benefiting greatly from the above-mentioned improvements in methods for characterizing the microbiology and chemistry of dechlorination in environmental systems. However, many of the core concepts were established around 50 years ago. Vogel, Criddle and McCarty (DOI: 10.1039/c9em00575g) recap some of this early work, in a paper that is partly a retrospective on the process that led to their seminal feature article in 1987 on the biological transformations of halogenated aliphatic compounds.16 This review, and the one by Mackay (Celsie et al., DOI: 10.1039/c9em00496c), stand out not only for the scientific contributions they summarize, but also for the sense they provide of the role that pioneering individuals have played in the development of this field.
Toxicity, health, and risk
The human health effects of X(S)VOCs depend on a number of factors including when the exposure occurs (e.g., during pregnancy or early life), dose, duration, and mode (e.g., inhalation or drinking water). For an industrial chemical like TCE, human exposures vary from the high doses that can arise from occupational uses (solvent, degreaser, dry cleaning) to much lower doses from contaminated water or soil. In laboratory animals and humans, exposure to TCE has long been linked to harmful effects on the central nervous system, liver, respiratory system, kidneys, immune system, the developing heart, and other adverse pregnancy outcomes. However, in 2012, an expert review panel at the International Agency for Research on Cancer (IARC)17 upgraded the classification of TCE to a Group 1 “known human carcinogen”, based on recent findings related to kidney cancer. Several studies have also linked TCE exposure to cancer of the liver, lung, testes, and certain hematopoietic cancers such as non-Hodgkin’s lymphoma.18The health effects of TCE exposure also are evident in several well-documented field sites, including Camp Lejeune, a U.S. Marine Corps base in North Carolina. From the 1950s until at least 1985, drinking water at the base was contaminated with TCE, tetrachloroethylene (PCE), vinyl chloride, and benzene. It has been estimated median monthly exposure levels from 1942–1987 were 336 ppb for TCE,19 which is more than 50-times the EPA’s Maximum Contaminant Level (MCL) for TCE (5 ppb). In 1989, the base was named a superfund site and, as mandated by law, the agency of toxic substances and disease registry (ATSDR, a part of the CDC) was required to conduct periodic health assessments.
Human exposure to TCE at Camp Lejeune was largely through drinking water, which is the primary exposure route for other halocarbons, ranging from the chlorinated solvent tetrachloroethene (PCE) to numerous disinfection byproducts (DBPs). Aschengrau et al. (DOI: 10.1039/c9em00590k) summarize the reproductive and developmental health effects of PCE based on epidemiological data from cities with recently improved drinking water distribution systems. McKenna et al. (DOI: 10.1039/c9em00468h) analyze the sensitivity of total toxicity indices to potential sampling biases in DBP measurements and recommend precautions that should minimize the risk of erroneous conclusions.
Several papers in this issue address health effects at a more mechanistic level. De Miranda et al. (DOI: 10.1039/c9em00578a) provide a comprehensive review to support the link between TCE exposure and Parkinson’s disease. Blossom et al. (DOI: 10.1039/c9em00514e) describe neural pathways that are potentially altered when TCE is combined with an additional environmental stressor (overnutrition/obesity) linked to abnormal brain development. Reproductive health effects of TCE that are described include human placental changes (DOI: 10.1039/c9em00537d) and developmental toxicity in zebrafish (DOI: 10.1039/c9em00565j). Perhaps one of the more controversial aspects of TCE toxicology concerns its effects on embryonic and fetal heart development. Here, Runyan et al. (DOI: 10.1039/c9em00597h) present compelling evidence that TCE alters transcriptional activity in the developing chick heart and provide further evidence that low dose TCE is capable of disrupting fetal heart development.
