How the 2010 Deepwater Horizon spill reshaped our understanding of crude oil photochemical weathering at sea: a past, present, and future perspective
Abstract
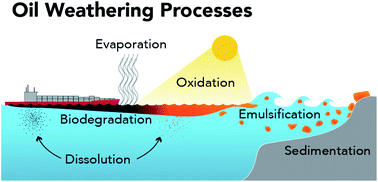
The weathering of crude oil at sea has been researched for nearly half a century. However, there have been relatively few opportunities to validate laboratory-based predictions about the rates, relative importance, and controls of oil weathering processes (e.g., evaporation, photo-oxidation, and emulsification) under natural field conditions. The 2010 Deepwater Horizon (DWH) spill in the Gulf of Mexico provided the oil spill science community with a unique opportunity to evaluate our laboratory-based predictions in nature. With a focus on photochemical weathering, we review what we knew prior to the DWH spill, what we learned from the DWH spill, and what priority gaps in knowledge remain. Three key findings from the DWH spill are discussed. First, the rate and extent of photochemical weathering was much greater for the floating surface oil than expected based on early conceptual models of oil weathering. Second, indirect photochemical processes played a major role in the partial oxidation of the floating surface oil. Third, the extensive and rapid changes to the physical and chemical properties of oil by sunlight may influence oil fate, transport, and the selection of response tools. This review also highlights findings and predictions about photochemical weathering of oil from several decades ago that appear to have escaped the broader scientific narrative and ultimately proved true for the DWH spill. By focusing on these early predictions and synthesizing the numerous findings from the DWH spill, we expect this review will better prepare the oil spill science community to respond to the next big spill in the ocean.
- This article is part of the themed collections: Environmental Science: Processes & Impacts Cover Art and Best Papers 2020 – Environmental Science: Processes & Impacts


 Please wait while we load your content...
Please wait while we load your content...