Engineered and built environments
The effects of X(S)VOCs on human health require exposure of individuals to the toxicant, and the main routes of direct human exposure usually involve sources that are associated with air, water, and surfaces in the built environment. Some recent work in this realm has emphasized non-target analysis of overall exposure (i.e., the exposome),20 but there are many specific aspects of the indoor air exposure scenario that merit further investigation.Perhaps the aspect that has received the most attention in recent years involves intrusion of vapor into buildings from groundwater that is contaminated with chlorinated solvents.21 Vapor intrusion has proven to be difficult to measure or model, because it is very sensitive to externalities like weather. This is demonstrated in the paper by Pennell et al. (DOI: 10.1039/c9em00567f), which goes further and models the effects of preferential transport pathways like land drains.
Far less attention has been devoted to characterization of potential exposures involving indoor air-borne contaminants that are not so characteristically volatile. A well-documented example involves phthalates22 – which are not X(S)VOCs – but the controlling physico-chemical process should also apply to heavy X(S)VOCs like PCBs or dioxins. Eichler et al. (DOI: 10.1039/c9em00556k) take this even further, and argue that indoor exposure to per- and poly-fluoroalkyl substances (PFAS) can be predicted using models that treat PFAS as SVOCs using partition coefficients to media such as dust and clothing.
Another major route of exposure to X(S)VOCs that is associated with the built-environment involves disinfection byproducts (DBPs) in tap water, either directly by ingestion or indirectly as vapor during bathing.23 The majority of research on this has focused on well-known DBPs like trihalomethanes (THMs; cf. the methyl halides discussed above), but modern analytical methods have led to identification of a wide range of previously undetected DBPs, some of which are X(S)VOCs. One example is the halogenated acetonitriles, which Linge et al. (DOI: 10.1039/c9em00603f) identify as DBPs formed from chloramination in the presence of natural organic matter.
In addition to minimizing exposure to X(S)VOCs by optimization of water disinfection processes to minimize DBP formation, a vast amount of research has been devoted to removing X(S)VOCs, and other contaminants, from the sources of drinking water supplies. The main concern is groundwater, which is commonly used as the source for drinking water (e.g., at Camp Lejeune, North Carolina (DOI: 10.1039/c9em00590k)), and is very difficult to clean-up once contaminated. In contrast to X(S)VOC degradation in air or surface water (with disinfection), where the reactions are mostly oxidative, dehalogenation in groundwater soils and sediments is often reductive. Reductive dehalogenation reactions often are microbially mediated (as summarized above), but they also can be purely chemical when driven directly by “abiotic” reductants such as ferrous iron or sulfide containing minerals or zerovalent metals such as Fe0 or Zn0.
The abiotic reduction of chlorinated solvents in groundwater by natural iron oxide and/or sulfide minerals has only recently become a process that engineers aspire to utilize for remediation of contaminated field sites. However, remedies based on addition of zerovalent iron (ZVI) are common, and there is a vast literature on fundamental, mechanistic aspects of this process and on engineering of the process for optimal outcomes. The former aspect is represented by the paper by Torralba-Sanchez et al. (DOI: 10.1039/c9em00557a), which demonstrates the uses of computational chemical methods to evaluate the possible pathways for reductive degradation of 1,2,3-trichloropropane by Zn0. The optimization of engineered dechlorination processes is exemplified by two papers that explore the benefits of ZVI modified with reduced sulfur (i.e., sulfidated). In one case, the study presents new data from experiments focused on clarifying the fundamental cause of the effects of sulfidation on halocarbon degradation (DOI: 10.1039/c9em00593e), whereas the other study presents a review with meta-analysis of previously published data in order to clarify how best to quantify the effects of sulfidation on the efficiency and selectivity of contaminant reduction by ZVI (DOI: 10.1039/c9em00592g).
While most of the X(S)VOCs included in this issue are C1–C3 compounds, some of their characteristics also apply to heavier halocarbons like DDT, PCBs, etc. Many studies have characterized the occurrence of these compounds in soils and sediments, and most of these studies indicate a strong correlation between halocarbon concentrations and organic carbon content. However, Otim (DOI: 10.1039/c9em00555b) found that this relationship is complicated by effects of particle size, and, in the process, he documented surprisingly large losses of SVOCs from samples during storage (DOI: 10.1039/c9em00545e).
This summary of the papers included in the ESPI themed issue on X(S)VOCs demonstrates how thoroughly this class of chemicals pervades all aspects of environmental science and its health and policy implications. Halogenated volatile and semivolatile organic compounds are produced in vast quantities, both naturally and by the chemical industry; have become distributed throughout the global environment; and have a wide range of adverse effects on human health and ecological health. The large role of anthropogenic X(S)VOCs in the environment requires further study of their sources, occurrence, fate, effects, and control, so that their beneficial uses can be compared systematically with their long-term impacts. Undesirable exposures to X(S)VOCs should be limited by adopting sustainable engineering practices that minimize new releases of these chemicals and control/remediation strategies that remove or contain existing contamination. To maximize the efficacy of these efforts, an integrated understanding is needed of the physico-chemical properties, biogeochemical cycling, health effects, and remediation of X(S)VOCs.
References
- M. MacLeod, T. Gouin and T. E. McKone, Modeling in environmental chemistry [Introduction to the themed issue on “Modeling in Environmental Chemistry”, ed. Matthew MacLeod, Todd Gouin and Thomas E. McKone], Environ. Sci.: Processes Impacts, 2018, 20, 10–11, 10.1039/c8em90001a.
- K. Fenner and P. G. Tratnyek, QSARs and computational chemistry methods in environmental chemical sciences [Introduction to the themed issue on “QSARs and computational chemistry methods in environmental chemical sciences”, ed. Paul G. Tratnyek and Kathrin Fenner], Environ. Sci.: Processes Impacts, 2017, 19, 185–187, 10.1039/c7em90008b.
- J. E. Lovelock, The electron-capture detector — A personal odyssey, in Journal of Chromatography Library, ed. A. Zlatkis and C. F. Poole, Elsevier, 1981, ch. 1, vol. 20, pp. 1–11, DOI:10.1016/s0301-4770(08)60125-6.
- M. J. Molina and F. S. Rowland, Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone, Nature, 1974, 249, 810–812, DOI:10.1038/249810a0.
- K. A. Read, A. S. Mahajan, L. J. Carpenter, M. J. Evans, B. V. E. Faria, D. E. Heard, J. R. Hopkins, J. D. Lee, S. J. Moller, A. C. Lewis, L. Mendes, J. B. McQuaid, H. Oetjen, A. Saiz-Lopez, M. J. Pilling and J. M. C. Plane, Extensive halogen-mediated ozone destruction over the tropical Atlantic Ocean, Nature, 2008, 453, 1232–1235, DOI:10.1038/nature07035.
- G. J. M. Velders, A. R. Ravishankara, M. K. Miller, M. J. Molina, J. Alcamo, J. S. Daniel, D. W. Fahey, S. A. Montzka and S. Reimann, Preserving Montreal Protocol Climate Benefits by Limiting HFCs, Science, 2012, 335, 922, DOI:10.1126/science.1216414.
- National Research Council, Alternatives for Managing the Nation’s Complex Contaminated Groundwater Sites, The National Academies Press, Washington, DC, 2013 DOI:10.17226/14668.
- M. Williams, L. Ingerman and L. McIlroy, Toxicological profile for 1,2,3-Trichloropropane: Draft for public comment, Center for Disease Control and Prevention, May 2019, https://stacks.cdc.gov/view/cdc/78828 Search PubMed.
- W. A. Chiu, J. Jinot, C. S. Scott, S. L. Makris, G. S. Cooper, R. C. Dzubow, A. S. Bale, M. V. Evans, K. Z. Guyton, N. Keshava, J. C. Lipscomb, S. Barone Jr, J. F. Fox, M. R. Gwinn, J. Schaum and J. C. Caldwell, Human health effects of trichloroethylene: key findings and scientific issues, Environ. Health Perspect., 2013, 121, 303–311, DOI:10.1289/ehp.1205879.
- D. Mackay, Finding fugacity feasible, Environ. Sci. Technol., 1979, 13, 1218–1223, DOI:10.1021/es60158a003.
- D. Leys, L. Adrian and H. Smidt, Organohalide respiration: microbes breathing chlorinated molecules [Introduction to the themed issue on “Organohalide respiration: Using halogenated hydrocarbons as the terminal electron acceptor”, ed. David Leys, Lorenz Adrian and Hauke Smidt], Philos. Trans. R. Soc., B, 2013, 368, 20120316, DOI:10.1098/rstb.2012.0316.
- A. Saiz-Lopez and R. von Glasow, Reactive halogen chemistry in the troposphere, Chem. Soc. Rev., 2012, 41, 6448–6472, 10.1039/c2cs35208g.
- C. A. Cooney, Epigenetic alterations due to trichloroethylene, in Trichloroethylene: Toxicity and Health Risks, ed. K. M. Gilbert and S. J. Blossom, Springer London, London, 2014, pp. 185–208 DOI:10.1007/978-1-4471-6311-4_10.
- WMO (World Meteorological Organization), Scientific Assessment of Ozone Depletion: 2018, Global Ozone Research and Monitoring Project Report No. 58, National Oceanic and Atmospheric Administration, Geneva, CH, 2018 Search PubMed.
- WMO (World Meteorological Organization), Scientific Assessment of Ozone Depletion: 2014, Global Ozone Research and Monitoring Project Report No. 55, National Oceanic and Atmospheric Administration, Geneva, CH, 2014 Search PubMed.
- T. M. Vogel, C. S. Criddle and P. L. McCarty, Transformations of halogenated aliphatic compounds, Environ. Sci. Technol., 1987, 21, 722–736, DOI:10.1021/es00162a001.
- N. Guha, D. Loomis, Y. Grosse, B. Lauby-Secretan, F. El Ghissassi, V. Bouvard, L. Benbrahim-Tallaa, R. Baan, H. Mattock and K. Straif, Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites, Lancet Oncol., 2012, 13, 1192–1193, DOI:10.1016/s1470-2045(12)70485-0.
- C. Siegel Scott and J. Jinot, Trichloroethylene and cancer: systematic and quantitative review of epidemiologic evidence for identifying hazards, Int. J. Environ. Res. Public Health, 2011, 8, 4238–4271 CrossRef PubMed.
- M. L. Maslia, M. M. Aral, P. Z. Ruckart and F. J. Bove, Reconstructing historical VOC concentrations in drinking water for epidemiological studies at a U.S. Military base: summary of results, Water, 2016, 8, 449 CrossRef PubMed.
- D. Dai, A. J. Prussin, L. C. Marr, P. J. Vikesland, M. A. Edwards and A. Pruden, Factors Shaping The Human Exposome In The Built Environment: Opportunities For Engineering Control, Environ. Sci. Technol., 2017, 51, 7759–7774, DOI:10.1021/acs.est.7b01097.
- R. Cernansky, It Came From Beneath: Detecting and Mitigating Vapor Intrusion, Environ. Health Perspect., 2016, 124, A141–A146, DOI:10.1289/ehp.124-a141.
- C. M. A. Eichler, E. A. Cohen Hubal and J. C. Little, Assessing Human Exposure to Chemicals in Materials, Products and Articles: The International Risk Management Landscape for Phthalates, Environ. Sci. Technol., 2019, 53, 13583–13597, DOI:10.1021/acs.est.9b03794.
- C. M. Villanueva, K. P. Cantor, J. O. Grimalt, G. Castaño-Vinyals, N. Malats, D. Silverman, A. Tardon, R. Garcia-Closas, C. Serra, A. Carrato, N. Rothman, F. X. Real, M. Dosemeci and M. Kogevinas, Assessment of lifetime exposure to trihalomethanes through different routes, Occup. Environ. Med., 2006, 63, 273, DOI:10.1136/oem.2005.023069.
| This journal is © The Royal Society of Chemistry 2020 |